Abstract
Background
A large number patients struggle with migraine which is classified as a chronic disorder. The relative efficacy, safety and tolerability of prophylactic medications for migraine play a key role in managing this disease.
Methods
We conducted an extensive literature search for popular prophylactic medications that are used for migraine patients. Pairwise meta-analysis and network meta-analysis (NMA) were carried out sequentially for determining the relative efficacy, safety and tolerability of prophylactic medications. Summary effect for migraine headache days, headache frequency, at least 50% reduction in headache attacks, all-adverse events, nausea, somnolence, dizziness, withdrawal and withdrawal due to adverse events were produced by synthesizing both direct and indirect evidence.
Results
Patients with three interventions exhibited significantly less average migraine headache days compared with those treated by placebo (topiramate, propranolol, divalproex). Moreover, topiramate and valproate exhibited a significantly increased likelihood of at least 50% reduction in migraine headache attacks compared to placebo. Patients with topiramate and propranolol also exhibited significantly reduced headache frequency compared to those with placebo. On the other hand, patients with divalproex exhibited significantly higher risk of nausea compared to those with placebo, topiramate, propranolol, gabapentin and amitriptyline. Finally, divalproex was associated with an increased risk of withdrawal compared to placebo and propranolol.
Conclusions
Topiramate, propranolol and divalproex may be more efficacious than other prophylactic medications. Besides, the safety and tolerability of divalproex should be further verified by future studies.
Similar content being viewed by others
Background
Migraine is a chronic neurological disorder with high prevalence. Females appeared to have a higher morbidity of migraine than males in developed countries [1]. Although a relatively small number of migraine cases were reported in Asia, the morbidity of migraine attack in this region can reach up to 9.3% [2]. Throbbing headache is usually accompanied with migraine, resulting in both poor productivity and unstable emotional state [3, 4]. Migraine patients are often managed by medications which are convenient and efficient. However, side effects such as nausea and dizziness resulted from these medications have been observed in patients who exhibited poor level of tolerance [5].
Two types of medications have been introduced to patients: abortive and preventative medications [6]. The above two types of medications differ considerably in their mechanisms: abortive treatments attenuate symptoms arise from acute migraine attacks whereas preventative medications specifically aim at reducing attack severity and frequency. Although several prophylactic medications have been developed for migraine patients, no consensus has been reached with respect to their relative efficacy, safety and tolerability [7]. Furthermore, some medications appear to provide inadequate relief since they are not effective to all migraine patients [8]. As a result, some meta-analysis has been designed to compare the relative efficacy between different medications and some conclusions have been obtained in the current literature. For instance, patients treated by sodium valproate were associated with a lower risk of headache compared to the control group [9]. Furthermore, triptans and non-triptans appear to provide patients with different levels of relief [10].
Nevertheless, the current literature does not contain adequate studies that are able to identify the most preferable prophylactic medication for migraine patients and there is an increasing demand for discriminating the available medications with respect to their efficacy, safety and tolerability. For this purpose, we compared several preventative medications for migraine patients by using the approach of network meta-analysis (NMA) and we expect this approach can provide more insights for the selection of prophylactic medications.
Methods
Search strategy
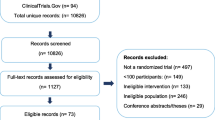
The medical literature for relevant studies in PubMed and EMBASE were systematically searched using electronic search strategies, and 1315 records were identified through searching the following key words, for example “migraine”, “topiramate”, “propranolol”, “gabapentin”, “amitriptyline”, “divalproex” and “valproate”. Two additional references were obtained from reviewers. As flow chart Fig. 1 illustrates, 556 duplicated records were identified and removed. Another 486 irrelevant studies were excluded from the remaining 761 records and a final 32 studies were subject to full-text review (Table 1).
Exclusion criteria
Articles were excluded in our study according to the following criteria: (1) the diagnose of migraine was not firmly confirmed in the study; (2) contain treatments that cannot form a closed network; (3) have no comparisons between different treatments; (4) contain outcomes without proper information; (5) does not have any relevant clinical outcomes or treatments; (6) studies without blinding procedures or studies with sample size less than 30; (7) non-randomized clinical trials such as reviews. A study was not considered to be eligible if any of the above criteria was fulfilled.
Outcome measures, data extraction and comparator network formation
We selected several clinical outcomes in order to measure the relative efficacy, safety and tolerability of prophylactic migraine medications: monthly migraine headache days, headache frequency, the percentages of patients with at least 50% reductions in migraine attacks (efficacy), the number of patients with all adverse events such as nausea, somnolence or dizziness (safety) and the number of patients who withdrew from studies (tolerability). The following data were extracted from eligible studies and shown in Table 1, including country of study, sample size, histology and clinical outcomes. The corresponding data were extracted into a database after two independent investigators reviewed the manuscripts of all the studies. A Jaded scale table was produced for the purpose of study quality assessment (Additional file 1: Table S1). After data extraction was performed for each study, a network plot with respect to each clinical outcome was produced for demonstrating direct and indirect comparisons (Figs. 2, 3 and 4).
Network plots of eligible comparisons of migraine intervention (monthly migraine headache days; headache frequency; ≥50% reduction in migraine headache attacks). A: Placebo; B: Topiramate; C: Propranolol; D: Gabapentin; E: Amitriptyline; F: Divalproex; G: Valproate. Direct comparisons were connected by solid lines whereas indirect comparisons were connected by dashed lines
Network plots of eligible comparisons for adverse events (all adverse events; nausea; somnolence; dizziness) A: Placebo; B: Topiramate; C: Propranolol; D: Gabapentin; E: Amitriptyline; F: Divalproex; G: Valproate. Direct comparisons were connected by solid lines whereas indirect comparisons were connected by dashed lines
Network plots of eligible comparisons for discontinued cases (all-cause withdrawal; withdrawal due to AEs). A: Placebo; B: Topiramate; C: Propranolol; D: Gabapentin; E: Amitriptyline; F: Divalproex; G: Valproate. Direct comparisons were connected by solid lines whereas indirect comparisons were connected by dashed lines
Statistical analysis
We implemented a two-step approach in our system review and evidence synthesis. Firstly, a conventional pairwise meta-analysis was carried out in order to pool all direct evidence in the current literature. For continuous outcomes such as monthly migraine headache days and headache frequency, raw mean differences (MD) between two groups were synthesized and a summary effect was produced based on raw mean differences, sample size and sample standard deviation. We selected raw mean differences for evidence synthesis since all eligible studies reported the above continuous outcomes in the same scale. On the other hand, the statistic of odds ratio (OR) was pooled from each eligible study and a summary OR was produced for each binary outcome. The pairwise meta-analysis was implemented based on the random-effects model because we did not have full knowledge of study participants or implementation for each eligible study [11].
The second step in our study involves conducting a NMA by synthesize both direct and indirect evidence. Similar to the pairwise meta-analysis, summary mean differences and summary ORs were produced using the Bayesian framework and the Markov Chain Monte Carlo (MCMC) sampling technique. The corresponding ranking of each intervention was obtained by using the surface under the cumulative ranking curve (SUCRA). If an intervention exhibited a higher SUCRA value compared to other interventions, then it is potentially more preferable than others with respect to an endpoint. After that, the assumption of consistency between direct and indirect evidence was assessed by using the node-splitting method whereas publication bias was visually inspected by using funnel plots [12, 13].
Results
Description of included studies
The prescribed searching strategy and exclusion criteria enabled us to identify and include 32 studies with a total number of 6052 subjects (Table 1) [14–45]. All included studies were carried out by using single or double blinding procedures. These studies were carried out between 1986 and 2015 with a maximum following-up duration of 1 year. The majority of the included studies belonged to typical randomized controlled trials (RCTs), however, we identified and included five crossover RCTs in which participants were randomized to receive a sequence of interventions.
Pairwise comparison using conventional meta-analysis
A total of ten direct comparisons with respect to each endpoint were produced by using pairwise meta-analysis (Table 2). Patients with topiramate exhibited significantly less average headache days, less headache frequency, a higher likelihood of at least 50% reduction compared to those with placebo (migraine headache days: −0.28, 95% CI = −0.53 to −0.03; headache frequency: −0.31, 95% CI = −0.45 to −0.17; ≥ 50% reduction: OR = 2.33, 95% CI = 1.58–3.42). However, patients with topiramate appeared to have significantly higher risk of all-adverse events and withdrawal due to adverse events compared to those with placebo (all-adverse events: OR = 1.35, 95% CI = 1.06–1.73, withdrawal due to adverse events: OR = 2.08, 95% CI = 1.56–2.78). Patients with propranolol exhibited a significantly less average headache days but higher risk of all-adverse events, somnolence and withdrawal due to adverse events compared to those with placebo (migraine headache days: −0.29, 95% CI = −0.49 to −0.09; all-adverse events: OR = 2.02, 95% CI = 1.05–4.08, somnolence: OR = 4.33, 95% CI = 1.21 to 15.53, withdrawal due to adverse events: OR = 1.87, 95% CI = 1.09 to 3.09). Although there is no significant differences in the average migraine days, headache frequency or the likelihood of at least 50% reduction in headache attacks between patients with gabapentin and those with placebo, gabapentin appeared to be associated with an increased risk of somnolence and dizziness (somnolence: OR = 2.23, 95% CI = 1.11 to 4.46; dizziness: OR = 3.13, 95% CI = 1.73 to 5.56). Patients treated with amitriptyline or divalproex exhibited a reduced headache days or headache frequency as well as a better performance in at least 50% reduction in headache attacks compared to those with placebo (amitriptyline: headache frequency: −0.36, 95% CI = −0.62 to −0.10; ≥ 50% reduction: OR = 1.81, 95% CI = 1.03–3.20; divalproex: migraine headache days: −0.40, 95% CI = −0.61 to −0.18; ≥ 50% reduction: OR = 4.27, 95% CI = 1.30–13.99), however, this was offset by an increased risk of all-adverse events or nausea (amitriptyline: all-adverse events: OR = 2.20, 95% CI = 1.04–4.66; divalproex: nausea: OR = 2.23, 95% CI = 1.21–4.10). Besides that, we were not able to identify any significant results between direct comparisons produced by conventional meta-analysis. Besides, propranolol was safer comparing to topiramate (all-adverse events: OR = 0.57, 95% CI = 0.36–0.90; withdrawal: OR = 0.66, 95% CI = 0.44–0.99; withdrawal due to adverse events: OR = 0.58, 95% CI = 0.37–0.91).
Including both direct and indirect evidence in the NMA
Results produced by NMA are displayed in Table 3 which determined the relative efficacy, safety and tolerability of prophylactic migraine interventions by using both direct and indirect evidence. Patients with three interventions exhibited significantly less average migraine headache days compared with those treated by placebo (topiramate: −1.20, 95% CrI = −1.83 to −0.70; propranolol: −0.98, 95% CrI = −1.86 to −0.07; divalproex: −1.28, 95% CrI = −2.44 to −0.27; Table 3, Fig. 5). Moreover, patients with topiramate and valproate exhibited a significantly increased likelihood of at least 50% reduction in migraine headache attacks compared to those with placebo (topiramate: OR = 4.28, 95% CrI = 1.35 to 14.70; valproate: 11.38, 95% CrI = 1.31 to 111.11; Table 3, Fig. 5). Patients with topiramate or propranolol also exhibited significantly reduced headache frequency compared to those with placebo (topiramate: −1.17, 95% CrI = −1.98 to −0.35; propranolol: −1.37, 95% CrI = −2.49 to −0.29; Table 3, Fig. 5).
Our NMA also provides results for the relative safety of migraine interventions. Patients with Divalproex exhibited significantly higher risk of nausea compared to those with placebo, topiramate, propranolol, gabapentin and amitriptyline (all ORs > 1). Furthermore, patients with amitriptyline exhibited a significantly elevated risk of all-adverse events compared to those with propranolol or placebo (all ORs > 1). However, patients with amitriptyline exhibited a significantly reduced risk of all-adverse events compared to those with divalproex (OR = 0.24, 95% CrI = 0.07 to 0.85). Patients with topiramate also exhibited significantly higher risk of all adverse events compared to those with placebo (OR = 2.44, 95% CrI = 1.55 to 3.88; Table 3, Fig. 6). Our NMA also discovered that patients with GABAPENTIN were associated with a significantly increase in the risk of dizziness in comparison to those received placebo (OR = 3.69, 95% CrI = 1.17 to 9.63; Table 3, Fig. 6). The relative tolerability of various migraine interventions were assessed by using the endpoints of all-cause withdrawal and withdrawal due to adverse events. As suggested by Table 3 and Fig. 7, patients with divalproex exhibited a significantly increased risk of all-case withdrawal compared to those with propranolol or placebo (propranolol: OR = 2.09, 95% CrI = 1.11 to 4.52; placebo: OR = 1.68, 95% CrI = 1.14 to 3.67). On the other hand, patients treated with topiramate or divalproex were associated with an increased risk of withdrawals due to adverse events compared to those with placebo (topiramate: OR = 2.33, 95% CrI = 1.55 to 3.45; divalproex: OR = 2.25, 95% CrI = 1.01 to 5.49).
Ranking of migraine interventions using SUCRA values
A ranking plot with respect to each endpoint was produced and a SUCRA table was created in order to differentiate the above migraine interventions (Figs. 8, 9 and 10, Additional file 2: Table S2). Divalproex appeared to have the largest SUCRA value with respect to migraine headache days. Propranolol, topiramate and gabapentin exhibited the largest three SUCRA values with respect to headache frequency. Moreover, valproate, topiramate and divalproex were more preferable than other interventions with respect to the endpoint of at least 50% reduction in migraine attacks. A similar ranking scheme was produced for the above interventions with respect to their safety and tolerability (Additional file 2: Table S2). We also conducted a cluster analysis for grouping the above prophylactic migraine interventions based on the SUCRA values of two endpoints. Overall, propranolol seemed to be the most desirable intervention when several endpoints were simultaneously considered (Fig. 11).
Assessing consistency between direct and indirect evidence
Since we implemented a consistency model when conducting the NMA, the node-splitting method was used to assess the validity of this assumption (Figs. 12, 13 and 14). Net heat plots were also produced by software in order to visualize the consistency pattern existed in each comparison (Figs. 15, 16 and 17). As suggested by both the node splitting method (P-value > 0.05) and net heat plots, there is no significant inconsistency between direct and indirect evidence for the majority of comparisons. Therefore, we concluded that the consistency model is valid in our NMA.
Net heat plot of study designs with respect to monthly migraine headache days and headache frequency. The area of the gray squares displays the contribution of the direct estimate in design d (shown in the column) to the network estimate in design d (shown in the row). The colors are associated with the change in inconsistency between direct and indirect evidence (shown in the row) after detaching the effect (shown in the column). Blue colors indicate an increase and warm colors indicate a decrease (the stronger the intensity of the color, the stronger the change)
Assessing publication bias using funnel plots
Potential publication bias contained in our NMA was evaluated by using the funnel plots produced by software (Additional file 3: Figures S1, Additional file 4: Figure S2 and Additional file 5: Figure S3). As suggested by the funnel plots, there is no significant asymmetry pattern and most studies were evenly distributed in the funnel plot. Therefore, we concluded that there is no significant publication bias in our study.
Discussion
Migraine is a chronic disabling disease accompanied with recurrent headache. Patients with migraine often suffer from throbbing headache and preventative therapies have been introduced to reduce the risk of migraine onset. Several medications have been applied to migraine patients as prophylaxis and most of these medications are able to reduce the monthly attack frequency by 50% [46]. We conducted an extensive literature review and NMA in order to determine the relative efficacy, safety and tolerability of six popular prophylactic migraine interventions: topiramate, propranolol, gabapentin, amitriptyline, divalproex and valproate.
Results of our NMA indicated that three interventions may be particularly efficacious for reducing the corresponding symptoms of migraine: divalproex, propranolol and valproate. In our study, divalproex ranked the highest with respect to the reduction of monthly headache days whereas propranolol appeared to be the most preferable intervention for reducing headache frequency. Moreover, our study also suggested that valproate exhibited superior performance with respect to at least 50% reduction in headache attacks. Accordingly to the American Academy of Neurology (AAN) and the American Society of Headache (AHS), divalproex is classified as level-A medication and it is offered to patients for migraine prophylaxis [47]. Another study conducted by Kaniecki et al. revealed that both divalproex and propranolol significantly reduced headache frequency and the number of headache days compared to placebo, however, there was no significant difference in the efficacy between the two interventions [48]. The above conclusions were verified by our NMA which did not suggest any significant difference in the efficacy between divalproex and propranolol. As suggested by AAN and AHS, valproate is also classified as level-A medication that should be offered to migraine patients [49]. The efficacy of valproate in reducing migraine attacks has been verified by several studies, for instance, Sørensen et al. was the first one who suggested that valproate exhibited a noteworthy effect on patients with severe migraine with respect to migraine prophylaxis [50]. Although our study suggested that patients with valproate were more likely to experience at least 50% reduction in migraine attacks than those with placebo, the wide confidence interval resulted from potential inconsistency or inadequate evidence should be addressed by conducting large-scale studies in order to verify the above conclusions.
Apart from efficacy, the safety of migraine medication is another predominating factor that must be considered by physicians when selecting an appropriate intervention. As suggested by previous studies, migraine patients treated by antiepileptic drugs may experience several side-effects, including nausea, dizziness and paresthesia [51]. One significant result produced by our NMA is that patients with divalproex exhibited a significantly increased risk of nausea compared to those with placebo or other interventions. This result was confirmed by our SUCRA ranking tables in which the SUCRA value of divalproex appeared to be the lowest with respect to the endpoint of nausea. Apart from that, our pairwise meta-analysis discovered that patients with divalproex were associated with a significantly increased risk of nausea compared to those with placebo. Furthermore, our NMA revealed that patients with divalproex may have poor medication compliance since they appeared to have an increased risk of withdrawal. We urged future researchers to design and conducted prospective studies in order to confirm the safety of divalproex.
Despite that some new findings have been suggested by our study, it is essential to discuss several key issues that may have impact on our conclusions. Firstly, we include both RCTs and crossover studies in our NMA; this may significantly increase the heterogeneity resulted from the design and implantation of different studies. For instance, crossover studies involves randomly assigning a sequence of interventions to different groups over the study period, therefore, the randomization technique used in crossover studies was completely different from that in RCTs. However, the inclusion of crossover studies did not enable us to adjust for the corresponding sequences where a serious of treatments was assigned. Furthermore, the inclusion of crossover studies produced some extra confounding factors that were not presented in RCTs. For instance, a wash-out period between interventions is often used in crossover studies and the duration of the wash-out period may have significant impact on medication compliance as well as on the corresponding endpoints. Our NMA did not adjust for the corresponding dose used for each intervention either. The above uncontrolled factors may independently affect our conclusion or interacted with each other, producing significant effect modification. Nevertheless, the corresponding conclusions and limitations underlying our study provide researchers with key guidelines for designing new trials or prospective studies for migraine patients.
Conclusions
Our NMA suggested that topiramate, propranolol and divalproex may be more efficacious than other prophylactic medications. However, based on the above limitations, our results need to be interpreted with caution. Besides, safety and tolerability of divalproex should be further verified by future studies.
References
Eyre HA, Air T, Pradhan A, Johnston J, Lavretsky H, Stuart MJ et al (2016) A meta-analysis of chemokines in major depression. Prog Neuropsychopharmacol Biol Psychiatry 68:1–8
Koenig J, Kemp AH, Feeling NR, Thayer JF, Kaess M (2016) Resting state vagal tone in borderline personality disorder: A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 64:18–26
Ren J, Li H, Palaniyappan L, Liu H, Wang J, Li C et al (2014) Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 51:181–189
Polesel DN, Fukushiro DF, Andersen ML, Nozoe KT, Mari-Kawamoto E, Saito LP et al (2014) Anxiety-like effects of meta-chlorophenylpiperazine in paradoxically sleep-deprived mice. Prog Neuropsychopharmacol Biol Psychiatry 49:70–77
Na KS, Lee KJ, Lee JS, Cho YS, Jung HY (2014) Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 48:79–85
Wang K, Song LL, Cheung EF, Lui SS, Shum DH, Chan RC (2013) Bipolar disorder and schizophrenia share a similar deficit in semantic inhibition: a meta-analysis based on Hayling Sentence Completion Test performance. Prog Neuropsychopharmacol Biol Psychiatry 46:153–160
Wu YL, Ding XX, Sun YH, Yang HY, Chen J, Zhao X et al. (2013) Association between MTHFR C677T polymorphism and depression: An updated meta-analysis of 26 studies. Prog Neuropsychopharmacol Biol Psychiatry 46:78–85
Schild AH, Pietschnig J, Tran US, Voracek M (2013) Genetic association studies between SNPs and suicidal behavior: a meta-analytical field synopsis. Prog Neuropsychopharmacol Biol Psychiatry 46:36–42
Niitsu T, Fabbri C, Bentini F, Serretti A (2013) Pharmacogenetics in major depression: a comprehensive meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 45:183–194
Yao L, Lui S, Liao Y, Du MY, Hu N, Thomas JA et al. (2013) White matter deficits in first episode schizophrenia: an activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 45:100–106
Bartley CA, Hay M, Bloch MH (2013) Meta-analysis: aerobic exercise for the treatment of anxiety disorders. Prog Neuropsychopharmacol Biol Psychiatry 45:34–39
Du MY, Wu QZ, Yue Q, Li J, Liao Y, Kuang WH et al. (2012) Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 36(1):11–16
Vederine FE, Wessa M, Leboyer M, Houenou J (2011) A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 35(8):1820–1826
Mikkelsen B, Pedersen KK, Christiansen LV (1986) Prophylactic treatment of migraine with tolfenamic acid, propranolol and placebo. Acta Neurol Scand 73(4):423–427
Pradalier A, Serratrice G, Collard M, Hirsch E, Feve J, Masson M et al. (1989) Long-acting propranolol in migraine prophylaxis: results of a double-blind, placebo-controlled study. Cephalalgia 9(4):247–253
Hering R, Kuritzky A (1992) Sodium valproate in the prophylactic treatment of migraine: a double-blind study versus placebo. Cephalalgia 12(2):81–84
Mathew NT, Saper JR, Silberstein SD, Rankin L, Markley HG, Solomon S et al. (1995) Migraine prophylaxis with divalproex. Arch Neurol 52(3):281–286
Bendtsen L, Jensen R, Olesen J (1996) A non-selective (amitriptyline), but not a selective (citalopram), serotonin reuptake inhibitor is effective in the prophylactic treatment of chronic tension-type headache. J Neurol Neurosurg Psychiatry 61(3):285–290
Diener HC, Foh M, Iaccarino C, Wessely P, Isler H, Strenge H et al. (1996) Cyclandelate in the prophylaxis of migraine: a randomized, parallel, double-blind study in comparison with placebo and propranolol. The Study group. Cephalalgia 16(6):441–447
Kaniecki RG (1997) A comparison of divalproex with propranolol and placebo for the prophylaxis of migraine without aura. Arch Neurol 54(9):1141–1145
Klapper J (1997) Divalproex sodium in migraine prophylaxis: a dose-controlled study. Cephalalgia 17(2):103–108
Mathew NT, Rapoport A, Saper J, Magnus L, Klapper J, Ramadan N et al (2001) Efficacy of gabapentin in migraine prophylaxis. Headache 41(2):119–128
Storey JR, Calder CS, Hart DE, Potter DL (2001) Topiramate in migraine prevention: a double-blind, placebo-controlled study. Headache 41(10):968–975
Freitag FG, Collins SD, Carlson HA, Goldstein J, Saper J, Silberstein S et al. (2002) A randomized trial of divalproex sodium extended-release tablets in migraine prophylaxis. Neurology 58(11):1652–1659
Brandes JL, Saper JR, Diamond M, Couch JR, Lewis DW, Schmitt J et al. (2004) Topiramate for migraine prevention: a randomized controlled trial. JAMA 291(8):965–973
Diener HC, Tfelt-Hansen P, Dahlöf C, Láinez MJA, Sandrini G, Wang SJ et al. (2004) Topiramate in migraine prophylaxis: Results from a placebo-controlled trial with propranolol as an active control. J Neurol 251(8):943–950
Mei D, Capuano A, Vollono C, Evangelista M, Ferraro D, Tonali P et al. (2004) Topiramate in migraine prophylaxis: a randomised double-blind versus placebo study. Neurol Sci 25(5):245–250
Silberstein SD, Neto W, Schmitt J, Jacobs D (2004) Topiramate in migraine prevention: results of a large controlled trial. Arch Neurol 61(4):490–495
Brandes JL, Kudrow DB, Rothrock JF, Rupnow MF, Fairclough DL, Greenberg SJ (2006) Assessing the ability of topiramate to improve the daily activities of patients with migraine. Mayo Clin Proc 81(10):1311–1319
Shaygannejad V, Janghorbani M, Ghorbani A, Ashtary F, Zakizade N, Nasr V (2006) Comparison of the effect of topiramate and sodium valporate in migraine prevention: a randomized blinded crossover study. Headache 46(4):642–648
Silberstein SD, Hulihan J, Karim MR, Wu SC, Jordan D, Karvois D et al (2006) Efficacy and tolerability of topiramate 200 mg/d in the prevention of migraine with/without aura in adults: a randomized, placebo-controlled, double-blind, 12-week pilot study. Clin Ther 28(7):1002–1011
de Tommaso M, Marinazzo D, Nitti L, Pellicoro M, Guido M, Serpino C et al. (2007) Effects of levetiracetam vs topiramate and placebo on visually evoked phase synchronization changes of alpha rhythm in migraine. Clin Neurophysiol 118(10):2297–2304
Diener HC, Bussone G, Van Oene JC, Lahaye M, Schwalen S, Goadsby PJ (2007) Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia 27(7):814–823
Diener HC, Kurth T, Dodick D (2007) Patent foramen ovale and migraine. Curr Pain Headache Rep 11(3):236–240
Gupta P, Singh S, Goyal V, Shukla G, Behari M (2007) Low-dose topiramate versus lamotrigine in migraine prophylaxis (the Lotolamp study). Headache 47(3):402–412
Silberstein SD, Lipton RB, Dodick DW, Freitag FG, Ramadan N, Mathew N et al. (2007) Efficacy and safety of topiramate for the treatment of chronic migraine: a randomized, double-blind, placebo-controlled trial. Headache 47(2):170–180
Ashtari F, Shaygannejad V, Akbari M (2008) A double-blind, randomized trial of low-dose topiramate vs propranolol in migraine prophylaxis. Acta Neurol Scand 118(5):301–305
Dodick DW, Freitag F, Banks J, Saper J, Xiang J, Rupnow M et al. (2009) Topiramate versus amitriptyline in migraine prevention: a 26-week, multicenter, randomized, double-blind, double-dummy, parallel-group noninferiority trial in adult migraineurs. Clin Ther 31(3):542–559
Holroyd KA, Cottrell CK, O’Donnell FJ, Cordingley GE, Drew JB, Carlson BW et al. (2010) Effect of preventive (β blocker) treatment, behavioural migraine management, or their combination on outcomes of optimised acute treatment in frequent migraine: Randomised controlled trial. BMJ 341(7776):769
Lipton RB, Silberstein S, Dodick D, Cady R, Freitag F, Mathew N et al. (2011) Topiramate intervention to prevent transformation of episodic migraine: the topiramate INTREPID study. Cephalalgia 31(1):18–30
Afshari D, Rafizadeh S, Rezaei M (2012) A comparative study of the effects of low-dose topiramate versus sodium valproate in migraine prophylaxis. Int J Neurosci 122(2):60–68
Silberstein S, Goode-Sellers S, Twomey C, Saiers J, Ascher J (2013) Randomized, double-blind, placebo-controlled, phase II trial of gabapentin enacarbil for migraine prophylaxis. Cephalalgia 33(2):101–111
Nofal WH, Mahmoud MS, Al Alim AA (2014) Does preoperative gabapentin affects the characteristics of post-dural puncture headache in parturients undergoing cesarean section with spinal anesthesia? Saudi J Anaesth 8(3):359–363
Sarchielli P, Messina P, Cupini LM, Tedeschi G, Di Piero V, Livrea P et al. (2014) Sodium valproate in migraine without aura and medication overuse headache: a randomized controlled trial. Eur Neuropsychopharmacol 24(8):1289–1297
Sadeghian H, Motiei-Langroudi R (2015) Comparison of Levetiracetam and sodium Valproate in migraine prophylaxis: A randomized placebo-controlled study. Ann Indian Acad Neurol 18(1):45–48
Trzesniak C, Kempton MJ, Busatto GF, de Oliveira IR, Galvao-de Almeida A, Kambeitz J et al. (2011) Adhesio interthalamica alterations in schizophrenia spectrum disorders: A systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 35(4):877–886
Farrer TJ, Hedges DW (2011) Prevalence of traumatic brain injury in incarcerated groups compared to the general population: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 35(2):390–394
Woon FL, Sood S, Hedges DW (2010) Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 34(7):1181–1188
Zai CC, Tiwari AK, Basile V, de Luca V, Muller DJ, Voineskos AN et al. (2010) Oxidative stress in tardive dyskinesia: genetic association study and meta-analysis of NADPH quinine oxidoreductase 1 (NQO1) and Superoxide dismutase 2 (SOD2, MnSOD) genes. Prog Neuropsychopharmacol Biol Psychiatry 34(1):50–56
Rahimi R, Nikfar S, Abdollahi M (2009) Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 33(1):118–127
Lin PY (2007) Meta-analysis of the association of serotonin transporter gene polymorphism with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 31(3):683–689
Funding
None.
Authors’ contributions
AH, DS, and CL initiated the concept and design of the study; collected data; prepared the manuscript; and finalized it according to the recommendations. AH, DS, and LZ initiated the study design, analyzed and interpreted the data. AH, DS, and LZ acquired the data and tested them for accuracy and integrity and interpreted the data. All authors read and approved the final manuscript.
Competing interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1:Table S1.
Jadad scale of 32 studies included. (DOCX 15 kb)
Additional file 2: Table S2.
Ranking of migraine interventions using SUCRA values. (DOCX 17 kb)
Additional file 3: Figure S1.
Funnel plot of studies assessing the efficacy of migraine interventions. (EPS 1495 kb)
Additional file 4: Figure S2.
Funnel plot of studies assessing the safety of migraine interventions. (EPS 1629 kb)
Additional file 5: Figure S3.
Funnel plot of studies assessing the tolerability of migraine interventions. (EPS 1394 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
He, A., Song, D., Zhang, L. et al. Unveiling the relative efficacy, safety and tolerability of prophylactic medications for migraine: pairwise and network-meta analysis. J Headache Pain 18, 26 (2017). https://doi.org/10.1186/s10194-017-0720-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-017-0720-7