Abstract
Background
Activation of the trigeminal-autonomic reflex, involving the trigeminal ganglion, the superior salivatory nucleus and the sphenopalatine ganglion (SPG) is crucial in the pathophysiology of cluster headache (CH). Since pituitary adenylate cyclase-activating polypeptide-38 (PACAP-38) is present both in the SPG and the trigeminal ganglion (TG) and its role in migraine has been described, our aim was to determine the plasma PACAP-38 levels in different phases of episodic CH (ECH).
Peripheral cubital fossa blood samples were taken during the ictal and inter-bout periods of male ECH patients and from age-matched healthy controls (n = 9). Plasma PACAP-38-like immunoreactivity (LI) was measured with specific and sensitive radioimmunoassay.
Findings
Significantly lower plasma PACAP-38-LI was detected in the inter-bout period of ECH patients than in healthy controls. However, PACAP-38 was significantly elevated in the plasma during CH attacks as compared to the inter-bout phase in the same subjects (n = 5).
Conclusions
This exploratory study suggests that PACAP-38 may be released during the attacks of ECH. Further patients and long-term follow-up are necessary to reveal its function.
Similar content being viewed by others
Background
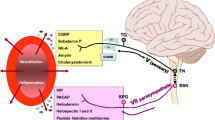
Cluster headache (CH) is a primary, trigeminal-autonomic headache disorder, manifesting in periodically occurring unilateral (supra/orbital, temporal) severe pain attacks in association with intense ipsilateral autonomic symptoms. The trigeminal-autonomic reflex (TAR) and the hypothalamic system (HS) are thought to play important roles in the mechanism of CH [1]. The significance of TAR is based on the structural and functional connections between the trigeminal ganglion (TG), the superior salivatory nucleus being the parasympathetic nucleus of the facial nerve, the sphenopalatine ganglion (SPG) and otic ganglion (OG). CH attacks involve activation of the TG and the SPG resulting in release of neuroactive neuropeptides such as calcitonin gene-related peptide (CGRP) and vasoactive intestinal polypeptide (VIP) [2]. These molecules reach the cranial vessels, the lacrimal glands and the nasal mucosa via the postganglionic fibers, leading to conjunctival injection, eyelid and nasal edema, and rhinorrhea. The circadian and circannual periodicity of the attacks is attributed to the activation of the HS.
Neurochemical studies have revealed that the VIP-related neuropeptide pituitary adenylate cyclase-activating polypeptide-38 (PACAP-38) co-localize [3] in the SPG and the OG [4], however some PACAP-38-immunoreactivity was also seen in the TG [5, 6]. It has recently been described that PACAP-38 mediates the activation of the trigeminovascular system (TS), and exerts modulatory function in the sensitization process and migraine headache [7–10]. Human studies have confirmed that intravenously administered PACAP-38 induces migraine-like headache with marked vascular effects in migraine patients without aura [11, 12], and that PACAP-38 concentrations in the systemic circulation are significantly altered depending on the phases of migraine headache [13, 14].
Therefore, PACAP-38 is likely to be an important factor in migraine pathophysiology; however, there is less evidence concerning its function in other primary headache disorders such as CH. It is suggested that PACAP-38 may be involved in the evolution of trigeminal-autonomic cephalalgias, since this peptide was presented in the TS [8, 9, 15, 16], and also in the SPG and OG [17, 18] of humans and rats. Moreover there is a clear increase of both trigeminal (CGRP) and parasympathetic (VIP) neuropeptides during CH attacks which is normalized when the pain was controlled in CH [19, 20], and it appears that activation of the VPAC1 and PAC1 receptors influences the TAR, the parasympathetic cranial outflow [17, 21]. Based on these data, our aim was to determine if CH patients are associated with alterations in plasma PACAP-38-like immunoreactivity (LI) in the inter-bout period and during the attack in patients suffering from ECH in comparison with age- and gender-matched healthy volunteers.
Materials and methods
Participants
Nine male ECH patients treated in our outpatient clinic (without other known neurological diseases) were selected in accordance with the criteria of the Headache Classification Committee of the International Headache Society. Nine age-matched male healthy volunteers screened for non-reported/non-treated headaches served as controls (age of healthy control subjects: 40.1 ± 12.0 years old, age of EHC patients: 40.3 ± 9.6 years old). Coexisting depression in patients was ruled out by Hamilton’s depression scale. A detailed questionnaire was used to assess the features of the headache disorder. The clinical and demographic data are summarized in Table 1.
Study design sampling procedures
The study was approved by the Ethics Committee of the Faculty of Medicine, University of Szeged (87/2009). All participants gave their written informed consent in accordance with the Declaration of Helsinki. There were no restrictions regarding food and drink intake. The study was performed between 2010 and 2012 years. Blood samples (6 ml per subject) were taken from the cubital vein in the routinely used clinical blood sampling plastic tubes containing 12 mg ethylenediaminetetraacetic acid (BD Vacutainer, Becton Dickinson Hungary Plc., Hungary) and 1200 IU peptidase inhibitor, aprotinin (Gordox, Richter Gedeon Plc., Hungary). A single blood sample was taken in supine position after a rest of 10 min from the controls, whereas samples were taken from ECH patients during the acute spontaneous attack (when they produced typical symptoms of CH [22]) and/or in the absolute attack-free, inter-bout period (out of the cluster episode—by more than a month) between 8 a.m. and 4 p.m. Samples from both periods (during attack and outside attack) could be obtained in 5 cases (it was unintentional selection), which could be used in self-controlled comparison. Patients were asked not to start their usual treatment before sampling even during the ictal sampling. Exact date and time of blood samplings are presented in the Table 2.
Analysis of PACAP-38 in plasma and data analysis
Blood collection, centrifugation, storage and PACAP-38 measurement were carried out with specific and sensitive radioimmunoassay (RIA). Briefly, following centrifugation of the plasma samples (2000 rpm at +4 °C for 10 min), the peptide was extracted from the plasma into three volumes of absolute alcohol. After precipitation and a second centrifugation, the samples were dried under a nitrogen flow and resuspended in 300 μl of assay buffer before RIA determination. The assay was prepared in 1 mL 0.05 mol/L (pH 7.4) phosphate buffer containing 0.1 mol/L sodium chloride, 0.25 % (w/v) bovine serum albumin and 0.05 % (w/v) sodium azide. The tracer mono-125I-labeled PACAP24–38 (5000 cpm/tube, 124 Bq/1.72 fmol) was prepared in our laboratory. Ovine PACAP38 was used as a RIA standard ranging from 0 to 1000 fmol/mL. The PACAP-38 antiserum (88111-3, dilution 1:10000 obtained from Abcam UK), the RIA tracer and the standard or unknown samples were measured into polypropylene tubes with the assay buffer. After incubation for 48–72 h at +4 °C, the antibody-bound peptide was separated from the free peptide by the addition of separating solution. Following centrifugation (3000 rpm at +4 °C for 20 min), the contents of the tubes were gently decanted and the radioactivity of the precipitates was measured in a gamma counter (Gamma, type: NZ310). The PACAP-38 concentrations of the unknown samples were read from calibration curves.
Statistical analysis
Data are expressed as median, interquartile range (IQR), minimum and maximum or individual values on the graphs. The distributions of data populations were checked with the Shapiro-Wilk normality test and the Levene’s test for analysis of the equality of variances was also applied.
Groups were compared with two sample unpaired and paired t-tests where appropriate via Monte-Carlo permutation (with 10000 random permutations) due to the small number of subjects, the unequal variances and the non-Gaussian distribution of the data of the control group. Correlations were revealed by the Pearson’s test. Statistical analyses were performed using the R software (R Development Core Team, 2002). Significance was accepted at p < 0.05.
Findings
Differences in plasma PACAP-38-LI between ECH patients and healthy controls
The level of plasma PACAP-38-LI in the inter-bout phase of ECH patients was significantly lower (n = 9; median = 24.4 fmol/ml, IQR = 2.68) than in age and gender matched healthy volunteers (n = 9; median = 30.5 fmol/ml, IQR = 8.84; p < 0.026).
Plasma samples from patients during an attack in the cluster episode exhibited higher PACAP-38 concentrations (n = 5; median = 28.8 fmol/ml, IQR = 3.40) as compared to inter-bout samples (n = 9; median = 24.4 fmol/ml, IQR = 2.68), but these groups were not statistically compared because of the partly dependent data between the self-controlled, paired data (n = 5) and the other inter-bout data (n = 4). No difference was found when ictal samples (n = 5; median = 28.8 fmol/ml, IQR = 3.40) were compared to healthy controls (n = 9; median = 30.5 fmol/ml, IQR = 8.84; p < 0.965) (Fig. 1/a). When we compared only those subjects that delivered plasma samples collected from both periods, a significant elevation was detected in ictal compared to the corresponding inter-bout samples in a paired comparison as well (n = 5; median = 24.4 fmol/ml, IQR = 5.20; p < 0.032). As seen in the illustration four patients showed a clear increase from inter-bout to ictal sampling and only one patient was with unchanged value of PACAP-38 (Fig. 1/b).
Positive correlation between inter-bout plasma PACAP-38 concentration and the age of patients
A positive correlation was revealed between the age of ECH patients and the inter-bout plasma PACAP-38-LI (n = 9; p < 0.026, r = 0.728) (Fig. 2/b). No correlation was found between the age of healthy controls and their plasma PACAP-38-LI (n = 9; p < 0.520, r = 0.248) (Fig. 2/a). There were no correlations between PACAP-38-LI and the clinical parameters of ECH patients (data not shown).
Discussion
This is the first study investigating alterations in plasma PACAP-38 concentration in CH patients; however limitation of our study is the sample size of ECH patients, especially the sample size of ictal blood samples. Our results agree with observations in plasma samples from migraine patients, obtained in the cubital fossa [11, 13] or the external jugular vein [14].
The source of PACAP-38 is unclear but two sites are to be discussed, the parasympathetic ganglia SPG and OT, and the TG, because neurons in ether contain PACAP-38. It is difficult to separate these sources of circulation PACAP-38, because they are intermingled. The stimulation of the superior sagittal sinus elicits release of both CGRP from the TG and VIP from the SPG/OT ganglion and the destruction of the trigeminal nerve aborts their release [10]. This shows interplay between the sensory and parasympathetic systems. It has been revealed that PACAP-38 may induce migraine-like attacks while VIP does not [11, 21, 23]. The role of PACAP-38 seems to differ from VIP, but there are similarities concerning theirs effects in the peripheral cutaneous nociception, which may be mediated by VPAC receptors [24].
Detailed study on distribution revealed that PACAP co-localize with CGRP in dura mater sensory fibers. The supply of parasympathetic innervation of the dura is scant as compared to that of the cerebral circulation [25]. Thus, it is possible that in both migraine and in CH the PACAP-38 release emanates mainly from the trigeminal system.
Our speculation is that the formation of PACAP-38 is depleted during the inter-bout period in certain nerve structures, which results in lower plasma PACAP-38 level outside the attacks of CH. Subsequently the chronically reduced peptide level may be a trigger for sensitization and altered nociception. During the evolution of nociception the concentration of PACAP-38 can increase and it exerts vasodilation, which is an essential trigger in conjunction with other unknown factors to the development of pain. During the attacks the level of PACAP-38 can reach the normal/control level, which may be a compensatory mechanism, but several further investigations are needed to affirm this theory.
It is also evidenced that the meningeal vasculature likely contributes to the propagation of the headache cascade of symptoms, but the vasodilator ability of PACAP-38 in these circumstances is questionable. It was showed that the PACAP-38 has high potency to induce dilation on pressurized rat meningeal arteries [26], while another study did not find any discernible effect of PACAP-38 on neither rat nor human samples [27].
Amin et al. have detected significantly elevated plasma PACAP-38 level following the PACAP-38 infusion in those migraine patients without aura, who later experienced migraine-like attack [11]. Although the half-life of this peptide in plasma is short (minutes), it was suggested that the administered PACAP-38 would induce long-lasting extracranial vasodilation and neurogenic inflammation and thereby provoke a migraine attack. The nature of such a mechanism is still unclear. An alternative mechanism might be that low levels of PACAP-38 is entering the CNS and interferes with central sensory mechanisms active in the migraine or CH brain.
Nevertheless, it is important to mention that elevation was detected in the plasma PACAP-38 level both in human migraine studies [11, 14] and experimentally, when the activation of the TS was provoked with electrical or chemical methods [7, 14]. However, these studies could not investigate the difference between the interictal and normal, healthy plasma PACAP-38 levels.
The main factor determining the levels of PACAP-38 in the systemic circulation is unidentified; however, it may originate from endogenous release, damaged elimination, or de novo synthesis [11]. Significant amount of peptide can be released from the peripheral (i.e., cranial meninges) and central branches (i.e., trigeminal caudal nucleus) of the TG and other PACAP-38-containing structures of the nervous system (i.e., SPG/OT) during the beginning of the headache. The activation of TAR is certainly involved in the neuropeptide overflow, which might result in middle meningeal artery vasodilation, mast cell degranulation, and peripheral and central sensitization; concomitantly it contributes to the development of headache [11, 13, 28].
Although PACAP-38 has not been previously investigated in CH, the concentrations of CGRP, VIP and substance P (SP) have already been examined. Regarding VIP a clinical study has revealed elevated VIP-like immunoreactivity in saliva of CH patients during attack period as compared with the VIP levels during the interictal phase [29]. Moreover, significantly increased CGRP and VIP levels were found in the plasma obtained from the ipsilateral external jugular vein during spontaneous attacks of ECH patients [19], and in the extracerebral circulation both during spontaneous and nitroglycerine-induced attack phase of CH [20, 30], whereas lower plasma SP levels were detected during histamine-induced and spontaneously occurring CH attacks when compared with controls [31]. Of note, similar alterations were found considering these neuropeptides in migraineurs, which parallels the concordant changes of PACAP-38-LI observed in migraine and CH disorders.
Additionally, it should be noted that the role of PACAP in CH may also be emphasized by the evidences, which showed that PACAP can modulate the melatonin synthesis, subsequently the circadian and circannual rhythm in animals (including mammals) [32–34]. However, we did not find any correlation between the plasma concentration of PACAP-38 and the date and time of blood sampling.
Moreover, we detected a positive linear correlation between the inter-bout plasma PACAP-38 level and the age of ECH patients, which suggests that older age is associated with smaller decrease in the concentration of PACAP-38 outside of cluster period. Our data can be related to clinical studies, which concluded that the development of chronic CH is much less frequent in older than in younger patients [35, 36], if we consider that a lower inter-bout plasma PACAP-38 concentration can be a triggering factor of attacks. However, it needs further cases and data collection to affirm our hypothesis regarding this association.
Conclusion
Our pilot study provides the first evidence supporting that PACAP-38 may influence the course of CH. Similar changes of this peptide in migraine suggest that PACAP-38 might serve as a marker of primary headache conditions. Further investigations are necessary to determine the exact functions, targets and signaling pathways of PACAP-38.
Abbreviations
CGRP, calcitonin gene-related peptide; CH, cluster headache; ECH, episodic cluster headache; EDTA, ethylenediaminetetraacetic acid; HS, hypothalamic system; LI, like immunoreactivity; OG, otic ganglion; PACAP-38, pituitary adenylate cyclase-activating polypeptide-38; RIA, radioimmunoassay; SP, substance P; SPG, sphenopalatine ganglion; TAR, trigeminal-autonomic reflex; TG, trigeminal ganglion; TS, trigeminovascular system; VIP, vasoactive intestinal polypeptide
References
Goadsby PJ (2002) Pathophysiology of cluster headache: a trigeminal autonomic cephalgia. Lancet Neurol 1(4):251–7
Goadsby PJ, Lipton RB (1997) A review of paroxysmal hemicranias, SUNCT syndrome and other short-lasting headaches with autonomic feature, including new cases. Brain 120(Pt 1):193–209
Fahrenkrug J, Hannibal J (2004) Neurotransmitters co-existing with VIP or PACAP. Peptides 25(3):393–401
Edvinsson L, Elsas T, Suzuki N, Shimizu T, Lee TJ (2001) Origin and Co-localization of nitric oxide synthase, CGRP, PACAP, and VIP in the cerebral circulation of the rat. Microsc Res Tech 53(3):221–8
Moller K, Zhang YZ, Hakanson R, Luts A, Sjolund B, Uddman R, Sundler F (1993) Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: immunocytochemical and immunochemical evidence. Neuroscience 57(3):725–32
Tajti J, Uddman R, Moller S, Sundler F, Edvinsson L (1999) Messenger molecules and receptor mRNA in the human trigeminal ganglion. J Auton Nerv Syst 76(2-3):176–83
Tuka B, Helyes Z, Markovics A, Bagoly T, Nemeth J, Mark L, Brubel R, Reglodi D, Pardutz A, Szolcsanyi J, Vecsei L, Tajti J (2012) Peripheral and central alterations of pituitary adenylate cyclase activating polypeptide-like immunoreactivity in the rat in response to activation of the trigeminovascular system. Peptides 33(2):307–16
Eftekhari S, Salvatore CA, Johansson S, Chen TB, Zeng Z, Edvinsson L (2015) Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res 1600:93–109
Palkovits M, Somogyvari-Vigh A, Arimura A (1995) Concentrations of pituitary adenylate cyclase activating polypeptide (PACAP) in human brain nuclei. Brain Res 699(1):116–20
Zagami AS, Goadsby PJ, Edvinsson L (1990) Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides 16(2):69–75
Amin FM, Hougaard A, Schytz HW, Asghar MS, Lundholm E, Parvaiz AI, de Koning PJ, Andersen MR, Larsson HB, Fahrenkrug J, Olesen J, Ashina M (2014) Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain 137(Pt 3):779–94
Schytz HW, Birk S, Wienecke T, Kruuse C, Olesen J, Ashina M (2009) PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 132(Pt 1):16–25
Tuka B, Helyes Z, Markovics A, Bagoly T, Szolcsanyi J, Szabo N, Toth E, Kincses ZT, Vecsei L, Tajti J (2013) Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia 33(13):1085–95
Zagami AS, Edvinsson L, Goadsby PJ (2014) Pituitary adenylate cyclase activating polypeptide and migraine. Ann Clin Transl Neurol 1(12):1036–40
Mulder H, Uddman R, Moller K, Zhang YZ, Ekblad E, Alumets J, Sundler F (1994) Pituitary adenylate cyclase activating polypeptide expression in sensory neurons. Neuroscience 63(1):307–12
Tajti J, Uddman R, Edvinsson L (2001) Neuropeptide localization in the “migraine generator” region of the human brainstem. Cephalalgia 21(2):96–101
Csati A, Tajti J, Kuris A, Tuka B, Edvinsson L, Warfvinge K (2012) Distribution of vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, nitric oxide synthase, and their receptors in human and rat sphenopalatine ganglion. Neuroscience 202:158–68
Uddman R, Tajti J, Moller S, Sundler F, Edvinsson L (1999) Neuronal messengers and peptide receptors in the human sphenopalatine and otic ganglia. Brain Res 826(2):193–9
Goadsby PJ, Edvinsson L (1994) Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain 117(Pt 3):427–34
Fanciullacci M, Alessandri M, Figini M, Geppetti P, Michelacci S (1995) Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain 60(2):119–23
Rahmann A, Wienecke T, Hansen JM, Fahrenkrug J, Olesen J, Ashina M (2008) Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia 28(3):226–36
Headache Classification Committee of the International Headache, S (2013) The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 33(9):629–808
Amin FM, Hougaard A, Magon S, Asghar MS, Ahmad NN, Rostrup E, Sprenger T, Ashina M (2016) Change in brain network connectivity during PACAP38-induced migraine attacks: A resting-state functional MRI study. Neurology 86(2):180–7
Schytz HW, Holst H, Arendt-Nielsen L, Olesen J, Ashina M (2010) Cutaneous nociception and neurogenic inflammation evoked by PACAP38 and VIP. J Headache Pain 11(4):309–16
Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L (2013) Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain 14(11):1289–303
Syed AU, Koide M, Braas KM, May V, Wellman GC (2012) Pituitary adenylate cyclase-activating polypeptide (PACAP) potently dilates middle meningeal arteries: implications for migraine. J Mol Neurosci 48(3):574–83
Grande G, Labruijere S, Haanes KA, MaassenVanDenBrink A, Edvinsson L (2014) Comparison of the vasodilator responses of isolated human and rat middle meningeal arteries to migraine related compounds. J Headache Pain 15:22
Vecsei L, Tuka B, Tajti J (2014) Role of PACAP in migraine headaches. Brain 137(Pt 3):650–1
Nicolodi M, Del Bianco E (1990) Sensory neuropeptides (substance P, calcitonin gene-related peptide) and vasoactive intestinal polypeptide in human saliva: their pattern in migraine and cluster headache. Cephalalgia 10(1):39–50
Fanciullacci M, Alessandri M, Sicuteri R, Marabini S (1997) Responsiveness of the trigeminovascular system to nitroglycerine in cluster headache patients. Brain 120(Pt 2):283–8
Sicuteri F, Fanciullacci M, Geppetti P, Renzi D, Caleri D, Spillantini MG (1985) Substance P mechanism in cluster headache: evaluation in plasma and cerebrospinal fluid. Cephalalgia 5(3):143–9
Schwartz C, Andrews MT (2013) Circannual transitions in gene expression: lessons from seasonal adaptations. Curr Top Dev Biol 105:247–73
Hannibal J, Ding JM, Chen D, Fahrenkrug J, Larsen PJ, Gillette MU, Mikkelsen JD (1997) Pituitary adenylate cyclase-activating peptide (PACAP) in the retinohypothalamic tract: a potential daytime regulator of the biological clock. J Neurosci 17(7):2637–44
Simonneaux V, Ouichou A, Pevet P (1993) Pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates melatonin synthesis from rat pineal gland. Brain Res 603(1):148–52
Donnet A, Lanteri-Minet M, Guegan-Massardier E, Mick G, Fabre N, Geraud G, Lucas C, Navez M, Valade D, C. Societe Francaise d’Etude des Migraines et (2007) Chronic cluster headache: a French clinical descriptive study. J Neurol Neurosurg Psychiatry 78(12):1354–8
Dong Z, Di H, Dai W, Pan M, Li Z, Liang J, Zhang M, Zhou Z, Liu R, Yu S (2013) Clinical profile of cluster headaches in China - a clinic-based study. J Headache Pain 14(1):27
Acknowledgements
This work was supported by the project TÁMOP-4.2.2.A-11/1/KONV-2012-0052, EUROHEADPAIN (Grant No. 602633), OTKA (PD 104715), the MTA-SZTE Neuroscience Research Group and the National Brain Research Programs (Grant No. KTIA_13_NAP-A-III/9., Chronic Pain Research Group: Grant No. KTIA_NAP_13-2014-0022; Z. Helyes, 888819). Dr. Árpád Párdutz and Dr. Nikoletta Szabó were supported by the Bolyai Scholarship Programme of the Hungarian Academy of Sciences.
Authors’ contributions
All authors have read the manuscript and agreed with the submission.
Competing interests
All authors declare that there are no conflicts of interest and they have no other relevant affiliations or financial involvement concerning this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tuka, B., Szabó, N., Tóth, E. et al. Release of PACAP-38 in episodic cluster headache patients – an exploratory study. J Headache Pain 17, 69 (2016). https://doi.org/10.1186/s10194-016-0660-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-016-0660-7