Abstract
Polysaccharides are promising renewable alternatives to petroleum-based plastics, and are high-value-added materials in various industries. In this work, we synthesized dextran (α-1,6-glucan) ester derivatives substituting acyl groups with different carbon numbers from acetate to laurate. We found that the thermal stability of dextran was improved by esterification. Moreover, using differential scanning calorimetry and X-ray diffraction, we revealed that dextran ester derivatives were amorphous. Self-standing, transparent, solvent-cast films of dextran ester derivatives were prepared. Dextran ester derivatives adhered to various materials, including polyvinyl alcohol (PVA) films, wood, glass, and aluminum. In addition, the adhesive interfaces were transparent, which is important for practical applications. The adhesive strength for PVA films increased with concentration, exceeding the breaking strength of the PVA film at 0.3 g/mL. Moreover, dextran valerate and dextran hexanoate behaved as hot-melt-type adhesives. These results demonstrate the potential of dextran ester derivatives as biomass-based adhesives.
Similar content being viewed by others

Introduction
Adhesives are an important type of polymer in industries ranging from electronics to construction. Although a variety of synthetic adhesives, such as polyvinyl acetate (PVAc), polyvinyl alcohol (PVA), and polyurethane, are used in industrial products, they are mainly derived from petrochemical resources. For a sustainable economy, it is necessary to develop bio-based adhesives from renewable resources.
Protein and polysaccharides are representative materials for bio-based adhesives. For example, casein [1] and soy protein [2] have been applied as protein-based adhesives for particle boards, and starch [3], chitosan [4, 5], and glucomannan [4] have been for particle boards and in wood products. Polysaccharide adhesives have particularly good adhesion to wood and wood products owing to their polar functional groups, such as hydroxyl groups. Therefore, they are promising as alternative adhesive materials. However, the widespread adoption of polysaccharide adhesives is limited by three main shortcomings. First, their application as adhesives is limited to wood and wood products. The second problem is their low processability: they have no solubility in common solvents except for water, which requires a long drying time, and they lack thermal plasticity. Finally, they have no water-resistance because of the high hydrophilicity derived from their hydroxyl groups. Therefore, novel bio-based adhesives are required to overcome these problems.
Esterification of hydroxyl groups of polysaccharides is an effective solution to achieve thermal plasticity, solubility in common organic solvents, and high water-resistance. We have previously reported the complete esterification of various polysaccharides, including curdlan and paramylon [6,7,8,9], pullulan [10, 11], and glucomannan [12, 13], all of which exhibited thermal plasticity. In addition, Copinet et al. reported that partial acetylation of starch improves its thermal processability [14]. Furthermore, it has been reported that microbial extracellular polysaccharide ester derivatives adhere to wood products [15].
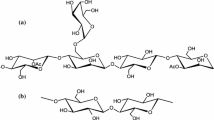
In the present study, we focused on the esterification of dextran (α-1,6-glucan) for application as a novel bio-based adhesive. Dextran is a natural polysaccharide, which is biologically synthesized by lactic acid bacteria, such as the Leuconostoc and Streptococcus species. Although neat dextran is widely used as a plasma augmentor, and as a binding agent in cosmetics because of its biocompatibility and biodegradability [16,17,18], the esterification of dextran for application as an industrial polymer has not been conducted. Dextran has flexibility owing to its main chain, which is composed the α-1,6-linkage of d-glucose [19]; therefore, we expected that it would form a flexible polymeric layer. We also expected that this would allow improvements of the Van der Waals force between materials and thus improve the adhesive properties toward both non-wood materials and wood materials.
The objective of this study was to synthesize dextran ester derivatives and evaluate their adhesive properties. Dextran ester derivatives with different alkyl chain lengths were synthesized and characterized, and their thermal properties were analyzed with thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) and dynamic mechanical analysis (DMA). In addition, we evaluated adhesion of dextran ester derivatives to PVA films, wood, glass, and aluminum.
Materials and methods
Materials
Dextran (weight-average molecular weights (Mw) = 150,000), trifluoroacetic anhydride (TFAA), acetic acid, propionic acid, butyric acid, valeric acid, hexanoic acid, octanoic acid, decanoic acid, lauric acid, ethanol, and chloroform were purchased from Wako Pure Chemicals (Tokyo, Japan). PVA films were provided by Kuraray. All reagents are used without further purification.
Preparation of dextran ester derivatives
Dextran ester derivatives were synthesized as shown Scheme 1. A representative procedure, for dextran acetate, is described as follows. A premixed solution of TFAA (40 mL) and acetic acid (40 mL), which had been stirred at 50 °C for 5 min, was added to freeze-dried dextran (2.0 g). The solution was stirred at 50 °C for 2.0 h. The solution was then poured into mixed solution of ethanol (1.0 L) and deionized water (1.0 L). The precipitate was filtered, dissolved in chloroform, and reprecipitated in the mixture of ethanol (1.0 L) and deionized water mixture, and dried to give dextran acetate (DexAc, n = 2) (2.2 g, 60% yield). For the other dextran esters, a comparable quantity of carboxylic acid was used (40 mL or 50 g; n = 3–12). The yields (%) of the dextran esters were as follows: dextran propionate (DexPr, n = 3) (2.3 g, 56%), dextran butylate (DexBu, n = 4) (3.2 g, 67%), dextran valerate (DexVa, n = 5) (4.2 g, 79%), dextran hexanoate (DexHe, n = 6) (4.7 g, 84%), dextran octanoate (DexOc, n = 8) (5.7 g, 86%), dextran decanoate (DexDe, n = 10) (6.7 g, 88%), and dextran laurate (DexLa, n = 12) (7.4 g, 78%).
Nuclear magnetic resonance (NMR) measurements
1H, 13C, double quantum filtered correlation (DQF-COSY), heteronuclear single quantum coherence (HSQC), and heteronuclear multiple bond correlation (HMBC) NMR spectra were recorded with a JEOL JNM-A-500 FT-NMR (500 MHz) spectrometer, using tetramethylsilane (TMS) as an internal standard. The degree of substitution (DS) of the dextran esters was calculated from the ratio of the integrated area of the methyl protons of the acyl group to the ring protons of glucose unit, as follows: DS = ([CH3]/3)/([ring-H]/7).
Gel-permeation chromatography (GPC) measurements
Number-average molecular weights (Mn) and Mw and polydispersity index values (Mw/Mn) were calculated using GPC (CBM-20A, DGU-20A3, LC-6AD, SIL-20ACHT, CTO-20A, RID-10A, Shimadzu) in chloroform at 40 °C. Shodex columns (K-806M, K-802) were used, and the flow rate was 0.8 mL/min. A calibration curve was obtained using polystyrene standards (Shodex).
Thermogravimetric analysis (TGA)
TGA was performed with a Thermo Plus TG 8120 (Rigaku, Tokyo, Japan) instrument under a nitrogen atmosphere. The temperature range of measurements was 30–450 °C, and the heating rate was 10 °C/min.
Differential scanning calorimetry (DSC)
DSC thermograms were investigated using a DSC8500 instrument (Perkin-Elmer, Massachusetts, U.S.) under a nitrogen atmosphere. Dextran ester powders (2 mg) were used as samples. The samples were first heated from − 50 to 250 °C at a rate of 100 °C/min, cooled to 0 °C at a rate of 100 °C/min, and then scanned with heating from 0 to 250 °C at a rate of 100 °C/min.
Dynamic mechanical analysis (DMA)
DMA measurements were carried out using a DVA-200S analyzer (IT Measurement Control, Osaka, Japan). The temperature range of the scans was 50–200 °C, and the heating rate was 5 °C/min. Measurements were performed under a nitrogen atmosphere. The specimens (20 × 5 mm2) were prepared from the solvent-cast films described in “Tensile testing of the solvent-cast films”.
Tensile testing of the solvent-cast films
Dextran ester derivatives (0.5 g; DexBu to DexLa) were dissolved in chloroform (7.5 mL) and poured onto plates (diameter 4.0 cm). The solvent was evaporated in an air atmosphere at room temperature for 24 h. Tensile tests for these prepared films were carried out at room temperature using an EZ-test machine (Shimadzu, Japan). The crosshead speed was 10 mm/min, and the initial gauge length was 10 mm. Five specimens (15 × 5 mm2) of each dextran ester derivatives were used for each measurement, and the data were averaged for each film.
Wide-angle X-ray diffraction analysis (WAXD)
WAXD was performed with a RINT 2000 instrument (Rigaku, Tokyo, Japan) operating at 40 kV and 40 mA. Ni-filtered Cu-Kα radiation (λ = 0.15418 nm) was collimated in a 1/2-degree divergence slit, 1/6-degree scatter slit and 0.15-mm receiving slit. Measurements were performed with a Bragg–Brentano type 2θ/θ goniometer in reflection mode. The range of scans was 2θ = 2°–40°. The scan rate was 0.5°/min and the step size was 0.1°. Solvent-cast films (DexPr, DexBu, DexVa, DexHe, and DexLa) and powders (DexAc, DexOc, and DexDe) of dextran ester derivatives were used as samples.
Preparation of adhesives
Dextran ester derivatives were dissolved in CHCl3 at 16.5 wt% (0.29 g/mL). In addition, DexBu was dissolved at four different concentrations: 1.9 wt% (0.033 g/mL), 7.5 wt% (0.13 g/mL), 11.3 wt% (0.20 g/mL), and 16.5 wt% (0.29 g/mL). These solutions were used as solvent-volatilization-type adhesives.
Adhesion tests
The adhesion to four kinds of material—birch wood, glass, aluminum, and PVA film—was tested for 16.5 wt% DexBu (0.1 g), which was prepared was described in “Preparation of adhesives”. The adhesive area of birch wood, glass, and aluminum was 2.5 × 2.5 cm2, and that of the PVA film was 2.5 × 2.5 cm2 or 0.5 × 15 mm2. The pressure of bonding was 1.0 kPa for 24 h in air at room temperature.
Tensile shear strength testing
To evaluate the adhesive strength, we performed tensile shear strength tests at room temperature using an EZ-test machine (Shimadzu, Japan). The crosshead speed was 10 mm/min, and the initial gauge length was 10 mm. Two PVA films (25 × 25 mm2) were adhered with an overlapping area of 25 × 8 mm2. The dextran ester derivative adhesives from DexPr to DexLa (0.1 mL; 0.29 g/mL) and DexBu adhesives at different concentrations (0.1 mL; 0.033, 0.13, 0.20, and 0.29 g/mL) prepared in “Preparation of adhesives” section were used for this test. The pressure of bonding was 1.0 kPa for 72 h in air at room temperature. After drying, these were cut into five strip specimens (5 × 8 mm2). Five specimens of the dextran ester derivatives were used for each measurement, and the data were averaged for each film.
Hot-melt-type adhesives
The solvent-cast films (7 × 5 mm2) of DexBu, DexVa, and DexHe were placed between two glass slides. We evaluated the adhesion of the different materials to the specimens as they were heated from 20 to 200 °C on a hot plate pressed at 20 kPa.
Results and discussion
Preparation of dextran esters
Mw, Mn, and degree of polydispersion (Mw/Mn) of the dextran ester derivatives were investigated using GPC. DS was estimated from the 1H-NMR spectra. The DS, Mw, Mn, and Mw/Mn are listed in Table 1. The Mw varied from 15 × 104 to 32 × 104 g/mol. Representative 1H-NMR spectra of DexBu and DexLa are shown in Fig. 1. The peaks of the ring protons appear at δ3.5–6.0 ppm, which is consistent with a previous report for dextran propionate acetate [19]. These peaks were assigned based on two-dimensional NMR. The peaks of the acyl groups appeared at δ0.9–2.5 ppm. Using these results, the DS values of all obtained dextran esters were calculated as 3.0. This indicates that three hydroxyl groups of dextran were fully substituted by acyl groups (n = 2–12).
Thermal properties
The thermal decomposition of dextran ester derivatives was measured using TGA. The thermal decomposition temperature at 5% weight loss (Td.5%) and 50% weight loss (Td.50%) are listed in Table 1, and the TGA curves are shown in Fig. 2. The Td.50% values of the dextran esters (382–400 °C) were higher than that of neat dextran (325 °C). This indicates that acylation improves the thermal stability. These observations are consistent with those of previous research on ester derivatives of other polysaccharides, such as starch [3, 14], pullulan [10], and glucomannan [12].
The results of the DSC measurements are shown in Fig. 3. Dextran ester derivatives did not exhibit any characteristics of crystallization and melting. This suggests that they are amorphous polymers, similar to pullulan ester derivatives [10] and glucomannan ester derivatives [12, 14]. Moreover, there was no peak in any of the dextran ester derivatives attributed to the glass transition temperature (Tg). This indicates that the difference in the molecular motion of the chains between the glass state and the undercooled state is small. This phenomenon is often observed in samples that have low thermal fluidity (e.g., thermosetting resin [20]).
Figure 4 shows the storage modulus (Eʹ) and loss tangent (tanδ) of the dextran ester derivatives measured using DMA. The films of DexPr were not measured because of their brittleness and weakness, and films of DexBu, DexVa, and DexOc broke during measurement. The storage modulus of the five dextran ester derivatives that did not break at the start of the measurement decreased at the tanδ peak temperature. However, the decrease in Eʹ was smaller than that of pullulan ester derivatives [10] and glucomannan ester derivatives [12]. This result supports out conclusion based on the DSC results above that there is a small difference in molecular motion between the glass and undercooled states. The peak of tanδ corresponds to the Tg. The Tg decreased with increasing acyl carbon number (n) in the short side-chain groups (n = 4–6). This phenomenon has also been reported in pullulan ester derivatives [10].
Preparation and tensile testing of solvent-cast films
Self-standing solvent-cast films of dextran ester derivatives were obtained by evaporating the chloroform solution. A solvent-cast film of DexAc was not prepared because DexAc did not dissolve in chloroform. Colorless and transparent films were obtained from the dextran ester derivatives except for DexOc, which was pale yellow. Figure 5 shows images of the DexBu, DexHe, and DexDe films.
In the tensile tests, the highest tensile strength of 8 MPa was observed for DexBu. The mechanical strength of dextran ester derivatives was lower than that of other polysaccharide ester derivatives, which ranges from 20 to 40 MPa [6, 10, 12].
X-ray diffraction measurements
Figure 6 shows the X-ray diffraction patterns of the dextran ester derivatives. All samples had a broad amorphous peak at 20°, and no crystalline peaks were observed. This result is in accordance with the absence of Tm in the DSC thermograms. Together, these results suggest that the dextran esters were amorphous polymers.
All diffractograms showed diffraction in the range of 2θ = 2–4°. The diffraction peaks shifted, depending on the side-chain length. Peaks with shorter side chains were observed in relatively wider-angle regions. Similar observations were reported in other amorphous polysaccharide ester derivatives, such as pullulan [10], and glucomannan [12]. In these previous reports, the peaks in the range of 2θ = 2–4° were attributed to alignment between main chains [10]. The d-spacing between main chains of dextran ester derivatives increases with increasing acyl carbon number (n).
Adhesion tests as solvent-volatilization-type adhesives
Figure 7 shows images of materials to which DexBu was adhered. As shown in Fig. 7b–e, the adhesive is transparent after drying, which is important for industrial applications. In addition, the adhesion to a range of materials (i.e., glass–aluminum, wood–aluminum, glass–wood, PVA film–aluminum, PVA film–PVA film, and wood–wood) further demonstrates its wide applicability. Moreover, Fig. 7f shows that the DexBu adhesive (applied to a PVA film) can lift a weight of 1.0 kg.
The adhesive strength for PVA films was investigated by tensile shear strength testing. From propionate to octanoate, the specimens broke at the PVA film rather than the adhesive layer (Fig. 8b). This suggests that the adhesive strength is higher than the tensile strength of PVA films. Therefore, in these cases, the adhesive strength could not be calculated. By contrast, the adhesive layers of DexDe and DexLa were broken at 0.15 and 0.18 MPa. This means that dextran ester derivatives with short side-chain groups have higher adhesive strength than those with long side-chain groups. Moreover, this result indicates that the adhesive strength can be controlled by the length of the side chain.
The adhesive strengths of different concentrations of DexBu are shown in Table 2 and Fig. 8a. In Table 2, ‘PVA failure’ indicates that the specimens were broken at the PVA film. This behavior was concentration-dependent: at a concentration of 0.20 g/mL, three specimens underwent PVA failure, whereas at 0.29 g/mL, all specimens underwent PVA failure. The adhesive strength of DexBu (0.29 g/mL; 0.1 mL) was approximately 0.5 MPa, which is equivalent to lifting 2.0 kg with the 40-mm2 adhesive area of these specimens.
Adhesive tests as hot-melt-type adhesives
Figure 9 shows images for the hot-melt adhesive testing of dextran ester derivatives with different side-chain lengths. DexVa and DexHe had adhesive properties at 60 and 20 °C, respectively. However, DexBu was not adhesive from 20 to 200 °C, despite its glass transition temperature being 75 °C. This is explainable in terms of the low thermal fluidity, which was confirmed by the high Eʹ. Figure 2a shows that the log(Eʹ) of DexVa at 60 °C and DexHe at room temperature was lower than 7.5, whereas the log(Eʹ) of DexBu was higher than 7.5 during the measurement. This result further confirms the influence of the storage modulus on the adhesive properties. More broadly, these results demonstrate the potential of dextran ester derivatives as environmentally benign, solvent-free adhesives.
Conclusion
We prepared dextran ester derivatives with different side-chain lengths (from acetate to laurate) and investigated their physical and adhesive properties. The products were amorphous, and adhered not only to PVA film, but also to birch wood, glass, and aluminum. The adhesive surface of the dextran esters was transparent. Furthermore, some dextran ester derivatives exhibited hot-melt-type adhesions. These biomaterials could have practical applications as environmentally benign adhesives.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- DexAc:
-
dextran acetate
- DexPr:
-
dextran propionate
- DexBu:
-
dextran butyrate
- DexVa:
-
dextran valerate
- DexHe:
-
dextran hexanoate
- DexOc:
-
dextran octanoate
- DexDe:
-
dextran decanoate
- DexLa:
-
dextran laurate
- TFAA:
-
trifluoroacetic anhydride
- PVAc:
-
poly vinyl acetate
- PVA:
-
poly vinyl alcohol
References
Mondragon G, Nygaard F (1979) The use of casein glue as an adhesive for tissue sections. Am J Dermatopathol 1:253–255
Kumar R, Choudhary V, Mishra S, Varma IK, Mattiason B (2002) Adhesives and plastics based on soy protein products. Ind Crops Prod 16:155–172
Imam HS, Gordon HS, Mao L, Chen L (2001) Environmentally friendly wood adhesive from a renewable plant polymer: characteristics and optimization. Polym Degrad Stab 73:529–533
Umemura K, Inoue A, Kawai S (2003) Development of new natural polymer-based wood adhesives I: dry bond strength and water resistance of konjac glucomannan, chitosan, and their composites. J Wood Sci 49:221–226
Umemura K, Kaiho K, Kawai S (2009) Characterization of bagasse-rind particleboard bonded with chitosan. J Appl Polym Sci 113:2103–2108
Marubayashi H, Yukinaka K, Enomoto-Rogers Y, Takemura A, Iwata T (2014) Curdlan ester derivatives: synthesis, structure, and properties. Carbohydr Polym 103:427–433
Zhai W, Danjo T, Iwata T (2018) Synthesis and physical properties of curdlan branched ester derivatives. J Polym Res 25:181
Zhai W, Danjo T, Iwata T (2019) Synthesis and properties of curdlan branched and linear mixed ester derivatives. Polym Degrad Stab 161:50–56
Gan H, Enomoto Y, Kabe T, Ishii D, Hikima T, Takata M, Iwata T (2017) Synthesis, properties and molecular conformation of paramylon ester derivatives. Polym Degrad Stab 145:142–149
Enomoto-Rogers Y, Iio N, Takemura A, Iwata T (2015) Synthesis and characterization of pullulan alkyl esters. Eur Polym 66:470–477
Danjo T, Enomoto Y, Shimada H, Nobukawa S, Yamaguchi M, Iwata T (2017) Zero birefringence films of pullulan ester derivatives. Sci Rep 7:46342
Enomoto-Rogers Y, Ohmomo Y, Takemura A, Iwata T (2014) Syntheses of glucomannan esters and their thermal and mechanical properties. Carbohydr Polym 101:592–599
Danjo T, Enomoto-Rogerse Y, Takemura A, Iwata T (2014) Syntheses and properties of glucomannan acetate butyrate mixed esters. Polym Degrad Stab 109:373–378
Copinet A, Bliard C, Onteniente J, Couturier Y (2001) Enzymatic degradation and deacetylation of native and acetylated starch-based extruded blends. Polym Degrad Stab 71:203–212
Haag Anthony P, Geesey Gill G, Mittleman Marc W (2006) Bacterially derived wood adhesive. Int J Adhes Adhes 26:177–183
DeGroot CJ, VanLuyn MJA, VanDiek-Wolthuis WNE, Cadée JA, Plantinga JA, Otter WD, Hennink WE (2001) In vitro biocompatibility of biodegradable dextran-based hydrogels tested with human fibroblasts. Biomaterials 22:1197
Cadee JA, VanLuyn MJA, Brouwer LA, Plantinga JA, VanWachem PB, DeGroot CJ, DenOtter W, Hennink WE (2000) In vivo biocompatibility of dextran-based hydrogels. J Biomed Mater Res 50:397
Funane K, Tokashiki T, Gibu S, Kawabata Y, Oguma T, Ito H, Nakachi M, Miyagi S, Kobayashi M (2007) A novel cyclic isomaltooligosaccharide (cycloisomaltodecaose, CI-10) produced by Bacillus circulans T-3040 displays remarkable inclusion ability compared with cyclodextrins. J Appl Glycosci 54:103–107
Heinze T, Liebert T, Heublein B, Hornig S (2006) Functional polymers based on dextran. Adv Polym Sci 205:199–291
Sbirrazzuoli N, Vyazovkin S, Mittelu A, Sladic C, Vincent L (2003) A study of epoxy-amine cure kinetics by combining isoconversional analysis with temperature modulated DSC and dynamic rheometry. Macromol Chem Phys 204(15):1815–1821
Funding
This work was carried out as part of the “Innovative Synthesis of High-Performance Bioplastics from Polysaccharides” project by JST ALCA Grant Number JPMJAL1502, Japan.
Author information
Authors and Affiliations
Contributions
AT, YE, AT, TI designed the experiments and performed syntheses and the analyses. AT was a major contributor in the experiments and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Togo, A., Enomoto, Y., Takemura, A. et al. Synthesis and characterization of dextran ester derivatives and their adhesive properties. J Wood Sci 65, 66 (2019). https://doi.org/10.1186/s10086-019-1845-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10086-019-1845-x