Abstract
Glucose-Regulated Protein 78 (GRP78) is a chaperone protein that is predominantly expressed in the lumen of the endoplasmic reticulum. GRP78 plays a crucial role in protein folding by assisting in the assembly of misfolded proteins. Under cellular stress conditions, GRP78 can translocate to the cell surface (csGRP78) were it interacts with different ligands to initiate various intracellular pathways. The expression of csGRP78 has been associated with tumor initiation and progression of multiple cancer types. This review provides a comprehensive analysis of the existing evidence on the roles of GRP78 in various types of cancer and other human pathology. Additionally, the review discusses the current understanding of the mechanisms underlying GRP78's involvement in tumorigenesis and cancer advancement. Furthermore, we highlight recent innovative approaches employed in downregulating GRP78 expression in cancers as a potential therapeutic target.
Similar content being viewed by others
Introduction
Glucose-Regulated Protein 78 (GRP78) is a heat shock protein chaperone that has garnered extensive attention in various disease progression (Gopal and Pizzo 2021). GRP78 primarily resides in the lumen of the endoplasmic reticulum, a cellular organelle responsible for protein synthesis and folding (Lee 2001). It plays a vital role in maintaining protein homeostasis by assisting in the assembly and proper folding of newly synthesized proteins (Pyrko et al. 2007).Under normal physiological conditions, GRP78 ensures the efficient folding of proteins and prevents the accumulation of misfolded or unfolded proteins in the endoplasmic reticulum (Fu et al. 2008). However, in response to cellular stress, such as nutrient deprivation, hypoxia, or exposure to toxins, the demand for proper protein folding increases. In these conditions, GRP78 expression is upregulated to meet the heightened protein-folding requirements (Koumenis et al. 2002). Interestingly, in addition to its role within the endoplasmic reticulum, GRP78 has been found to undergo translocation to the cell surface. This cell surface localization gives rise to a distinct form of GRP78 known as cell surface GRP78 (csGRP78) (Misra et al. 2005). The translocation of GRP78 to the cell surface is believed to be a stress response mechanism that allows cells to adapt and survive adverse conditions (Lee 2001).
csGRP78 has been implicated in the development and progression of various types of cancer (Dong et al. 2005). It has been observed that csGRP78 expression is often upregulated in cancer cells compared to normal cells (Dong et al. 2011). This overexpression is associated with several hallmarks of cancer, including increased cell survival, enhanced proliferation, angiogenesis (formation of new blood vessels to support tumor growth), and resistance to chemotherapy and radiation therapy (Sedighzadeh et al. 2021). At the cell surface, csGRP78 interacts with specific ligands, such as hormones, growth factors, and extracellular matrix proteins. These interactions trigger signaling pathways that promote cell survival, activate pro-survival and pro-growth signaling cascades, and contribute to the acquisition of malignant traits by cancer cells (Dong et al. 2005; Fu et al. 2008). Understanding the intricate involvement of GRP78, particularly csGRP78, in cancer biology would therefore be crucial in developing effective therapeutic strategies. Targeting GRP78 or its interactions with ligands holds promise for disrupting the survival and growth pathways that drive cancer progression (Fu et al. 2008). Therefore, numerous research efforts have been directed towards unraveling the underlying mechanisms of GRP78 in cancer and exploring innovative approaches to modulate its expression or function (Ninkovic et al. 2020; Qiao et al. 2020).
In this review, we aim to provide a systematic breakdown of the correlation between GRP78 and various types of cancer. By compiling and analyzing relevant research findings, we seek to elucidate the extensive role of GRP78 in cancer pathogenesis and shed light on recent advances in downregulating GRP78 as a potential therapeutic avenue.
Discovery and types of GRPs
In the 1970s, researchers (Pouysségur et al. 1977; Hightower 1980) identified a group of proteins that were constitutively produced and activated in response to glucose deprivation, and they were given the moniker glucose-regulated proteins (GRPs). One of the most extensively studied GRPs is GRP78. The chaperone protein GRP78 was first identified and described by Stone et al. in 1974 (Stone et al. 1974). Initially, in 1976, it was believed that GRP78 was a 73 kDa membrane protein associated with viral transformation. However, subsequent studies by Shiu et al. in 1977 demonstrated that this membrane protein was primarily involved in glucose regulation within cells rather than viral transformation (Shiu et al. 1977).
Over the years, the understanding of GRPs has expanded, and various types of GRPs have been identified with their distinct functions. These GRPs act as chaperones and are activated in response to cellular stress. Here are some of the key types of GRPs and their important functions (Table 1):
A comprehensive literature search using the terms "GRP78" or "BiP" reveals a vast number of publications on the topic, reflecting the extensive research interest in GRP78. Most references cited in this article are derived from reliable sources such as PubMed, Web of Science, and reputable scientific publishers like Elsevier and Wiley. This study provides a deeper insight into the role of GRP78 and various diseases, particularly cancer. GRP78 has been implicated in the pathogenesis and progression of various cancers, highlighting its significance as a potential therapeutic target (Luo et al. 2018). Investigating the subcellular processes and functions of GRP78 offers valuable insights into the development of novel strategies for the treatment of associated diseases.
Description of GRP78 coupled with its roles
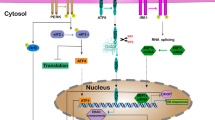
GRP78, also known as glucose-regulated protein 78, is a chaperone protein that primarily resides in the lumen of the endoplasmic reticulum (ER) due to its carboxyl KDEL retention motif, but it can also be found on the cell surface (Lee 2014). It is encoded by the Hsp5a gene and belongs to the heat shock protein-70 (Hsp70) family. Although GRP78 is the most common member of the Hsp70 family, it lacks a heat shock element in its promoter, preventing activation by heat shock (Casas 2017). In normal cell states, GRP78 remains inactive while bound to transmembrane stress detectors of the unfolded protein response (UPR) pathway, including PRK-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme (IRE1), and activating transcription factor 6 (ATF6) (Pfaffenbach and Lee 2011) (Fig. 1). This binding keeps GRP78 in an inactive state, ready to respond to ER stress. The regulation of GRP78 involves the binding of nuclear transcription factor Y (NFY), SP1, and histone deacetylase 1 (HDAC1) to stress response elements (EREs) present in its promoter region, thereby maintaining its low basal transcription level (Fig. 1) (Yoshida et al. 1998; Roy and Lee 1999; Baumeister et al. 2005). Interestingly, GRP78 plays a dual role within the endoplasmic reticulum. Firstly, it functions as a resident chaperone, facilitating proper protein folding and preventing protein aggregation. This chaperone activity of GRP78 ensures the correct folding and assembly of newly synthesized polypeptides. Secondly, GRP78 acts as a key regulator of the UPR pathway. During ER stress, the accumulation of unfolded proteins sequesters GRP78 away from PERK, IRE1, and ATF6, thereby activating these transmembrane proteins (Fig. 2) (Hendershot 2004). The UPR is a cellular response mechanism that aims to restore ER homeostasis under stress conditions. It is divided into two phases: the early, pro-survival UPR, which activates adaptive responses to mitigate ER stress, and the late, pro-apoptotic UPR, which triggers cell death if ER stress becomes overwhelming(Roller and Maddalo 2013a). It is noteworthy that the persistent activation of the UPR pathway and the dysregulation of GRP78 have been implicated in various malignancies. Genes upstream or downstream of the UPR are frequently upregulated in cancer cells, suggesting that the sustained activation of this pathway provides tumors with a growth advantage(Roller and Maddalo 2013a).
Illustration of the inactive state of GRP78 and the presence of stress response elements (ERES) positioned upstream of the TATA element. In cells that are not experiencing stress, the ERES region is bound by NFY, SP1, and HDAC1, leading to the maintenance of low transcription levels for GRP78. Additionally, GRP78, located within the lumen of the endoplasmic reticulum (ER), interacts with the ER stress sensors (IRE1, ATF6, and PERK) and acts as a signaling component for the unfolded protein response (UPR)
Representation of the general mechanism by which GRP78 functions within the endoplasmic reticulum (ER). GRP78 plays a crucial role in assisting ER proteins by promoting their stability and facilitating the process of protein folding. This is achieved through its interaction with misfolded proteins and unassembled complexes, thereby initiating the ER-associated degradation (ERAD) pathway. Within this pathway, GRP78 targets the misfolded proteins for degradation through a series of events that involve ubiquitination and subsequent proteasomal degradation
Studies investigating the transcriptional activation mechanism of GRP78 have contributed to the identification of novel intracellular signaling pathways that transmit ER stress signals to the nucleus, initiating the transcription of genes involved in the unfolded protein response (UPR) (Luo and Lee 2013; Mori 2000; Chang et al. 1989). Figure 3 illustrates the cascade of downstream pathways initiated by UPR stress sensors when unfolded peptides accumulate. Upon activation, PERK forms homodimers and undergoes autophosphorylation, enabling the alpha subunit of eukaryotic translation initiation factor 2 (elf2) to be phosphorylated (Ibrahim et al. 2019; Li et al. 2008; Liu et al. 2020). Phosphorylated elf2 inhibits translation initiation, triggering the activation of the transcription factor ATF4 and its target gene C/EBP homologous protein (CHOP). CHOP, in turn, induces apoptosis by downregulating BCL-2 (an antiapoptotic outer mitochondrial membrane protein) and activating the expression of Bax and Bim genes (Roller and Maddalo 2013a). IRE1 also homodimerizes and auto phosphorylates before cleaving and splicing the mRNA encoding X-box binding protein 1. Additionally, IRE1 recruit’s tumor necrosis factor α receptor-associated factor 2 (TRAF2), which activates JUN amino-terminal kinases (JNK), ultimately leading to apoptosis. Furthermore, ATF6 translocate to the Golgi apparatus after dissociation from GRP78 and subsequently undergoes cleavage, allowing its active form, cATF6, to enter the nucleus. In the nucleus, cATF6 acts as a transcription factor, upregulating proteins essential for enhancing the ER's capacity for protein folding (Ibrahim et al. 2019). The pathways mediated by PERK, IRE1, and ATF6 intersect with each other and elicit cellular responses that can be both pro-survival and pro-apoptotic. Importantly, the induction of GRP78 transcription is not solely triggered by ER stress. Some scholarly articles have demonstrated the activation of GRP78 transcription by histone deacetylase inhibitors (HDAC1) without concurrently inducing the overall stress response (Baumeister et al. 2009). Additionally, impaired autophagy in tumor cells has been shown to upregulate ER chaperones in response to metabolic stress (Mathew et al. 2009).
In tumor microenvironments where GRP78 is overexpressed, it localizes to the cell membrane surface (Roller and Maddalo 2013b). Referred to as cell surface GRP78 (csGRP78), this protein plays a significant role in various signaling events and acts as a co-receptor in studies involving different tumor types, influencing tumor cell survival, proliferation, and motility. The relocation of GRP78 to the cell surface has been strongly associated with drug resistance and cellular transformation (Roller and Maddalo 2013b; Chen et al. 2022). Additionally, alterations in cell surface GRP78 have been shown to impact the behavior of cancer stem cell populations in diverse tumor types (Chen et al. 2018a, b). One well-studied pathway involving cell surface GRP78 is its interaction with the tumorigenic PI3K/AKT pathway, facilitated by complex formation with PI3K (phosphoinositide-3-kinase) (Lee et al. 2022a) (Fig. 4). This interaction has been observed in prostate cancer (Zhang et al. 2013). In a study conducted by Zhang et al. in 2013 (Zhang et al. 2013), they discovered an insertion mutation in the N-terminus domain of GRP78. This mutation resulted in a decrease in complex formation, while the protein's expression and ability to move to the cell surface remained stable, similar to the normal, unmutated protein. The researchers concluded that blocking csGRP78 could present a distinct strategy to inhibit PI3K activity and potentially overcome treatment resistance in cancer cells.
A breakdown of cell surface GRP78 (csGRP78) roles in AKT/P13K/ mTOR pathway. AKT and phosphoinositide-dependent kinase-1 (PDK1) are both drawn to the membrane after PI3K produces the second messenger PIP3. Due to proximity, PDK1 can phosphorylate AKT at position T308 of the activation loop (T-loop). The rapamycin-resistant mTOR complex 2 then phosphorylates AKT at residue S473 of the hydrophobic motif (mTORC2), a key step in the complete activation of AKT's kinase activity. TSC2, a negative regulator of mTORC1 activity, is one of several additional substrates that AKT itself has the ability to phosphorylate
GRP78 role in various diseases
GRP78, a master chaperone protein of the unfolded protein response (UPR), plays a crucial role in various diseases, mainly cancer. In cancer, GRP78 has been implicated in chemotherapy resistance and the tumor virulence (Cook and Clarke 2015). Tumor cells often face a hostile metabolic environment characterized by low glucose levels, acidity, and nutrient deprivation (Ferraresi et al. 2020; Wang et al. 2009). Interestingly, the UPR, which involves the activation of GRP78, is triggered in response to oxygen or glucose starvation to promote cell survival (Wojtkowiak et al. 2012; Ferraresi et al. 2020; Wang et al. 2009; Koumenis 2006). Elevated expression levels of GRP78 have been observed in initial tumors compared to benign tissues, indicating its involvement in tumor progression and aggressiveness (Wojtkowiak et al. 2012). GRP78 acts as a multifunctional protein in cancer, contributing to diverse cellular processes such as protein folding, ER homeostasis, regulation of apoptosis, and protection against cellular stress (Ferraresi et al. 2020).
The role of GRP78 extends beyond cancer. Here, we highlighted some diseases where GRP78 has been implicated: (Fig. 5 and Table2 below):
Novel approaches employed in downregulating GRP78 implicated in various diseases
Several researchers have been actively exploring the possibilities of targeting GRP78 downregulation in cancer therapeutic strategy. These efforts aim to restore normal cellular function and counteract the detrimental effects associated with GRP78 dysregulation. Recent publications have shed light on various innovative approaches employed in targeting GRP78 expression or function. Downregulation of GRP78 expression has been investigated as a potential therapeutic strategy in several diseases(Luo et al. 2018; Wang et al. 2017a, b). In prostate cancer, Lu et al. utilized RNA interference technology to downregulate GRP78 and GRP74 expression in the PC-3 cell line, and this result in reduced cell migration and induction of apoptosis(Lu et al. 2019). Another study focused on inhibiting the VEGF/GRP78 axis using the sFLT01 protein in human prostate cancer cells (DU145). The researchers observed downregulation of GRP78 and matrix metallopeptidase proteins 2&9 (MMP2&9) transcript levels, along with increased expression of tissue inhibitor of metalloproteinase proteins 1&2 (TIMP1&2), suggesting that sFLT01 inhibited cancer cell proliferation and invasiveness (Taghizadeh et al. 2021).
In breast cancer, a proteomic approach identified cell surface GRP78 and Dermcidin (DCD) as cooperative regulators of breast cancer cell migration through Wnt signaling. The interaction between GRP78 and DCD was found to be involved in the regulation of stem cell and cancer cell migration(Lager et al. 2021). Additionally, upregulating ATAD3A in colorectal cancer cells was shown to stabilize GRP78, reduce endoplasmic reticulum (ER) stress, and decrease chemotherapy-induced cancer cell death(Huang et al. 2021a, b). Cardiac glycosides (CGs), such as Lanatoside C (LanC), have been investigated for their inhibitory effect on GRP78 activation induced by ER stress in pancreatic cancer cells. The inhibition of GRP78 activation was associated with apoptosis induction in pancreatic cancer cells(Ha et al. 2021). Furthermore, Suppressor of cytokine signaling-3 (SOCS3) was found to target GRP78 ubiquitination for proteasomal degradation, thereby blocking ER stress and mitophagy pathways in cardiac hypertrophy, which could potentially prevent heart failure(Liu et al. 2021).
Traditional Chinese Medicine (TCM) treatments have also been explored for their ability to downregulate GRP78 expression. TCMSini's San (SNS), used for depression-related symptoms, was shown to attenuate GRP78 expression, preventing its interaction with LRP5 on the cell surface and inhibiting breast cancer stem cell signaling through the Wnt and β-catenin pathway(Zheng et al. 2021; Liu et al. 2022; Sadeghipour et al. 2022). Another TCM remedy, Ai Du Qing (ADQ), downregulated GRP78 expression, leading to the degradation of β-catenin and attenuating chemoresistance in breast cancer cells (Liao et al. 2021). Palmatine (Fig. 6), a plant-derived compound with various protective properties, was found to downregulate GRP78 expression in a streptozotocin-induced diabetic rat model. Palmatine treatment inhibited the upregulation of GRP78 in the pancreas, suggesting its potential as a therapeutic agent for diabetes(Okechukwu et al. 2021). In the field of immunotherapy, chimeric antigen receptor (CAR) T cells targeting cell surface GRP78 (GRP78.1x, GRP78.2x, and GRP78.3 × CAR T cells) demonstrated potent anti-tumor activity against acute myeloid leukemia (AML) cells. The CAR T cells induced the production of anti-tumor cytokines and effectively suppressed tumor progression in preclinical models (Yu et al. 2022) (Fig. 6).Recently, the activation of GRP78 ATPase by ZBM-H has been shown to suppress A549 lung cancer cell migration by promoting the degradation of integrin β4 (ITGB4). ZBM-H treatment resulted in decreased ITGB4 protein levels and inhibited the epithelial-mesenchymal transition (EMT) process in A549 cells. The activation of GRP78 ATPase by ZBM-H also facilitated the interaction between annexin A7 (ANXA7) and heat shock cognate 70 kDa protein (Hsc70), which contributed to the regulation of selective autophagy and degradation of ITGB4(Ning et al. 2022a, b).
These studies highlight various novel approaches employed to downregulate GRP78 expression and investigate its implications in different diseases. The use of RNA interference technology, protein inhibitors, traditional Chinese medicine, cardiac glycosides, and immunotherapy strategies targeting cell surface GRP78 have shown promising results in inhibiting tumor cell proliferation, migration, and inducing apoptosis. Additionally, the modulation of GRP78 expression has been explored in the context of diabetes, cardiac hypertrophy, and psychological stress-related diseases. Targeting GRP78 and exploring its regulatory mechanisms offer potential avenues for therapeutic interventions in cancer, diabetes, cardiovascular diseases, and other conditions associated with GRP78 dysregulation. Further research is needed to elucidate the underlying mechanisms and optimize the therapeutic strategies targeting GRP78 to improve patient outcomes.
Agents that target cell surface GRP78
Agents that target cell surface GRP78 include specific peptides, both conjugated and unconjugated, as well as the human plasminogen factor kringle 5. These agents have demonstrated efficient targeting of cell surface GRP78. Additionally, antibodies against cell surface GRP78 have been developed with diverse mechanisms to induce cancer cell death and suppress GRP78-mediated oncogenic signaling(Lee 2014; Hernandez and Cohen 2022). Numerous strategies have been employed to reduce cell surface GRP78 levels, and various inhibitory agents have been investigated. The table below provides a brief overview of phytochemicals, peptides, and antibodies that have shown inhibitory effects against cell surface GRP78 (Table 3) (Elfiky et al. 2020; Shimizu et al. 2022).
Despite, various strategies employed, there are some compounds that are site specific when binding. Several compounds like Honokiol, Salicylate and epigallocatechin gallate; directly bind to ATP binding domain to inactivate GRP78 in cancer(Ermakova et al. 2006; Martin et al. 2013). More so, alpha 2 macroglobulin and Mouse C38 and C107 binds with cell surface GRP78 at the N and C terminal respectively (Misra et al. 2009)(Fig. 7 and Table 4).
Clinical significance of GRP78
The clinical significance of glucose-regulated protein 78 (GRP78) in cancer is profound, as it participates in several tumor biology-related processes, suggesting its potential as a therapeutic target. GRP78 holds significance in three key aspects: tumor therapy, enhancement of therapeutic benefits, and as a prognostic biomarker.
Firstly, GRP78 can serve as a target for tumor therapy. Its overexpression in cancer cells has been consistently observed and is associated with various aspects of tumor progression, including cell survival, resistance to apoptosis, angiogenesis promotion, and facilitation of epithelial-mesenchymal transition (EMT). Targeting GRP78 with selective inhibitors or antibodies offers the potential to disrupt its pro-survival functions, sensitize cancer cells to conventional therapies, and overcome treatment resistance. By inhibiting GRP78, it becomes possible to interfere with critical cellular processes essential for cancer cell survival and growth, thereby enhancing the effectiveness of cancer treatment. Secondly, the creation of unfolded protein response (UPR)-related proteins, particularly GRP78, can enhance the therapeutic benefits of drugs commonly used in clinical practice. The UPR is a cellular stress response mechanism activated in cancer cells under conditions of increased protein folding demand and nutrient deprivation. GRP78 is a key regulator of the UPR and assists in maintaining cellular homeostasis during stress. Modulating the UPR through GRP78 can improve the efficacy of anticancer drugs by increasing their cytotoxic effects on cancer cells. This approach has the potential to enhance treatment outcomes and overcome drug resistance. Lastly, GRP78 can serve as a biomarker for evaluating cancer patients and predicting prognosis. The expression levels of GRP78 in tumor tissues or body fluids can be indicative of disease progression and patient outcomes. Assessing GRP78 as a diagnostic or prognostic biomarker holds promise for early cancer detection and risk stratification. By evaluating GRP78 expression, it becomes possible to identify high-risk patients who may benefit from aggressive treatment strategies or personalized therapies. Additionally, monitoring changes in GRP78 levels during the course of treatment can provide valuable information on treatment response and disease status.
In preclinical studies, selective inhibitors or antibodies against GRP78 have shown promising results in disrupting its pro-survival functions and sensitizing cancer cells to conventional therapies (Lee 2007). For instance, a study by Chen et al. (2022) demonstrated that an anti-GRP78 antibody, PAT-SM6, effectively inhibited tumor growth in melanoma mouse models(Chen et al. 2022). Furthermore, combination therapies involving GRP78 targeting have been investigated to enhance treatment response. Combining GRP78 inhibition with conventional therapies or emerging treatment modalities, such as immunotherapy, has shown synergistic effects, leading to improved outcomes (Lev et al. 2017). These approaches hold great potential for overcoming treatment resistance and improving patient survival. One approach to improving therapeutic outcomes is by utilizing UPR-related proteins, including GRP78, to enhance drug delivery and sensitize cancer cells to treatment. For instance, Zhao et al. (2015) developed a nanoparticle-based drug delivery system that specifically targets GRP78-expressing cancer cells, resulting in improved drug efficacy and reduced systemic toxicity (Zhao et al. 2015). By exploiting the UPR pathway, such strategies have the potential to optimize the effectiveness of conventional chemotherapeutic agents and overcome drug resistance mechanisms.
Despite the challenges in comprehending the precise role of csGRP78 in cancer, the targeting of csGRP78 has shown promise in preclinical and early clinical studies. PAT-SM6, an anti-GRP78 antibody, has demonstrated potent anti-tumoral activity in preclinical models and has progressed to phase I clinical trials in patients with malignant melanoma (Hensel et al. 2013). Further investigations are warranted to explore the therapeutic potential of csGRP78-targeted strategies and to gain a deeper understanding of its functional implications in different cancer types.
Conclusion and a Glimpse into the future
In conclusion, this study has provided a systematic breakdown and pictorial evidence showcasing the correlation between glucose-regulated protein 78 (GRP78) and various types of cancer. The consistent upregulation of GRP78 in cancer cells has been associated with tumor initiation, growth, metastasis, and resistance to therapy. The visual representation of GRP78 expression patterns in different cancer types strengthens its clinical relevance. GRP78 plays a crucial role in cancer development and progression by promoting cell survival, inhibiting apoptosis, facilitating angiogenesis, inducing epithelial-mesenchymal transition (EMT), and conferring therapy resistance. The extensive research supporting these observations highlights the potential of GRP78 as a therapeutic target in cancer treatment. The findings discussed here pave the way for several exciting future perspectives that could contribute to the development of effective cancer therapies. Firstly, there is a need to explore the therapeutic targeting of GRP78. The development of selective inhibitors or antibodies against GRP78 could disrupt its pro-survival functions and sensitize cancer cells to conventional therapies, potentially overcoming treatment resistance and improving patient outcomes. Secondly, investigating combination therapies involving GRP78 targeting is crucial. Assessing the synergistic effects of GRP78 inhibition with conventional therapies or emerging treatment modalities like immunotherapy may enhance treatment response and minimize the likelihood of relapse. Thirdly, exploring the utility of GRP78 as a diagnostic or prognostic biomarker holds promise. Further research is required to evaluate the clinical applicability of GRP78 in early cancer detection and risk stratification, enabling timely intervention and improved patient outcomes. Additionally, unraveling the intricate molecular mechanisms underlying GRP78's role in cancer progression is imperative. This knowledge will provide valuable insights into potential downstream targets and signaling pathways that can be exploited for therapeutic intervention. Finally, preclinical studies using in vitro and in vivo models should be conducted to validate the efficacy and safety of GRP78-targeted therapies before clinical translation. These studies will help optimize treatment strategies and assess potential side effects. In summary, the correlation between GRP78 and various cancers presents a promising avenue for future research and therapeutic interventions. By focusing on GRP78 as a target, we have the potential to overcome treatment resistance and improve outcomes for cancer patients. However, further investigation and validation are required to fully harness the therapeutic potential of GRP78 inhibition in clinical practice.
It is worth noting that GRP78 and other components of the unfolded protein response (UPR) also play important roles in autoimmune disorders and neurological disorders. Understanding the processes through which cell surface GRP78 functions will provide crucial insights into not only cancer but also these other disease areas. The overexpression of cell surface GRP78 in cancer makes it a potential target for tumor therapy(Farshbaf et al. 2020). Exploring new approaches that combine drugs promoting GRP78 translocation to the cell surface with peptides or small compounds targeting GRP78 could significantly enhance the efficacy of cancer treatments. Currently, two GRP78 antibodies, PAT-SM6 and Bold-100, have undergone testing in human clinical trials (Hensel et al. 2013; Rasche et al. 2015; Burris et al. 2016). Initial studies on PAT-SM6 in melanoma tumor-bearing mice showed potent anti-tumoral activity, and a phase I clinical trial was conducted in patients with malignant melanoma to assess the safety and anti-tumor effectiveness of the antibody (Hensel et al. 2013).
Furthermore, Bold-100 also known as IT-139, NKP1339) is a small ruthenium-based compound that specifically targets GRP78. By inhibiting GRP78, Bold-100 aims to disrupt the protective mechanisms of cancer cells, making them more vulnerable to standard cancer treatments such as chemotherapy and radiation therapy (Burris et al. 2016). The rationale behind targeting GRP78 is that it is overexpressed on the surface of cancer cells, providing them with enhanced survival advantages, including resistance to apoptosis (programmed cell death) and increased tolerance to stress conditions. By blocking GRP78, Bold-100 intends to sensitize cancer cells to treatment-induced cell death. Bold Therapeutics has conducted preclinical and early clinical studies to evaluate the safety and efficacy of Bold-100 in various types of cancers (Yoo et al. 2012).
Bold-100 is currently undergoing a global Phase 2 clinical trial for the treatment of advanced gastrointestinal cancers, including bile duct, colon, gastric, and pancreatic cancers. The trial, registered as NCT04421820, involves six sites in Canada, two in the United States, and five in South Korea. Promising results from the Phase 2 study in advanced colorectal cancer were presented at the American Association for Cancer Research (AACR) conference in April 2023. The data demonstrated a clinically significant improvement when compared to standard of care treatments. Specifically, among patients in the 3rd line and beyond with advanced colorectal cancer (n = 17), the study revealed a median Progression-Free Survival (PFS) of 4.7 months, Overall Survival (OS) of 9.8 months, and an Overall Response Rate (ORR) of 13%. The potential of Bold-100 has been recognized by the U.S. Food and Drug Administration (FDA), which has granted Orphan Drug Designations (ODDs) for gastric and pancreatic cancers. It is anticipated that in 2023, Bold-100 will also receive breakthrough therapy designations (BTDs) for colorectal cancer and potentially other indications. As demonstrated in this review, various solid tumor cell types express GRP78 on their surface, making them potential candidates for future clinical studies. Utilizing the GRP78 antibody in tumors where other antibody-based therapies are already available could offer clinical benefits through the development of mechanisms that enable targeted drug delivery.
Conclusively, further research and exploration are needed to fully exploit the therapeutic potential of GRP78 inhibition in cancer treatment, as well as to understand its implications in autoimmune and neurological disorders.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. Resources: https://www.clinicaltrials.gov/ct2/show/NCT01727778, https://classic.clinicaltrials.gov/ct2/show/NCT04421820.
Abbreviations
- AD:
-
Alzheimer's disease
- AML:
-
Acute myeloid leukemia
- ATF6:
-
Activating transcription factor 6
- BiP:
-
Binding immunoglobulin Protein
- CAR:
-
Chimeric antigen receptor
- CsGRP78:
-
Cell surface glucose regulatory protein
- DCD:
-
Dermcidin
- EGCG:
-
Epigallocatechin-3-gallate
- EMT:
-
Epithelial-mesenchymal transition
- ER:
-
Endoplasmic reticulum
- GnRHa:
-
Gonadotropin-releasing hormone analogs
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- GRP78:
-
Glucose regulatory protein
- GSK-3:
-
Glycogen synthase kinase-3
- HBV:
-
Hepatitis B virus
- HDAC1:
-
Histone deacetylase 1
- HPCs:
-
Hematopoietic progenitor cells
- HSP70:
-
Heat shock protein 70
- IRE1:
-
Inositol requiring enzyme 1
- JNK:
-
JUN amino-terminal kinases
- LanC:
-
Lanatoside C
- MDR:
-
Multidrug resistance
- MI:
-
Myocardial infarction
- MMPs:
-
Matrix metalloproteinases
- OA:
-
Osteoarthritis
- PERK:
-
Protein kinase R-like endoplasmic reticulum kinase
- P-gp:
-
P-glycoprotein
- PI3K:
-
Phosphoinositide 3-kinase
- pSS:
-
Primary Sjögren's syndrome
- SNc:
-
Substantia nigra pars compacta
- TCM:
-
Traditional Chinese Medicine
- TNFα:
-
Tumor necrosis factor α
- UPR:
-
Unfolded protein response
References
Abdel Malek MA, Jagannathan S, Malek E, Sayed DM, Elgammal SA, Abd El-Azeem HG, Thabet NM, Driscoll JJ. Molecular chaperone GRP78 enhances aggresome delivery to autophagosomes to promote drug resistance in multiple myeloma. Oncotarget. 2015;6:3098–110.
Abhishek A, Benita S, Kumari M, Ganesan D, Paul E, Sasikumar P, Mahesh A, Yuvaraj S, Ramprasath T, Selvam GS. Molecular analysis of oxalate-induced endoplasmic reticulum stress mediated apoptosis in the pathogenesis of kidney stone disease. J Physiol Biochem. 2017;73:561–73.
Agyemang AF, Harrison SR, Siegel RM, McDermott MF. Protein misfolding and dysregulated protein homeostasis in autoinflammatory diseases and beyond. Semin Immunopathol. 2015;37:335–47.
Al-Hashimi AA, Lebeau P, Majeed F, Polena E, Lhotak Š, Collins CAF, Pinthus JH, Gonzalez-Gronow M, Hoogenes J, Pizzo SV. Autoantibodies against the cell surface–associated chaperone GRP78 stimulate tumor growth via tissue factor. J Biol Chem. 2017;292:21180–92.
Amodio N, Stamato MA, Juli G, Morelli E, Fulciniti M, Manzoni M, Taiana E, Agnelli L, Cantafio MEG, Romeo E, Raimondi L, Caracciolo D, Zuccalà V, Rossi M, Neri A, Munshi NC, Tagliaferri P, Tassone P. Drugging the lncRNA MALAT1 via LNA gapmeR ASO inhibits gene expression of proteasome subunits and triggers anti-multiple myeloma activity. Leukemia. 2018;32:1948–57.
Arap MA, Lahdenranta J, Mintz PJ, Hajitou A, Sarkis AS, Arap W, Pasqualini R. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6:275–84.
Baumeister P, Luo S, Skarnes WC, Sui G, Seto E, Shi Y, Lee AS. Endoplasmic reticulum stress induction of the Grp78/BiP promoter: activating mechanisms mediated by YY1 and its interactive chromatin modifiers. Mol Cell Biol. 2005;25:4529–40.
Baumeister P, Dong D, Yong Fu, Lee AS. Transcriptional induction of GRP78/BiP by histone deacetylase inhibitors and resistance to histone deacetylase inhibitor–induced apoptosisresistance to HDAC inhibitors by GRP78 induction. Mol Cancer Ther. 2009;8:1086–94.
Beddoe T, Paton AW, Le Nours J, Rossjohn J, Paton JC. Structure, biological functions and applications of the AB5 toxins. Trends Biochem Sci. 2010;35:411–8.
Bellucci A, Navarria L, Zaltieri M, Falarti E, Bodei S, Sigala S, Battistin L, Spillantini M, Missale C, Spano P. Induction of the unfolded protein response by α-synuclein in experimental models of Parkinson’s disease. J Neurochem. 2011;116:588–605.
Best S, Hashiguchi T, Kittai A, Bruss N, Paiva C, Okada C, Liu T, Berger A, Danilov AV. Targeting ubiquitin-activating enzyme induces ER stress–mediated apoptosis in B-cell lymphoma cells. Blood Adv. 2019;3:51–62.
Bhattacharjee G, Ahamed J, Pedersen B, El-Sheikh A, Mackman N, Ruf W, Liu C, Edgington TS. Regulation of tissue factor–mediated initiation of the coagulation cascade by cell surface grp78. Arterioscler Thromb Vasc Biol. 2005;25:1737–43.
Bosco DA, Morfini G, Karabacak NM, Song Y, Gros-Louis F, Pasinelli P, Goolsby H, Fontaine BA, Lemay N, McKenna-Yasek D, Frosch MP, Agar JN, Julien JP, Brady ST, Brown RH Jr. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13:1396–403.
Burris HA, Bakewell S, Bendell JC, Infante J, Jones SF, Spigel DR, Weiss GJ, Ramanathan RK, Ogden A, Von Hoff D. Safety and activity of IT-139, a ruthenium-based compound, in patients with advanced solid tumours: a first-in-human, open-label, dose-escalation phase I study with expansion cohort. ESMO Open. 2016;1: e000154.
Carlos AJ, Ha DP, Yeh DW, Van Krieken R, Tseng CC, Zhang P, Gill P, Machida K, Lee AS. The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection. J Biol Chem. 2021;296: 100759.
Casas C. GRP78 at the centre of the stage in cancer and neuroprotection. Front Neurosci. 2017;11:177.
Celli CM, Jaiswal AK. Role of GRP58 in mitomycin C-induced DNA cross-linking. Cancer Res. 2003;63:6016–25.
Chang SC, Erwin AE, Lee AS. Glucose-regulated protein (GRP94 and GRP78) genes share common regulatory domains and are coordinately regulated by common trans-acting factors. Mol Cell Biol. 1989;9:2153–62.
Chen X, Easton D, Oh HJ, Lee-Yoon DS, Liu X, Subjeck J. The 170 kDa glucose regulated stress protein is a large HSP70-, HSP110-like protein of the endoplasmic reticulum. FEBS Lett. 1996;380:68–72.
Chen H-Y, Chang J-C, Chien K-Y, Lee Y-S, You G-R, Cheng A-J. The endogenous GRP78 interactome in human head and neck cancers: a deterministic role of cell surface GRP78 in cancer stemness. Sci Rep. 2018a;8:1–13.
Chen S, Wu J, Jiao K, Wu Q, Ma J, Chen D, Kang J, Zhao G, Shi Y, Fan D, Zhao G. MicroRNA-495-3p inhibits multidrug resistance by modulating autophagy through GRP78/mTOR axis in gastric cancer. Cell Death Dis. 2018b;9:1070.
Chen J, Lynn EG, Yousof TR, Sharma H, MacDonald ME, Byun JH, Shayegan B, Austin RC. Scratching the surface-an overview of the roles of cell surface GRP78 in cancer. Biomedicines. 2022;10:1098.
Chern YJ, Wong JCT, Cheng GSW, Yu A, Yin Y, Schaeffer DF, Kennecke HF, Morin G, Tai IT. The interaction between SPARC and GRP78 interferes with ER stress signaling and potentiates apoptosis via PERK/eIF2α and IRE1α/XBP-1 in colorectal cancer. Cell Death Dis. 2019;10:504.
Cook KL, Clarke R. Role of GRP78 in promoting therapeutic-resistant breast cancer. Future Med Chem. 2015;7:1529–34.
Crane ED, Al-Hashimi AA, Chen J, Lynn EG, Won KD, Lhoták Š, Naeim M, Platko K, Lebeau P, Byun JH, Shayegan B, Krepinsky JC, Rayner KJ, Marchiò S, Pasqualini R, Arap W, Austin RC. Anti-GRP78 autoantibodies induce endothelial cell activation and accelerate the development of atherosclerotic lesions. JCI Insight. 2018;3:e99363.
D’Angelo S, Staquicini FI, Ferrara F, Staquicini DaI, Sharma G, Tarleton CA, Nguyen H, Naranjo LA, Sidman RL, Arap W. Selection of phage-displayed accessible recombinant targeted antibodies (SPARTA): methodology and applications. JCI Insight. 2018;3:e98305.
de Ridder GG, Ray R, Pizzo SV. A murine monoclonal antibody directed against the carboxyl-terminal domain of GRP78 suppresses melanoma growth in mice. Melanoma Res. 2012;22:225–35.
Dong D, Ko B, Baumeister P, Swenson S, Costa F, Markland F, Stiles C, Patterson JB, Bates SE, Lee AS. Vascular targeting and antiangiogenesis agents induce drug resistance effector GRP78 within the tumor microenvironment. Cancer Res. 2005;65:5785–91.
Dong D, Stapleton C, Luo B, Xiong S, Ye W, Zhang Y, Jhaveri N, Zhu G, Ye R, Liu Z, Bruhn KW, Craft N, Groshen S, Hofman FM, Lee AS. A critical role for GRP78/BiP in the tumor microenvironment for neovascularization during tumor growth and metastasis. Cancer Res. 2011;71:2848–57.
Du T, Li H, Fan Y, Yuan L, Guo X, Zhu Q, Yao Y, Li X, Liu C, Yu X, Liu Z, Cui CP, Han C, Zhang L. The deubiquitylase OTUD3 stabilizes GRP78 and promotes lung tumorigenesis. Nat Commun. 2019;10:2914.
Duan XF, Xin YW. Overexpression of molecule GRP94 favors tumor progression in lung adenocarcinoma by interaction with regulatory T cells. Thorac Cancer. 2020;11:704–12.
Elfiky AA. Natural products may interfere with SARS-CoV-2 attachment to the host cell. J Biomol Struct Dyn. 2021;39:3194–203.
Elfiky AA, Ibrahim IM. Zika virus envelope—heat shock protein A5 (GRP78) binding site prediction. J Biomol Struct Dyn. 2021;39:5248–60.
Elfiky AA, Baghdady AM, Ali SA, Ahmed MI. GRP78 targeting: Hitting two birds with a stone. Life Sci. 2020;260: 118317.
Ermakova SP, Kang BS, Choi BY, Choi HS, Schuster TF, Ma WY, Bode AM, Dong Z. (-)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66:9260–9.
Farshbaf M, Khosroushahi AY, Mojarad-Jabali S, Zarebkohan A, Valizadeh H, Walker PR. Cell surface GRP78: an emerging imaging marker and therapeutic target for cancer. J Control Release. 2020;328:932–41.
Ferraresi A, Girone C, Esposito A, Vidoni C, Vallino L, Secomandi E, Dhanasekaran DN, Isidoro C. How autophagy shapes the tumor microenvironment in ovarian cancer. Front Oncol. 2020;10: 599915.
Frickel EM, Frei P, Bouvier M, Stafford WF, Helenius A, Glockshuber R, Ellgaard L. ERp57 is a multifunctional thiol-disulfide oxidoreductase. J Biol Chem. 2004;279:18277–87.
Fu Y, Wey S, Wang M, Ye R, Liao CP, Roy-Burman P, Lee AS. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci U S A. 2008;105:19444–9.
Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5: a013169.
Ghribi O, Herman MM, Pramoonjago P, Savory J. MPP+ induces the endoplasmic reticulum stress response in rabbit brain involving activation of the ATF-6 and NF-kappaB signaling pathways. J Neuropathol Exp Neurol. 2003;62:1144–53.
Girona J, Rodríguez-Borjabad C, Ibarretxe D, Vallvé J-C, Ferré R, Heras M, Rodríguez-Calvo R, Guaita-Esteruelas S, Martínez-Micaelo N, Plana N. The circulating GRP78/BiP is a marker of metabolic diseases and atherosclerosis: bringing endoplasmic reticulum stress into the clinical scenario. J Clin Med. 2019;8:1793.
Gopal U, Pizzo SV. Cell surface GRP78 signaling: an emerging role as a transcriptional modulator in cancer. J Cell Physiol. 2021;236:2352–63.
Ha DP, Tsai YL, Lee AS. Suppression of ER-stress induction of GRP78 as an anti-neoplastic mechanism of the cardiac glycoside Lanatoside C in pancreatic cancer: Lanatoside C suppresses GRP78 stress induction. Neoplasia. 2021;23:1213–26.
Hafiza WA, Latifah SY. Potential implications of GRP58 expression and susceptibility of cervical cancer to cisplatin and thymoquinone-based therapy. Onco Targets Ther. 2014;7:1375–87.
Han J, Li E, Chen L, Zhang Y, Wei F, Liu J, Deng H, Wang Y. The CREB coactivator CRTC2 controls hepatic lipid metabolism by regulating SREBP1. Nature. 2015;524:243–6.
Hardy B, Raiter A. Peptide-binding heat shock protein GRP78 protects cardiomyocytes from hypoxia-induced apoptosis. J Mol Med. 2010;88:1157–67.
Hebbar N, Epperly R, Vaidya A, Thanekar U, Moore SE, Umeda M, Ma J, Patil SL, Langfitt D, Huang S, Cheng C, Klco JM, Gottschalk S, Velasquez MP. CAR T cells redirected to cell surface GRP78 display robust anti-acute myeloid leukemia activity and do not target hematopoietic progenitor cells. Nat Commun. 2022;13:587.
Hebert-Schuster M, Rotta BE, Kirkpatrick B, Guibourdenche J, Cohen M. The interplay between glucose-regulated protein 78 (GRP78) and steroids in the reproductive system. Int J Mol Sci. 2018;19:1842.
Hendershot LM. The ER function BiP is a master regulator of ER function. Mt Sinai J Med. 2004;71:289–97.
Hendershot LM, Wei JY, Gaut JR, Lawson B, Freiden PJ, Murti KG. In vivo expression of mammalian BiP ATPase mutants causes disruption of the endoplasmic reticulum. Mol Biol Cell. 1995;6:283–96.
Hensel F, Eckstein M, Rosenwald A, Brändlein S. Early development of PAT-SM6 for the treatment of melanoma. Melanoma Res. 2013;23:264–75.
Hernandez I, Cohen M. Linking cell-surface GRP78 to cancer: from basic research to clinical value of GRP78 antibodies. Cancer Lett. 2022;524:1–14.
Hightower LE. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol. 1980;102:407–27.
Hirano N, Shibasaki F, Sakai R, Tanaka T, Nishida J, Yazaki Y, Takenawa T, Hirai H. Molecular cloning of the human glucose-regulated protein ERp57/GRP58, a thiol-dependent reductase. Identification of its secretory form and inducible expression by the oncogenic transformation. Eur J Biochem. 1995;234:336–42.
Hsu WM, Hsieh FJ, Jeng YM, Kuo ML, Tsao PN, Lee H, Lin MT, Lai HS, Chen CN, Lai DM, Chen WJ. GRP78 expression correlates with histologic differentiation and favorable prognosis in neuroblastic tumors. Int J Cancer. 2005;113:920–7.
Huang H, Gao Y, Liu A, Yang X, Huang F, Xu L, Danfeng X, Chen L. EIF3D promotes sunitinib resistance of renal cell carcinoma by interacting with GRP78 and inhibiting its degradation. EBioMedicine. 2019;49:189–201.
Huang KC, Chiang SF, Yang PC, Ke TW, Chen TW, Lin CY, Chang HY, Chen WT, Chao KC. ATAD3A stabilizes GRP78 to suppress ER stress for acquired chemoresistance in colorectal cancer. J Cell Physiol. 2021a;236:6481–95.
Huang L, Liu Q, Zhou T, Zhang J, Tian Q, Zhang Q, Wei W, Wu H. Deficiency of β-arrestin2 alleviates apoptosis through GRP78-ATF6-CHOP signaling pathway in primary Sjögren’s syndrome. Int Immunopharmacol. 2021b;101: 108281.
Ibrahim IM, Abdelmalek DH, Elfiky AA. GRP78: a cell’s response to stress. Life Sci. 2019;226:156–63.
Jin R, Zhao A, Han S, Zhang D, Sun H, Li M, Su D, Liang X. The interaction of S100A16 and GRP78 actives endoplasmic reticulum stress-mediated through the IRE1α/XBP1 pathway in renal tubulointerstitial fibrosis. Cell Death Dis. 2021;12:942.
Johnson JL. Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim Biophys Acta. 2012;1823:607–13.
Kabakov AE, Gabai VL. HSP70s in breast cancer: promoters of tumorigenesis and potential targets/tools for therapy. Cells. 2021;10:3446.
Kaira K, Toyoda M, Shimizu A, Mori K, Shino M, Sakakura K, Takayasu Y, Takahashi K, Oyama T, Asao T, Chikamatsu K. Expression of ER stress markers (GRP78/BiP and PERK) in patients with tongue cancer. Neoplasma. 2016;63:588–94.
Kelber JA, Panopoulos AD, Shani G, Booker EC, Belmonte JC, Vale WW, Gray PC. Blockade of cripto binding to cell surface GRP78 inhibits oncogenic cripto signaling via MAPK/PI3K and Smad2/3 pathways. Oncogene. 2009;28:2324–36.
Kempen V, Tessa S, Wenink MH, Leijten EFA, Radstake TRDJ, Boes M. Perception of self: distinguishing autoimmunity from autoinflammation. Nat Rev Rheumatol. 2015;11:483–92.
Khongwichit S, Sornjai W, Jitobaom K, Greenwood M, Greenwood MP, Hitakarun A, Wikan N, Murphy D, Smith DR. A functional interaction between GRP78 and Zika virus E protein. Sci Rep. 2021;11:393.
Kikuchi H, Almer G, Yamashita S, Guégan C, Nagai M, Xu Z, Sosunov AA, McKhann GM 2nd, Przedborski S. Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model. Proc Natl Acad Sci U S A. 2006;103:6025–30.
Kim SY, Kim HJ, Kim HJ, Kim DH, Han JH, Byeon HK, Lee K, Kim CH. HSPA5 negatively regulates lysosomal activity through ubiquitination of MUL1 in head and neck cancer. Autophagy. 2018;14:385–403.
Kim JW, Cho YB, Lee S. Cell surface GRP94 as a novel emerging therapeutic target for monoclonal antibody cancer therapy. Cells. 2021;10:670.
Koch G, Smith M, Macer D, Webster P, Mortara R. Endoplasmic reticulum contains a common, abundant calcium-binding glycoprotein, endoplasmin. J Cell Sci. 1986;86:217–32.
Koumenis C. ER stress, hypoxia tolerance and tumor progression. Curr Mol Med. 2006;6:55–69.
Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol. 2002;22:7405–16.
Kroeger H, Chiang WC, Felden J, Nguyen A, Lin JH. ER stress and unfolded protein response in ocular health and disease. Febs j. 2019;286:399–412.
Kuang XY, Jiang HS, Li K, Zheng YZ, Liu YR, Qiao F, Li S, Hu X, Shao ZM. The phosphorylation-specific association of STMN1 with GRP78 promotes breast cancer metastasis. Cancer Lett. 2016;377:87–96.
Kumar M, Garg H, Gupta N, Sharma A, Kaushal S, Kumar R, Dinda AK. Glucose- regulated protein 78 (GRP78) in renal cell carcinoma: a novel biomarker for predicting tumor behavior. Heliyon. 2021;7: e07300.
Kuwabara K, Matsumoto M, Ikeda J, Hori O, Ogawa S, Maeda Y, Kitagawa K, Imuta N, Kinoshita T, Stern DM, Yanagi H, Kamada T. Purification and characterization of a novel stress protein, the 150-kDa oxygen-regulated protein (ORP150), from cultured rat astrocytes and its expression in ischemic mouse brain. J Biol Chem. 1996;271:5025–32.
Lager TW, Conner C, Keating CR, Warshaw JN, Panopoulos AD. Cell surface GRP78 and Dermcidin cooperate to regulate breast cancer cell migration through Wnt signaling. Oncogene. 2021;40:4050–9.
Lebeau PF, Wassef H, Byun JH, Platko K, Ason B, Jackson S, Dobroff J, Shetterly S, Richards WG, Al-Hashimi AA, Won KD, Mbikay M, Prat A, Tang A, Paré G, Pasqualini R, Seidah NG, Arap W, Chrétien M, Austin RC. The loss-of-function PCSK9Q152H variant increases ER chaperones GRP78 and GRP94 and protects against liver injury. J Clin Invest. 2021;131:e128650.
Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–10.
Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Can Res. 2007;67:3496–9.
Lee AS. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev Cancer. 2014;14:263–76.
Lee CH, Chiang CF, Kuo FC, Su SC, Huang CL, Liu JS, Lu CH, Hsieh CH, Wang CC, Lee CH, Shen PH. High-molecular-weight hyaluronic acid inhibits IL-1β-induced synovial inflammation and macrophage polarization through the GRP78-NF-κB signaling pathway. Int J Mol Sci. 2021;22:11917.
Lee WJ, Jung EJ, Hwang JM, Bae JW, Kwon WS. GRP78 plays a key role in sperm function via the PI3K/PDK1/AKT pathway. Reprod Toxicol. 2022a;113:103–9.
Lev A, Lulla AR, Wagner J, Ralff MD, Kiehl JB, Zhou Y, Benes CH, Prabhu VV, Oster W, Astsaturov I, Dicker DT, El-Deiry WS. Anti-pancreatic cancer activity of ONC212 involves the unfolded protein response (UPR) and is reduced by IGF1-R and GRP78/BIP. Oncotarget. 2017;8:81776–93.
Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–71.
Li Z, Zhang L, Li H, Shan S, Li Z. Glucose regulated protein 78 promotes cell invasion via regulation of uPA production and secretion in colon cancer cells. BMB Rep. 2014;47:445–50.
Li J, Qi F, Su H, Zhang C, Zhang Q, Chen Y, Chen P, Su L, Chen Y, Yang Y, Chen Z, Zhang S. GRP75-faciliated mitochondria-associated ER Membrane (MAM) Integrity controls cisplatin-resistance in ovarian cancer patients. Int J Biol Sci. 2022;18:2914–31.
Liao M, Wang C, Yang B, Huang D, Zheng Y, Wang S, Wang X, Zhang J, Tang C, Xu Z, He Y, Huang R, Zhang F, Wang Z, Wang N. Autophagy blockade by ai du Qing formula promotes chemosensitivity of breast cancer stem cells via GRP78/β-Catenin/ABCG2 axis. Front Pharmacol. 2021;12: 659297.
Lin CY, Chen WH, Liao CT, Chen IH, Chiu CC, Wang HM, Yen TC, Lee LY, Chang JT, Cheng AJ. Positive association of glucose-regulated protein 78 during oral cancer progression and the prognostic value in oral precancerous lesions. Head Neck. 2010;32:1028–39.
Liu C, Bhattacharjee G, Boisvert W, Dilley R, Edgington T. In vivo interrogation of the molecular display of atherosclerotic lesion surfaces. Am J Pathol. 2003;163:1859–71.
Liu R, Li X, Gao W, Zhou Y, Wey S, Mitra SK, Krasnoperov V, Dong D, Liu S, Li D, Zhu G, Louie S, Conti PS, Li Z, Lee AS, Gill PS. Monoclonal antibody against cell surface GRP78 as a novel agent in suppressing PI3K/AKT signaling, tumor growth, and metastasis. Clin Cancer Res. 2013;19:6802–11.
Liu ZC, Chu J, Lin L, Song J, Ning LN, Luo HB, Yang SS, Shi Y, Wang Q, Qu N, Zhang Q, Wang JZ, Tian Q. SIL1 rescued bip elevation-related tau hyperphosphorylation in ER stress. Mol Neurobiol. 2016;53:983–94.
Liu K, Tsung K, Attenello FJ. Characterizing cell stress and GRP78 in glioma to enhance tumor treatment. Front Oncol. 2020;10: 608911.
Liu S, Sun WC, Zhang YL, Lin QY, Liao JW, Song GR, Ma XL, Li HH, Zhang B. SOCS3 negatively regulates cardiac hypertrophy via targeting GRP78-mediated ER stress during pressure overload. Front Cell Dev Biol. 2021;9: 629932.
Liu M, Yang J, Lv W, Wang S, Du T, Zhang K, Wu Y, Feng X. Down-regulating GRP78 reverses pirarubicin resistance of triple negative breast cancer by miR-495-3p mimics and involves the p-AKT/mTOR pathway. 2022. Biosci Rep. https://doi.org/10.1042/BSR20210245.
Lizardo MM, Morrow JJ, Miller TE, Hong ES, Ren L, Mendoza A, Halsey CH, Scacheri PC, Helman LJ, Khanna C. Upregulation of glucose-regulated protein 78 in metastatic cancer cells is necessary for lung metastasis progression. Neoplasia. 2016;18:699–710.
Lu T, Wang Y, Xu K, Zhou Z, Gong J, Zhang Y, Gong H, Dai Q, Yang J, Xiong B, Song Z, Yang G. Co-downregulation of GRP78 and GRP94 induces apoptosis and inhibits migration in prostate cancer cells. Open Life Sci. 2019;14:384–91.
Lu G, Luo H, Zhu X. Targeting the GRP78 pathway for cancer therapy. Front Med (lausanne). 2020;7:351.
Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32:805–18.
Luo C, Xiong H, Chen L, Liu X, Zou S, Guan J, Wang K. GRP78 promotes hepatocellular carcinoma proliferation by increasing FAT10 expression through the NF-κB pathway. Exp Cell Res. 2018;365:1–11.
Luo D, Fan N, Zhang X, Ngo FY, Zhao J, Zhao W, Huang M, Li D, Wang Y, Rong J. Covalent inhibition of endoplasmic reticulum chaperone GRP78 disconnects the transduction of ER stress signals to inflammation and lipid accumulation in diet-induced obese mice. Elife. 2022;11:e72182.
Mahadevan NR, Rodvold J, Sepulveda H, Rossi S, Drew AF, Zanetti M. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc Natl Acad Sci U S A. 2011;108:6561–6.
Maki RG, Old LJ, Srivastava PK. Human homologue of murine tumor rejection antigen gp96: 5’-regulatory and coding regions and relationship to stress-induced proteins. Proc Natl Acad Sci U S A. 1990;87:5658–62.
Martin S, Hill DS, Paton JC, Paton AW, Birch-Machin MA, Lovat PE, Redfern CP. Targeting GRP78 to enhance melanoma cell death. Pigment Cell Melanoma Res. 2010;23:675–82.
Martin S, Lamb HK, Brady C, Lefkove B, Bonner MY, Thompson P, Lovat PE, Arbiser JL, Hawkins AR, Redfern CP. Inducing apoptosis of cancer cells using small-molecule plant compounds that bind to GRP78. Br J Cancer. 2013;109:433–43.
Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen H-Y, Bray K, Reddy A, Bhanot G, Gelinas C. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–75.
Mazzarella RA, Green M. ERp99, an abundant, conserved glycoprotein of the endoplasmic reticulum, is homologous to the 90-kDa heat shock protein (hsp90) and the 94-kDa glucose regulated protein (GRP94). J Biol Chem. 1987;262:8875–83.
Mintz PJ, Kim J, Do KA, Wang X, Zinner RG, Cristofanilli M, Arap MA, Hong WK, Troncoso P, Logothetis CJ, Pasqualini R, Arap W. Fingerprinting the circulating repertoire of antibodies from cancer patients. Nat Biotechnol. 2003;21:57–63.
Misra UK, Gonzalez-Gronow M, Gawdi G, Wang F, Pizzo SV. A novel receptor function for the heat shock protein Grp78: silencing of Grp78 gene expression attenuates alpha2M*-induced signalling. Cell Signal. 2004;16:929–38.
Misra UK, Deedwania R, Pizzo SV. Binding of activated alpha2-macroglobulin to its cell surface receptor GRP78 in 1-LN prostate cancer cells regulates PAK-2-dependent activation of LIMK. J Biol Chem. 2005;280:26278–86.
Misra UK, Mowery Y, Kaczowka S, Pizzo SV. Ligation of cancer cell surface GRP78 with antibodies directed against its COOH-terminal domain up-regulates p53 activity and promotes apoptosis. Mol Cancer Ther. 2009;8:1350–62.
Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–4.
Mozos A, Roué G, López-Guillermo A, Jares P, Campo E, Colomer D, Martinez A. The expression of the endoplasmic reticulum stress sensor BiP/GRP78 predicts response to chemotherapy and determines the efficacy of proteasome inhibitors in diffuse large b-cell lymphoma. Am J Pathol. 2011;179:2601–10.
Mufrrih M, Chen B, Chan SW. Zika virus induces an atypical tripartite unfolded protein response with sustained sensor and transient effector activation and a blunted BiP response. Msphere. 2021;6:e0036121.
Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–51.
Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (-)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int J Cancer. 2005;114:513–21.
Ning X, Shu J, Du Y, Ben Q, Li Z. Therapeutic strategies targeting cancer stem cells. Cancer Biol Ther. 2013;14:295–303.
Ning J, Cui X, Li N, Li N, Zhao B, Miao J, Lin Z. Activation of GRP78 ATPase suppresses A549 lung cancer cell migration by promoting ITGB4 degradation. Cell Adh Migr. 2022a;16:107–14.
Ning J, Wang X, Li N, Cui X, Li N, Zhao B, Miao J, Lin Z. ZBM-H-induced activation of GRP78 ATPase promotes apoptosis via annexin A7 in A549 lung cancer cells. J Cell Biochem. 2022b;123:798–806.
Ninkovic S, Harrison SJ, Quach H. Glucose-regulated protein 78 (GRP78) as a potential novel biomarker and therapeutic target in multiple myeloma. Expert Rev Hematol. 2020;13:1201–10.
Nourbakhsh M, Sharifi R, Heydari N, Nourbakhsh M, Ezzati-Mobasser S, Zarrinnahad H. Circulating TRB3 and GRP78 levels in type 2 diabetes patients: crosstalk between glucose homeostasis and endoplasmic reticulum stress. J Endocrinol Invest. 2022;45:649–55.
Nuss JE, Choksi KB, DeFord JH, Papaconstantinou J. Decreased enzyme activities of chaperones PDI and BiP in aged mouse livers. Biochem Biophys Res Commun. 2008;365:355–61.
Okechukwu PN, Ekeuku SO, Chan HK, Eluri K, Froemming GRA. Palmatine inhibits up-regulation of GRP78 and CALR protein in an STZ-induced diabetic rat model. Curr Pharm Biotechnol. 2021;22:288–98.
Pan D, Yang Y, Nong A, Tang Z, Li QX. GRP78 activity moderation as a therapeutic treatment against obesity. Int J Environ Res Public Health. 2022;19:15965.
Papalas JA, Vollmer RT, Gonzalez-Gronow M, Pizzo SV, Burchette J, Youens KE, Johnson KB, Selim MA. Patterns of GRP78 and MTJ1 expression in primary cutaneous malignant melanoma. Mod Pathol. 2010;23:134–43.
Park K, Lee SE, Shin KO, Uchida Y. Insights into the role of endoplasmic reticulum stress in skin function and associated diseases. Febs j. 2019;286:413–25.
Park YS, Kim HL, Lee SH, Zhang Y, Kim IB. Expression of the endoplasmic reticulum stress marker GRP78 in the normal retina and retinal degeneration induced by blue LED stimuli in mice. Cells. 2021;10:995.
Paz Gavilán M, Vela J, Castaño A, Ramos B, del Río JC, Vitorica J, Ruano D. Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiol Aging. 2006;27:973–82.
Pfaffenbach KT, Lee AS. The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol. 2011;23:150–6.
Pouysségur J, Shiu RP, Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977;11:941–7.
Pyrko P, Schönthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–16.
Qiao Y, Dsouza C, Matthews AA, Jin Y, He W, Bao J, Jiang F, Chandna R, Ge R, Fu L. Discovery of small molecules targeting GRP78 for antiangiogenic and anticancer therapy. Eur J Med Chem. 2020;193: 112228.
Rabek JP, Boylston WH 3rd, Papaconstantinou J. Carbonylation of ER chaperone proteins in aged mouse liver. Biochem Biophys Res Commun. 2003;305:566–72.
Rasche L, Duell J, Castro IC, Dubljevic V, Chatterjee M, Knop S, Hensel F, Rosenwald A, Einsele H, Topp MS, Brändlein S. GRP78-directed immunotherapy in relapsed or refractory multiple myeloma - results from a phase 1 trial with the monoclonal immunoglobulin M antibody PAT-SM6. Haematologica. 2015;100:377–84.
Roller C, Maddalo D. The Molecular chaperone GRP78/BiP in the development of chemoresistance: mechanism and possible treatment. Front Pharmacol. 2013a;4:10.
Roy B, Lee AS. The mammalian endoplasmic reticulum stress response element consists of an evolutionarily conserved tripartite structure and interacts with a novel stress-inducible complex. Nucleic Acids Res. 1999;27:1437–43.
Royle J, Ramírez-Santana C, Akpunarlieva S, Donald CL, Gestuveo RJ, Anaya JM, Merits A, Burchmore R, Kohl A, Varjak M. Glucose-regulated protein 78 interacts with zika virus envelope protein and contributes to a productive infection. Viruses. 2020;12:524.
Rozas P, Bargsted L, Martínez F, Hetz C, Medinas DB. The ER proteostasis network in ALS: determining the differential motoneuron vulnerability. Neurosci Lett. 2017;636:9–15.
Sabirli R, Koseler A, Goren T, Turkcuer I, Kurt O. High GRP78 levels in Covid-19 infection: a case-control study. Life Sci. 2021;265: 118781.
Sadeghipour MM, Torabizadeh SA, Karimabad MN. The Glucose-Regulated Protein78 (GRP78) in the unfolded protein response (UPR) pathway: a potential therapeutic target for breast cancer. Anticancer Agents Med Chem; 2022
Sadeghipour MM, Torabizadeh SA, Karimabad MN. The glucose-regulated protein78 (GRP78) in the unfolded protein response (UPR) pathway: a potential therapeutic target for breast cancer. Anticancer Agents Med Chem. 2023;23:505–24.
Sato M, Yao VJ, Arap W, Pasqualini R. GRP78 signaling hub a receptor for targeted tumor therapy. Adv Genet. 2010a;69:97–114.
Schild H, Rammensee HG. gp96–the immune system’s Swiss army knife. Nat Immunol. 2000;1:100–1.
Schneider M, Winkler K, Kell R, Pfaffl MW, Atkinson MJ, Moertl S. The chaperone protein GRP78 promotes survival and migration of head and neck cancer after direct radiation exposure and extracellular vesicle-transfer. Front Oncol. 2022;12: 842418.
Sciandra JJ, Subjeck JR, Hughes CS. Induction of glucose-regulated proteins during anaerobic exposure and of heat-shock proteins after reoxygenation. Proc Natl Acad Sci U S A. 1984;81:4843–7.
Sedighzadeh SS, Khoshbin AP, Razi S, Keshavarz-Fathi M, Rezaei N. A narrative review of tumor-associated macrophages in lung cancer: regulation of macrophage polarization and therapeutic implications. Transl Lung Cancer Res. 2021;10:1889–916.
Sha Q, Jiang H. Protective effect of dexmedetomidine pre treatment against myocardial ischemia reperfusion injury in rats. Trop J Pharm Res. 2023;22:349–54.
Shahriari-Felordi M, Alikhani HK, Hashemian SR, Hassan M, Vosough M. Mini review ATF4 and GRP78 as novel molecular targets in ER-Stress modulation for critical COVID-19 patients. Mol Biol Rep. 2022;49:1545–9.
Shen K, Vesey DA, Ellis RJ, Vecchio SJD, Cho Y, Teixeira-Pinto A, McGuckin MA, Johnson DW, Gobe GC. GRP78 expression in tumor and perinephric adipose tissue is not an optimal risk stratification marker for clear cell renal cell carcinoma. PLoS ONE. 2019;14: e0210246.
Shi Q, Jin Xi, Fan R, Xing M, Guo J, Zhang Z, Zhang J, Shiwen Xu. Cadmium-mediated miR-30a-GRP78 leads to JNK-dependent autophagy in chicken kidney. Chemosphere. 2019;215:710–5.
Shimizu F, Ogawa R, Mizukami Y, Watanabe K, Hara K, Kadono C, Takahashi T, Misu T, Takeshita Y, Sano Y, Fujisawa M, Maeda T, Nakashima I, Fujihara K, Kanda T. GRP78 antibodies are associated with blood-brain barrier breakdown in anti-myelin oligodendrocyte glycoprotein antibody-associated disorder. Neurol Neuroimmunol Neuroinflamm. 2022;9:e1038.
Shin J, Toyoda S, Nishitani S, Fukuhara A, Kita S, Otsuki M, Shimomura I. Possible involvement of adipose tissue in patients with older age, obesity, and diabetes with SARS-CoV-2 infection (COVID-19) via GRP78 (BIP/HSPA5): significance of hyperinsulinemia management in COVID-19. Diabetes. 2021;70:2745–55.
Shin J, Toyoda S, Fukuhara A, Shimomura I. GRP78, a novel host factor for SARS-CoV-2: the emerging roles in COVID-19 related to metabolic risk factors. Biomedicines. 2022a;10:1995.
Shin WJ, Ha DP, Machida K, Lee AS. The stress-inducible ER chaperone GRP78/BiP is upregulated during SARS-CoV-2 infection and acts as a pro-viral protein. Nat Commun. 2022b;13:6551.
Shiu RP, Pouyssegur J, Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977;74:3840–4.
Stone KR, Smith RE, Joklik WK. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974;58:86–100.
Su R, Li Z, Li H, Song H, Bao C, Wei J, Cheng L. Grp78 promotes the invasion of hepatocellular carcinoma. BMC Cancer. 2010;10:20.
Suwanmanee Y, Wada M, Ueda K. Functional roles of GRP78 in hepatitis B virus infectivity and antigen secretion. Microbiol Immunol. 2021;65:189–203.
Sykes EK, Mactier S, Christopherson RI. Melanoma and the unfolded protein response. Cancers (basel). 2016;8:30.
Taghizadeh S, Soheili ZS, Sadeghi M, Samiei S, Ranaei Pirmardan E, Kashanian A, Zakeri F, Latifi-Navid H, Shams Najafabadi H. sFLT01 modulates invasion and metastasis in prostate cancer DU145 cells by inhibition of VEGF/GRP78/MMP2&9 axis. BMC Mol Cell Biol. 2021;22:30.
Tran Q, Lee H, Jung JH, Chang SH, Shrestha R, Kong G, Park J, Kim SH, Park KS, Rhee HW, Yun J, Cho MH, Kim KP, Park J. Emerging role of LETM1/GRP78 axis in lung cancer. Cell Death Dis. 2022;13:543.
Trink J, Ahmed U, O’Neil K, Li R, Gao B, Krepinsky JC. Cell surface GRP78 regulates TGFβ1-mediated profibrotic responses via TSP1 in diabetic kidney disease. Front Pharmacol. 2023;14:1098321.
Tsai YC, Mendoza A, Mariano JM, Zhou M, Kostova Z, Chen B, Veenstra T, Hewitt SM, Helman LJ, Khanna C, Weissman AM. The ubiquitin ligase gp78 promotes sarcoma metastasis by targeting KAI1 for degradation. Nat Med. 2007;13:1504–9.
Turpin J, Frumence E, Harrabi W, Haddad JG, El Kalamouni C, Desprès P, Krejbich-Trotot P, Viranaïcken W. Zika virus subversion of chaperone GRP78/BiP expression in A549 cells during UPR activation. Biochimie. 2020;175:99–105.
Udagawa T, Nemoto N, Wilkinson CR, Narashimhan J, Jiang L, Watt S, Zook A, Jones N, Wek RC, Bähler J, Asano K. Int6/eIF3e promotes general translation and Atf1 abundance to modulate Sty1 MAPK-dependent stress response in fission yeast. J Biol Chem. 2008;283:22063–75.
Wadhwa R, Kaul SC, Ikawa Y, Sugimoto Y. Identification of a novel member of mouse hsp70 family. Its association with cellular mortal phenotype. J Biol Chem. 1993;268:6615–21.
Wadhwa R, Taira K, Kaul SC. An Hsp70 family chaperone, mortalin/mthsp70/PBP74/Grp75: what, when, and where? Cell Stress Chaperones. 2002;7:309–16.
Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6.
Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–16.
Wang H, Pezeshki AM, Yu X, Guo C, Subjeck JR, Wang XY. The endoplasmic reticulum chaperone GRP170: from immunobiology to cancer therapeutics. Front Oncol. 2014;4:377.
Wang X, Zhou Y, Xia L, Zhao C, Chen L, Yi D, Chang J, Huang L, Zheng X, Zhu H, Xie Y, Xu Y, Lin K. Fabrication of nano-structured calcium silicate coatings with enhanced stability, bioactivity and osteogenic and angiogenic activity. Colloids Surf B Biointerfaces. 2015;126:358–66.
Wang C, Cai L, Liu J, Wang G, Li H, Wang X, Xu W, Ren M, Feng L, Liu P, Zhang C. MicroRNA-30a-5p inhibits the growth of renal cell carcinoma by modulating GRP78 expression. Cell Physiol Biochem. 2017a;43:2405–19.
Wang J, Li Y, Ma F, Zhou H, Ding R, Lu B, Zou L, Li J, Lu R. Inhibitory effect of Par-4 combined with cisplatin on human Wilms’ tumor cells. Tumour Biol. 2017b;39:1010428317716689.
Wang X, Bi X, Zhang G, Deng Y, Luo X, Xu L, Scherer PE, Ferdous A, Fu G, Gillette TG, Lee AS, Jiang X, Wang ZV. Glucose-regulated protein 78 is essential for cardiac myocyte survival. Cell Death Differ. 2018;25:2181–94.
Weng H, Liu F, Hu S, Li L, Wang Y. GnRH agonists induce endometrial epithelial cell apoptosis via GRP78 down-regulation. J Transl Med. 2014;12:306.
Wojtkowiak JW, Rothberg JM, Kumar V, Schramm KJ, Haller E, Proemsey JB, Lloyd MC, Sloane BF, Gillies RJ. Chronic autophagy is a cellular adaptation to tumor acidic ph microenvironmentschronic autophagic response to extracellular acidosis. Can Res. 2012;72:3938–47.
Wu Y, Guo Q, Ju X, Hu Z, Xia L, Deng Y, Zhao P, Zhang M, Shao Y, Huang S, He X, Wen H, Wu X. HNRNPH1-stabilized LINC00662 promotes ovarian cancer progression by activating the GRP78/p38 pathway. Oncogene. 2021;40:4770–82.
Xi J, Chen Y, Huang S, Cui F, Wang X. Suppression of GRP78 sensitizes human colorectal cancer cells to oxaliplatin by downregulation of CD24. Oncol Lett. 2018;15:9861–7.
Xia S, Duan W, Liu W, Zhang X, Wang Q. GRP78 in lung cancer. J Transl Med. 2021;19:118.
Yoo SA, You S, Yoon HJ, Kim DH, Kim HS, Lee K, Ahn JH, Hwang D, Lee AS, Kim KJ, Park YJ, Cho CS, Kim WU. A novel pathogenic role of the ER chaperone GRP78/BiP in rheumatoid arthritis. J Exp Med. 2012;209:871–86.
Yoon AR, Wadhwa R, Kaul SC, Yun CO. Why is mortalin a potential therapeutic target for cancer? Front Cell Dev Biol. 2022;10: 914540.
Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998;273:33741–9.
Yu W, Zhang H, Yuan Y, Tang J, Chen X, Liu T, Zhao X. Chimeric antigen receptor T cells targeting cell surface GRP78 to eradicate acute myeloid leukemia. Front Cell Dev Biol. 2022;10: 928140.
Yuan H, Zhao Z, Guo Z, Ma L, Han J, Song Y. A novel ER stress mediator TMTC3 promotes squamous cell carcinoma progression by activating GRP78/PERK signaling pathway. Int J Biol Sci. 2022;18:4853–68.
Zhang C. Roles of Grp78 in female mammalian reproduction. Adv Anat Embryol Cell Biol. 2017;222:129–55.
Zhang LH, Yang XL, Zhang X, Cheng JX, Zhang W. Association of elevated GRP78 expression with increased astrocytoma malignancy via Akt and ERK pathways. Brain Res. 2011;1371:23–31.
Zhang Yi, Tseng C-C, Tsai Y-L, Xiaoyong Fu, Schiff R, Lee AS. Cancer cells resistant to therapy promote cell surface relocalization of GRP78 which complexes with PI3K and enhances PI (3, 4, 5) P3 production. PLoS ONE. 2013;8: e80071.
Zhang J, Wang J, Liu Q, Gao J, Wang Qi. Polymorphisms of glucose-regulated protein 78 and clinical relevance of neuroblastoma: risk and prognosis. J Cancer Res Ther. 2016a;12:1178–83.
Zhang L, Zhang Q, Huang G. A transient quasi-3D entire time scale line source model for the fluid and ground temperature prediction of vertical ground heat exchangers (GHEs). Appl Energy. 2016b;170:65–75.
Zhang C, Luo Y, Zhang Z, Zhang Z, Zhang G, Wang F, Che Y, Fang L, Zhang Y, Sun N, He J. Identification of a prognostic immune signature for esophageal squamous cell carcinoma to predict survival and inflammatory landscapes. Front Cell Dev Biol. 2020;8: 580005.
Zhang Q, Ali M, Wang Y, Sun QN, Zhu XD, Tang D, Wang W, Zhang CY, Zhou HH, Wang DR. Galectin-1 binds GRP78 to promote the proliferation and metastasis of gastric cancer. Int J Oncol. 2022a;61:141.
Zhang T, Li J, Yang M, Ma X, Wang Z, Ma X, Sun M, Sun W, Xu J, Hua Y, Cai Z. CDK7/GRP78 signaling axis contributes to tumor growth and metastasis in osteosarcoma. Oncogene. 2022b;41:4524–36.
Zhao L, Li H, Shi Y, Wang G, Liu L, Su C, Su R. Nanoparticles inhibit cancer cell invasion and enhance antitumor efficiency by targeted drug delivery via cell surface-related GRP78. Int J Nanomedicine. 2015;10:245–56.
Zhao G, Kang J, Guanghui Xu, Wei J, Wang X, Jing X, Zhang L, Yang A, Wang K, Wang J, Wang Li, Hou J, Liu Q, Jiao K, Gao B. Tunicamycin promotes metastasis through upregulating endoplasmic reticulum stress induced GRP78 expression in thyroid carcinoma. Cell Biosci. 2020;10:115.
Zheng HC, Gong BC, Zhao S. The meta and bioinformatics analysis of GRP78 expression in gastric cancer. Oncotarget. 2017;8:73017–28.
Zheng Y, Zhang J, Huang W, Zhong LLD, Wang N, Wang S, Yang B, Wang X, Pan B, Situ H, Lin Y, Liu X, Shi Y, Wang Z. Sini san inhibits chronic psychological stress-induced breast cancer stemness by suppressing cortisol-mediated GRP78 activation. Front Pharmacol. 2021;12: 714163.
Zhu G, Lee AS. Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J Cell Physiol. 2015;230:1413–20.
Acknowledgements
We would like to express our sincere gratitude to Professor Tianyan Gao and Professor Qiou Wei for their invaluable guidance and insightful suggestions throughout the development of this manuscript. Their expertise and support have greatly enhanced the quality of our research. We would also like to acknowledge the Toxicology and Cancer Biology Department for their assistance and resources provision of enabling resources to undertake this study.
Funding
The work was generously supported by the National Institutes of Health (NIH) (No. R01 CA266579 to Zhiguo Li) and partially supported by the UK CARES Career Development Program (No. P30ES026529) and the American Cancer Society (No. IRG 19–140-31).
Author information
Authors and Affiliations
Contributions
The authors collectively contributed to the writing of this manuscript and have approved its submission in its final form.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors state that they have no known conflicts of interest, whether financial or personal, that could have influenced the work presented in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akinyemi, A.O., Simpson, K.E., Oyelere, S.F. et al. Unveiling the dark side of glucose-regulated protein 78 (GRP78) in cancers and other human pathology: a systematic review. Mol Med 29, 112 (2023). https://doi.org/10.1186/s10020-023-00706-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10020-023-00706-6