Abstract
Circular RNAs (circRNAs) have become a research hotspot in recent years with their universality, diversity, stability, conservativeness, and spatiotemporal specificity. N6-methyladenosine (m6A), the most abundant modification in the eukaryotic cells, is engaged in the pathophysiological processes of various diseases. An increasing amount of evidence has suggested that m6A modification is common in circRNAs and is associated with their biological functions. This review summarizes the effects of m6A modification on circRNAs and their regulation mechanisms in cancers, providing some suggestions of m6A-modified circRNAs in cancer therapy.
Similar content being viewed by others
Background
Circular RNAs (CircRNAs) were first discovered in the 1970s and were initially used to represent splicing errors before serving as a by-product of splicing (Sanger et al. 1976). Subsequently, a large number of biologically significant circRNAs have merged and come to the attention of scholars. Abnormally expressed circRNAs are commonly linked to various human diseases such as cardiovascular diseases (CVDs), kidney diseases, immunity, and cancers (Gomes et al. 2020; Jan van Zonneveld et al. 2021; Chen et al. 2019a; Shang et al. 2019). Therefore, circRNAs hold great promise for cancer diagnosis and treatment thanks to their universality, diversity, stability, conservativeness, and spatiotemporal specificity (Kristensen et al. 2019).
More than 170 chemically distinct types of modifications have been identified in messenger RNAs (mRNAs) and a few non-coding RNAs (ncRNAs) of eukaryotes, bacteria and archaea, giving rise to RNA epigenetics (Boccaletto et al. 2022). The most popular RNA modifications include N6-methyladenosines (m6A), 5-methylcytosines (m5C), 5-hydroxymethylcytosine (5hmC), N1-methyladenosines (m1A), N6, 2′-Odimethyladenosine (m6Am), 7-methylguanine (m7G), and pseudouridine (Ψ) (Nombela et al. 2021). Among them, the m6A modification is the most abundant base modification in eukaryotic cells with a typical consensus sequence RRACH motif (R = G or A; H = A, C, or U) (Dominissini et al. 2012). Generally, those bases are enriched in the coding sequence (CDS), 3′-untranslated regions (3′-UTRs), and near stop codons of mRNAs (Meyer et al. 2012).
Recently, the m6A modification in the N6 position of adenosine has been found in circRNAs (Yang et al. 2017). However, the regulatory network between m6A modification and circRNAs remains complex. This review, centered on the roles of m6A modification on circRNAs, summarizes the existing detection methods and databases for m6A-modified circRNAs. The regulatory mechanisms of m6A-modified circRNAs in cancers and their effects on chemoradiotherapy resistance are reviewed to provide a comprehensive understanding of cancer diagnosis and treatment.
Biogenesis, characteristics and biological functions of circRNAs
Biogenesis of circRNAs
CircRNAs have proliferated and are primarily generated by the back-splicing of pre-mRNAs. Four biogenesis models of circRNAs have been discovered, including lariat-driven circularization, intron pairing-driven circularization, RNA binding proteins (RBPs)-driven circularization, and intronic lariat (Kristensen et al. 2019). Besides, a small fraction of intron-derived circRNAs can also be generated by pre-tRNA. Briefly, the tRNA splicing nucleic acid endonuclease (TSEN) complex cleaves the intron-containing pre-tRNA at a typical bulge-helix-bulge (BHB) motif and then the resultant intron termini are joined by RtcB ligase to form a stable circRNA (Lu et al. 2015; Schmidt et al. 2019) (Fig. 1A). CircRNAs can thus be divided into four types based on their origins, including: exonic circRNAs (EcircRNAs), exon–intron circRNAs (EIciRNAs), intronic circRNAs (CiRNAs), and others, such as tRNA intronic circular RNAs (TricRNAs) (Schmidt et al. 2019; Zhang et al. 2013) (Fig. 1B).
Biogenesis and biological functions of circRNAs. A The biogenesis models of circRNAs include lariat-driven circularization, intron pairing-driven circularization, RBP-driven circularization, intronic lariat, and splicing of pre-tRNA. B Based on the origin of circRNA, it can be divided into four categories, namely EcircRNA, EIciRNA, CiRNA, and TricRNA. C CircRNAs serve four main biological functions, including acting as miRNA sponges, interacting with RBPs, translating into proteins, and regulating gene transcription
Characteristics of circRNAs
CircRNAs are found in nearly all mammals (Ji et al. 2019), plants (Wang et al. 2014), parasites (Broadbent et al. 2015), archaea (Danan et al. 2012), and viruses (Nahand et al. 2020). Particularly approximately 9% of expressed genes in human tissues can generate corresponding circRNAs in human heart, and 20% of genes can produce circRNAs in the brain (Aufiero et al. 2018; Rybak-Wolf et al. 2015). Researchers have validated more than 25,000 human fibroblast RNAs with backsplices as circRNAs (Jeck et al. 2013). Furthermore, the same genes can generate various types of circRNAs through alternative circularization (Salzman et al. 2012). Unlike linear RNAs with 5′ and 3′ ends, circRNAs have a covalently closed loop structure generated from primary transcripts by back-splicing (Jeck et al. 2013). CircRNAs are more stable than linear RNAs because the former ones do not have free ends, and therefore are resistant to foreign chemicals or exonuclease interference, and they have a long half-life of more than 48 h (Suzuki et al. 2006; Enuka et al. 2016). In this sense, circRNA can affect cell functions by accumulating in cells with slower division rates. CircRNAs are also highly conserved. One study has shown that approximately 20% of human circRNAs are homologous to mouse circRNAs (Guo et al. 2014). Another study discovered that approximately 20% of porcine splice sites involved in circRNA production are functionally conserved between mice and humans (Venø et al. 2015). Last but not least, circRNAs, which are dynamically expressed in a spatiotemporal manner, especially during mammalian brain development, have varied expression levels during the developmental process and at different regulation levels, making them more likely to be a disease biomarker (Venø et al. 2015; You et al. 2015).
Biological functions of circRNAs
As research advances, circRNAs have received increased attention for their biological functions, as evidenced by the following aspects. (i) Being as microRNA (miRNA) sponges. Many circRNAs have specific binding sites to miRNAs that can reduce the activity of miRNAs while increasing that of miRNA target genes. CircRNAs, as competing endogenous RNAs (ceRNAs) remain the most classical mechanism of tumor regulation (Hansen et al. 2013). (ii) Interacting with RBPs. Some circRNAs contain specific protein binding sites that bind to RBPs and regulate target RNA, thus fostering the linear splicing of the gene and parental gene transcription (Ashwal-Fluss et al. 2014). (iii) Being translated into proteins. Some circRNAs have proven to be translated by the IRES-dependent mechanism, and ribosomes can be recruited by IRES-transacting factors (ITAFs) to initiate translation in the absence of typical translation initiation factors (Jiang et al. 2021; Xia et al. 2019). Besides, m6A-modified circRNAs can function in cap-independent translation, which will be discussed further below. (iv) Regulating gene transcription. Some researchers claim that some circRNAs in the nucleus can regulate gene transcription and thus perform specific physiological functions. For example, some CiRNAs and EIciRNAs, such as Ci-ankrd52, EIciPAIP2, and EIciEIF3J, are abundant in the nucleus and associated with RNA Pol II to promote transcription of their parental genes (Li et al. 2015). It is worth mentioning that circRNAs can also act as regulators affecting mRNA translation and stability (Wu et al. 2019a; Huang et al. 2020) (Fig. 1C). Therefore, circRNAs have wide range of biological functions that need further exploration.
M6A writers, erasers, and readers
M6A modifications on circRNAs can be installed, removed, and recognized by the same m6A regulators in mRNAs, known as “writers” (methyltransferases), “erasers” (demethylases), and “readers” (recognitions).
M6A writers/methyltransferases
Generally, m6A modification are installed by various methyltransferases acting on specific RNAs, but most of them are installed by the multicomponent m6A methyltransferases complex (MTC, also named “writers”), with methyltransferase-like 3 and 14 proteins (METTL3 and METTL14) as its core components (Wang et al. 2016). Other MTC components, such as Wilms Tumor 1 Associated Protein (WTAP) (Ping et al. 2014), Vir-like m6A methyltransferase associated (VIRMA, also called “Virilizer” or “KIAA1429”) (Schwartz et al. 2014), RNA recognition motif 15/15B (RBM15/15B) (Patil et al. 2016), Zinc finger CCCH domain-containing protein 13 (ZC3H13) (Knuckles et al. 2018), and Cbl proto-oncogene-like 1 (CBLL1, also known as “HAKAI”) (Bawankar et al. 2021), also play roles in facilitating the complex’s recruitment to specific sites and maintaining its stability. Aside from the enzymes mentioned above involved in MTC formation, methyltransferase-like 16 (METTL16) (Pendleton et al. 2017), methyltransferase-like 5 (METTL5) (Tran et al. 2019), and Zinc finger CCCH-Type containing 4 (ZCCHC4) (Ma et al. 2019) have been discovered to be independent RNA methyltransferases. However, these methyltransferases can only catalyze a few m6A residues in RNAs (Pendleton et al. 2017; Tran et al. 2019; Pinto et al. 2020).
M6A erasers/demethylases
M6A methylation is a dynamic, multi-layered, and reversible process that can be removed by erasers (also known as “demethylases”). Fat mass and obesity-associated protein (FTO, also known as “ALKBH9”) and AlkB homolog 5 (ALKBH5) belong to the AlkB subfamily of Fe (II)/α-ketoglutaric acid (αKG) dioxygenase, and they can catalyze the demethylation of m6A in both αKG and Fe (II) dependence(Jia et al. 2011; Zheng et al. 2013).
M6A readers/recognitions
Numerous studies have revealed that m6A modifications can be recognized by various binding proteins (also called readers) to perform specific biological functions. To date, several readers have been extensively studied. Take YT521-B homology (YTH) domain family for example. It contains five proteins: YTH domain family protein 1 (YTHDF1), YTH domain family protein 2 (YTHDF2), YTH domain family protein 3 (YTHDF3), YTH domain containing 1 (YTHDC1), and YTH domain containing 2 (YTHDC2) (Liu et al. 2015). The first three are typically found in the cytoplasm to perform their functions. Among them, YTHDF2 can interact with the carbon catabolite repressor 4-negative on TATA (CCR4-NOT) complex to transport RNA to the processing body (P-body), thereby degrading RNA (Du et al. 2016). Besides, YTHDF1 and YTHDF3 have been found to act synergically to mediate m6A modifications in RNAs and affect the initiate translation of RNA with eukaryotic initiation factor 3, 4E, and 4G (eIF3, eIF4E, and eIF4G), poly(A) binding protein (PABP), and the 40S ribosomal subunit in a cap-dependent manner (Wang et al. 2015; Shi et al. 2017). However, a recent study has found that YTHDF2 can also exist in the nucleus, interact with m6A modifications on RNA within R-loops, and destabilize the RNA: DNA hybrids, thus regulating the accumulation of R-loops, and playing a role in safeguarding genomic stability (Abakir et al. 2020). YTHDC1 is also nuclear enriched and primarily involved in the selective splicing and nuclear transport of m6A transcripts (Widagdo et al. 2022). YTHDC2, which occurs in the cytoplasm and plays a vital role in RNA decay via interactions with adaptor proteins, and in RNA translation efficiency (Wojtas et al. 2017; Mao et al. 2019). In addition to the YTH domain family, heterogeneous nuclear ribonucleoprotein C1/C2 (HNRNPC), heterogeneous nuclear ribonucleoprotein G (HNRNPG), and heterogeneous nuclear ribonucleoprotein A2B1 (HNRNPA2B1), as part of the heterogeneous nuclear ribonucleoprotein (HNRNP) family are involved in alternative splicing and nuclear RNA processing (Alarcón et al. 2015; Liu et al. 2017). Furthermore, it has been proposed that eIF3 initiates translation in a cap-independent manner by binding to the m6A sites in the 5′-UTR of mRNAs (Meyer et al. 2015), while insulin-like growth factor 2 mRNA-binding protein 1/2/3 (IGF2BP1/2/3) can enhance the stability and translation of the target RNAs in the cytoplasm (Zhang et al. 2018; Wu et al. 2019b). Similar to IGF2BP1/2/3, fragile X mental retardation protein (FMRP) and proline-rich spiral coil 2A (PRRC2A) can also maintain the stability of their target RNAs. Furthermore, it is worth noting that FMRP can also occur in the nucleus and take part in the nuclear export of m6A-enriched RNAs (Hsu et al. 2019) (Fig. 2).
The dynamic and reversible process of m6A modification. The m6A modification can be installed by the multicomponent m6A methyltransferases complex (writers) which includes METTL3, METTL14, WTAP, VIRMA, RBM15/15B, ZC3H13 and CBLL1, as well as independent RNA methyltransferases such as METTL16, METTL5, ZCCHC4, and removed by demethylases (erasers) FTO and ALKBH5. Various binding proteins (readers) can then recognize the m6A modification to perform specific biological functions. In the nucleus, m6A can be identified by YTHDC1, HNRNPC/G, HNRNPA2B1, YTHDF2 and FMRP, and is involved in RNA alternative splicing, nuclear RNA processing, R-loop degradation, and RNA export. In the cytoplasm, m6A can be identified by YTHDF1/2/3, YTHDC2, IGF2BPs, eIF3, FMRP and PRRC2A, and regulates RNA stability, translation, and degradation
In summary, those m6A regulators, particularly readers, are complex and diverse. Their effects on m6A-modified circRNAs in cancers are discussed in detail below.
Detection methods and databases for m6A-modified circRNAs
Over the past decades, further research into the functions of m6A-mediated circRNAs has been limited by a lack of suitable detection methods and databases. However, with the continuous improvement of multiple detection methods and databases, especially the emergence of the next-generation sequencing (NGS), the field of m6A methylation has seen a dramatic shift.
Quantitative and semi-quantitative detection of m6A-modified circRNAs
As a semi-quantitative method for determining the level of overall m6A-modified circRNAs, dot blot is easy to operate and time-saving, but it only can confirm the presence of m6A or compare the amount of m6A in different groups, but cannot quantify or locate m6A (Zhou et al. 2017). In addition, the m6A level detection is a colorimetric method for quantifying the overall level of m6A RNA methylation in total RNAs, mRNAs, and ncRNAs. The concept of the test is similar to enzyme-linked immunosorbent assay (ELISA) and easy to operate (Ge et al. 2020). However, Ribonuclease R (RNase R) should be first used to de-linearize for quantifying the overall m6A level in total circRNAs and more research papers will be required to validate the method in the future. Besides, Methylated RNA immunoprecipitation (MeRIP) assay (m6A RIP), is a method for enriching m6A-modified circRNAs by using an anti-m6A antibody and quantitative real-time polymerase chain reaction (qPCR) to identify the enriched circRNAs. This method is convenient and only requires a kit to perform an experiment, but it lacks specificity (Chen et al. 2021). Moreover, the m6A-circRNA epitranscriptomic microarray in combination with a dual-color fluorescence microarray labeling system and RNA modification immunoprecipitation, allows for the quantitative detection of the percentage of epigenetic modifications in each transcript with a low total RNA requirement and high specificity. This method, however, is not widely used and deserves more attention (Fan et al. 2022) (Table 1).
The detection of m6A modification sites in circRNAs
Although most relevant methods focus on detecting m6A modifications in linear RNAs, the precise detection of m6A modification sites in circRNAs remains uncommon. Methylated RNA immunoprecipitation and sequencing (MeRIP-seq/m6A-seq), is a predominant method for detecting m6A modifications in RNAs. It mainly combines anti-m6A antibody with m6A-containing RNA fragments for NGS. The m6A-seq approach has some limitations: (i) It can only identify m6A hypermethylation enrichment regions on RNAs with a resolution of about 100nt, but cannot locate individual m6A sites; (ii) It requires a large number of total RNA samples due to its low sensitivity; (iii) Antibodies to m6A can recognize modifications similar to m6A, such as m6Am, with less specificity (Dominissini et al. 2012; Antanaviciute et al. 2017). Notably, a variety of antibody-independent methods for detecting m6A modifications have been discovered in recent years. For example, MazF PCR is a single-base m6A detection method that uses the m6A-sensitive RNA endonuclease MazF, which has been found to cleave RNAs with non-methylated ACA sequence, but not those with the methylated m6ACA sequence. However, to cover all the RRACH motifs in the transcriptome, new enzymes that recognize more universal sequence motifs must be explored (Imanishi et al. 2017). Besides, the T3 DNA ligase-dependent PCR assay is a highly sensitive and selective single-base detection that can locate m6A modification fraction at any specific RNA site. It is worth noting that both MazF PCR and ligase-dependent PCR assays for detecting m6A sites in circRNAs require RNase R to digest linear RNA before performing such validations (Liu et al. 2018). Furthermore, nanopore-based direct RNA sequencing (nanopore DRS) is another single-base detection method that locate m6A modifications in circRNAs by enriching circRNAs in samples, fragmenting and sequencing them on nanopore platforms, with high efficiency and simplicity (Wang et al. 2020). However, more studies are needed to validate the application of the aforementioned antibody-independent methods in m6A-related fundamental studies and clinical diagnosis (Table 1).
Databases for predicting m6A-modified circRNAs
The databases for predicting m6A methylation sites of circRNAs include Ensembl (Howe et al. 2021), Circm6A (Ye et al. 2021), TransCirc (Huang et al. 2021), SRAMP (Zhou et al. 2016), RMVar (Luo et al. 2021), RMBase V2.0 (Xuan et al. 2018), circBank (Liu et al. 2019), and DeepM6ASeq (Zhang and Hamada 2018). These databases can predict not only m6A modifications but also circRNAs with miRNA binding sites, protein-coding potential, conservations, mutations, etc. Notably, m6A2Target is a novel comprehensive database for exploring the target genes of writers, erasers, and readers of m6A modification (Deng et al. 2021). Thanks to their convenience, simplicity, and data visualization, those databases facilitate scientific research (Table 2).
Role of m6A modifications on circRNAs
M6A modification mediates circRNAs translation
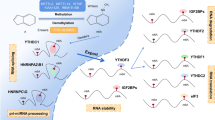
Accumulating evidence indicates that circRNAs code mainly through the IRES-driven translation and m6A-driven translation. Studies found that circRNAs containing m6A residues can be translated cap-independently. For example, Yang et al. discovered that the m6A-driven translation of circRNAs relies on the reading protein YTHDF3, as well as eukaryotic translation initiation factor 4 gamma 2 (eIF4G2) and eukaryotic initiation factor 3A (eIF3A) and that this process can be enhanced by methyltransferase METTL3/14 and inhibited by demethylase FTO. Moreover, further assays have indicated that an m6A site is sufficient to initiate translation and identify 33 peptides encoded by the back-splice junctions of m6A-modified circRNAs. These 33 peptides do not match any known proteins in the UniProt database but can be identified through proteomic analyses, suggesting that the m6A-driven translation of circRNAs widespread in the human transcriptome (Yang et al. 2017). Similarly, in human papillomavirus (HPV), circE7 with m6A modification can be translated into the E7 tumor protein (Zhao et al. 2019). Besides, studies have pointed out that m6A modifications can initiate and regulate circRNAs translation. Previous studies have discovered that circ-ZNF609 can be translated through the IRES-driven manner, while the latest one has identified that m6A-modified circ-ZNF609 can drive cap-independent translation through YTHDF3 and elF4G2. The above-mentioned findings suggest that the possibility of an interaction between the two forms that drive the translation of circRNAs. However, the specific correlation between them needs to be further explored (Legnini et al. 2017; Timoteo et al. 2020) (Fig. 3A).
Role of M6A modifications on circRNAs. A M6A modification mediates circRNAs translation. M6A-driven translation of circRNAs relies on YTHDF3, eIF4G2 and eIF3A. Meanwhile, the process can be enhanced by METTL3/14 and inhibited by FTO. Besides, it is suggested that an interaction may exist between the IRES-driven translation and m6A-driven translation. B M6A modification mediates circRNAs nucleoplasmic transport. The m6A readers, such as YTHDC1 and FMRP, could induce the nuclear and cytoplasmic transport of circRNAs. C M6A modification regulates the stability of circRNAs. M6A-modified circRNAs can be endoribonuclease-cleaved via YTHDF2-HRSP12-RNase P/MRP axis
To summarize, all those findings provide more possibilities for exploring the translation of m6A-driven circRNAs.
M6A modification mediates nucleoplasmic transport of circRNAs
In recent years, many published articles have shown that individual circRNAs can be transported into the cytoplasm during biogenesis and development, competing with other RNAs for binding by RBPs or miRNAs (Memczak et al. 2013). Therefore, it is crucial to understand how circRNAs export from nucleus to the cytoplasm. In drosophila, researchers have found that the Drosophila DExH/D-box helicase at 25E (Hel25E) interference significantly enriches circRNAs in the nucleus. In human cells, circRNAs have been discovered to be transported from the nucleus to the cytoplasm in a transcript-length-dependent manner via drosophila Hel25E and its human homologs, ATP-dependent RNA helicase DDX39A (also termed as nuclear RNA helicase URH49) and spliceosomal RNA helicase DDX39B (also termed as dead box protein UAP56) (Huang et al. 2018). Besides, Chen et al. identified that circ1662 overexpression increases the nuclear yes-associated protein 1 (YAP1) and decreases the cytoplasmic YAP1, indicating that circ1662 could promote YAP1 nuclear transport. Further function assays have confirmed that circ1662 promotes colorectal cancer (CRC) invasion and migration by accelerating YAP1 nuclear transport (Chen et al. 2021). In addition, the m6A reader YTHDC1 can bind to circNSUN2 and facilitate circNSUN2 to export from the nucleus to the cytoplasm in an m6A-dependent manner, and to promote colorectal liver metastasis through the circNSUN2-IGF2BP2-High Mobility Group AT-Hook 2 (HMGA2) RNA–protein ternary complex in the cytoplasm (Chen et al. 2019b). Furthermore, YTHDC1 and FMRP have been identified as readers to recognize HBV transcripts with m6A methylation modification and facilitate their transport to the cytoplasm (Kim et al. 2021) (Fig. 3B).
Consequently, the m6A modification can affect the nuclear and cytoplasmic transport of circRNAs by interacting with proteins.
M6A modification regulates the stability of circRNAs
RNase R and actinomycin D assays have shown that circRNAs are more stable than their origin genes, because they are not easily degraded by nucleic acid exonucleases and have a long half-life. Nonetheless, a recent study has pointed out that circRNAs can be degraded in some unique manners. For example, Hansen et al. revealed that the removal of the circular cerebellar degeneration associated protein 1 (CDR1) antisense transcripts with perfect complementary miRNA target sites could be mediated by miR-671 in an Argonaute2 (Ago2)-slicer-dependent manner. However, it does not work for circRNAs that lack miRNA sponge function or specific miRNA target sites (Hansen et al. 2011). Another study has reported that the depletion of GW182, a key component of P-body and RNA interference (RNAi) machine, can accumulate steady-state circRNA transcripts. However, that of other P-body components or RNAi machine factors does not affect circRNA levels, indicating that GW182 is a major factor in circRNA degradation. Nevertheless, the specific mechanisms remain to be further investigated (Jia et al. 2019). Aside from the above-mentioned findings, YTHDF2-heat-responsive protein 12 (HRSP12)-ribonuclease P (RNase P)/mitochondrial RNA processing (MRP) is the most common way of endoribonucleolytic cleavage of m6A-modified circRNAs. HRSP12 acts as an adapter protein that links YTHDF2 and RNase P/MRP, rapidly degrading YTHDF2-bound circRNAs (Park et al. 2019) (Fig. 3C).
Therefore, the complexities in the degradation of m6A circRNAs could contribute to the more dynamic regulation of m6A-modified circRNAs during various biological and physiological processes.
M6A-modified circRNAs in cancers
Colorectal cancer
Colorectal cancer (CRC) has been reported to rank third in incidence, and second in mortality according to the latest research. It accounts for about one in ten cancer cases and deaths (Sung et al. 2021). Therefore, specific mechanisms must be explored to better understand the CRC progression.
By using the MeRIP assay, Gene Expression Omnibus (GEO), and The Cancer Genome Atlas (TCGA) databases, researchers have found that circ3823 is enriched in the m6A precipitated fraction and have speculated that YTHDF3 and ALKBH5 cooperate with YTHDF2 to degrade circ3823, demonstrating that circ3823 might promote CRC growth, metastasis, and angiogenesis via circ3823/miR-30c-5p/Transcription factor 7 (TCF7) axis (Guo et al. 2021). Besides, refractory metastatic CRC is usually the leading cause of death in CRC patients (Hofheinz and Stintzing 2019). For example, Chen et al. demonstrated that METTL3 can induce circ1662 formation by installing m6A modifications in its flanking reverse complementary sequences via MeRIP assay, thus promoting epithelial-mesenchymal transition (EMT) and accelerating lung metastases of CRC via the YAP1-mothers against decapentaplegic homolog 3 (SMAD3) axis (Chen et al. 2021). Additionally, another study has identified that m6A-modified circNSUN2 is frequently upregulated in CRC patients with liver metastasis (LM), indicating a lower patient survival. MeRIP assay and other assays first verified that circNSUN2 is highly enriched in the m6A precipitated fraction and YTHDC1 can promote cytoplasmic export of m6A-modified circNSUN2. Further assays have indicated that circNSUN2 enhances the stability of HMGA2 mRNA by forming a circNSUN2/IGF2BP2/HMGA2 ternary complex in the cytoplasm, thus leading to the LM of CRC (Chen et al. 2019b).
In conclusion, those findings suggest that m6A-modified circRNAs may play a vital role in the CRC progression and serve as a potential diagnostic and therapeutic target for CRC, especially the metastasis-related CRC.
Gastric cancer
In the latest global cancer report, gastric cancer (GC) is the fifth most common cancer and the fourth leading cause of cancer death worldwide (Sung et al. 2021). Therefore, further study of the molecular mechanism underlying GC is required.
Zhang et al. first predicted the potential m6A sites of the top 20 differentiated expressed circRNAs (DECs) by adopting the SRAMP database and m6A RIP assays, indicating that the m6A level of DECs is positively correlated with the DEC expression level in gastric tissues and may be closely related to circRNA functionality. Nevertheless, more research into the potential functions and mechanisms of m6A modification on identified DECs in poorly differentiated gastric adenocarcinoma (PDGA) is needed (Zhang et al. 2020). M6A-circRNA epitranscriptomic microarray and MeRIP assays have revealed that METTL14 can regulate the m6A level and expression of circORC5, and that METTL14-mediated circORC5 can sponge miR-30c-2-3p to regulate AKT1 substrate 1 (AKT1S1) and eukaryotic translation initiation factor 4B (EIF4B) expression in GC cells, thereby promoting GC progression (Fan et al. 2022).
Overall, those findings shed light on how m6A-modified circRNAs contribute to GC.
Liver cancer
Liver cancer is the sixth most common cancer and the third leading cause of cancer death worldwide, among which hepatocellular carcinoma (HCC) comprises 75–85% (Sung et al. 2021). Several studies have shown that m6A-modified circRNAs are involved in HCC regulation.
In the study of Chi et al., circMAP2K4 was validated to promote HCC biogenesis via the miR-139-5p/YTHDF1 axis. Then, the expression and prognostic value of all m6A RNA methylation modulators and the biological pathways were evaluated by TCGA and International Cancer Genome Consortium (ICGC) databases, indicating that the circRNA regulatory network based on hsa-miR-139-5p/YTHDF1 axis is involved in regulating m6A RNA methylation modulators (Chi et al. 2021). Besides, Liu et al. observed that KIAA1429 is negatively correlated with m6A-modified circDLC1 after the intersection of RNA-seq and m6A-seq approaches. Further assays have found that circDLC1 binds to Human Antigen R (HuR) and blocks the interaction between HuR and matrix metalloproteinase 1 (MMP1) mRNAs, suggesting that m6A-regulated circDLC1 may serve as a therapeutic target for HCC (Liu et al. 2021). Additionally, MeRIP-seq, SRAMP database, and m6A RIP assays have confirmed that circHPS5 is highly m6A-modified, and METTL3 can mediate the circHPS5 formation. YTHDC1 can expedite the cytoplasmic output of m6A-modified circHPS5, making circHPS5 act as a miR-370 sponge to regulate HMGA2 expression and accelerate HCC cell development (Rong et al. 2021).
Hence, those findings convincingly indicated that m6A regulated-circRNAs may serve as potential therapeutic targets for liver cancer.
Breast cancer
Breast cancer (BC) is the fifth leading cause of cancer mortality, surpassing lung cancer as the leading cause worldwide (Sung et al. 2021). Therefore, identifying novel mechanisms and therapeutic targets is crucial for BC treatment.
Fortunately, the circBank database and m6A RIP assays have revealed that circMETTL3 is highly enriched in m6A precipitated fraction, and its expression is affected by the m6A modification. CircMETTL3 can sponge miR-31-5p to upregulate cyclin-dependent kinases (CKD1) expression, thus promoting BC progression (Li et al. 2021a).
Those findings indicated that circMETTL3 may act as a potential therapeutic target for BC. Nevertheless, the role of m6A-modified circRNAs in BC is rarely reported and deserves more attention.
Cervical cancer
Cervical cancer (CC) is the fourth most commonly diagnosed cancer and the fourth leading cause of cancer death in women (Sung et al. 2021).
M6A-RIP assay has confirmed that METTL3 can mediate the m6A modification level of human papillomavirus (HPV)-derived circE7. Further assays have revealed that circE7 can encode E7 oncoprotein in a heat-shock regulated manner and that the mutation of the potential m6A motifs of circE7 can strongly inhibit E7 oncoprotein expression, implying that m6A-modified circE7 plays a vital role in the translation mechanism (Zhao et al. 2019). Besides, another study has found that m6A-modified circARHGAP12 can interact with the m6A reader IGF2BP2 to enhance forkhead box M1 (FOXM1) mRNA stability and thus allow CC cells to proliferate and migrate(Ji et al. 2021).
In summary, those achievements might provide ideas for the targeted therapy based on the mechanisms of m6A-modified circRNAs regulating CC tumorigenesis.
Lung cancer
Lung cancer remains the leading cause of cancer morbidity and mortality worldwide, with non-small cell lung cancer (NSCLC) accounting for about 80–85% (Sung et al. 2021). Despite recent advances in NSCLC treatment, the overall cure and survival rates remain low (Hirsch et al. 2017). Therefore, it is crucial to study and figure out the molecular mechanism of NSCLC to improve its prognosis.
In the study by Li et al., the MeRIP assay revealed that circNDUFB2 is considerably enriched in m6A modification, and that METTL3/14 plays a significant role in affecting the interactions between circNDUFB2 and IGF2BPs. CircNDUFB2 not only acts as a scaffold by forming a tripartite motif containing 25 (TRIM25)/circNDUFB2/IGF2BPs ternary complex to facilitate the degradation of IGF2BPs, but it also triggers cellular immune responses by activating retinoic acid-inducible gene-I (RIG-I), thereby regulating NSCLC progression (Li et al. 2021b).
To sum up, their study broadens the knowledge of m6A-modified circRNAs action in NSCLC progression, implying that circNDUFB2 may have immunotherapy potentials for NSCLC.
Glioma
Glioma, an intracranial malignant tumor, has a high mortality and morbidity rate (Ostrom et al. 2014). Recent research into the molecular mechanism of glioma malignant proliferation has sparked widespread concern.
By using m6A level detection and MeRIP assays, Wu et al. discovered that METTL3-mediated m6A modification can enhance the stability and expression of circDLC1, thereby promoting the competitive binding of circDLC1 and miR-671-5p, facilitating Catenin Beta Interacting Protein 1 (CTNNBIP1) transcription, and ultimately suppressing the malignant proliferation of glioma cells (Wu et al. 2022).
This study first reported the mechanism of METTL3-mediated m6A modification of circDLC1 on the malignant proliferation of glioma cells, shedding light on glioma treatment.
M6A-modified circRNAs and tumor chemoradiotherapy resistance
Increasing evidence suggests that m6A-modified circRNAs may also contribute to cancer chemotherapy resistance. For example, in sorafenib-resistant HCC cells, Xu et al. demonstrated that the m6A modification can increase its stability to regulate circRNA-SORE expression by using SRAMP, RMBase v2.0 database, and MeRIP assays, and that increased circRNA-SORE can sponge miR-103a-2-5p and miR-660-3p to activate Wingless-types/beta-catenin (Wnt/β-catenin) pathway and induce sorafenib resistance (Xu et al. 2020). Besides, the SRAMP database and MeRIP assays discovered that circMAP3K4 is highly enriched in the m6A modification, and further investigations revealed that IGF2BP1-mediated m6A recognition can translate circMAP3K4 into circMAP3K4 translation produced a 455 amino acid protein (circMAP3K4-455aa), thus preventing HCC cells from cisplatin-induced death (Duan et al. 2022). Additionally, recent research has explored how radiotherapy affects hypopharyngeal squamous cell carcinoma (HPSCC) prognosis. Diagnostics and treatments based on molecular biology are urgently needed to mitigate toxicity and adverse effects. For example, one study using MeRIP assays confirmed that METTL3 could stabilize the expression of circCUX1 through m6A modification in head and neck tumor cell lines. Notably, circCUX1 can bind to caspase 1 mRNA and inhibit its expression, thereby inhibiting caspase 1 mediated inflammation and developing tolerance to radiotherapy (Wu et al. 2021) (Table 3).
To sum up, those findings suggest that m6A-modified circRNAs may act as a potential therapeutic target for tumor chemotherapy and radiotherapy tolerance.
Conclusion and remarks
Much evidence supports that epigenetic modification can affect RNAs involved in cellular processes. The m6A modification on circRNAs has been gradually identified and is also critical for human development and disease progression. Similar to the modification in mRNAs, the m6A modification in circRNAs can be written, removed, and read by the same regulators and perform specific biological functions. In terms of the biological function, m6A modification can regulate circRNA translation, nuclear-cytoplasmic transport, and degradation. Most importantly, m6A-modified circRNAs can participate in various physiological and pathological processes, particularly in cancers. That means m6A-modified circRNAs have a wide range of biological functions and a broad research space in the future.
Previous studies have shown that circRNAs are stable in blood and body fluids due to their unique structure of single-stranded, covalently closed circular transcripts, which can help them avoid exonuclease degradation. Hence, abnormal-expressed circRNAs in peripheral blood or body fluids have been proven useful as biomarkers for tumor diagnosis (Ge et al. 2022). One recent study has found that the m6A level in peripheral blood RNA combined with current tumor markers such as carcinoembryonic antigen (CEA) or m6A demethylases ALKBH5 and FTO can improve the diagnostic value of m6A, revealing that the m6A level in peripheral blood RNA can be a potential biomarker for GC diagnosis and follow-up (Ge et al. 2020). Additionally, several cancer treatments, including surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy, have been widely applied over the past few decades, generally prolonging disease-free survival (PFS) and overall survival (OS) rates among cancer patients (Maji et al. 2018; Esfahani et al. 2020). However, due to the enormous tumor heterogeneity, cancer cells typically show primary or acquired drug resistance, leading to cancer treatment failure. For this reason, an increasing amount of research is focusing on less toxic therapies based on molecular biology. Aside from the m6A-modified circRNAs as therapeutic targets for tumor chemotherapy and radiotherapy resistance, the m6A regulators have also become therapeutic targets for tumors. For example, one research has revealed that ALKBH5-mediated alterations in m6A density can regulate the splicing and expression of mRNAs with potential roles in controlling tumor growth, thus suggesting that ALKBH5, the m6A demethylase, can be a potential therapeutic target for cancer treatment alone or in combination with immune checkpoint blockade (ICB) (Li et al. 2020). Nevertheless, more research is needed to comprehensively understanding how m6A regulatory factors function in cancer therapy. Furthermore, some methods for detecting m6A-modified circRNAs, such as dot blot, MeRIP assay, and MeRIP-seq, are widely used. Other methods, such as m6A-circRNA epitranscriptomic microarray, MazF PCR, and nanopore DRS, will require more proof-of-concept studies in the future.
Briefly, more studies on the biological functions and mechanisms of m6A-modified circRNAs are needed, especially in the following aspects: (i) Detecting whether the m6A level of m6A-modified circRNAs in peripheral blood or other liquid biopsy samples can serve as biomarkers or not; (ii) Determining how much m6A regulators and m6A-modified circRNAs play essential roles in cancer therapy and offer potential therapeutic targets; and (iii) Overcoming the technical obstacles and challenges in studying m6A-modified circRNAs. Based on previous research, we believe m6A-modified circRNAs will advance the field of the epigenome, provide novel potential targets for cancer progression, and generate more serendipity.
Availability of data and materials
Not applicable.
Abbreviations
- circRNAs:
-
Circular RNAs
- m6A:
-
N6-methyladenosine
- CVDs:
-
Cardiovascular diseases
- mRNAs:
-
Messenger RNAs
- ncRNAs:
-
Non-coding RNAs
- m5C:
-
5-Methylcytosines
- 5hmC:
-
5-Hydroxymethylcytosine
- m1A:
-
N1-methyladenosines
- m6Am:
-
N6, 2′-Odimethyladenosine
- m7G:
-
7-Methylguanine
- Ψ:
-
Pseudouridine
- CDS:
-
Coding sequence
- 3′-UTRs:
-
3′-Untranslated regions
- RBP:
-
RNA binding protein
- TSEN:
-
TRNA splicing endonuclease
- BHB:
-
Bulge-helix-bulge motif
- EcircRNAs:
-
Exonic circRNAs
- EIciRNAs:
-
Exon–intron circRNAs
- CiRNAs:
-
Intronic circRNAs
- TricRNAs:
-
TRNA intronic circular RNAs
- miRNA:
-
MicroRNA
- ceRNA:
-
Competing endogenous RNA
- IRES:
-
Internal ribosome entry site
- ITAFs:
-
IRES-transacting factors
- MTC, also named “writers”:
-
Methyltransferases complex
- METTL3:
-
Methyltransferase-like 3 protein
- METTL14:
-
Methyltransferase-like 14 protein
- WTAP:
-
Wilms Tumor 1 Associated Protein
- VIRMA, also called “Virilizer” or “KIAA1429”:
-
Vir-like m6A methyltransferase associated
- RBM15/15B:
-
RNA recognition motif 15/15B
- ZC3H13:
-
Zinc finger CCCH domain-containing protein 13
- CBLL1, also known as “HAKAI”:
-
Cbl proto-oncogene-like 1
- METTL16:
-
Methyltransferase-like 16
- METTL5:
-
Methyltransferase-like 5
- ZCCHC4:
-
Zinc finger CCCH-Type containing 4
- FTO, also known as “ALKBH9”:
-
Fat mass and obesity-associated protein
- ALKBH5:
-
AlkB homolog 5
- αKG:
-
α-Ketoglutaric acid
- YTH:
-
YT521-B homology
- YTHDF1:
-
YTH domain family protein 1
- YTHDF2:
-
YTH domain family protein 2
- YTHDF3:
-
YTH domain family protein 3
- YTHDC1:
-
YTH domain containing 1
- YTHDC2:
-
YTH domain containing 2
- CCR4-NOT:
-
Carbon catabolite repressor 4-negative on TATA
- P-body:
-
Processing body
- eIF3:
-
Eukaryotic initiation factor 3
- eIF4E:
-
Eukaryotic initiation factor 4E
- eIF4G:
-
Eukaryotic initiation factor 4G
- PABP:
-
Poly(A) binding protein
- HNRNPC:
-
Heterogeneous nuclear ribonucleoprotein C1/C2
- HNRNPG:
-
Heterogeneous nuclear ribonucleoprotein G
- HNRNPA2B1:
-
Heterogeneous nuclear ribonucleoprotein A2B1
- HNRNP:
-
Heterogeneous nuclear ribonucleoprotein
- IGF2BP1/2/3:
-
Insulin-like growth factor 2 mRNA-binding protein 1/2/3
- FMRP:
-
Fragile X mental retardation protein
- PRRC2A:
-
Proline-rich spiral coil 2A
- NGS:
-
Next-generation sequencing
- ELISA:
-
Enzyme-linked immunosorbent assay
- RNase R:
-
Ribonuclease R
- MeRIP:
-
Methylated RNA immunoprecipitation
- RIP:
-
RNA immunoprecipitation
- qPCR:
-
Quantitative real-time polymerase chain reaction
- MeRIP-seq:
-
Methylated RNA immunoprecipitation and sequencing
- nanopore DRS:
-
Nanopore-based direct RNA sequencing
- eIF4G2:
-
Eukaryotic translation initiation factor 4 gamma 2
- eIF3A:
-
Eukaryotic initiation factor 3A
- HPV:
-
Human papillomavirus
- Hel25E:
-
Helicase at 25E
- nuclear RNA helicase URH49:
-
ATP-dependent RNA helicase DDX39A
- dead box protein UAP56:
-
Spliceosomal RNA helicase DDX39B
- YAP1:
-
Yes-associated protein 1
- HMGA2:
-
High Mobility Group AT-Hook 2
- CDR1:
-
Cerebellar degeneration associated protein 1
- Ago2:
-
Argonaute2
- RNAi:
-
RNA interference
- HRSP12:
-
Heat-responsive protein 12
- RNase P:
-
Ribonuclease P
- MRP:
-
Mitochondrial RNA processing
- CRC:
-
Colorectal carcinoma
- GEO:
-
Gene Expression Omnibus
- TCGA:
-
The Cancer Genome Atlas
- TCF7:
-
Transcription factor 7
- EMT:
-
Epithelial-mesenchymal transition
- SMAD3:
-
Mothers against decapentaplegic homolog 3
- LM:
-
Liver metastasis
- RNA-EMSA:
-
RNA electrophoretic mobility shift assay
- FISH:
-
Fluorescence in situ hybridization
- GC:
-
Gastric cancer
- DECs:
-
Differentiated expressed circRNAs
- PDGA:
-
Poorly differentiated gastric adenocarcinoma
- AKT1S1:
-
AKT1 substrate 1
- EIF4B:
-
Eukaryotic translation initiation factor 4B
- HCC:
-
Hepatocellular carcinoma
- ICGC:
-
International Cancer Genome Consortium
- HuR:
-
Human Antigen R
- MMP1:
-
Matrix metalloproteinase 1
- BC:
-
Breast cancer
- CKD1:
-
Cyclin-dependent kinases
- CC:
-
Cervical cancer
- HPV:
-
Human papillomavirus
- FOXM1:
-
Forkhead box M1
- NSCLC:
-
Non-small cell lung cancer
- TRIM25:
-
Tripartite motif containing 25
- RIG-I:
-
Retinoic acid-inducible gene-I
- CTNNBIP1:
-
Catenin Beta Interacting Protein 1
- Wnt/β-catenin:
-
Wingless-types/beta-catenin
- circMAP3K4-455aa:
-
CircMAP3K4 translation produced a 455 amino acid protein
- HPSCC:
-
Hypopharyngeal squamous cell carcinoma
- CEA:
-
Carcinoembryonic antigen
- PFS:
-
Disease-free survival rate
- OS:
-
Overall survival rate
- ICB:
-
Immune checkpoint blockade
References
Abakir A, Giles T, Cristini A, Foster J, Dai N, Starczak M, Rubio-Roldan A, Li M, Eleftheriou M, Crutchley J, Flatt L, Young L, Gaffney D, Denning C, Dalhus B, Emes R, Gackowski D, Corrêa I, Garcia-Perez J, Klungland A, Gromak N, Ruzov A. N-methyladenosine regulates the stability of RNA:DNA hybrids in human cells. Nat Genet. 2020;52:48–55.
Alarcón C, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie S. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 2015;162:1299–308.
Antanaviciute A, Baquero-Perez B, Watson C, Harrison S, Lascelles C, Crinnion L, Markham A, Bonthron D, Whitehouse A, Carr I. Nm6aViewer: software for the detection, analysis, and visualization of N-methyladenosine peaks from mA-seq/ME-RIP sequencing data. RNA. 2017;23:1493–501.
Ashwal-Fluss R, Meyer M, Pamudurti N, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55–66.
Aufiero S, van den Hoogenhof M, Reckman Y, Beqqali A, van der Made I, Kluin J, Khan M, Pinto Y, Creemers E. Cardiac circRNAs arise mainly from constitutive exons rather than alternatively spliced exons. RNA. 2018;24:815–27.
Bawankar P, Lence T, Paolantoni C, Haussmann I, Kazlauskiene M, Jacob D, Heidelberger J, Richter F, Nallasivan M, Morin V, Kreim N, Beli P, Helm M, Jinek M, Soller M, Roignant J. Hakai is required for stabilization of core components of the mA mRNA methylation machinery. Nat Commun. 2021;12:3778.
Boccaletto P, Stefaniak F, Ray A, Cappannini A, Mukherjee S, Purta E, Kurkowska M, Shirvanizadeh N, Destefanis E, Groza P, Avşar G, Romitelli A, Pir P, Dassi E, Conticello S, Aguilo F, Bujnicki J. MODOMICS: a database of RNA modification pathways. Nucleic Acids Res. 2022;50:D231–5.
Broadbent K, Broadbent J, Ribacke U, Wirth D, Rinn J, Sabeti P. Strand-specific RNA sequencing in Plasmodium falciparum malaria identifies developmentally regulated long non-coding RNA and circular RNA. BMC Genomics. 2015;16:454.
Chen X, Yang T, Wang W, Xi W, Zhang T, Li Q, Yang A, Wang T. Circular RNAs in immune responses and immune diseases. Theranostics. 2019a;9:588–607.
Chen R, Chen X, Xia L, Zhang J, Pan Z, Ma X, Han K, Chen J, Judde J, Deas O, Wang F, Ma N, Guan X, Yun J, Wang F, Xu R, Xie D. N-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat Commun. 2019b;10:4695.
Chen C, Yuan W, Zhou Q, Shao B, Guo Y, Wang W, Yang S, Guo Y, Zhao L, Dang Q, Yang X, Wang G, Kang Q, Ji Z, Liu J, Sun Z. N6-methyladenosine-induced circ1662 promotes metastasis of colorectal cancer by accelerating YAP1 nuclear localization. Theranostics. 2021;11:4298–315.
Chi F, Cao Y, Chen Y. Analysis and validation of circRNA-miRNA network in regulating mA RNA methylation modulators reveals CircMAP2K4/miR-139–5p/YTHDF1 axis involving the proliferation of hepatocellular carcinoma. Front Oncol. 2021;11:560506.
Danan M, Schwartz S, Edelheit S, Sorek R. Transcriptome-wide discovery of circular RNAs in Archaea. Nucleic Acids Res. 2012;40:3131–42.
Deng S, Zhang H, Zhu K, Li X, Ye Y, Li R, Liu X, Lin D, Zuo Z, Zheng J. M6A2Target: a comprehensive database for targets of m6A writers, erasers and readers. Brief Bioinform. 2021;2:bbaa055.
Di Timoteo G, Dattilo D, Centrón-Broco A, Colantoni A, Guarnacci M, Rossi F, Incarnato D, Oliviero S, Fatica A, Morlando M, Bozzoni I. Modulation of circRNA metabolism by mA modification. Cell Rep. 2020;31: 107641.
Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, Sorek R, Rechavi G. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–6.
Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, Ma J, Wu L. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016;7:12626.
Duan J, Chen W, Xie J, Zhang M, Nie R, Liang H, Mei J, Han K, Xiang Z, Wang F, Teng K, Chen R, Deng M, Yin Y, Zhang N, Xie D, Cai M. A novel peptide encoded by N6-methyladenosine modified circMAP3K4 prevents apoptosis in hepatocellular carcinoma. Mol Cancer. 2022;21:93.
Enuka Y, Lauriola M, Feldman M, Sas-Chen A, Ulitsky I, Yarden Y. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 2016;44:1370–83.
Esfahani K, Roudaia L, Buhlaiga N, Del Rincon S, Papneja N, Miller W. A review of cancer immunotherapy: from the past, to the present, to the future. Curr Oncol. 2020;27:S87–97.
Fan H, Chen Z, Chen X, Chen M, Yi Y, Zhu J, Zhang J. METTL14-mediated mA modification of circORC5 suppresses gastric cancer progression by regulating miR-30c-2–3p/AKT1S1 axis. Mol Cancer. 2022;21:51.
Ge L, Zhang N, Chen Z, Song J, Wu Y, Li Z, Chen F, Wu J, Li D, Li J, Wang C, Wang H, Wang J. Level of N6-methyladenosine in peripheral blood RNA: a novel predictive biomarker for gastric cancer. Clin Chem. 2020;66:342–51.
Ge L, Sun Y, Shi Y, Liu G, Teng F, Geng Z, Chen X, Xu H, Xu J, Jia X. Plasma circRNA microarray profiling identifies novel circRNA biomarkers for the diagnosis of ovarian cancer. J Ovarian Res. 2022;15:58.
Gomes C, Schroen B, Kuster G, Robinson E, Ford K, Squire I, Heymans S, Martelli F, Emanueli C, Devaux Y. Regulatory RNAs in heart failure. Circulation. 2020;141:313–28.
Guo J, Agarwal V, Guo H, Bartel D. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014;15:409.
Guo Y, Guo Y, Chen C, Fan D, Wu X, Zhao L, Shao B, Sun Z, Ji Z. Circ3823 contributes to growth, metastasis and angiogenesis of colorectal cancer: involvement of miR-30c-5p/TCF7 axis. Mol Cancer. 2021;20:93.
Hansen T, Wiklund E, Bramsen J, Villadsen S, Statham A, Clark S, Kjems J. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011;30:4414–22.
Hansen T, Jensen T, Clausen B, Bramsen J, Finsen B, Damgaard C, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–8.
Hirsch F, Scagliotti G, Mulshine J, Kwon R, Curran W, Wu Y, Paz-Ares L. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389:299–311.
Hofheinz R, Stintzing S. Study evidence confirms current clinical practice in refractory metastatic colorectal cancer: the ReDOS trial. Lancet Oncol. 2019;20:1036–7.
Howe K, Achuthan P, Allen J, Allen J, Alvarez-Jarreta J, Amode M, Armean I, Azov A, Bennett R, Bhai J, Billis K, Boddu S, Charkhchi M, Cummins C, Da Rin Fioretto L, Davidson C, Dodiya K, El Houdaigui B, Fatima R, Gall A, Garcia Giron C, Grego T, Guijarro-Clarke C, Haggerty L, Hemrom A, Hourlier T, Izuogu O, Juettemann T, Kaikala V, Kay M, Lavidas I, Le T, Lemos D, Gonzalez Martinez J, Marugán J, Maurel T, McMahon A, Mohanan S, Moore B, Muffato M, Oheh D, Paraschas D, Parker A, Parton A, Prosovetskaia I, Sakthivel M, Salam A, Schmitt B, Schuilenburg H, Sheppard D, Steed E, Szpak M, Szuba M, Taylor K, Thormann A, Threadgold G, Walts B, Winterbottom A, Chakiachvili M, Chaubal A, De Silva N, Flint B, Frankish A, Hunt S, Iisley G, Langridge N, Loveland J, Martin F, Mudge J, Morales J, Perry E, Ruffier M, Tate J, Thybert D, Trevanion S, Cunningham F, Yates A, Zerbino D, Flicek P. Ensembl. Nucleic Acids Res. 2021;49:D884–91.
Hsu P, Shi H, Zhu A, Lu Z, Miller N, Edens B, Ma Y, He C. NThe RNA-binding protein FMRP facilitates the nuclear export of -methyladenosine-containing mRNAs. J Biol Chem. 2019;294:19889–95.
Huang C, Liang D, Tatomer D, Wilusz J. A length-dependent evolutionarily conserved pathway controls nuclear export of circular RNAs. Genes Dev. 2018;32:639–44.
Huang Q, Guo H, Wang S, Ma Y, Chen H, Li H, Li J, Li X, Yang F, Qiu M, Zhao S, Wang J. A novel circular RNA, circXPO1, promotes lung adenocarcinoma progression by interacting with IGF2BP1. Cell Death Dis. 2020;11:1031.
Huang W, Ling Y, Zhang S, Xia Q, Cao R, Fan X, Fang Z, Wang Z, Zhang G. TransCirc: an interactive database for translatable circular RNAs based on multi-omics evidence. Nucleic Acids Res. 2021;49:D236–42.
Imanishi M, Tsuji S, Suda A, Futaki S. Detection of N-methyladenosine based on the methyl-sensitivity of MazF RNA endonuclease. Chem Commun. 2017;53:12930–3.
Jan van Zonneveld A, Kölling M, Bijkerk R, Lorenzen J. Circular RNAs in kidney disease and cancer. Nat Rev Nephrol. 2021;17(12):814–26.
Jeck W, Sorrentino J, Wang K, Slevin M, Burd C, Liu J, Marzluff W, Sharpless N. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–57.
Ji P, Wu W, Chen S, Zheng Y, Zhou L, Zhang J, Cheng H, Yan J, Zhang S, Yang P, Zhao F. Expanded expression landscape and prioritization of circular RNAs in mammals. Cell Rep. 2019;26:3444-60.e5.
Ji F, Lu Y, Chen S, Yu Y, Lin X, Zhu Y, Luo X. IGF2BP2-modified circular RNA circARHGAP12 promotes cervical cancer progression by interacting mA/FOXM1 manner. Cell Death Discov. 2021;7:215.
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang Y, He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–7.
Jia R, Xiao M, Li Z, Shan G, Huang C. Defining an evolutionarily conserved role of GW182 in circular RNA degradation. Cell Discov. 2019;5:45.
Jiang T, Xia Y, Lv J, Li B, Li Y, Wang S, Xuan Z, Xie L, Qiu S, He Z, Wang L, Xu Z. A novel protein encoded by circMAPK1 inhibits progression of gastric cancer by suppressing activation of MAPK signaling. Mol Cancer. 2021;20:66.
Kim G, Imam H, Siddiqui A. The RNA binding proteins YTHDC1 and FMRP regulate the nuclear export of -methyladenosine-modified hepatitis B virus transcripts and affect the viral life cycle. J Virol. 2021;95:e0009721.
Knuckles P, Lence T, Haussmann I, Jacob D, Kreim N, Carl S, Masiello I, Hares T, Villaseñor R, Hess D, Andrade-Navarro M, Biggiogera M, Helm M, Soller M, Bühler M, Roignant J. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the mA machinery component Wtap/Fl(2)d. Genes Dev. 2018;32:415–29.
Kristensen L, Andersen M, Stagsted L, Ebbesen K, Hansen T, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–91.
Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade M, Laneve P, Rajewsky N, Bozzoni I. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66:22-37.e9.
Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, Zhong G, Yu B, Hu W, Dai L, Zhu P, Chang Z, Wu Q, Zhao Y, Jia Y, Xu P, Liu H, Shan G. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22:256–64.
Li N, Kang Y, Wang L, Huff S, Tang R, Hui H, Agrawal K, Gonzalez G, Wang Y, Patel S, Rana T. ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proc Natl Acad Sci USA. 2020;117:20159–70.
Li Z, Yang H, Dai X, Zhang X, Huang Y, Shi L, Wei J, Ding Q. CircMETTL3, upregulated in a m6A-dependent manner, promotes breast cancer progression. Int J Biol Sci. 2021a;17:1178–90.
Li B, Zhu L, Lu C, Wang C, Wang H, Jin H, Ma X, Cheng Z, Yu C, Wang S, Zuo Q, Zhou Y, Wang J, Yang C, Lv Y, Jiang L, Qin W. circNDUFB2 inhibits non-small cell lung cancer progression via destabilizing IGF2BPs and activating anti-tumor immunity. Nat Commun. 2021b;12:295.
Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–4.
Liu N, Zhou K, Parisien M, Dai Q, Diatchenko L, Pan T. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017;45:6051–63.
Liu W, Yan J, Zhang Z, Pian H, Liu C, Li Z. Identification of a selective DNA ligase for accurate recognition and ultrasensitive quantification of N-methyladenosine in RNA at one-nucleotide resolution. Chem Sci. 2018;9:3354–9.
Liu M, Wang Q, Shen J, Yang B, Ding X. Circbank: a comprehensive database for circRNA with standard nomenclature. RNA Biol. 2019;16:899–905.
Liu H, Lan T, Li H, Xu L, Chen X, Liao H, Chen X, Du J, Cai Y, Wang J, Li X, Huang J, Yuan K, Zeng Y. Circular RNA circDLC1 inhibits MMP1-mediated liver cancer progression via interaction with HuR. Theranostics. 2021;11:1396–411.
Lu Z, Filonov G, Noto J, Schmidt C, Hatkevich T, Wen Y, Jaffrey S, Matera A. Metazoan tRNA introns generate stable circular RNAs in vivo. RNA. 2015;21:1554–65.
Luo X, Li H, Liang J, Zhao Q, Xie Y, Ren J, Zuo Z. RMVar: an updated database of functional variants involved in RNA modifications. Nucleic Acids Res. 2021;49:D1405–12.
Ma H, Wang X, Cai J, Dai Q, Natchiar S, Lv R, Chen K, Lu Z, Chen H, Shi Y, Lan F, Fan J, Klaholz B, Pan T, Shi Y, He C. N-Methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat Chem Biol. 2019;15:88–94.
Maji S, Panda S, Samal S, Shriwas O, Rath R, Pellecchia M, Emdad L, Das S, Fisher P, Dash R. Bcl-2 antiapoptotic family proteins and chemoresistance in cancer. Adv Cancer Res. 2018;137:37–75.
Mao Y, Dong L, Liu X, Guo J, Ma H, Shen B, Qian S. mA in mRNA coding regions promotes translation via the RNA helicase-containing YTHDC2. Nat Commun. 2019;10:5332.
Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak S, Gregersen L, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–8.
Meyer K, Saletore Y, Zumbo P, Elemento O, Mason C, Jaffrey S. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell. 2012;149:1635–46.
Meyer K, Patil D, Zhou J, Zinoviev A, Skabkin M, Elemento O, Pestova T, Qian S, Jaffrey S. 5’ UTR m(6)A promotes cap-independent translation. Cell. 2015;163:999–1010.
Nahand J, Jamshidi S, Hamblin M, Mahjoubin-Tehran M, Vosough M, Jamali M, Khatami A, Moghoofei M, Baghi H, Mirzaei H. Circular RNAs: new epigenetic signatures in viral infections. Front Microbiol. 2020;11:1853.
Nombela P, Miguel-López B, Blanco S. The role of mA, mC and Ψ RNA modifications in cancer: novel therapeutic opportunities. Mol Cancer. 2021;20:18.
Ostrom Q, Bauchet L, Davis F, Deltour I, Fisher J, Langer C, Pekmezci M, Schwartzbaum J, Turner M, Walsh K, Wrensch M, Barnholtz-Sloan J. The epidemiology of glioma in adults: a “state of the science” review. Neuro-Oncol. 2014;16:896–913.
Park O, Ha H, Lee Y, Boo S, Kwon D, Song H, Kim Y. Endoribonucleolytic cleavage of mA-containing RNAs by RNase P/MRP complex. Mol Cell. 2019;74:494-507.e8.
Patil D, Chen C, Pickering B, Chow A, Jackson C, Guttman M, Jaffrey S. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–73.
Pendleton K, Chen B, Liu K, Hunter O, Xie Y, Tu B, Conrad N. The U6 snRNA mA methyltransferase METTL16 Regulates SAM synthetase intron retention. Cell. 2017;169:824–35.
Ping X, Sun B, Wang L, Xiao W, Yang X, Wang W, Adhikari S, Shi Y, Lv Y, Chen Y, Zhao X, Li A, Yang Y, Dahal U, Lou X, Liu X, Huang J, Yuan W, Zhu X, Cheng T, Zhao Y, Wang X, Rendtlew Danielsen J, Liu F, Yang Y. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–89.
Pinto R, Vågbø C, Jakobsson M, Kim Y, Baltissen M, O’Donohue M, Guzmán U, Małecki J, Wu J, Kirpekar F, Olsen J, Gleizes P, Vermeulen M, Leidel S, Slupphaug G, Falnes P. The human methyltransferase ZCCHC4 catalyses N6-methyladenosine modification of 28S ribosomal RNA. Nucleic Acids Res. 2020;48:830–46.
Rong D, Wu F, Lu C, Sun G, Shi X, Chen X, Dai Y, Zhong W, Hao X, Zhou J, Xia Y, Tang W, Wang X. m6A modification of circHPS5 and hepatocellular carcinoma progression through HMGA2 expression. Mol Ther Nucleic Acids. 2021;26:637–48.
Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, Herzog M, Schreyer L, Papavasileiou P, Ivanov A, Öhman M, Refojo D, Kadener S, Rajewsky N. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell. 2015;58:870–85.
Salzman J, Gawad C, Wang P, Lacayo N, Brown P. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE. 2012;7: e30733.
Sanger H, Klotz G, Riesner D, Gross H, Kleinschmidt A. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci USA. 1976;73:3852–6.
Schmidt C, Giusto J, Bao A, Hopper A, Matera A. Molecular determinants of metazoan tricRNA biogenesis. Nucleic Acids Res. 2019;47:6452–65.
Schwartz S, Mumbach M, Jovanovic M, Wang T, Maciag K, Bushkin G, Mertins P, Ter-Ovanesyan D, Habib N, Cacchiarelli D, Sanjana N, Freinkman E, Pacold M, Satija R, Mikkelsen T, Hacohen N, Zhang F, Carr S, Lander E, Regev A. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5’ sites. Cell Rep. 2014;8:284–96.
Shang Q, Yang Z, Jia R, Ge S. The novel roles of circRNAs in human cancer. Mol Cancer. 2019;18:6.
Shi H, Wang X, Lu Z, Zhao B, Ma H, Hsu P, Liu C, He C. YTHDF3 facilitates translation and decay of N-methyladenosine-modified RNA. Cell Res. 2017;27:315–28.
Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71:209–49.
Suzuki H, Zuo Y, Wang J, Zhang M, Malhotra A, Mayeda A. Characterization of RNase R-digested cellular RNA source that consists of lariat and circular RNAs from pre-mRNA splicing. Nucleic Acids Res. 2006;34: e63.
van Tran N, Ernst F, Hawley B, Zorbas C, Ulryck N, Hackert P, Bohnsack K, Bohnsack M, Jaffrey S, Graille M, Lafontaine D. The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res. 2019;47:7719–33.
Venø M, Hansen T, Venø S, Clausen B, Grebing M, Finsen B, Holm I, Kjems J. Spatio-temporal regulation of circular RNA expression during porcine embryonic brain development. Genome Biol. 2015;16:245.
Wang P, Bao Y, Yee M, Barrett S, Hogan G, Olsen M, Dinneny J, Brown P, Salzman J. Circular RNA is expressed across the eukaryotic tree of life. PLoS ONE. 2014;9: e90859.
Wang X, Zhao B, Roundtree I, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–99.
Wang P, Doxtader K, Nam Y. structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol Cell. 2016;63:306–17.
Wang Y, Wang H, Xi F, Wang H, Han X, Wei W, Zhang H, Zhang Q, Zheng Y, Zhu Q, Kohnen M, Reddy A, Gu L. Profiling of circular RNA N-methyladenosine in moso bamboo (Phyllostachys edulis) using nanopore-based direct RNA sequencing. J Integr Plant Biol. 2020;62:1823–38.
Widagdo J, Anggono V, Wong J. The multifaceted effects of YTHDC1-mediated nuclear mA recognition. Trends Genet. 2022;38:325–32.
Wojtas M, Pandey R, Mendel M, Homolka D, Sachidanandam R, Pillai R. Regulation of mA transcripts by the 3’→5’ RNA helicase YTHDC2 is essential for a successful meiotic program in the mammalian germline. Mol Cell. 2017;68:374-87.e12.
Wu N, Yuan Z, Du K, Fang L, Lyu J, Zhang C, He A, Eshaghi E, Zeng K, Ma J, Du W, Yang B. Translation of yes-associated protein (YAP) was antagonized by its circular RNA via suppressing the assembly of the translation initiation machinery. Cell Death Differ. 2019a;26:2758–73.
Wu R, Li A, Sun B, Sun J, Zhang J, Zhang T, Chen Y, Xiao Y, Gao Y, Zhang Q, Ma J, Yang X, Liao Y, Lai W, Qi X, Wang S, Shu Y, Wang H, Wang F, Yang Y, Yuan Z. A novel mA reader Prrc2a controls oligodendroglial specification and myelination. Cell Res. 2019b;29:23–41.
Wu P, Fang X, Liu Y, Tang Y, Wang W, Li X, Fan Y. N6-methyladenosine modification of circCUX1 confers radioresistance of hypopharyngeal squamous cell carcinoma through caspase1 pathway. Cell Death Dis. 2021;12:298.
Wu Q, Yin X, Zhao W, Xu W, Chen L. Molecular mechanism of mA methylation of circDLC1 mediated by RNA methyltransferase METTL3 in the malignant proliferation of glioma cells. Cell Death Discov. 2022;8:229.
Xia X, Li X, Li F, Wu X, Zhang M, Zhou H, Huang N, Yang X, Xiao F, Liu D, Yang L, Zhang N. A novel tumor suppressor protein encoded by circular AKT3 RNA inhibits glioblastoma tumorigenicity by competing with active phosphoinositide-dependent Kinase-1. Mol Cancer. 2019;18:131.
Xu J, Wan Z, Tang M, Lin Z, Jiang S, Ji L, Gorshkov K, Mao Q, Xia S, Cen D, Zheng J, Liang X, Cai X. N-methyladenosine-modified CircRNA-SORE sustains sorafenib resistance in hepatocellular carcinoma by regulating β-catenin signaling. Mol Cancer. 2020;19:163.
Xuan J, Sun W, Lin P, Zhou K, Liu S, Zheng L, Qu L, Yang J. RMBase v2.0: deciphering the map of RNA modifications from epitranscriptome sequencing data. Nucleic Acids Res. 2018;46:D327–34.
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen L, Wang Y, Wong C, Xiao X, Wang Z. Extensive translation of circular RNAs driven by N-methyladenosine. Cell Res. 2017;27:626–41.
Ye Y, Feng W, Zhang J, Zhu K, Huang X, Pan L, Su J, Zheng Y, Li R, Deng S, Bai R, Zhuang L, Wei L, Deng J, Li M, Chen R, Lin D, Zuo Z, Zheng J. Genome-wide identification and characterization of circular RNA mA modification in pancreatic cancer. Genome Med. 2021;13:183.
You X, Vlatkovic I, Babic A, Will T, Epstein I, Tushev G, Akbalik G, Wang M, Glock C, Quedenau C, Wang X, Hou J, Liu H, Sun W, Sambandan S, Chen T, Schuman E, Chen W. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci. 2015;18:603–10.
Zhang Y, Hamada M. DeepM6ASeq: prediction and characterization of m6A-containing sequences using deep learning. BMC Bioinform. 2018;19:524.
Zhang Y, Zhang X, Chen T, Xiang J, Yin Q, Xing Y, Zhu S, Yang L, Chen L. Circular intronic long noncoding RNAs. Mol Cell. 2013;51:792–806.
Zhang F, Kang Y, Wang M, Li Y, Xu T, Yang W, Song H, Wu H, Shu Q, Jin P. Fragile X mental retardation protein modulates the stability of its m6A-marked messenger RNA targets. Hum Mol Genet. 2018;27:3936–50.
Zhang C, Wang J, Geng X, Tu J, Gao H, Li L, Zhou X, Wu H, Jing J, Pan W, Mou Y. Circular RNA expression profile and m6A modification analysis in poorly differentiated adenocarcinoma of the stomach. Epigenomics. 2020;12:1027–40.
Zhao J, Lee E, Kim J, Yang R, Chamseddin B, Ni C, Gusho E, Xie Y, Chiang C, Buszczak M, Zhan X, Laimins L, Wang R. Transforming activity of an oncoprotein-encoding circular RNA from human papillomavirus. Nat Commun. 2019;10:2300.
Zheng G, Dahl J, Niu Y, Fedorcsak P, Huang C, Li C, Vågbø C, Shi Y, Wang W, Song S, Lu Z, Bosmans R, Dai Q, Hao Y, Yang X, Zhao W, Tong W, Wang X, Bogdan F, Furu K, Fu Y, Jia G, Zhao X, Liu J, Krokan H, Klungland A, Yang Y, He C. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29.
Zhou Y, Zeng P, Li Y, Zhang Z, Cui Q. SRAMP: prediction of mammalian N6-methyladenosine (m6A) sites based on sequence-derived features. Nucleic Acids Res. 2016;44:e91.
Zhou C, Molinie B, Daneshvar K, Pondick J, Wang J, Van Wittenberghe N, Xing Y, Giallourakis C, Mullen A. Genome-wide maps of m6A circRNAs identify widespread and cell-type-specific methylation patterns that are distinct from mRNAs. Cell Rep. 2017;20:2262–76.
Acknowledgements
Not applicable.
Funding
This project was supported by grants from the National Natural Science Foundation of China [Grant Number: 81871720], Postgraduate Research & Practice Innovation Program of Jiangsu Province [Grant Number: KYCX21_3116].
Author information
Authors and Affiliations
Contributions
SQ and SJ conceived the structure of the manuscript and drafted the initial manuscript and charts. QZ collated the literature and proofread the manuscript. YX, SM, and TW provided valuable advice and participated in the final revision of the manuscript. YH checked and revised the final manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have read and approved the final manuscript.
Competing interests
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qin, S., Zhang, Q., Xu, Y. et al. m6A-modified circRNAs: detections, mechanisms, and prospects in cancers. Mol Med 28, 79 (2022). https://doi.org/10.1186/s10020-022-00505-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10020-022-00505-5