Abstract
Atherosclerosis (AS) is widely accepted to be a multistep pathophysiological process associated with several other processes such as angiogenesis and inflammatory response. Long non-coding RNAs (lncRNAs) are non-protein coding RNAs (more than 200 nucleotides in length) and can regulate gene expression at the transcriptional and post-transcriptional levels. Recent studies suggest that lncRNA-H19 plays important roles in the regulation of angiogenesis, adipocyte differentiation, lipid metabolism, inflammatory response, cellular proliferation and apoptosis. In this review, we primarily discuss the roles of lncRNA-H19 in atherosclerosis-related pathophysiological processes and the potential mechanisms by which lncRNA-H19 regulates the development of atherosclerosis, to help provide a better understanding of the biological functions of lncRNA-H19 in atherosclerosis.
Similar content being viewed by others
Introduction
Atherosclerosis (AS) drives cardiovascular disease, which is one of the main causes of mortality in the world (Libby et al. 2019; Barquera et al. 2015). Atherogenesis is a slowly progressive process that is characterized by multifocal structural alterations in the wall of large and medium-sized arteries and the subsequent formation of atherosclerotic plaques. Many pathophysiological processes are mechanistically involved in the pathogenesis and development of atherosclerosis, such as angiogenesis, inflammatory and immune responses, adipogenesis, lipid metabolism, cellular proliferation and apoptosis. These processes are critical for the development of atherosclerosis and ultimately trigger thrombotic plaque complications, such as myocardial infarction (MI), stroke, and cardiovascular death (Libby et al. 2019; Camaré et al. 2017; Ross 1999).
Long non-coding RNAs (lncRNAs) are widely classified as transcripts > 200 nucleotides with limited coding potential (Klattenhoff et al. 2013). Numerous functions of lncRNAs in many biological activities have been found, such as: (i) serving as precursors for shorter functional RNAs as exemplified by primary transcripts for microRNAs (miRNAs); (ii) regulating transcription, translation, imprinting, genome rearrangement and chromatin modification; (iii) regulating protein activities; (iv) producing scaffolds for higher-order complexes, such as Polycomb repressive complex 2 (PRC2); (v) working as competing endogenous RNAs (ceRNAs) or natural miRNA sponges. All RNA transcripts that contain miRNA-binding sites can communicate with and regulate each other by competing specifically for shared miRNAs and thus lncRNAs can impact target gene mRNA expression by acting as miRNA molecular sponges (Fatica and Bozzoni 2014; St Laurent et al. 2015; Tay et al. 2014).
Recently, emerging evidence shows that lncRNAs are crucial regulators in many pathophysiological processes of atherosclerosis (Aryal and Suarez 2019; Kim and Kook 2019; Yu and Wang 2018). For example, lncRNA-p21 expression, which is significantly decreased in atherosclerotic plaques of ApoE−/− mice, suppresses proliferation and facilitates apoptosis of vascular smooth muscle cells (VSMCs) by increasing p53 activity (Wu et al. 2014). In addition, the lncRNA SMILR, as another example, regulates the proliferation of VSMCs, and its expression is significantly upregulated in unstable human atherosclerotic plaques (Ballantyne et al. 2016).
The H19 gene, which encodes lncRNA-H19, is localized near the telomeric region of chromosome 11p15, within a unique locus shared with the insulin-like growth factor 2 (IGF2) gene. The H19 gene is 2.3 kb in length and is highly evolutionarily conserved, suggesting that it may have some important biological functions (Hurst and Smith 1999). Based on their functions, lncRNAs can be classified as ncRNA-activating, ceRNAs and precursors for shorter functional RNAs as exemplified by primary transcripts for miRNAs and piRNAs (St Laurent et al. 2015). Importantly, lncRNA-H19 can act as a miRNA primary transcript to generate miRNA or as a ceRNA to sponge miRNA (St Laurent et al. 2015; Cai and Cullen 2007; Yan et al. 2015). Some previous trials found that lncRNA-H19 is highly expressed in human atherosclerotic plaques and injured carotid arteries in rat model but was barely expressed in normal coronary arteries (Han et al. 1996; Kim et al. 1994). Recent studies have shown that increased plasma level of lncRNA-H19 is associated with an increased risk of coronary artery disease (Bitarafan et al. 2019; Zhang et al. 2017). In patients with atherosclerosis, a high level of lncRNA-H19 is detected and overexpression of lncRNA-H19 promotes proliferation and inhibits apoptosis of VSMCs (Pan 2017). Furthermore, Huang et al. reported that overexpression of lncRNA-H19 contributes to the occurrence of atherosclerosis (Huang et al. 2019). These findings indicate that lncRNA-H19 may be involved in the onset and progression of atherosclerosis.
In this review, we summarized the current studies and discussed the roles of lncRNA-H19 in atherosclerosis to demonstrate the potential value of lncRNA-H19 in atherosclerosis therapy and provide a basis for further investigations.
LncRNA-H19 and the pathophysiology of atherosclerosis
The development of native atherosclerosis is mechanistically considered to be the consequence of dysregulation of numerous biological phenomena including angiogenesis, adipogenesis, lipid metabolism, inflammatory response, cellular proliferation and apoptosis (Camaré et al. 2017; Ross 1999). Many studies have shown that lncRNA-H19 is involved in these phenomena via various mechanisms.
LncRNA-H19 regulates angiogenesis
Angiogenesis significantly influences plaque growth and instability in atherosclerotic lesions. Increased neovascularization enhances the local supply of nutrients and O2 in atherosclerotic areas, thereby promoting plaque progression and remodeling. The incomplete maturation and the fragility of neocapillaries likely promote intraplaque hemorrhages, leading to plaque instability and rupture that are often associated with athero-thrombotic events (Camaré et al. 2017; Falk 2006).
Some studies have shown that lncRNA-H19 participates in vascular physiopathology and angiogenesis. It was reported that inhibition of lncRNA-H19 results in dramatic reductions in endothelial cell (EC) growth and capillary-like structure formation (Voellenkle et al. 2016). Moreover, a recent study demonstrated that endothelial-specific knockdown of lncRNA-H19 impairs angiogenesis while exogenous lncRNA-H19 partially rescues this effect (Hofmann 2018).
Vascular endothelial growth factor (VEGF) is considered as one of the crucial factors associated with angiogenesis (Di Stefano et al. 2009). The VEGF family includes several members: VEGF-A, −B, −C, −D, −E, and -F and placental growth factor (PlGF) (Hoeben et al. 2004). In aortic tissues of atherosclerotic mice, knockdown of lncRNA-H19 prevents intraplaque angiogenesis and downregulates the expression of the angiogenesis-related factors matrix metalloproteinase 2 (MMP-2) and VEGF (Yang et al. 2019). In contrast, lncRNA-H19 overexpression recruits CCCTC-binding factor (CTCF) to repress the polycystic kidney disease 1 (PKD1) gene (Yang et al. 2019), mutations of which may lead to angiogenesis (Li et al. 2003). VEGF-A has been certified as a target of microRNA-199a-5p (miR-199a-5p) (Hsu et al. 2014). Interestingly, lncRNA-H19 serves as a ceRNA to inhibit miR-199a-5p, resulting in the upregulation of VEGF-A expression. Moreover, lncRNA-H19 enhances the survival of mesenchymal stem cells and their angiogenic potential in vitro 31. Some studies have shown that VEGF-A is also a target of miR-29a and miR-29c (Chen et al. 2014; Liu et al. 2017). Knockdown of lncRNA-H19 suppresses glioma-induced EC proliferation, migration and tube formation by upregulating miR-29a (Jia et al. 2016). In addition, lncRNA-H19 can enhance corneal neovascularization by binding directly to miR-29c, which negatively regulates VEGF-A (Sun et al. 2019a). Moreover, Zhu et al. revealed that lncRNA-H19 overexpression exerts pro-angiogenic effects in human dermal vascular endothelial cells (HMEC-1) (Zhu et al. 2019a). LncRNA-H19 overexpression also increases the protein levels of VEGF and endothelial NO synthase (eNOS) in HMEC-1 cells, suggesting that lncRNA-H19 promotes tube formation by regulating VEGF and eNOS. Further experiments showed that the underlying mechanism is associated with lncRNA-H19-mediated downregulation of miR-181a, and subsequent activation of the c-Jun N-terminal kinase (JNK) and AMP-activated protein kinase (AMPK) pathways (Zhu et al. 2019a). Human amniotic mesenchymal stem cells (HAMSCs) express and secrete significantly high levels of representative pro-angiogenic factors including VEGF-A (Kim et al. 2012). A recent study found that lncRNA-H19 knockdown in HAMSCs suppresses angiogenesis. Mechanistically, lncRNA-H19 interacts with the histone methyltransferase enhancer of zeste homolog 2 (EZH2). LncRNA-H19 knockdown inhibits EZH2 from recruiting methyl groups to the promoter region of the angiogenesis inhibitor vasohibin1 (VASH1) gene, thus increasing VASH1 expression and secretion in HAMSCs, and suppressing angiogenesis (Yuan et al. 2019). In addition, Hofmann et al. showed that lncRNA-H19 is decreased during aging and controls EC senescence, proliferation and angiogenic sprouting by inhibiting the activation of signal transducer and activator of transcription 3 (STAT3) (Hofmann et al. 2019), while STAT3 activation upregulates the expression of VEGF (Kujawski et al. 2008; Niu et al. 2002).
In summary, these studies suggest that lncRNA-H19 promotes angiogenesis and accelerates the development of atherosclerosis.
LncRNA-H19 regulates adipocyte differentiation
White adipose tissue (WAT), brown adipose tissue (BAT) and perivascular adipose tissue (PVAT) play differential roles in atherosclerosis. WAT, acting as a lipid sink, prevents the accumulation of lipids in circulation but active BAT helps combust lipids. PVAT has properties of both WAT and BAT. Evidence suggests a critical role for PVAT in regulating the focal inflammatory state and vessel homeostasis via pro-atherogenic or anti-atherogenic mechanisms such as releasing inflammatory cytokines and adipokines, depending on the PVAT state in health and disease (Ahmadieh et al. 2020; van Dam et al. 2017; Verhagen and Visseren 2011).
Bone marrow mesenchymal stem cells (BMSCs) are precursor cells of adipocytes (Pittenger et al. 1999), and lncRNA-H19 is a primary miRNA precursor for miR-675 (Smits et al. 2008). Huang et al. found that in human BMSCs, lncRNA-H19 and lncRNA-H19-derived miR-675 inhibits BMSCs differentiation into adipocytes. Mechanistically, miR-675 directly binds to the 3′ UTRs of class II histone deacetylase (HDAC) 4–6 transcripts and downregulates their expression (Huang et al. 2016). Class II HDACs play an essential role in adipocyte differentiation (Nebbioso et al. 2010), thus their downregulation inhibits adipocyte differentiation of human BMSCs (Huang et al. 2016). In a more recent trial using mouse BMSCs, knockdown of lncRNA-H19 markedly increases miR-188 expression. MiR-188 overexpression promotes adipocyte differentiation of mouse BMSCs by directly removing the effects of ligand-dependent corepressor (LCoR) (Wang et al. 2018), and LCoR is a negative regulator of adipogenesis (Cao et al. 2017). Therefore, through this lncRNA-H19/miR-188/LCoR pathway, lncRNA-H19 knockdown subsequently induces adipocyte differentiation in mouse BMSCs (Wang et al. 2018). Taken together, these findings suggest that lncRNA-H19 may have a negative correlation with adipocyte differentiation of BMSCs by regulating its targeted genes.
LncRNA-H19 regulates lipid metabolism
The lipid accumulation occupies an important position in the progression of atherosclerosis. For example, foam cell formation is a vital process in atherogenesis and involves phagocytosis of matrix-retained lipoproteins and fluid-phase pinocytosis of aggregated lipoproteins by macrophages (Libby et al. 2019; Weber and Noels 2011). Han et al. observed that lncRNA-H19 knockdown in foam cells counteracts the increased amount of triglyceride (TG), total cholesterol (TC), and low density lipoprotein-cholesterol (LDL-C) and the decreased amount of high density lipoprotein-cholesterol (HDL-C). Moreover, the Oil red O staining revealed that lncRNA-H19 knockdown decreases lipid accumulation. These results suggested that lncRNA-H19 induces lipid metabolic disorders in foam cells by suppressing lipid metabolism and increasing lipid accumulation, which contribute to the progression of atherosclerosis. In addition, it was found that miR-130b is downregulated in foam cells compared with the normal macrophages (Han et al. 2018), and is also involved in lipid metabolic disorders (Lv et al. 2015). Therefore, miR-130b may be a potential target of lncRNA-H19, which is involved in lipid metabolism and atherosclerosis (Han et al. 2018).
Nonalcoholic fatty liver disease (NAFLD) is strongly associated with atherosclerosis (Sookoian and Pirola 2008; Zheng et al. 2018). Peroxisome proliferator-activated receptor γ (PPARγ) was reported to be correlated with NAFLD (Zhu et al. 2019b). In hepatocytes, it was reported that miR-130a directly binds with lncRNA-H19 and PPARγ. By directly upregulating miR-130a, lncRNA-H19 knockdown inhibits PPARγ expression to alleviate lipid accumulation in hepatocytes. Hence, lncRNA-H19 promotes hepatic lipogenesis via the lncRNA-H19/miR-130a/PPARγ axis (Liu et al. 2019). Sterol regulatory element-binding protein 1c (SREBP1c) is an endoplasmic reticulum membrane-bound protein that functions as a transcription factor in the liver and the induction of lipogenesis is mainly controlled by SREBP1c (Watanabe et al. 2004). Liu et al. found that overexpression of lncRNA-H19 in hepatocytes increases the endogenous nuclear SREBP1 protein, thereby enhancing lipid accumulation. Further analysis showed that lncRNA-H19 interacts with polypyrimidine tract-binding protein 1 (PTBP1, also known as PTB, or hnRNP I) to enhance the binding of PTBP1 to SREBP1c mRNA. The combination of lncRNA-H19 and PTBP1 increases the stability and transcriptional activity of SREBP1c mRNA (Liu et al. 2018).
BAT activation lowers plasma TG and cholesterol levels, attenuating atherosclerosis (Berbee et al. 2015). Schmidt et al. reported that lncRNA-H19 possesses positive effects on the differentiation and mature fat cell function in BAT (Schmidt et al. 2018). This group showed that overexpression of lncRNA-H19 enhances, while its silence impairs adipogenesis, oxidative metabolism and mitochondrial respiration in brown adipocytes but not white adipocytes. Moreover, they found that lncRNA-H19 recruits the chromatin modifier methyl-CpG–binding domain protein 1 (MBD1) to form H19-MBD1 chromatin modifier complexes. These complexes specifically repress paternally expressed genes (PEGs) in brown adipocytes (Schmidt et al. 2018), and PEGs negatively affect BAT (Peters 2014), thereby lncRNA-H19 acting as the selective PEG gatekeeper in BAT (Schmidt et al. 2018). This finding may also shed light on the role of lncRNA-H19 in adipogenesis.
Collectively, lncRNA-H19 appears to play an important role in regulating lipid metabolism. In this way, lncRNA-H19 participates in atherosclerosis and its function is possibly tissue-specific while the underlying mechanisms need to be further studied.
LncRNA-H19 regulates inflammatory response
The inflammatory response is critically involved in the pathogenesis of atherosclerosis (Libby et al. 2019; Ross 1999; Weber and Noels 2011). Several mechanisms underlying the roles of inflammation in atherosclerosis have been identified, such as inflammatory activation of ECs, foam cell formation, foam cell secretion of inflammatory cytokines, and macrophage cell death (Geovanini and Libby 2018).
Previous studies have revealed that lncRNA-H19 is involved in several kinds of inflammatory responses. For example, in a rat model of diabetic cardiomyopathy, overexpression of lncRNA-H19 reduces the concentrations of inflammatory cytokines in myocardial tissues (Li et al. 2016). Moreover, Hu et al. demonstrated that pro-inflammatory factors (IL-1β, IL-6 and TNF-α) are reduced after the knockdown of lncRNA-H19 in lipopolysaccharide (LPS)-treated C28/I2 cells (Hu et al. 2019). Hence, there is a good reason to propose that lncRNA-H19 plays a role in inflammatory responses.
As a major transcription factor of inflammatory responses, nuclear factor-κB (NF-κB) was first discovered in 1986 (Sen and Baltimore 1986). Many NF-κB activators and NF-κB-regulated genes have been identified to be involved directly or indirectly in the process of atherosclerosis (Pamukcu et al. 2011).
Compelling evidence has revealed that mitogen-activated protein kinase (MAPK) signaling is a vital regulator of NF-κB-mediated inflammatory responses (Yuan et al. 2014; Shi et al. 2016; He et al. 2018). Overexpression of lncRNA-H19 leads to an increase of p38 and p65 in human umbilical vein endothelial cells (HUVECs), which are both key factors in the MAPK and NF-κB signaling pathways (Pan 2017). Similar results were reported in a recent trial also using HUVECs, after overexpression of lncRNA-H19, the NF-κB pathway is activated, p38 and p65 are increased (Li et al. 2019a), which further supports that lncRNA-H19 can increase the NF-κB-mediated inflammatory responses in vascular ECs.
In addition, knockdown of lncRNA-H19 in foam cells effectively decreases the expression of pro-inflammatory factors (TNF-α and IL-β) and increases the expression of anti-inflammatory factors (IL-4 and IL-10) (Han et al. 2018). The authors hypothesized that miR-130b is a target of lncRNA-H19. By upregulating miR-130b expression, lncRNA-H19 knockdown removes the facilitating effects of the miR-130b inhibitor on inflammatory responses, thereby lncRNA-H19 knockdown alleviates the inflammatory responses (Han et al. 2018). The miR-130 family negatively regulates metabolism-related inflammatory processes through several pathways including the NF-κB signaling pathway (Song et al. 2016; Zheng et al. 2016). Thus, it can be inferred that lncRNA-H19 plays a pro-inflammatory role via the potential lncRNA-H19/miR-130b/NF-κB pathway.
NF-κB possesses a causative function in inflammation, when in response to inflammatory signals, it promotes interleukin-6 (IL-6) expression by downregulating microRNA let-7. IL-6, which is a pro-inflammatory cytokine, activates NF-κB, thereby completing a positive feedback loop of the NF-κB/let-7/IL-6 pathway (Iliopoulos et al. 2009). Interestingly, in addition to regulating NF-κB, lncRNA-H19 can directly target let-7 to regulate inflammatory responses. In a recent study using a mouse model of abdominal aortic aneurysm (AAA), by sponging let-7a as a ceRNA, overexpression of lncRNA-H19 in VSMCs increases IL-6 expression, and ultimately promotes vascular inflammation and induces AAA formation. Moreover, the authors detected high levels of lncRNA-H19 in human and mouse AAA tissue samples (Sun et al. 2019b). Furthermore, Cao et al. showed that lncRNA-H19/let-7 axis participates in the regulation of oxygenized low density lipoprotein (ox-LDL)-induced EC injury. LncRNA-H19 knockdown in HUVECs reduces ox-LDL-induced secretion of inflammatory cytokines, such as IL-6 and TNF-α. The underlying mechanism may be that lncRNA-H19 inhibits periostin expression at least partially by sponging let-7 (Cao et al. 2019), and periostin acts as a regulator of inflammatory diseases including atherosclerosis (Koh et al. 2016; Schwanekamp et al. 2016). Taken together, these studies demonstrate a possible lncRNA-H19/let-7/IL-6 pathway for lncRNA-H19 regulation of atherosclerosis-related inflammatory responses, suggesting that lncRNA-H19 regulates inflammatory responses through several mechanisms.
LncRNA-H19 regulates cellular proliferation and apoptosis
The major cells involved in atherosclerosis such as VSMCs and ECs, are considered to undergo abnormal cellular proliferation and apoptosis to develop atherosclerosis. Aberrant apoptosis of VSMCs, for example, promotes both atherogenesis and multiple features of plaque instability (Ross 1999; Bennett et al. 2016).
Overexpression of lncRNA-H19 in VSMCs and HUVECs induces an increase in proliferation and a decrease in apoptosis (Pan 2017). Moreover, it has been reported that ox-LDL upregulates the expression of lncRNA-H19 in VSMCs and promotes the proliferation of VSMCs (Xu et al. 2015). Thus it can be inferred that lncRNA-H19 may have a role in cellular proliferation and apoptosis. In a recent trial using VSMCs and HUVECs, lentivirus-mediated lncRNA-H19-forced expression upregulates acid phosphatase 5 (ACP5) protein levels and subsequently promotes cellular proliferation and suppresses apoptosis. The authors hypothesized that ACP5, as a direct target of lncRNA-H19, causes atherosclerosis by affecting cellular proliferation and apoptosis (Huang et al. 2019).
Many studies have reported that lncRNA-H19 functions as a ceRNA to sponge its target miRNAs such as microRNA let-7 (Hsu et al. 2014; Sun et al. 2019b; Cao et al. 2019). Sun et al. found that lncRNA-H19 sponges let-7a, which binds to the 3′ UTR of cyclin D1 mRNA to exert negative regulation; therefore, lncRNA-H19 positively regulates cyclin D1, which is a key factor promoting the proliferation of VSMCs. Hence, lncRNA-H19 promotes the proliferation of VSMCs through the lncRNA-H19/let-7a/cyclin D1 axis (Sun et al. 2019c). Similarly, it was reported that lncRNA-H19 sponges let-7b to stimulate the expression of Ang II type 1 receptor (AT1R), which is a critical regulator of vasoconstriction and proliferation of arteries. Through the lncRNA-H19/let-7b/AT1R axis, lncRNA-H19 also promotes the proliferation of VSMCs (Su et al. 2018). In addition, Zhang et al. found that lncRNA-H19 functions as a ceRNA of miR-148b to enhance WNT1 expression, and the WNT/β-catenin signaling pathway is a vital pathway in the proliferation and apoptosis of VSMCs. Thus, lncRNA-H19 could facilitate proliferation and inhibit apoptosis of VSMCs through the lncRNA-H19/miR-148b/WNT/β-catenin axis (Zhang et al. 2018a). Taken together, these trials indicate that lncRNA-H19 promotes the proliferation and suppresses apoptosis of VSMCs by acting as a ceRNA to regulate its target genes.
In addition to working as a ceRNA, lncRNA-H19 can also regulate the proliferation and apoptosis of VSMCs by generating miR-675. Lv et al. found that lncRNA-H19 was overexpressed in balloon-injured carotid arteries. Their further results suggested that overexpression of lncRNA-H19 accelerates human aortic VSMC (HA-VSMC) proliferation in a miR-675-dependent manner. They identified that phosphatase and tensin homology deleted on chromosome ten (PTEN) is a target of miR-675 and PTEN is a well-known tumor suppressor that mediates VSMC proliferation. Hence, a lncRNA-H19/miR-675/PTEN axis was uncovered, through which lncRNA-H19 promotes the proliferation of VSMCs (Lv et al. 2018). Also via a miR-675-dependent pathway, another study reported that in balloon-injured rat carotid arteries, loss of lncRNA-H19 led to an increase of VSMC apoptosis. The authors found that H19-derived miR-675-5p targets and downregulates Mitofusin-2 (Mfn2) (Xu and Sun 2018), which has pro-apoptotic and anti-proliferative effects (Jin et al. 2011; Wang et al. 2012). However, in a study using a rat model of AAA, the authors found miR-675-independent pro-apoptosis effects of lncRNA-H19 on HA-VSMCs. Mechanistically, lncRNA-H19 enhances hypoxia-inducible factor 1α (HIF1α) expression and retains it within the cytoplasm. Increased cytoplasmic HIF1α directly interacts with mouse double minute 2 homolog (Mdm2) and inhibits Mdm2-mediated reduction of p53, leading to downregulation of the antiapoptotic mediator B cell lymphoma 2 (Bcl-2) and upregulation of the proapoptotic protein Bcl-2 associated X (BAX) (Li et al. 2018). Therefore, it is possible that lncRNA-H19 induces pro-proliferation and anti-apoptosis effects on VSMCs by generating miR-675 but it also induces pro-apoptosis effects on VSMCs when it functions via other mechanisms independent of miR-675.
Additionally, homocysteine (Hcy) induces proliferation of VSMCs. It has been reported that Hcy treatment results in the demethylation of differentially methylated regions between the H19 gene and IGF2 gene, which increases the expression of H19 and decreases the expression of IGF2. The proliferation of VSMCs induced by Hcy may be related to this mechanism (Li et al. 2009).
LncRNA-H19 and atherosclerosis-related cardiac dysfunction
Atherosclerosis is the major cause of coronary artery disease, leading to myocardial ischemia and infarction (Barquera et al. 2015; de Valk and Marx 1999). In addition to the regulation of vascular function, lncRNA-H19 has been suggested to participate in cardiomyocyte injury induced by ischemia/reperfusion (I/R) or infarction (Zhang et al. 2020; Li et al. 2019b).
The mRNA level of lncRNA-H19, which is significantly increased in acute myocardial infarction (AMI) patients, is positively correlated with cardiac biomarkers, such as creatine kinase (CK), suggesting a potential role for lncRNA-H19 in AMI diagnosis (Wang et al. 2019). It was found that lncRNA-H19 expression is significantly decreased in the infarcted myocardium of mice, and overexpression of lncRNA-H19 in AMI mice reduces infarct size and improve cardiac function by activating autophagy (Zhou et al. 2018). Using a rat model of AMI, Zhang et al. revealed that lncRNA-H19 sponges miR-22-3p to ameliorate AMI-induced myocardial damage by upregulating lysine (K)-specific demethylase 3A (KDM3A) (Zhang et al. 2020). It was also reported that lncRNA-H19 protects H9C2 cells against hypoxia-induced injury by sponging miR-139, which targets Sex determining region Y (SRY)-related high-mobility group box 8 (Sox8) (Gong et al. 2017). In addition, Choong et al. demonstrated that lncRNA-H19 and its interacting protein Y-box-binding protein-1 (YB-1) are involved in extracellular matrix (ECM) regulation during cardiac remodeling after infarction, and lncRNA-H19 directly antagonizes YB-1 under hypoxia, resulting in de-repression of Collagen 1A1 expression and cardiac fibrosis (Choong et al. 2019).
With regard to I/R injury, it was reported that rat lncRNA-H19 levels were increased following myocardial I/R injury (Rajagopalan et al. 2017). Li et al. showed that lncRNA-H19 upregulates Bcl-2 expression by sponging miR-877-3p and consequently alleviate myocardial I/R in mice and cardiomyocyte injury induced by H2O2 (often utilized to establish an in vitro model for I/R injury) (Li et al. 2019b). Similarly, lncRNA-H19 sponges miR-103 and miR-107, suppressing their expression and targeting Fas-associated protein with death domain (FADD) to antagonize cardiomyocyte necrosis in H2O2-induced H9C2 cells and a mouse I/R model (Wang et al. 2015). Furthermore, Chen et al. found that lncRNA-H19 protects against H2O2-induced cardiomyocyte injury by increasing the stability of nucleolin protein (Chen et al. 2020). In another study using hypoxic postconditioning (H/Post)-treated aged cardiomyocytes, lncRNA-H19 inhibits aged myocardial apoptosis and relieve H/Post-associated injury by sponging miR-29b-3p targeting cellular inhibitor of apoptosis protein 1 (cIAP1) (Zhang et al. 2019). However, a recent study showed that knockdown of lncRNA-H19 markedly improves the alterations of cardiac structure and function in myocardial I/R, at least partially due to the regulation of the miR-675/PPARα axis (Luo et al. 2019).
Conclusion
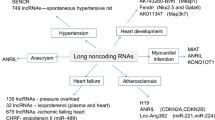
LncRNA-H19 plays important roles in the onset, development and progression of atherosclerosis by regulating its targets. Both clinical and laboratory research data suggest emerging roles for lncRNA-H19 and its pathways in various functions (Table 1), which are involved in the regulation of angiogenesis, adipocyte differentiation, lipid metabolism, inflammatory responses, cellular proliferation and apoptosis (Fig. 1).
Compared with the normal healthy control subjects, increased levels of lncRNA-H19 have been detected in the serum of atherosclerosis patients. Hence, the serum levels of lncRNA-H19 could be proposed as risk factors or diagnostic markers for atherosclerosis (Pan 2017; Huang et al. 2019; Han et al. 2018; Li et al. 2019a; Zhang et al. 2018a). Studies have shown that lncRNA-H19 upregulates VEGF-A by targeting miR-199a-5p, miR-29a and miR-29c. Thus, it can be inferred that lncRNA-H19 promotes angiogenesis by regulating multiple targets (Hou et al. 2018; Jia et al. 2016; Sun et al. 2019a). However, the exact role of lncRNA-H19 in adipogenesis and lipid metabolism remains to be elucidated. Ongoing studies should further clarify its relationship with different kinds of tissue and cells such as adipose tissue and precursor cells of adipocytes. With regard to the atherosclerosis-related inflammatory response, current studies have shown pro-inflammatory effects of lncRNA-H19 through several mechanisms including the NF-κB signaling pathway. Although the exact mechanisms are complicated, there is compelling evidence that lncRNA-H19 plays a critical role in the proliferation and apoptosis of VSMCs and ECs. Moreover, it has been shown that lncRNA-H19 is involved in cardiomyocyte injury induced by I/R injury or infarction (Fig. 2). Additionally, knockdown of lncRNA-H19 inhibits abnormal differentiation of small intestinal epithelial cells in diabetic mice and elevated hepatic expression of lncRNA-H19 contributes to hyperglycemia in type 2 diabetes, suggesting its potential role in the modulation of type 2 diabetes (Zhang et al. 2018b; Shan et al. 2018).
It is important to note that lncRNA-H19 mainly exerts its function by acting as a ceRNA to sponge miRNA, generating miRNA or directly regulating the downstream target. Experimental results have suggested that one miRNA could target more than 100 mRNAs and one miRNA could be targeted by many upstream factors (Chen et al. 2013; Najafi-Shoushtari et al. 2010; Rayner et al. 2010). Thus, how the single lncRNA-H19 regulates multiple targets in vitro and in vivo should be precisely identified in ongoing studies.
In summary, many studies currently suggest the pathophysiological contribution of lncRNA-H19 in the process of atherosclerosis. Based on increasing evidence, there has been a good reason to propose that lncRNA-H19 may serve as a potential indicator or a novel target for developing therapeutic strategies for atherosclerosis. In the future, much more research will be necessary before novel lncRNA-H19-based therapeutics are utilized into clinical practice.
Future perspectives and challenges
Many questions have been raised from the current studies about lncRNA-H19. It will be necessary to perform further studies to elucidate the following questions: (i) What influences lncRNA-H19 levels in the onset, development and progression of atherosclerosis? (ii) How does lncRNA-H19 strictly regulate multiple targets in vitro and in vivo? (iii) Are there any other lncRNA-H19 targets that can increase the risk of atherosclerosis? (iv) Does lncRNA-H19 influence the process of atherosclerosis by targeting different pathways in various cell types and tissues? (v) Why and when lncRNA-H19 may serve pro-apoptotic vs. anti-apoptotic roles and pro-survival vs. deleterious roles in I/R injury? Note that there are still some technical challenges that make future lncRNA-H19-based therapeutics difficult. Importantly, the potential significance of lncRNA-H19 requires careful studies that complement the development of reliable strategies to specifically target different genes and proteins in atherosclerosis-related cells. Understanding the molecular mechanisms and cellular pathways controlled by lncRNA-H19 will be warranted.
Availability of data and materials
Not applicable.
Abbreviations
- AS:
-
Atherosclerosis
- MI:
-
Myocardial infarction
- lncRNAs:
-
Long non-coding RNAs
- miRNAs:
-
microRNAs
- PRC2:
-
Polycomb repressive complex 2
- ceRNAs:
-
Competing endogenous RNAs
- VSMCs:
-
Vascular smooth muscle cells
- IGF2:
-
Insulin-like growth factor 2
- ECs:
-
Endothelial cells
- VEGF:
-
Vascular endothelial growth factor
- PlGF:
-
Placenta growth factor
- MMP-2:
-
Matrix metalloproteinase 2
- CTCF:
-
CCCTC-binding factor
- PKD1:
-
Polycystic kidney disease 1
- miR-199a-5p:
-
microRNA-199a-5p
- HMEC-1:
-
Human dermal vascular endothelial cells
- eNOS:
-
Endothelial NO synthase
- JNK:
-
C-Jun N-terminal kinase
- AMPK:
-
AMP-activated protein kinase
- HAMSCs:
-
Human amoiotic mesenchymal stem cells
- EZH2:
-
Enhancer of zeste homolog 2
- VASH1:
-
Vasohibin1
- STAT3:
-
Signal transducer and activator of transcription 3
- WAT:
-
White adipose tissue
- BAT:
-
Brown adipose tissue
- PVAT:
-
Perivascular adipose tissue
- BMSCs:
-
Bone marrow mesenchymal stem cells
- HDAC:
-
Histone deacetylase
- LCoR:
-
Ligand-dependent corepressor
- TG:
-
Triglycerides
- TC:
-
Total cholesterol
- LDL-C:
-
Lipoprotein-cholesterol
- HDL-C:
-
High density lipoprotein-cholesterol
- NAFLD:
-
Nonalcoholic fatty liver disease
- PPARγ:
-
Peroxisome proliferator-activated receptor γ
- SREBP1c:
-
Sterol regulatory element-binding protein 1c
- PTBP1:
-
Polypyrimidine tract-binding protein 1
- MBD1:
-
Methyl-CpG–binding domain protein 1
- PEGs:
-
Paternally expressed genes
- LPS:
-
Lipopolysaccharide
- NF-κB:
-
Nuclear factor-κB
- MAPK:
-
Mitogen-activated protein kinase
- HUVECs:
-
Human umbilical vein endothelial cells
- IL-6:
-
Interleukin-6
- AAA:
-
Abdominal aortic aneurysm
- ox-LDL:
-
Oxygenized low density lipoprotein
- ACP5:
-
Acid phosphatase 5
- AT1R:
-
Ang II type 1 receptor
- HA-VASC:
-
Human aortic vascular smooth muscle cell
- PTEN:
-
Phosphatase and tensin homology deleted on chromosome ten
- Mfn2:
-
Mitofusin-2
- HIF1α:
-
Hypoxia-inducible factor 1α
- Mdm2:
-
Mouse double minute 2 homolog
- Bcl-2:
-
B cell lymphoma 2
- BAX:
-
Bcl-2 associated X
- Hcy:
-
Homocysteine
- I/R:
-
Ischemia/reperfusion
- AMI:
-
Acute myocardial infarction
- CK:
-
Creatine kinase
- KDM3A:
-
Lysine (K)-specific demethylase 3A
- Sox8:
-
Sex determining region Y (SRY)-related high-mobility group box 8
- YB-1:
-
Y-box-binding protein-1
- ECM:
-
Extracellular matrix
- FADD:
-
Fas-associated protein with death domain
- H/Post:
-
Hypoxic postconditioning
- cIAP1:
-
Cellular inhibitor of apoptosis protein 1
References
Ahmadieh S, Kim HW, Weintraub NL. Potential role of perivascular adipose tissue in modulating atherosclerosis. Clin Sci (London, England : 1979). 2020;134:3–13.
Aryal B, Suarez Y. Non-coding RNA regulation of endothelial and macrophage functions during atherosclerosis. Vasc Pharmacol. 2019;114:64–75.
Ballantyne MD, Pinel K, Dakin R, Vesey AT, Diver L, Mackenzie R, et al. Smooth muscle enriched Long noncoding RNA (SMILR) regulates cell proliferation. Circulation. 2016;133:2050–65.
Barquera S, Pedroza-Tobías A, Medina C, Hernández-Barrera L, Bibbins-Domingo K, Lozano R, et al. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. 2015;46:328–38.
Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692–702.
Berbee JF, Boon MR, Khedoe PP, Bartelt A, Schlein C, Worthmann A, et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun. 2015;6:6356.
Bitarafan S, Yari M, Broumand MA, Ghaderian SMH, Rahimi M, Mirfakhraie R, et al. Association of Increased Levels of lncRNA H19 in PBMCs with risk of coronary artery disease. Cell J. 2019;20:564–8.
Cai X, Cullen BR. The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA (New York, N.Y.). 2007;13:313–6.
Camaré C, Pucelle M, Nègre-Salvayre A, Salvayre R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017;12:18–34.
Cao H, Zhang S, Shan S, Sun C, Li Y, Wang H, et al. Ligand-dependent corepressor (LCoR) represses the transcription factor C/EBPbeta during early adipocyte differentiation. J Biol Chem. 2017;292:18973–87.
Cao L, Zhang Z, Li Y, Zhao P, Chen Y. LncRNA H19/miR-let-7 axis participates in the regulation of ox-LDL-induced endothelial cell injury via targeting periostin. Int Immunopharmacol. 2019;72:496–503.
Chen C, Liu M, Tang Y, Sun H, Lin X, Liang P, et al. LncRNA H19 is involved in myocardial ischemic preconditioning via increasing the stability of nucleolin protein. J Cell Physiol. 2020;235:5985-94.
Chen L, Xiao H, Wang ZH, Huang Y, Liu ZP, Ren H, et al. miR-29a suppresses growth and invasion of gastric cancer cells in vitro by targeting VEGF-a. BMB Rep. 2014;47:39–44.
Chen WJ, Zhang M, Zhao GJ, Fu Y, Zhang DW, Zhu HB, et al. MicroRNA-33 in atherosclerosis etiology and pathophysiology. Atherosclerosis. 2013;227:201–8.
Choong OK, Chen CY, Zhang J, Lin JH, Lin PJ, Ruan SC, et al. Hypoxia-induced H19/YB-1 cascade modulates cardiac remodeling after infarction. Theranostics. 2019;9:6550–67.
de Valk B, Marx JJ. Iron, atherosclerosis, and ischemic heart disease. Arch Intern Med. 1999;159:1542–8.
Di Stefano R, Felice F, Balbarini A. Angiogenesis as risk factor for plaque vulnerability. Curr Pharm Des. 2009;15:1095–106.
Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47:C7–12.
Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21.
Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci (London, England : 1979). 2018;132:1243–52.
Gong LC, Xu HM, Guo GL, Zhang T, Shi JW, Chang C. Long non-coding RNA H19 protects H9c2 cells against hypoxia-induced injury by targeting MicroRNA-139. Cell Physiol Biochem. 2017;44:857–69.
Han DK, Khaing ZZ, Pollock RA, Haudenschild CC, Liau G. H19, a marker of developmental transition, is reexpressed in human atherosclerotic plaques and is regulated by the insulin family of growth factors in cultured rabbit smooth muscle cells. J Clin Invest. 1996;97:1276–85.
Han Y, Ma J, Wang J, Wang L. Silencing of H19 inhibits the adipogenesis and inflammation response in ox-LDL-treated Raw264.7 cells by up-regulating miR-130b. Yonsei Med J. 2018;93:107–14.
He P, Yan S, Zheng J, Gao Y, Zhang S, Liu Z, et al. Eriodictyol attenuates LPS-induced Neuroinflammation, Amyloidogenesis, and cognitive impairments via the inhibition of NF-kappaB in male C57BL/6J mice and BV2 microglial cells. J Agric Food Chem. 2018;66:10205–14.
Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–80.
Hofmann P. The Long Noncoding RNA H19 Controls Endothelial Cell Functions by STAT3 Repression. In: Johann Wolfgang Goethe-Universität Frankfurt am Main; 2018.
Hofmann P, Sommer J, Theodorou K, Kirchhof L, Fischer A, Li Y, et al. Long non-coding RNA H19 regulates endothelial cell aging via inhibition of STAT3 signalling. Cardiovasc Res. 2019;115:230–42.
Hou J, Wang L, Wu Q, Zheng G, Long H, Wu H, et al. Long noncoding RNA H19 upregulates vascular endothelial growth factor a to enhance mesenchymal stem cells survival and angiogenic capacity by inhibiting miR-199a-5p. Stem Cell Res Ther. 2018;9:109.
Hsu CY, Hsieh TH, Tsai CF, Tsai HP, Chen HS, Chang Y, et al. miRNA-199a-5p regulates VEGFA in endometrial mesenchymal stem cells and contributes to the pathogenesis of endometriosis. J Pathol. 2014;232:330–43.
Hu Y, Li S, Zou Y. Knockdown of LncRNA H19 relieves LPS-induced damage by modulating miR-130a in osteoarthritis. Yonsei Med J. 2019;60:381–8.
Huang Y, Wang L, Mao Y, Nan G. Long noncoding RNA-H19 contributes to atherosclerosis and induces ischemic stroke via the Upregulation of acid phosphatase 5. Front Neurol. 2019;10:32.
Huang Y, Zheng Y, Jin C, Li X, Jia L, Li W. Long non-coding RNA H19 inhibits adipocyte differentiation of bone marrow Mesenchymal stem cells through epigenetic modulation of histone deacetylases. Sci Rep. 2016;6:28897.
Hurst LD, Smith NG. Molecular evolutionary evidence that H19 mRNA is functional. Trends Genet. 1999;15:134–5.
Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706.
Jia P, Cai H, Liu X, Chen J, Ma J, Wang P, et al. Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Cancer Lett. 2016;381:359–69.
Jin B, Fu G, Pan H, Cheng X, Zhou L, Lv J, et al. Anti-tumour efficacy of mitofusin-2 in urinary bladder carcinoma. Med Oncol (Northwood, London, England). 2011;28(Suppl 1):S373–80.
Kim DK, Zhang L, Dzau VJ, Pratt RE. H19, a developmentally regulated gene, is reexpressed in rat vascular smooth muscle cells after injury. J Clin Invest. 1994;93:355–60.
Kim SW, Zhang HZ, Kim CE, An HS, Kim JM, Kim MH. Amniotic mesenchymal stem cells have robust angiogenic properties and are effective in treating hindlimb ischaemia. Cardiovasc Res. 2012;93:525–34.
Kim YK, Kook H. Diverse roles of noncoding RNAs in vascular calcification. Arch Pharm Res. 2019;42:244–51.
Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152:570–83.
Koh SJ, Choi Y, Kim BG, Lee KL, Kim DW, Kim JH, et al. Matricellular protein Periostin mediates intestinal inflammation through the activation of nuclear factor kappaB signaling. PLoS One. 2016;11:e0149652.
Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–77.
Li DY, Busch A, Jin H, Chernogubova E, Pelisek J, Karlsson J, et al. H19 induces abdominal aortic aneurysm development and progression. Circulation. 2018;138:1551–68.
Li L, Xie J, Zhang M, Wang S. Homocysteine harasses the imprinting expression of IGF2 and H19 by demethylation of differentially methylated region between IGF2/H19 genes. Acta Biochim Biophys Sin. 2009;41:464–71.
Li Q, Shen PY, Wu G, Chen XZ. Polycystin-2 interacts with troponin I, an angiogenesis inhibitor. Biochemistry. 2003;42:450–7.
Li X, Luo S, Zhang J, Yuan Y, Jiang W, Zhu H, et al. lncRNA H19 alleviated myocardial I/RI via suppressing miR-877-3p/Bcl-2-mediated mitochondrial apoptosis. Mol Ther Nucleic Acids. 2019b;17:297–309.
Li X, Wang H, Yao B, Xu W, Chen J, Zhou X. lncRNA H19/miR-675 axis regulates cardiomyocyte apoptosis by targeting VDAC1 in diabetic cardiomyopathy. Sci Rep. 2016;6:36340.
Li ZF, Shu XJ, Chang YW, Liu SY, Wang WH. Effect of lncRNA H19 on the apoptosis of vascular endothelial cells in arteriosclerosis obliterans via the NF-kappaB pathway. Eur Rev Med Pharmacol Sci. 2019a;23:4491–7.
Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56.
Liu C, Yang Z, Wu J, Zhang L, Lee S, Shin DJ, et al. Long noncoding RNA H19 interacts with polypyrimidine tract-binding protein 1 to reprogram hepatic lipid homeostasis. Hepatology (Baltimore, Md.). 2018;67:1768–83.
Liu J, Tang T, Wang GD, Liu B. LncRNA-H19 promotes hepatic lipogenesis by directly regulating miR-130a/PPARgamma axis in non-alcoholic fatty liver disease. Biosci Rep. 2019;39.
Liu L, Bi N, Wu L, Ding X, Men Y, Zhou W, et al. MicroRNA-29c functions as a tumor suppressor by targeting VEGFA in lung adenocarcinoma. Mol Cancer. 2017;16:50.
Luo H, Wang J, Liu D, Zang S, Ma N, Zhao L, et al. The lncRNA H19/miR-675 axis regulates myocardial ischemic and reperfusion injury by targeting PPARalpha. Mol Immunol. 2019;105:46–54.
Lv C, Zhou YH, Wu C, Shao Y, Lu CL, Wang QY. The changes in miR-130b levels in human serum and the correlation with the severity of diabetic nephropathy. Diabetes Metab Res Rev. 2015;31:717–24.
Lv J, Wang L, Zhang J, Lin R, Wang L, Sun W, et al. Long noncoding RNA H19-derived miR-675 aggravates restenosis by targeting PTEN. Respir Res. 2018;497:1154–61.
Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science (New York, N.Y.). 2010;328:1566–9.
Nebbioso A, Dell'Aversana C, Bugge A, Sarno R, Valente S, Rotili D, et al. HDACs class II-selective inhibition alters nuclear receptor-dependent differentiation. J Mol Endocrinol. 2010;45:219–28.
Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–8.
Pamukcu B, Lip GY, Shantsila E. The nuclear factor--kappa B pathway in atherosclerosis: a potential therapeutic target for atherothrombotic vascular disease. Thromb Res. 2011;128:117–23.
Pan JX. LncRNA H19 promotes atherosclerosis by regulating MAPK and NF-kB signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21:322–8.
Peters J. The role of genomic imprinting in biology and disease: an expanding view. Nat Rev Genet. 2014;15:517–30.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science (New York, N.Y.). 1999;284:143–7.
Rajagopalan V, Zhang Y, Pol C, Costello C, Seitter S, Lehto A, et al. Modified low-dose Triiodo-L-thyronine therapy safely improves function following myocardial ischemia-reperfusion injury. Front Physiol. 2017;8:225.
Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science (New York, N.Y.). 2010;328:1570–3.
Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26.
Schmidt E, Dhaouadi I, Gaziano I, Oliverio M. LincRNA H19 protects from dietary obesity by constraining expression of monoallelic genes in brown fat. Nat Commun. 2018;9:3622.
Schwanekamp JA, Lorts A, Vagnozzi RJ, Vanhoutte D, Molkentin JD. Deletion of periostin protects against atherosclerosis in mice by altering inflammation and extracellular matrix remodeling. Arterioscler Thromb Vasc Biol. 2016;36:60–8.
Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921–8.
Shan TD, Lv SY, Tian ZB, Liu XS, Liu FG, Sun XG. Knockdown of lncRNA H19 inhibits abnormal differentiation of small intestinal epithelial cells in diabetic mice. J Cell Physiol. 2018;234:837–48.
Shi ZM, Han YW, Han XH, Zhang K, Chang YN, Hu ZM, et al. Upstream regulators and downstream effectors of NF-kappaB in Alzheimer's disease. J Neurol Sci. 2016;366:127–34.
Smits G, Mungall AJ, Griffiths-Jones S, Smith P, Beury D, Matthews L, et al. Conservation of the H19 noncoding RNA and H19-IGF2 imprinting mechanism in therians. Nat Genet. 2008;40:971–6.
Song CL, Liu B, Shi YF, Liu N, Yan YY, Zhang JC, et al. MicroRNA-130a alleviates human coronary artery endothelial cell injury and inflammatory responses by targeting PTEN via activating PI3K/Akt/eNOS signaling pathway. Oncotarget. 2016;7:71922–36.
Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008;49:600–7.
St Laurent G, Wahlestedt C, Kapranov P. The landscape of long noncoding RNA classification. Trends Genet. 2015;31:239–51.
Su H, Xu X, Yan C, Shi Y, Hu Y, Dong L, et al. LncRNA H19 promotes the proliferation of pulmonary artery smooth muscle cells through AT1R via sponging let-7b in monocrotaline-induced pulmonary arterial hypertension. Respir Res. 2018;19:254.
Sun B, Ding Y, Jin X, Xu S, Zhang H. Long non-coding RNA H19 promotes corneal neovascularization by targeting microRNA-29c. Biosci Rep. 2019a;39.
Sun W, Lv J, Duan L, Lin R, Li Y, Li S, et al. Long noncoding RNA H19 promotes vascular remodeling by sponging let-7a to upregulate the expression of cyclin D1. Biochem Biophys Res Commun. 2019c;508:1038–42.
Sun Y, Zhong L, He X, Wang S, Lai Y, Wu W, et al. LncRNA H19 promotes vascular inflammation and abdominal aortic aneurysm formation by functioning as a competing endogenous RNA. J Mol Cell Cardiol. 2019b;131:66-81.
Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–52.
van Dam AD, Boon MR, Berbée JFP, Rensen PCN, van Harmelen V. Targeting white, brown and perivascular adipose tissue in atherosclerosis development. Eur J Pharmacol. 2017;816:82–92.
Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214:3–10.
Voellenkle C, Garcia-Manteiga JM, Pedrotti S, Perfetti A, De Toma I, Da Silva D, et al. Implication of Long noncoding RNAs in the endothelial cell response to hypoxia revealed by RNA-sequencing. Sci Rep. 2016;6:24141.
Wang J-X, Zhang X-J, Li Q, Wang K, Wang Y, Jiao J-Q, et al. MicroRNA-103/107 regulate programmed necrosis and myocardial ischemia/reperfusion injury through targeting FADD. Circ Res. 2015;117:352–63.
Wang W, Lu J, Zhu F, Wei J, Jia C, Zhang Y, et al. Pro-apoptotic and anti-proliferative effects of mitofusin-2 via Bax signaling in hepatocellular carcinoma cells. Med Oncol (Northwood, London, England). 2012;29:70–6.
Wang XM, Li XM, Song N, Zhai H, Gao XM, Yang YN. Long non-coding RNAs H19, MALAT1 and MIAT as potential novel biomarkers for diagnosis of acute myocardial infarction. Biomed Pharmacother = Biomed Pharmacother. 2019;118:109208.
Wang Y, Liu W, Liu Y, Cui J, Zhao Z, Cao H, et al. Long noncoding RNA H19 mediates LCoR to impact the osteogenic and adipogenic differentiation of mBMSCs in mice through sponging miR-188. J Cell Physiol. 2018;233:7435–46.
Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–18.
Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–22.
Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen C, et al. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. 2014;130:1452–65.
Xu H, Zhang K, Wang Y, Duan C, Li L. The effect of ox-LDL on the proliferation and expression of H19 and IGF2 in VSMCs. J Zunyi Med Univ. 2015;38:374-8.
Xu X, Sun S. H19 promotes vascular smooth muscle cell proliferation by releasing miR-675-5p to target mitofusin-2. Atheroscler Suppl. 2018;32:103.
Yan L, Zhou J, Gao Y, Ghazal S, Lu L, Bellone S, et al. Regulation of tumor cell migration and invasion by the H19/let-7 axis is antagonized by metformin-induced DNA methylation. Oncogene. 2015;34:3076–84.
Yang Y, Tang F, Wei F, Yang L, Kuang C, Zhang H, et al. Silencing of long non-coding RNA H19 downregulates CTCF to protect against atherosclerosis by upregulating PKD1 expression in ApoE knockout mice. Aging. 2019;11:10016–30.
Yu B, Wang S. Angio-LncRs: LncRNAs that regulate angiogenesis and vascular disease. Theranostics. 2018;8:3654–75.
Yuan L, Wu Y, Ren X, Liu Q, Wang J, Liu X. Isoorientin attenuates lipopolysaccharide-induced pro-inflammatory responses through down-regulation of ROS-related MAPK/NF-kappaB signaling pathway in BV-2 microglia. Mol Cell Biochem. 2014;386:153–65.
Yuan Z, Bian Y, Ma X, Tang Z, Chen N, Shen M. LncRNA H19 knockdown in human amniotic mesenchymal stem cells suppresses angiogenesis by associating with EZH2 and activating VASH1. Stem Cells Dev. 2019;28:781-90.
Zhang BF, Jiang H, Chen J, Hu Q, Yang S, Liu XP, et al. LncRNA H19 ameliorates myocardial infarction-induced myocardial injury and maladaptive cardiac remodelling by regulating KDM3A. J Cell Mol Med. 2020;24:1099–115.
Zhang L, Cheng H, Yue Y, Li S, Zhang D, He R. H19 knockdown suppresses proliferation and induces apoptosis by regulating miR-148b/WNT/beta-catenin in ox-LDL-stimulated vascular smooth muscle cells. J Biomed Sci. 2018a;25:11.
Zhang N, Geng T, Wang Z, Zhang R, Cao T, Camporez JP, et al. Elevated hepatic expression of H19 long noncoding RNA contributes to diabetic hyperglycemia. JCI Insight. 2018b;3:e120304.
Zhang X, Cheng L, Xu L, Zhang Y, Yang Y, Fu Q, et al. The lncRNA, H19 Mediates the protective effect of hypoxia postconditioning against hypoxia-reoxygenation injury to senescent cardiomyocytes by targeting microRNA-29b-3p. Shock (Augusta, Ga.). 2019;52:249–56.
Zhang Z, Gao W, Long QQ, Zhang J, Li YF, Liu DC, et al. Increased plasma levels of lncRNA H19 and LIPCAR are associated with increased risk of coronary artery disease in a Chinese population. Sci Rep. 2017;7:7491.
Zheng H, Dong X, Liu N, Xia W, Zhou L, Chen X, et al. Regulation and mechanism of mouse miR-130a/b in metabolism-related inflammation. Int J Biochem Cell Biol. 2016;74:72–83.
Zheng J, Zhou Y, Zhang K, Qi Y, An S, Wang S, et al. Association between nonalcoholic fatty liver disease and subclinical atherosclerosis: a cross-sectional study on population over 40 years old. BMC Cardiovasc Disord. 2018;18:147.
Zhou M, Zou YG, Xue YZ, Wang XH, Gao H, Dong HW, et al. Long non-coding RNA H19 protects acute myocardial infarction through activating autophagy in mice. Eur Rev Med Pharmacol Sci. 2018;22:5647–51.
Zhu A, Chu L, Ma Q, Li Y. Long non-coding RNA H19 down-regulates miR-181a to facilitate endothelial angiogenic function. Artif Cells, Nanomed Biotechnol. 2019a;47:2698–705.
Zhu P, Lu H, Jing Y, Zhou H, Ding Y, Wang J, et al. Interaction between AGTR1 and PPARgamma gene polymorphisms on the risk of nonalcoholic fatty liver disease. Genet Testing Mol Biomark. 2019b;23:166–75.
Acknowledgments
Not applicable.
Funding
This work is supported by National Natural Sciences Foundation of China (81870337, China).
Author information
Authors and Affiliations
Contributions
XS and G-JZ conceived the article. XS is the major contributor in writing the manuscript, as well as in preparing the figure and Table. Y-TW contributed to writing some sections and preparing the Table. HL, TJ, X-LZ, KY and G-JZ contributed to revision of the manuscript. KY and G-JZ are corresponding authors. G-JZ is the supervisor for the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, X., Wei, YT., Li, H. et al. Long non-coding RNA H19 in atherosclerosis: what role?. Mol Med 26, 72 (2020). https://doi.org/10.1186/s10020-020-00196-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10020-020-00196-w





 : promote,
: promote, : inhibit
: inhibit
 : aggravate,
: aggravate, : alleviate
: alleviate