Abstract
Swine production has been an important part of our lives since the late Mesolithic or early Neolithic periods, and ranks number one in world meat production. Pig production also contributes to high-value-added medical markets in the form of pharmaceuticals, heart valves, and surgical materials. Genetic engineering, including the addition of exogenous genetic material or manipulation of the endogenous genome, holds great promise for changing pig phenotypes for agricultural and medical applications. Although the first transgenic pigs were described in 1985, poor survival of manipulated embryos; inefficiencies in the integration, transmission, and expression of transgenes; and expensive husbandry costs have impeded the widespread application of pig genetic engineering. Sequencing of the pig genome and advances in reproductive technologies have rejuvenated efforts to apply transgenesis to swine. Pigs provide a compelling new resource for the directed production of pharmaceutical proteins and the provision of cells, vascular grafts, and organs for xenotransplantation. Additionally, given remarkable similarities in the physiology and size of people and pigs, swine will increasingly provide large animal models of human disease where rodent models are insufficient. We review the challenges facing pig transgenesis and discuss the utility of transposases and recombinases for enhancing the success and sophistication of pig genetic engineering. 'The paradise of my fancy is one where pigs have wings.' (GK Chesterton).
Similar content being viewed by others
Introduction
Pigs are ungulates native to Eurasia collectively grouped under the genus Sus within the Suidae family. Phylogeographic analysis reveals that pigs were domesticated independently at least seven times around the globe, first at least 9,000 years ago [1, 2]. Our longstanding affinity for pigs stems from their omnivorous ability to convert even our scraps into healthy and nutritious pork. Since their domestication, pigs have also captured our cultural imagination. Their intelligence and almost human behavior finds pigs intertwined with us in mythology, language, and art. The meat pig represents a significant commodity worldwide, in 2004 producing more than 89 million tons of meat [3] and contributing more than $50 billion to the US economy alone [4]. Co-products from hogs play a vital although less visible role in maintaining and improving the quality of human life, being the primary source of more than 20 drugs and pharmaceuticals [5]. Pig insulin, which differs from the human protein by a single amino acid, had saved the lives of innumerable type 1 diabetic patients before the development of recombinant human insulin. Pig heart valves are used to repair damaged or diseased human hearts, and pig skin is used to treat severe burn victims and to produce collagen scaffolds, gels, and other surgical materials.
The anthropomorphism of pigs in our culture seems almost prescient, given what we now know to be extensive similarities between human and pig molecular, cellular, and systems physiology [6, 7]. Pigs were Galen's preferred models in his quest for truth about human anatomy during an era that forbade human dissection [8]. Christian Barnard, who performed the worlds first heart transplant in 1967, once remarked that, 'Strange as it may seem, in several anatomic aspects the pig is closer to the human being than any other animal' [9], a view that motivated the development of the Minnesota minipig at the Hormel Institute in Austin, Minnesota [9, 10]. Improvements in our ability to manipulate the pig genome will increase the importance of pigs in biomedicine, both as models of human disease and as donors of cells, tissues, and organs for xenotransplantation.
Goals and applications of pig genome modification
Since their domestication, producers have striven to improve the performance of pigs by the selection and improvement of pig genetics, and by engineering of systems for their production. Significant contemporary efforts are focused on genetic improvement using genetic marker assisted selection [11, 12] and genetical genomics [13, 14]. With the emergence of technologies for animal transgenesis and genetic engineering, scientists have also sought to improve the performance or change the phenotype of pigs based on directed genetic modification. Agricultural objectives include enhancing growth and nutrient partitioning [15–17], changing pork composition [18, 19], supplementing milk composition for piglet consumption [20, 21], improving pig resistance to pathogens [22], and even reducing the environmental impact of pig waste [23]. Efforts to expand the utility of pigs as bioreactors for pharmaceutical production have targeted the expression of therapeutic proteins in their milk [24–26], blood [27, 28], urine [29], and potentially semen [30, 31].
A survey of the US National Institutes of Health CRISP (Computer Retrieval of Information on Scientific Projects) database reveals that pigs are currently the subjects of more than 450 active research projects. Among these, a handful aim to alter pigs genetically and so develop large animal models of human disease. Nearly a decade ago, a pig model of retinitis pigmentosa was created by germline transgenesis with a dominant mutant rhodopsin gene (Pro347Leu) [32]. This model provided important data regarding the earliest stages in photoreceptor degeneration in this condition. Contemporary targets include models of arteriosclerosis and cystic fibrosis [33, 34], diseases in which animal size and physiology diminish the utility of mouse models.
Xenotransplantation - the transplantation of cells, tissue, and organs from one species to another - may be the most important application of pig genetic engineering. According to the United Network for Organ Sharing, nearly 94,000 people are currently on the waiting list for organ transplants in the USA alone, with only 20% likely to receive this life saving procedure (Table 1) because of a shortage of suitable organs or tissues. Targets for the genetic modification of pigs for xenotransplantation have thus far emphasized reducing the immunogenicity of pig cells and tissues, and preventing the hyperacute rejection (HAR) and acute vascular rejection responses that are observed within minutes and days, respectively, after transplantation of pig organs to non-human primates (NHPs).
HAR of porcine organs by old world primate recipients is mediated through preformed antibodies against galactosyl-α-1,3-galactose epitopes expressed on the surface of pig cells. Antigen recognition leads to complement activation and assembly of membrane attack complexes on the surface of donor tissue endothelium, causing cell lysis, hemorrhage, and clotting that occludes the donor tissue blood supply. Transgenic pigs have been developed that express regulators of the complement cascade, including CD55 (decay accelerating factor), CD59, and CD46 (membrane co-factor protein), which are intended to suppress the assembly of membrane attack complexes on donor tissues [35–37]. Xenogenic transplants of organs from these pigs into NHPs have indeed exhibited significant improvement in terms of controlling HAR. A complementary approach has focused on eliminating the galactosyl-α-1,3-galactose antigen from the surface of donor cells. Several groups achieved this feat by generating pigs without the gene encoding α-1,3-galactosyltransferase, which is the enzyme that is required for this sugar modification [38]. This was accomplished by the serial 'knockout' of the gene in cultured pig fibroblasts, followed by somatic cell nuclear transfer (SCNT) to generate pigs. This revolutionary accomplishment marks the beginning of a new era in pig genetic engineering, providing a path to the generation of pigs based on both gene supplementation and ablation.
Pig cells are also a promising resource to counter the limited supply of human tissues for cell-based therapy, particularly neurologic disorders and diabetes. Recent clinical and pre-clinical trials of islet cell transplantation and xenotransplantation, respectively, suggest that xenogeneic cellular therapy may indeed provide a viable option for the treatment of diabetes. Serendipitously, adult pig islets do not express the galactosyl-α-1,3-galactose epitope. Instead, rejection [39] of xenogeneic islets in NHPs results from direct or indirect activation of T cells by donor pig xenopeptides. Targeted prevention of T cell co-stimulation has led to great strides in pig islet xenotransplantation to NHPs [40, 41]. However, maintenance of immunosuppression puts patients at risk for opportunistic infections, and can cause significant cardiovascular, renal, hematologic, gastrointestinal, and (in female patients) reproductive toxicity [42, 43]. Pig transgenesis could provide an alternative approach to systemic T-cell co-stimulation blockade, instead relying on the local provision of immunotherapeutic proteins by the xenograft [44, 45].
Prevention of zoonotic transmission of pathogens from donor pigs to patients is also crucial for clinical application of porcine xenotransplantation. Although husbandry in a biosecure environment can eliminate most risk, endogenous agents such as porcine endogenous retroviruses (PERVs) require special attention. Indeed, upon co-cultivation of pig and human cells, PERVs inefficiently traverse the species barrier [46–48]. Although no evidence of pig to human transfer has ever been observed in vivo [49–51], it is prudent to develop pigs with a reduced genetic potential for PERV transmission [52–56].
Casting pearls unto swine (porcine transgenesis)
Generation of transgenic pigs, like that of other mammals, has traditionally relied on the introduction of exogenous DNA expression constructs into the pig genome by pronuclear injection (PNI) [57]. Although PNI remains the primary method of mouse transgenesis, low rates of germline transmission and expensive husbandry costs have interfered with the widespread application of this technology to livestock. SCNT has emerged as an excellent alternative to PNI, boasting transgenesis rates of 100% depending on selection of donor nuclei. Despite the success of both PNI and SCNT in pig transgenesis, both methods suffer from an extremely low transgenesis rate per embryo/ova processed. Recent successes in the application of lentiviral transduction to pig transgenesis have demonstrated it to be quite efficient, and improvements in embryo survival result in transgenesis efficiencies of about 80% of live-born animals with a concomitant increase in the rate of transgenesis per embryo processed [58, 59]. Recent results in mice suggest that coupling PNI with transposon systems also provides a viable alternative to transgenic pig production. We discuss the strengths and weaknesses of each of these approaches, and present an analysis of the value of transposons as tools for mammalian transgenesis, with an emphasis on pigs (also see Table 2).
A poke in a pig (pronuclear injection)
PNI was the first method used to produce transgenic pigs [57]. Generally, this involves surgical harvest of pronuclear staged embryos from the oviduct of donor animals, injection of a DNA solution into the male pronuclei, and then transfer of injected embryos into the oviduct of a recipient female at a similar stage of estrus. Significant challenges in coordinating the reproductive cycles of donors and recipients have been countered with the development of excellent methods for estrous synchronization and superovulation [60]. Pronuclear microinjection is further complicated by the presence of a lipid-laden cytoplasm that obfuscates visualization of the pronucleus. However, brief centrifugation stratifies the cytoplasm, revealing the pronucleus in 66% to 85% of embryos [61]. Tail-docks, ear-clips, or blood of live-born piglets is usually screened by either polymerase chain reaction or Southern blotting to identify transgenic founders and to eliminate nontransgenic animals from further husbandry.
There are two primary bottlenecks that limit the efficiency of this approach: embryo survival and the efficiency of transgene integration. In vitro culture and manipulation severely reduce the survival of injected embryos. Unfortunately, simply transferring embryos from one pig oviduct to another results in live-birth rates of only 35% to 40% of transferred embryos [62]. Microinjection results in only 10% to 15% of transferred embryos surviving to term [63, 64], with increased losses probably due to physical perturbation of the cell and toxicity of DNA and associated impurities [65, 66]. Transgenesis frequencies per injected embryo have ranged between 0.24% and 2.6% [67, 68], although a transgenesis rate as high as 4.2% following optimization of DNA concentration was recently reported [66]. A compromise between embryo survival and transgenesis is required to obtain the greatest overall efficiency of transgenic offspring per injected embryo, because increasing the concentration of injected DNA enhances transgenesis but reduces the number of animals born [66]. As discussed below, enzymatic delivery of transgenes to the genome by transposons may permit the use of low DNA concentrations, thereby maximizing live-birth rate without compromising rates of transgenesis.
A notable limitation of PNI is an inability to create allelic substitution (so-called knock-out or knock-in) by homologous recombination (HR). Therefore, alternative methods are required to generate hypomorphic, loss-of-function, or null pigs depleted of specific gene products. One approach successfully used in pigs relied on PNI-mediated transgenesis with a dominant negative transgene [32]. As mentioned above, Petters and coworkers [32] developed an informative swine model of retinitis pigmentosa based on directed expression of a dominant negative allele of the human rhodopsin gene. However, dominant negative alleles will not be available for every target and so are likely to be limiting. RNA interference (RNAi), on the other hand, provides a seemingly universal method for depleting gene function in swine (for review [69]).
RNAi is an evolutionarily conserved surveillance mechanism that responds to double-stranded RNA by sequence-specific silencing of gene expression. Stable expression of short hairpin RNA in eukaryotic cells using H1, U6, and 7S K polymerase III promoters [70, 71] as well as polymerase II promoters [72], has proven effective in eliminating mRNA transcribed from targeted genes. Peng and coworkers [73] recently observed RNAi-mediated mouse phenotypes after PNI transgenesis without toxicity. Indeed, we were able to generate gastrointestinal phenocopies of cystic fibrosis in mice by PNI transgenesis with transposons expressing short hairpin RNA directed against the cystic fibrosis transmembrane regulator (Carlson and coworkers, unpublished data). These observations, coupled with the demonstrated efficacy of RNAi in pig cells [33], suggest that RNAi represents an efficient, dominant, and specific approach to developing transgenic pigs by PNI or SCNT.
Turning a sow's ear into a silk purse (somatic cell nuclear transfer)
SCNT, or cloning, involves the transfer of a somatic cell nucleus from a donor cell into an enucleated oocyte, fusion and activation of the reconstructed embryo, and subsequent transfer to surrogate females to establish pregnancy. Since its introduction, SCNT has become a popular alternative to PNI for the addition of transgenes to the pig genome for several reasons (Table 2). Two of the more notable advantages of SCNT in producing transgenic offspring by gene addition are the rate of transgenesis among live-born offspring and the possibility of screening nuclear donor cells for transgenesis and gene expression before embryo reconstruction. Depending on the method of donor cell transfection and selection, the transgenesis rate in SCNT piglets can be 100%. However, considering that only 0.05% to 1.2% [74, 75] of reconstructed embryos will produce live offspring, the overall rate of transgenesis per reconstructed embryo is similar to that for PNI. Another advantage of SCNT is the ready commercial availability of oocytes, which can be matured in vitro and then enucleated before receiving nuclei from donor cells.
Although the ability to screen for transgene expression in donor cells before cloning provides some advantage, given their restricted lineage, transgene expression in porcine fetal fibroblasts (PFFs) is frequently not expected to be indicative of expression in animals derived from them. The most striking advantage of SCNT is the ability to achieve HR in cultured donor cells [74, 76–79], demonstrated by several groups focused on eliminating the α-(1,3)-galactosyltransferase locus. This has important implications for the knockout or allelic replacement of target genes, although other loci may be more challenging, given that loci vary in the efficiency with which they can be targeted [80, 81]. Additionally, unlike murine embryonic stem cells, the window of opportunity for isolating recombined cellular clones, and thus the complexity of manipulations possible, is limited by PFF cellular senescence. The limited lifespan of PFFs has prohibited serial transgenesis, genetic manipulation, or selection cassette recycling in vitro. Although serial genetic manipulations in pig could be achieved by standard breeding, this is slow and implies excessive husbandry costs (>10 months from impregnation to sexual maturity). Instead, researchers have used an iterative cloning approach, in which each round of genetic modification requires isolation of fetal fibroblasts, genetic manipulation, re-cloning, reimplantation, and fetal development [74, 79, 82]. Despite this clever solution, inefficiencies in nuclear reprogramming and SCNT render this approach to creating pigs with complex genetic manipulations or multiple transgenes difficult and time consuming. A porcine cellular resource more amenable to genetic manipulation, less susceptible to cellular senescence, and more effectively reprogrammed would dramatically improve the efficiency of complex genetic manipulation in vitro before SCNT.
Given their potential in terms of long-term culture and their superiority as nuclear donors [83, 84], embryonic stem cells are a highly desirable resource for pig transgenesis and cloning. Indeed, successful derivation of germline competent embryonic stem cells from livestock species has been an actively pursued goal for many years [85]. Although many groups have reported isolation of embryonic stem-like cells, far fewer have produced cells demonstrated to contribute to chimeric piglets when injected into an early blastocyst [86, 87], and to date no evidence of germline chimerism from porcine embryonic stem cells has been reported. However, the recent isolation of multipotent cells from pigs by several groups may provide alternative cellular resources with many of the desirable features of embryonic stem cells [88–94], with the potential to increase the efficiency and complexity of genetic manipulations by SCNT.
The naked truth about DNA integration
Stable integration and expression of a transgene in the pig genome requires that several conserved, fundamental barriers be overcome. The initial barrier is entry of the transgene into a cell, embryo, or ova. This has been accomplished by either direct microinjection of DNA into cells or ova, by transfection of cells with DNA complexed with cationic lipids, polycations, or other conjugating substances, or by electroporation. Subsequent trafficking of DNA into the nucleus is not understood, but it may require dissolution of the nuclear membrane when a cell divides (for review [95]). Once within the nucleus, the transgene must rely on cellular machinery to serendipitously insert the transgene into host chromosomes.
Linearized DNA integrates with an efficiency fivefold greater than that of supercoiled DNA [96], and so it is preferred for the generation of transgenic cells and animals. This observation makes sense, considering that the DNA double strand break (DSB) repair machinery is responsible for transgene integration, with nonhomologous end joining (NHEJ) being the most prominent mechanism [97]. As the name implies, NHEJ responds to DNA DSBs in cells by nonhomologous ligation of available DSBs. The introduction of 104 copies of a transgene into a cell (in the case of PNI) provides a great deal of substrate for NHEJ, giving rise to head to tail, multicopy gene arrays (concatemers) of extrachromosomal DNA before or simultaneous with integration into chromosomes. NHEJ acts very rapidly in mouse embryos, with concatemers observed in 100% of embryos only 5 to 10 min after DNA injection [98]. These concatemers are either degraded or find their way into the genome, presumably at a DSB [98], resulting in transgenic mice carrying a transgene concatemer at one or more loci in the genome [98–101].
Although use of naked DNA has provided an effective method for producing transgenic cells and animals, significant complications associated with un-facilitated integration have been described. Concatemerized transgenes are prone to silencing by the host for several reasons. Flanking GC-rich bacterial sequences may accompany the transgene cassette, causing hypermethylation and resulting in transgene silencing [102, 103]. Additionally, the nature of a concatemer itself (multiple tandem copies of a transgene at a single locus) can stimulate transgene silencing [104, 105] - a phenomenon that is partially ameliorated by the use of viral and transposon systems that deliver precise single copies of transgenes to the genome.
Genetic lesions and instability have also been encountered with un-facilitated integration of DNA, resulting in deletions adjacent to the insertion site, chromosomal translocations, and insertion of additional genomic sequence within a transgene concatemer [106–110]. These types of genomic alterations may not be overtly detected, but they could certainly affect the health of animals produced by PNI or from genetically modified cells by SCNT. Furthermore, valuable transgenic animal lines may suffer from transgene instability, giving rise to rearrangements at the transgene locus that can result in loss of transgene concatemers (possibly including flanking DNA), lower than expected transmission to offspring, somatic mosaicism of F1 progeny, or increased morbidity [111–113]. In contrast, the precise integration of transgenes by viral and transpositional transgenesis provides for reduced concatemer-associated transgene instability.
Viral transgenesis
Recent publications [58, 59] reported a highly efficient method for transgenic swine production using pseudotyped lentiviruses. Like PNI, current methods for lentiviral transgenesis rely on surgical procurement of early embryos and implantation into the reproductive tract of a synchronized recipient after treatment. Then, concentrated pseudotyped lentivirus is microinjected into the peri-vitelline space of the early embryo, whereupon the viral machinery mediates transport of the transgene to the nucleus and integration of provirus into the pig genome. Peri-vitelline injection is minimally invasive to the embryo, probably accounting for enhanced embryo survival (18% to 27%) compared with PNI and SCNT (Table 2) [58]. In addition, reported rates of live-born pig transgenesis of 70% [59] and 92% [58] rival those observed with SCNT, providing an overall transgenesis efficiency of 13% and 25%, respectively, of transferred embryos resulting in transgenic piglets. These overall transgenesis rates are about tenfold better than those with PNI or SCNT on a per embryo basis. Furthermore, most transgenic F0 animals have multiple copies of the proviral insert (up to 20 in the report by Hofmann and coworkers [59]).
Inserting this many transgenes is both good and bad. The good news is that, with patience, there are many chances to identify a transgene with an appropriate expression domain. The bad news is that if anything other than ubiquitous expression is desired, then identification of a transgene with an appropriate expression pattern requires breeding to segregate away other transgene loci. A further complication is the tendency of lentiviruses to insert into or near transcriptional units [114, 115], increasing the likelihood of insertional mutagenesis or position effects from nearby endogenous enhancer elements. Several studies have also noted an increased likelihood of transgene silencing in the context of the retroviral genome [116]. In agreement with this tendency, Hofmann and coworkers [117] observed loss of transgene expression in one-third of outbred F1 animals attributed to transgene methylation. However, transgene expression was consistent between sibling animals carrying the same insertion, suggesting that expression was fixed for a specific insertion before germline transmission. Constraints on lentiviral cargo capacity (Table 2), the potential use of cryptic splice signals in the gene expression cassette during reverse transcription of the viral genome, and a requirement for viral titers of 109 to 1010 particles per milliliter all complicate the construction and preparation of lentiviral transgene vectors. Nevertheless, the efficiency of transgenesis using this technique is the greatest thus far reported; it is therefore likely to remain a valuable implement in the pig genetic engineering toolbox.
Transposons in vertebrates
Transposable elements, especially DNA transposons, have been used extensively for germline transformation of invertebrates and plants. Efficient integration of DNA into the genome is one of the reasons why transposon-based insertional mutagenesis is an essential component of large-scale functional genomic efforts in many species, including bacteria, yeast, insects, and plants [118–123]. The application of transposons to vertebrate biology began in 1997 with the 'reawakening' of the Sleeping Beauty transposon [124]. Ivics and coworkers [124] reconstructed the SB10 transposase based on the consensus sequence of inactive transposons littered throughout several salmonid genomes. The refurbished SB10 transposase facilitated efficient gene transfer in cultured cells from many vertebrate species [125]. Since the restoration of Sleeping Beauty, other transposon systems including Tol2 [126, 127], piggyBac [128, 129], Frog Prince [130], Minos [131], Himar1 [132], and Passport [133] (Clark and coworkers, unpublished data) have been used to transpose DNA into vertebrate cells. DNA 'cut and paste' transposons are capable of enzymatically moving a gene expression cassette from a delivery vector into a host genome. The transposase binds to the inverted terminal repeats of the transposon, excises it from its original location, and integrates it into the genome. Domestication of transposon systems generally finds them operating as a binary system: the transposon vector containing the transgene expression cassette flanked by terminal repeats of the transposon; and the transposase enzyme, which can be provided by a second gene expression cassette on the same (cis) or separate vector (trans), as mRNA [134–136] or potentially as recombinant protein.
The Tc1/mariner family of transposons [137], whose members include Sleeping Beauty, Frog Prince, Minos, Himar1, and Passport, randomly integrate into TA dinucleo-tides distributed around the genome. Upon integration, the TA dinucleotide is duplicated at each exterior end of the inverted terminal repeats. The piggyBac transposon, the founding member of the piggyBac family of transposons [138], integrates into a TTAA tetranucleotide, which is duplicated at each end of the transposon. Tol2, a member of the hAT family of transposons [139], does not integrate into a specific target sequence, instead relying on local DNA deformation [140]; it nonetheless also creates a target site duplication of eight base pairs at the junction between transposon and genome. Transposons mobilized by transposase result in a DSB at the excision site that is repaired by cellular machinery. The major repair pathway for Sleeping Beauty is NHEJ, which most often results in conversion of the original TA dinucleotide to a TACA/TGTA, although other repair sequences have been observed, including small insertions and deletions [141, 142]. This canonical footprint results in a five-nucleotide insertion that would disrupt the coding sequence of an interrupted open reading frame. Tol2 repair also relies on NHEJ without a predominant repair sequence because of variance in target-site sequences. Insertions and deletions have also been observed after Tol2 excision [143, 144]. Mobilization of the piggyBac transposon, on the other hand, generally results in restoration of the duplicated TTAA back to a single TTAA, leaving no disruption at the excision site [128]. The clean repair of excised transposons, as well as piggyBac's proclivity for landing in genes [145, 146] (see below), suggest that it will be valuable as a reagent for functional genomics. Sleeping Beauty, Tol2, and piggyBac systems have all been used to produce transgenic animals, including fish, frogs, mice, and rats [135, 136, 145, 147, 148] (Guerts and coworkers, unpublished data) by pronuclear or cytoplasmic DNA microinjection. Transposons have also been remobilized in vivo from chromosomal locations, often leading to their vacating the original locus and taking up residence at a new one. For example, expression of Sleeping Beauty transposase in the germline of mice has been used to mobilize transposons previously introduced into the mouse genome [149–152]. Gene and enhancer trap vectors have been developed and used for germline mutagenesis in fish and mice for functional genomic applications [152–158]. Similarly, Sleeping Beauty vectors have been used to identify genes that are involved in cancer genesis by causing activation of proto-oncogenes or interruption of tumor suppressor genes by remobilization of transposons in somatic tissues of mice [159, 160].
Despite the benefits of transposition, there are perhaps some limitations. There have been several reports indicating a decrease in transposition efficiency with increasing transposon size [125, 161, 162]. However, in all of these cases the influence of plasmid size on transfection was not accounted for, despite the fact that even small differences in plasmid size can alter transfection efficiency [163]. Where transposition can be observed without being confounded by transfection, for example in PNI or upon mobilization from a genomic context, large transposons appear to mobilize with nearly the same efficiency as do smaller ones [145, 158]. As mentioned above, some transposons prefer to integrate into transcription units. This can be either a benefit or a disadvantage, depending on whether the goal is to mutate genes or to safely deliver a transgene. In this case, having multiple transposon systems available may permit selection based on the application and the temperament of a particular transposon. For instance, at first glance piggyBac appears to integrate preferentially into or very near transcription units, landing in them as much as 67% of the time [145, 146]. By contrast, Sleeping Beauty does not integrate into transcription units at a rate much higher than what would be expected by random integration [164].
Genetic engineering with site-specific recombinases
Site-specific recombinases, such as the P1 bacteriophage cyclization recombinase enzyme (Cre) and flippase (Flp) from Saccharomyces cerevisiae, have revolutionized genetic engineering by allowing efficient and accurate manipulation of the genome by site-directed deletion, inversion, insertion, or chromosomal exchange (for review [165]). The use of recombinases and their recognition sites in trans has allowed the development of 'genetic switches' for the conditional activation or inactivation of gene expression. Specific and complex control of transgene expression can be achieved in a manner that is dependent on the spatiotemporal expression domain of the recombinase(s). The ability to express the recombinase from tightly regulated spatially or temporally restricted promoters has allowed investigation of gene function beyond their initial developmental role, potentially lethal as a null, and to examine the role played by a gene product in specific tissues in late-stage embryos or adults.
Application of transposons and recombinases for genetic engineering of pigs
Transposons and recombinases for mobilizing transgenes in pig cells
We recently reported, for the first time, transpositional transgenesis in pig cells using Sleeping Beauty, Passport, Tol2, and piggyBac transposon systems [166]. Initial assessment of these transposons relied primarily on a porcine endometrial glandular epithelial (PEGE) cell line [167], which is one of very few immortalized cellular resources available for pigs. In PEGE cells, transposons increased cellular transgenesis from 5-fold to 28-fold above background, depending on which transposon system was used. In addition to the baseline enhancement of transgenesis measured by clone formation, transposons differed in their robustness of integration, as indicated by the number of integrations per clone, which ranged from 1 to 15. Southern analysis of cellular clones revealed that the vast majority of transgene insertions resulted from transposition, a fact borne out by analysis of the junctions between transgene and the genome for each class of transposon. Without optimization in PEGE cells, piggyBac and Tol2 transposon systems were more active than Sleeping Beauty, which was more active than Passport. The Passport transposon system relies on wild-type sequences isolated from the Pleuronectes plattesa genome, representing the only vertebrate Tc1-type transposon thus far found to be active in its native form (Clark and coworkers, unpublished data). Its activity could probably be improved by engineering of its inverted terminal repeats or transposase, analogous to improvements made to the Sleeping Beauty system [168]. Additional hyperactive mutants of Sleeping Beauty might also be more active in pig cells [169–171]. However, although it may be possible to further improve transposon systems for application to PEGE cells, transposon efficiency varies depending on the cell type [125, 129, 130]. Therefore, the relative activity of any transposon system in different pig cells, including pig embryos, requires further investigation. It is likely that, depending on the application, there will be distinct and overlapping roles for a variety of transposon systems in swine genetics (Figure 1). It is therefore quite promising that four unique transposon systems result in enhanced transgenesis as well as precise integration of expression cassettes into one or more genomic locus in swine.
Applications of transposition to porcine transgenesis. Presented is flow diagram of the primary steps involved in the production of transgenic pigs by somatic cell nuclear transfer (SCNT), pronuclear injection (PNI), and lentiviral transduction (LVT). Each procedure requires the surgical isolation of oocytes or embryos. SCNT requires the production of transgenic donor cells, which can be augmented by transposon-mediated transgenesis (TnT). The donor cells are injected into enucleated oocytes, which are then fused and activated before embryo transfer into a recipient. PNI involves the injection of DNA into the male pronuclei before nuclei fusion. PNI can be augmented by TnT. LVT occurs by injection into the peri-vitelline space of staged embryos. In all cases manipulated embryos are surgically implanted into a synchronized recipient sow. A portion of the recipient sows will maintain pregnancy until parturition. The piglets can then be screened for the presence of the transgene by polymerase chain reaction, Southern hybridization, or detection of marker gene expression.
In addition to characterizing the activity of four vertebrate transposons in porcine cells, Clark and coworkers [166] also demonstrated for the first time the ability of Cre and Flp recombinases to mediate site-specific recombination of the pig genome. Both Cre and Flp recombinase were functional in pig cells, as indicated by their ability to remove a positive-negative selection cassette from episomal and numerous genomic locations. In addition, a Cre-dependent genetic switch was demonstrated to be effective in mediating conditional gene expression from episomal and genome-resident transposons. This study provides the basis for developing transposon and recombinase based tools for genetic engineering of the swine genome.
Transposition and recombination for porcine somatic cell nuclear transfer
The first step in creating transgenic pigs by SCNT involves the transgenesis of cells that will serve as nuclear donors. This generally involves transfecting or electroporating PFFs or another suitable cell type with DNA expression constructs. Most if not all transgenesis by SCNT involves the co-delivery (in cis or trans) of a selectable marker for enrichment of transgenic cells destined to serve as nuclear donors. Certainly, this in not the limiting step in producing transgenic pigs by SCNT. However, the routine use of transposons would increase the efficiency of cellular transgenesis while avoiding concatemerization and integration of CpG-rich vector sequences. Since the production of transgenic swine can be quite expensive, any advantage with regard to stable transgene expression should be exploited. In addition, the introduction of multiple, unlinked transgenes by transposition could increase the value of founder pigs, although breeding would be required to segregate these loci.
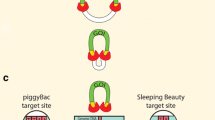
The most compelling application of recombinases in porcine SCNT relates to selection cassette recycling. Elimination of selectable marker genes from prospective donor cells simplifies genotype-phenotype correlations and eliminates the potential for selection cassette interference [172] on transgene expression. It would of course be important to minimize the presence of extraneous DNA (especially antibiotic resistance genes) from genetically modified pigs were they ever to be considered for entry into the food chain. The use of a positive/negative selectable transgene such as PuroΔTK or HygroCodA [173, 174] flanked with recombinase recognition site (RRS) provides a facile substrate for marker removal using site-specific recombinases before SCNT (Figure 2). Unfortunately, because their tendency toward senescence, the most commonly used cellular resource for SCNT (PFFs) are not amenable to the extended culture required for multiple rounds of drug selection. Recently developed mesenchymal and multipotent stem cells from pigs may provide a solution to this dilemma, because they appear to be amenable to extended culture [89–91, 175, 176], genetic manipulation [88, 92, 177], and use as nuclear donors for SCNT [92–94]. Elimination of RRS flanked selection cassettes could also await breeding of cloned transgenic pigs to a line of pigs that express Cre or Flp recombinase in their germline (Figure 2).
Selection cassette recycling of selectable marker in pigs. A transposon containing two genes, a transgene of interest ('gene', red) and a selectable marker ('marker', green) can be used to construct a transgenic pig. The promoters can differ for the two genes. Generally, the marker will be driven by a ubiquitous promoter (Ub), allowing selection for expressing donor cells (somatic cell nuclear transfer [SCNT]) or piglets (pronuclear injection [PNI] or lentiviral transduction [LVT]), whereas the promoter for the transgene could be a ubiquitous promoter or tissue-specific promoter (TSP). In the diagram the 'gene' is driven by a TSP. Expression in an F0 animal is depicted by the pig on the left with the marker being expressed ubiquitously and the transgene being expressed in a tissue-specific manner, shown here as pancreatic expression. Crossing this pig to a pig that ubiquitously expresses Cre recombinase (blue) would result in F1 progeny that lost expression of the ubiquitous marker and retained expression of the transgene in the pancreas. Ubiquitous Cre expression would occur in the F1 (50% or 100%, depending on whether the Cre pig was heterozygous or homozygous), but this would be irrelevant to analysis of the F1 phenotype. RRS, recombinase recognition site.
Similar strategies for selection cassette recycling can be used after homologous recombination if gene targeting vectors are designed with RRS flanking positive/negative selectable markers (Figure 3). A simple case of selection cassette recycling requires flanking a positive/negative selectable marker, such as PuroΔTK or HygroCodA [173, 174], with RRS sites (Figure 3b). In addition to RRS sites, the gene targeting vector must contain a unique negative marker (not part of the positive/negative marker) for counter-selection against random integration; this could be CodA, TK, or diphtheria toxin [178]. After selection of homologous recombinants, the cells can be transfected with Cre and selected for loss of the positive/negative marker (gancyclovir for PuroΔTK). Cells that lose the selection cassette will grow and can be used for nuclear donors before SCNT. Alternatively, the selection cassette can be removed after SCNT by crossing transgene carriers to pigs expressing Cre recombinase or by delivering recombinase transiently in carrier embryos by micro-injection of Cre mRNA or protein.
Selection cassette recycling after homologous recombination. (a) An illustration of a typical homologous recombination event utilizing a positive/negative selection scheme. The gene sequence is shown with exons 2, 3, and 4. The targeting vector replaces exon 3 with a positive selectable marker, such as the PGK driven neomycin resistance cassette (phosphoglycerate kinase [PGK]-neoR), and utilizes a negative selectable marker, such as the herpes simplex virus promoter driven thymidine kinase gene (HSV-TK), to counter-select against random integration of the targeting vector. Homologous recombination results in replacement of exon 3 with the PGK-neoR cassette. (b) The use of site-specific recombinases such as Cre or Flp allows removal of a selection cassette before or after the production of an animal by SCNT. In order to accomplish this, positive marker is flanked by recombinase recognition sites like loxP. After homologous recombination (HR) the selection cassette can be removed by Cre recombinase in culture or in vivo. In order to select efficiently for removal of the selection cassette in vitro, a positive/negative selectable marker such as PuroΔTK or hygroCodA, with the negative selection marker outside the homology arms (for example, CodA, TK, or diphtheria toxin). (c) Schematic for generating conditional knockout alleles using site-specific recombinases Cre and Flp. A targeting construct is generated that leaves each exon intact, but includes loxP sites flanking an exon critical for gene function, in this case exon 3 (wild-type or an alternative allele). The selectable marker, flanked by frt sites, can be removed from the targeted allele either in vitro or in vivo to avoid selection cassette interference of the modified allele. Animals carrying this targeted modification can be crossed to animals that express Cre ubiquitously or in a specific tissue, resulting in progeny with a deletion of exon 3 in the whole animal or in a specific tissue.
In addition to selection cassette recycling, recombinases can be used to create conditional nulls following gene targeting (Figure 3c). In this case two sets of RRSs are used. The first set flanks the positive/negative selectable marker for selection cassette recycling to ensure that there is no impairment of gene function at the locus. Ideally, the selection cassette would be removed in vitro before SCNT, but this could also be done in pig, as described above. The second set of RRS flanks a critical element of the locus, for example an exon within the coding region. The conditional knockdown of the locus can then be achieved by crossing the conditional null carrier to pigs expressing Cre in a desired manner.
Transposition and recombination for porcine pronuclear injection
The combination of transposons and recombinases may also greatly increase the efficiency and complexity of transgenic pig production by PNI. Sleeping Beauty, Tol2, and piggyBac transposons have all been used for germline transformation of multiple species by PNI and cytoplasmic microinjection, at a rate far superior to unfacilitated DNA injection. In particular Sleeping Beauty and piggyBac transposons have been used for the generation of transgenic mice by PNI. Dupuy and coworkers [136] saw the rate of transgenic live-born pups increase from 29% up to 45% using the Sleeping Beauty transposon system. Ding and colleagues [145] saw increases in mouse embryo transgenesis rates from 10% to 35%, from 18% to 66%, and from 5% to 46% after PNI with three transposons when piggyBac transposase was included.
In addition, with reports of increased transposition using methylated Sleeping Beauty transposons that were methylated in vitro before transfection [179], Geurts and coworkers (unpublished data) tested the influence of this treatment on the efficiency of mouse transgenesis by PNI. This preliminary experiment yielded an unprecedented live-born transgenesis rate of 90%, with integrations that were later transmitted to F1 mice and shown to express in a locus-dependent manner. The fact that four transposon systems were recently demonstrated to be active in pig cells bodes well for their application to porcine transgenesis by PNI. A modest improvement in the rate of swine embryo transgenesis using transposons could have a significant impact on the efficiency of swine engineering for agricultural and medical applications. The observation of multiple transposed integrations in pig cells (1 to 15) and in transgenic mouse embryos and pups (1 to 10) also suggests that it will be possible to create pigs with multiple stable, unlinked, and reliably expressed transgenes using one or more transposon system [145] (Clark and coworkers, unpublished data).
There are a number of reasons to include RRS in transposons to be used for PNI. Selection cassette recycling is mentioned above. In addition, to circumvent unsuspected deleterious effects of ubiquitously expressed transgenes, it may be desirable to include conditional (recombinase-activated) gene expression cassettes (Figure 4). For example, a transgene encoding a visibly or systemically detectable protein (for example, green fluorescent protein or secreted alkaline phosphatase) could be expressed in the default state (either ubiquitously or in specific tissues), potentially facilitating the identification of transgenic piglets. Conditional juxtaposition of the downstream transgene could be activated in later generations by crossing to a pig that expresses Cre recombinase ubiquitously or in a tissue-specific manner.
Conditional activation of transgenes in pigs. A transposon containing a Cre-activatable transgene ('gene', red) interrupted by a selectable marker gene ('marker', green) can be used to obtain transgenic pigs with conditional expression of a transgene. The promoter driving expression of the marker/gene can be either ubiquitous (Ub) or tissue-specific (TSP), which would result in the ubiquitous or tissue-specific expression of the marker in F0 pigs, as shown (green) to the left. Tissue-specific activation of the transgene can be accomplished in two ways: by crossing pigs that ubiquitously express the marker-interrupted transgene with a pig expressing Cre (blue) in a tissue-specific manner, or by crossing pigs that express the marker-interrupted transgene in a tissue-specific manner with a pig that ubiquitously expresses Cre. Controlled expression of the transgene or controlled excision of the marker allows expression of the transgene (to the right, red) in a specific tissue, shown here to represent the pancreas.
A role for transposons in somatic cell therapies
Porcine models of gene therapy
In addition to the germline transformation, transposon systems can increase the stable integration of transgenes into somatic cells. In fact, the Sleeping Beauty transposon system is actively being developed for several gene therapy applications. Currently, much of this work is being done in rodent models with successful long-term expression of therapeutic transgenes [180–188]. However, the methodology of gene delivery, clinical dosage, and efficacy of treatments in the mouse may not be directly applicable to treatment of human patients. It is therefore likely that large animal models will be important in advancing clinically relevant gene therapy protocols. Pigs have been used to improve surgical techniques for years because of their similarity in size and physiology to humans, as well as their widespread availability as an accepted part of the human food chain. It is therefore quite reasonable to test gene therapy protocols in pigs. For example, hydrodynamic delivery of DNA by the injection of a large volume of DNA solution into the tail-vein of mice results in significant DNA uptake into the liver [180]; however, this technique is unlikely to be directly scalable to large animals or humans. DNA has successfully been delivered by local hydrodynamic injection into pig arterial vessels [189] and muscle [190], although - as expected for naked DNA - the expression was short lived. Perhaps similar local hydrodynamic delivery coupled with transposons could allow selective uptake and maintained expression by these tissues or other targets, such as liver, without the need for systemic injection of large volumes of fluid. Pigs may also provide an ideal large animal model for testing the efficacy of reagents being developed for systemic delivery of therapeutic genes to specific tissues or organs.
The potential tractability of pigs for development of large animal models of human disease makes them an attractive system not only for developing gene delivery protocols but also for testing the efficacy of these regimens in curing disease. For example, the National Swine Research and Resource Center is currently developing pig models of cystic fibrosis based on gene knockout and transposon-based RNAi [33]. These pigs not only may provide the first animal model of the cystic fibrosis pulmonary phenotype, but they may also be ideal for the development of gene therapy protocols to treat this devastating disease.
DNA vaccination
The evolutionary speed of viruses and bacteria challenges our ability to develop efficacious protein-based vaccines. Molecular biology, on the other hand, provides a rapid approach to the cloning and expression of potential antigens. The promise of DNA as a pharmaceutical has been actively pursued since the observation that naked DNA injection into muscle can direct the production of protein [191]. Applications in gene therapy and vaccination have been extensively explored, stimulated by the fact that DNA can be prepared in large quantities in compliance with cGMP standards, and in a lyophilized form independent of the traditional cold chain. Although both humoral and cellular immune responses can be mobilized with DNA vaccines, problems with DNA delivery and the intercellular trafficking of antigen have limited their success [192]. To date, only two DNA vaccines have been licensed for use in animals; a DNA vaccine to protect farmed salmon and trout from infectious hematopoietic necrosis virus, and one to protect horses from West Nile virus [193, 194]. Recent findings suggest that transposons may provide for more efficient and longer lasting cellular transgenesis to increase the expression and intercellular trafficking of antigens. Indeed, in the context of developing transposon-based reagents for gene therapy, a robust immune response to the expression of genes from Sleeping Beauty transposons encoding either clotting factor VIII [186] or iduronidase [195] have been observed in mice. Given that transposons are active in pig cells, swine could serve as excellent preclinical models for human vaccine development, in addition to their obvious importance in the development of vaccines targeted against pathogens important to swine production.
High on the hog (conclusions and horizons)
The relevance of pigs to agriculture and medicine makes them unique among large animal models. With the complete sequence of their genome soon to be delivered, pigs are likely to play an increasing role in defining gene function in human disease using reverse genetic approaches. The use of enzymatic approaches such as transposition and recombination should expand the ease and complexity of genetic modifications available with which to engineer the pig to model human disease and to produce agricultural and biomedical products.
In addition, pigs may also be amenable to forward genetic screens because of reproductive fecundity (about ten piglets per litter) that rivals that of mice. With appropriate planning and coordination, and the use of clever molecular reagents, conducting a mutagenesis screen in pigs could provide important information about gene function in large animals. Some cancers and age-related disease etiologies, as well as therapies for treating them, might be better studied in pigs, which commonly live to be ten years old and, in rare exceptions, into their second decade.
Transposons are ideal for use as insertional mutagens, particularly piggyBac, which tends to land in transcription units and can later be excised for reversion analysis. Specialized 'trapping' vectors based on transposons are able to cause mutations efficiently upon insertion into a transcription unit, and make identification of the interrupted gene straightforward [152, 153, 156, 158, 196, 197]. Transposon-based mutagenesis screens in mice have generally relied first on the generation of two mouse strains: one transgenic for a mutagenic transposon vector (usually in the form of a concatemer) and another strain transgenic for the corresponding transposase expression construct [149, 150, 158]. Breeding these lines together provides doubly transgenic 'seed' mice, in which germline mobilization of the transposon provides for the recovery of mutated loci in an out-crossed generation. However, with a 4-month gestation period and 6 months to sexual maturity, mutagenesis in pigs using this strategy would require a minimum of 4 years before mutations could be bred to homozygosity and a screen initiated.
More immediate would be to use a strategy recently applied in zebrafish [157, 197], which treats the injected generation as seed stock by supplying both transposon and transposase. Given a reasonable rate of transgenesis by transposon-based PNI of pig embryos, mutant alleles could be bred to homozygosity and a screen initiated within 2 years. Each F0 could be a source of 1 to 15 transposon insertions, with about 12% to 25% of the integrations 'trapping' a transcription unit [145, 153, 164]. The direct injection method will provide proof of principle in the shortest amount of time. However, the longer initial investment required for the production of double-transgenic 'seed' boars would be rewarded by a nearly constant supply of novel gene traps due to re-mobilization of transposons in the male germline. Additionally, improvement in the efficiency of cloning and the availability of porcine stem cells allows another attractive approach. Development of a library of 'trapped', characterized, and catalogued pig stem cell clones could provide an on-demand resource for the generation of pigs by SCNT, analogous to strategies used for generating mice from 'trapped' embryonic stem cell clones [198]. Using this approach, transposon-trapped alleles could be bred to homozygosity and a phenotypic analysis begun in pigs in less than 1 year. In the woven words of Charlotte the spider, the unique contributions of such pigs would surely reveal dear Wilbur to represent 'Some Pig' [199].
References
Epstein J, Bichard M: Pigs. Evolution of Domesticated Animals. Edited by: Mason IL. 1984, New York: Longman, 145-162.
Larson G, Dobney K, Albarella U, Fang M, Matisoo-Smith E, Robins J, Lowden S, Finlayson H, Brand T, Willerslev E, et al: Worldwide phylogeography of wild boar reveals multiple centers of pig domestication. Science. 2005, 307: 1618-1621. 10.1126/science.1106927.
Global Livestock Health and Production Atlas. [http://www.fao.org/ag/aga/glipha/index.jsp]
Otto D, Lawrence J: The United States pork industry 2003: patterns and economic importance. [http://www.mnpork.com/producer/presentations/other/uslawrence2003.pdf]
Pork facts - everything but the oink: pharmaceutical co-products. [http://www.pork.org/newsandinformation/quickfacts/PorkFacts16.aspx]
McCrea MR, Tribe DE: The baby pig as a laboratory animal. J Physiol. 1954, 124: 52P-
Bustad LK, McClellan RO: Miniature swine: development, management, and utilization. Lab Anim Care. 1968, 280-287. Suppl
Patel G, Duffin J: History of Medicine: A Scandalously Short Introduction. 2000, Toronto, Canada: University of Toronto Press
Watson L: The Whole Hog: Exploring the Extraordinary Potential of Pigs. 2004, Washington DC: Smithsonian Books
England DC, Winters LM, Carpenter LE: The development of a breed of miniature swine: a preliminary report. Growth. 1954, 18: 207-214.
Rothschild MF: From a sow's ear to a silk purse: real progress in porcine genomics. Cytogenet Genome Res. 2003, 102: 95-99. 10.1159/000075732.
Rothschild MF: Porcine genomics delivers new tools and results: this little piggy did more than just go to market. Genet Res. 2004, 83: 1-6. 10.1017/S0016672303006621.
Swine Protein-Annotated Oligonucleotide Microarray. [http://www.pigoligoarray.org]
Haley C, de Koning DJ: Genetical genomics in livestock: potentials and pitfalls. Anim Genet. 2006, 10-12. 10.1111/j.1365-2052.2006.01470.x. Suppl 1
Vize PD, Michalska AE, Ashman R, Lloyd B, Stone BA, Quinn P, Wells JR, Seamark RF: Introduction of a porcine growth hormone fusion gene into transgenic pigs promotes growth. J Cell Sci. 1988, 90: 295-300.
Wieghart M, Hoover JL, McGrane MM, Hanson RW, Rottman FM, Holtzman SH, Wagner TE, Pinkert CA: Production of transgenic pigs harbouring a rat phosphoenolpyruvate carboxykinase-bovine growth hormone fusion gene. J Reprod Fertil Suppl. 1990, 41: 89-96.
Pursel V, Wall RJ, Mitchell AD, Elsasser TH, Solomon MB, Coleman ME, DeMayo F, Schwartz RJ: Expression of Insulin-like Growth Factor I in Skeletal Muscle of Transgenic Swine. 1999, Wallingford, UK: CAB International
Lai L, Kang JX, Li R, Wang J, Witt WT, Yong HY, Hao Y, Wax DM, Murphy CN, Rieke A, et al: Generation of cloned transgenic pigs rich in omega-3 fatty acids. Nat Biotechnol. 2006, 24: 435-436. 10.1038/nbt1198.
Saeki K, Matsumoto K, Kinoshita M, Suzuki I, Tasaka Y, Kano K, Taguchi Y, Mikami K, Hirabayashi M, Kashiwazaki N, et al: Functional expression of a Delta12 fatty acid desaturase gene from spinach in transgenic pigs. Proc Natl Acad Sci USA. 2004, 101: 6361-6366. 10.1073/pnas.0308111101.
Noble MS, Rodriguez-Zas S, Cook JB, Bleck GT, Hurley WL, Wheeler MB: Lactational performance of first-parity transgenic gilts expressing bovine alpha-lactalbumin in their milk. J Anim Sci. 2002, 80: 1090-1096.
Monaco MH, Gronlund DE, Bleck GT, Hurley WL, Wheeler MB, Donovan SM: Mammary specific transgenic over-expression of insulin-like growth factor-I (IGF-I) increases pig milk IGF-I and IGF binding proteins, with no effect on milk composition or yield. Transgenic Res. 2005, 14: 761-773. 10.1007/s11248-005-7219-8.
Muller M, Brenig B, Winnacker EL, Brem G: Transgenic pigs carrying cDNA copies encoding the murine Mx1 protein which confers resistance to influenza virus infection. Gene. 1992, 121: 263-270. 10.1016/0378-1119(92)90130-H.
Golovan SP, Meidinger RG, Ajakaiye A, Cottrill M, Wiederkehr MZ, Barney DJ, Plante C, Pollard JW, Fan MZ, Hayes MA, et al: Pigs expressing salivary phytase produce low-phosphorus manure. Nat Biotechnol. 2001, 19: 741-745. 10.1038/90788.
Park JK, Lee YK, Lee P, Chung HJ, Kim S, Lee HG, Seo MK, Han JH, Park CG, Kim HT, et al: Recombinant human erythropoietin produced in milk of transgenic pigs. J Biotechnol. 2006, 122: 362-371. 10.1016/j.jbiotec.2005.11.021.
Paleyanda RK, Velander WH, Lee TK, Scandella DH, Gwazdauskas FC, Knight JW, Hoyer LW, Drohan WN, Lubon H: Transgenic pigs produce functional human factor VIII in milk. Nat Biotechnol. 1997, 15: 971-975. 10.1038/nbt1097-971.
Velander WH, Johnson JL, Page RL, Russell CG, Subramanian A, Wilkins TD, Gwazdauskas FC, Pittius C, Drohan WN: High-level expression of a heterologous protein in the milk of transgenic swine using the cDNA encoding human protein C. Proc Natl Acad Sci USA. 1992, 89: 12003-12007. 10.1073/pnas.89.24.12003.
Sharma A, Martin MJ, Okabe JF, Truglio RA, Dhanjal NK, Logan JS, Kumar R: An isologous porcine promoter permits high level expression of human hemoglobin in transgenic swine. Biotechnology (NY). 1994, 12: 55-59. 10.1038/nbt0194-55.
Swanson ME, Martin MJ, O'Donnell JK, Hoover K, Lago W, Huntress V, Parsons CT, Pinkert CA, Pilder S, Logan JS: Production of functional human hemoglobin in transgenic swine. Biotechnology (NY). 1992, 10: 557-559. 10.1038/nbt0592-557.
Lee GS, Kim HS, Hyun SH, Lee SH, Jeon HY, Nam DH, Jeong YW, Kim S, Kim JH, Han JY, et al: Production of transgenic cloned piglets from genetically transformed fetal fibroblasts selected by green fluorescent protein. Theriogenology. 2005, 63: 973-991. 10.1016/j.theriogenology.2004.04.017.
Dyck MK, Gagne D, Ouellet M, Senechal JF, Belanger E, Lacroix D, Sirard MA, Pothier F: Seminal vesicle production and secretion of growth hormone into seminal fluid. Nat Biotechnol. 1999, 17: 1087-1090. 10.1038/15067.
Dyck MK, Lacroix D, Pothier F, Sirard MA: Making recombinant proteins in animals - different systems, different applications. Trends Biotechnol. 2003, 21: 394-399. 10.1016/S0167-7799(03)00190-2.
Petters RM, Alexander CA, Wells KD, Collins EB, Sommer JR, Blanton MR, Rojas G, Hao Y, Flowers WL, Banin E, et al: Genetically engineered large animal model for studying cone photoreceptor survival and degeneration in retinitis pigmentosa. Nat Biotechnol. 1997, 15: 965-970. 10.1038/nbt1097-965.
Palmer ML, Lee SY, Carlson D, Fahrenkrug S, O'Grady SM: Stable knockdown of CFTR establishes a role for the channel in P2Y receptor-stimulated anion secretion. J Cell Physiol. 2006, 206: 759-770. 10.1002/jcp.20519.
Bruscia E, Sangiuolo F, Sinibaldi P, Goncz KK, Novelli G, Gruenert DC: Isolation of CF cell lines corrected at DeltaF508-CFTR locus by SFHR-mediated targeting. Gene Ther. 2002, 9: 683-685. 10.1038/sj.gt.3301741.
Bucher P, Morel P, Buhler LH: Xenotransplantation: an update on recent progress and future perspectives. Transpl Int. 2005, 18: 894-901. 10.1111/j.1432-2277.2005.00124.x.
Cox A, Zhong R: Current advances in xenotransplantation. Hepatobiliary Pancreat Dis Int. 2005, 4: 490-494.
Houdebine LM: Use of transgenic animals to improve human health and animal production. Reprod Domest Anim. 2005, 40: 269-281. 10.1111/j.1439-0531.2005.00596.x.
Zhong R: Gal knockout and beyond. Am J Transplant. 2007, 7: 5-11. 10.1111/j.1600-6143.2006.01615.x.
Rayat GR, Rajotte RV, Hering BJ, Binette TM, Korbutt GS: In vitro and in vivo expression of Galalpha-(1,3)Gal on porcine islet cells is age dependent. J Endocrinol. 2003, 177: 127-135. 10.1677/joe.0.1770127.
Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, Bello-Laborn H, Hacquoil B, Strobert E, Gangappa S, et al: Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006, 12: 304-306. 10.1038/nm1375.
Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, Ansite JD, Nakano M, Cheng J, Li W, et al: Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006, 12: 301-303. 10.1038/nm1369.
Rother KI, Harlan DM: Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J Clin Invest. 2004, 114: 877-883. 10.1172/JCI200423235.
Cure P, Pileggi A, Froud T, Norris PM, Baidal DA, Cornejo A, Hafiz MM, Ponte G, Poggioli R, Yu J, et al: Alterations of the female reproductive system in recipients of islet grafts. Transplantation. 2004, 78: 1576-1581. 10.1097/01.TP.0000147301.41864.C0.
Martin C, Plat M, Nerriere-Daguin V, Coulon F, Uzbekova S, Venturi E, Conde F, Hermel JM, Hantraye P, Tesson L, et al: Transgenic expression of CTLA4-Ig by fetal pig neurons for xenotransplantation. Transgenic Res. 2005, 14: 373-384. 10.1007/s11248-004-7268-4.
Sutherland RM, Brady JL, Georgiou HM, Thomas HE, Lew AM: Protective effect of CTLA4Ig secreted by transgenic fetal pancreas allografts. Transplantation. 2000, 69: 1806-1812. 10.1097/00007890-200005150-00013.
Martin U, Winkler ME, Id M, Radeke H, Arseniev L, Takeuchi Y, Simon AR, Patience C, Haverich A, Steinhoff G: Productive infection of primary human endothelial cells by pig endogenous retrovirus (PERV). Xenotransplantation. 2000, 7: 138-142. 10.1034/j.1399-3089.2000.00052.x.
Blusch JH, Patience C, Takeuchi Y, Templin C, Roos C, Von Der Helm K, Steinhoff G, Martin U: Infection of nonhuman primate cells by pig endogenous retrovirus. J Virol. 2000, 74: 7687-7690. 10.1128/JVI.74.16.7687-7690.2000.
Patience C, Takeuchi Y, Weiss RA: Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997, 3: 282-286. 10.1038/nm0397-282.
Winkler ME, Winkler M, Burian R, Hecker J, Loss M, Przemeck M, Lorenz R, Patience C, Karlas A, Sommer S, et al: Analysis of pig-to-human porcine endogenous retrovirus transmission in a triple-species kidney xenotransplantation model. Transpl Int. 2005, 17: 848-858. 10.1007/s00147-005-0808-x.
Fishman JA, Patience C: Xenotransplantation: infectious risk revisited. Am J Transplant. 2004, 4: 1383-1390. 10.1111/j.1600-6143.2004.00542.x.
Patience C, Patton GS, Takeuchi Y, Weiss RA, McClure MO, Rydberg L, Breimer ME: No evidence of pig DNA or retroviral infection in patients with short-term extracorporeal connection to pig kidneys. Lancet. 1998, 352: 699-701. 10.1016/S0140-6736(98)04369-4.
Bartosch B, Stefanidis D, Myers R, Weiss R, Patience C, Takeuchi Y: Evidence and consequence of porcine endogenous retrovirus recombination. J Virol. 2004, 78: 13880-13890. 10.1128/JVI.78.24.13880-13890.2004.
Scobie L, Taylor S, Wood JC, Suling KM, Quinn G, Meikle S, Patience C, Schuurman HJ, Onions DE: Absence of replication-competent human-tropic porcine endogenous retroviruses in the germ line DNA of inbred miniature swine. J Virol. 2004, 78: 2502-2509. 10.1128/JVI.78.5.2502-2509.2004.
Miyagawa S, Nakatsu S, Nakagawa T, Kondo A, Matsunami K, Hazama K, Yamada J, Tomonaga K, Miyazawa T, Shirakura R: Prevention of PERV infections in pig to human xenotransplantation by the RNA interference silences gene. J Biochem (Tokyo). 2005, 137: 503-508.
Karlas A, Kurth R, Denner J: Inhibition of porcine endogenous retroviruses by RNA interference: increasing the safety of xenotransplantation. Virology. 2004, 325: 18-23. 10.1016/j.virol.2004.04.022.
Jonsson SR, Hache G, Stenglein MD, Fahrenkrug SC, Andresdottir V, Harris RS: Evolutionarily conserved and non-conserved retrovirus restriction activities of artiodactyl APOBEC3F proteins. Nucleic Acids Res. 2006, 34: 5683-5694. 10.1093/nar/gkl721.
Hammer RE, Pursel VG, Rexroad CE, Wall RJ, Bolt DJ, Ebert KM, Palmiter RD, Brinster RL: Production of transgenic rabbits, sheep and pigs by microinjection. Nature. 1985, 315: 680-683. 10.1038/315680a0.
Whitelaw CB, Radcliffe PA, Ritchie WA, Carlisle A, Ellard FM, Pena RN, Rowe J, Clark AJ, King TJ, Mitrophanous KA: Efficient generation of transgenic pigs using equine infectious anaemia virus (EIAV) derived vector. FEBS Lett. 2004, 571: 233-236. 10.1016/j.febslet.2004.06.076.
Hofmann A, Kessler B, Ewerling S, Weppert M, Vogg B, Ludwig H, Stojkovic M, Boelhauve M, Brem G, Wolf E, et al: Efficient transgenesis in farm animals by lentiviral vectors. EMBO Rep. 2003, 4: 1054-1060. 10.1038/sj.embor.7400007.
Martin MJ, Pinkert CA: Production of transgenic swine byDNA microinjection. Transgenic Animal Technology: A Laboratory Handbook. Edited by: Pinkert CA. 2002, Amsterdam, Boston: Academic Press, 307-336. 2
Wall RJ, Pursel VG, Hammer RE, Brinster RL: Development of porcine ova that were centrifuged to permit visualization of pronuclei and nuclei. Biol Reprod. 1985, 32: 645-651. 10.1095/biolreprod32.3.645.
Youngs CR: Factors influencing the success of embryo transfer in the pig. Theriogenology. 2001, 56: 1311-1320. 10.1016/S0093-691X(01)00632-X.
Springmann K, Brem G: Embryo transfer in swine in relation to a gene transfer program [in German]. Tierarztl Prax Suppl. 1989, 4: 21-25.
Niemann H, Rath D: Progress in reproductive biotechnology in swine. Theriogenology. 2001, 56: 1291-1304. 10.1016/S0093-691X(01)00630-6.
Wall RJ, Paleyanda RK, Foster JA, Powell A, Rexroad C, Lubon H: DNA preparation method can influence outcome of transgenic animal experiments. Anim Biotechnol. 2000, 11: 19-32.
Nottle MB, Haskard KA, Verma PJ, Du ZT, Grupen CG, McIlfatrick SM, Ashman RJ, Harrison SJ, Barlow H, Wigley PL, et al: Effect of DNA concentration on transgenesis rates in mice and pigs. Transgenic Res. 2001, 10: 523-531. 10.1023/A:1013007329936.
Pinkert CA, Kooyman DL, Dyer TJ: Enhanced growth performance in transgenic swine. Biotechnology. 1991, 16: 251-258.
Kues WA, Schwinzer R, Wirth D, Verhoeyen E, Lemme E, Herrmann D, Barg-Kues B, Hauser H, Wonigeit K, Niemann H: Epigenetic silencing and tissue independent expression of a novel tetracycline inducible system in double-transgenic pigs. FASEB J. 2006, 20: 1200-1202. 10.1096/fj.05-5415fje.
Palmer ML, Fahrenkrug SC, O'Grady SM: RNA interference and ion channel physiology. Cell Biochem Biophys. 2006, 46: 175-192. 10.1385/CBB:46:2:175.
Brummelkamp TR, Bernards R, Agami R: A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002, 296: 550-553. 10.1126/science.1068999.
Koper-Emde D, Herrmann L, Sandrock B, Benecke BJ: RNA interference by small hairpin RNAs synthesised under control of the human 7S K RNA promoter. Biol Chem. 2004, 385: 791-794. 10.1515/BC.2004.103.
Denti MA, Rosa A, Sthandier O, De Angelis FG, Bozzoni I: A new vector, based on the PolII promoter of the U1 snRNA gene, for the expression of siRNAs in mammalian cells. Mol Ther. 2004, 10: 191-199. 10.1016/j.ymthe.2004.04.008.
Peng S, York JP, Zhang P: A transgenic approach for RNA interference-based genetic screening in mice. Proc Natl Acad Sci USA. 2006, 103: 2252-2256. 10.1073/pnas.0511034103.
Takahagi Y, Fujimura T, Miyagawa S, Nagashima H, Shigehisa T, Shirakura R, Murakami H: Production of alpha 1,3-galactosyltransferase gene knockout pigs expressing both human decay-accelerating factor and N-acetylglucosaminyltransferase III. Mol Reprod Dev. 2005, 71: 331-338. 10.1002/mrd.20305.
Watanabe S, Iwamoto M, Suzuki S, Fuchimoto D, Honma D, Nagai T, Hashimoto M, Yazaki S, Sato M, Onishi A: A novel method for the production of transgenic cloned pigs: electroporation-mediated gene transfer to non-cultured cells and subsequent selection with puromycin. Biol Reprod. 2005, 72: 309-315. 10.1095/biolreprod.104.031591.
Harrison SJ, Guidolin A, Faast R, Crocker LA, Giannakis C, D'Apice AJ, Nottle MB, Lyons I: Efficient generation of alpha(1,3) galactosyltransferase knockout porcine fetal fibroblasts for nuclear transfer. Transgenic Res. 2002, 11: 143-150. 10.1023/A:1015262108526.
Jin DI, Lee SH, Choi JH, Lee JS, Lee JE, Park KW, Seo JS: Targeting efficiency of a-1,3-galactosyl transferase gene in pig fetal fibroblast cells. Exp Mol Med. 2003, 35: 572-577.
Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, et al: Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002, 295: 1089-1092. 10.1126/science.1068228.
Ramsoondar JJ, Machaty Z, Costa C, Williams BL, Fodor WL, Bondioli KR: Production of alpha 1,3-galactosyltransferase-knockout cloned pigs expressing human alpha 1,2-fucosylosyltransferase. Biol Reprod. 2003, 69: 437-445. 10.1095/biolreprod.102.014647.
Hasty P, Crist M, Grompe M, Bradley A: Efficiency of insertion versus replacement vector targeting varies at different chromosomal loci. Mol Cell Biol. 1994, 14: 8385-8390.
Schultes NP, Szostak JW: A poly(dA.dT) tract is a component of the recombination initiation site at the ARG4 locus in Saccharomyces cerevisiae. Mol Cell Biol. 1991, 11: 322-328.
Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, et al: Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003, 299: 411-414. 10.1126/science.1078942.
Rideout WM, Wakayama T, Wutz A, Eggan K, Jackson-Grusby L, Dausman J, Yanagimachi R, Jaenisch R: Generation of mice from wild-type and targeted ES cells by nuclear cloning. Nat Genet. 2000, 24: 109-110. 10.1038/72753.
Eggan K, Akutsu H, Loring J, Jackson-Grusby L, Klemm M, Rideout WM, Yanagimachi R, Jaenisch R: Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc Natl Acad Sci USA. 2001, 98: 6209-6214. 10.1073/pnas.101118898.
Wheeler MB, Walters EM: Transgenic technology and applications in swine. Theriogenology. 2001, 56: 1345-1369. 10.1016/S0093-691X(01)00635-5.
Chen LR, Shiue YL, Bertolini L, Medrano JF, BonDurant RH, Anderson GB: Establishment of pluripotent cell lines from porcine preimplantation embryos. Theriogenology. 1999, 52: 195-212. 10.1016/S0093-691X(99)00122-3.
Wheeler MB: Development and validation of swine embryonic stem cells: a review. Reprod Fertil Dev. 1994, 6: 563-568. 10.1071/RD9940563.
Bosch P, Pratt SL, Stice SL: Isolation, characterization, gene modification, and nuclear reprogramming of porcine mesenchymal stem cells. Biol Reprod. 2006, 74: 46-57. 10.1095/biolreprod.105.045138.
Dyce PW, Zhu H, Craig J, Li J: Stem cells with multilineage potential derived from porcine skin. Biochem Biophys Res Commun. 2004, 316: 651-658. 10.1016/j.bbrc.2004.02.093.
Ringe J, Kaps C, Schmitt B, Buscher K, Bartel J, Smolian H, Schultz O, Burmester GR, Haupl T, Sittinger M: Porcine mesenchymal stem cells. Induction of distinct mesenchymal cell lineages. Cell Tissue Res. 2002, 307: 321-327. 10.1007/s00441-002-0525-z.
Kues WA, Petersen B, Mysegades W, Carnwath JW, Niemann H: Isolation of murine and porcine fetal stem cells from somatic tissue. Biol Reprod. 2005, 72: 1020-1028. 10.1095/biolreprod.104.031229.
Colleoni S, Donofrio G, Lagutina I, Duchi R, Galli C, Lazzari G: Establishment, differentiation, electroporation, viral transduction, and nuclear transfer of bovine and porcine mesenchymal stem cells. Cloning Stem Cells. 2005, 7: 154-166. 10.1089/clo.2005.7.154.
Faast R, Harrison SJ, Beebe LF, McIlfatrick SM, Ashman RJ, Nottle MB: Use of adult mesenchymal stem cells isolated from bone marrow and blood for somatic cell nuclear transfer in pigs. Cloning Stem Cells. 2006, 8: 166-173. 10.1089/clo.2006.8.166.
Tomii R, Kurome M, Ochiai T, Wako N, Ueda H, Hirakawa K, Kano K, Nagashima H: Production of cloned pigs by nuclear transfer of preadipocytes established from adult mature adipocytes. Cloning Stem Cells. 2005, 7: 279-288.
Lechardeur D, Lukacs GL: Nucleocytoplasmic transport of plasmid DNA: a perilous journey from the cytoplasm to the nucleus. Hum Gene Ther. 2006, 17: 882-889. 10.1089/hum.2006.17.882.
Brinster RL, Chen HY, Trumbauer ME, Yagle MK, Palmiter RD: Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci USA. 1985, 82: 4438-4442. 10.1073/pnas.82.13.4438.
Bishop JO, Smith P: Mechanism of chromosomal integration of microinjected DNA. Mol Biol Med. 1989, 6: 283-298.
Burdon TG, Wall RJ: Fate of microinjected genes in pre-implantation mouse embryos. Mol Reprod Dev. 1992, 33: 436-442. 10.1002/mrd.1080330410.
Gordon JW, Ruddle FH: DNA-mediated genetic transformation of mouse embryos and bone marrow: a review. Gene. 1985, 33: 121-136. 10.1016/0378-1119(85)90087-3.
Palmiter RD, Brinster RL: Germ-line transformation of mice. Annu Rev Genet. 1986, 20: 465-499. 10.1146/annurev.ge.20.120186.002341.
Pawlik KM, Sun CW, Higgins NP, Townes TM: End joining of genomic DNA and transgene DNA in fertilized mouse eggs. Gene. 1995, 165: 173-181. 10.1016/0378-1119(95)00519-C.
Townes TM, Chen HY, Lingrel JB, Palmiter RD, Brinster RL: Expression of human beta-globin genes in transgenic mice: effects of a flanking metallothionein-human growth hormone fusion gene. Mol Cell Biol. 1985, 5: 1977-1983.
Dalle B, Rubin JE, Alkan O, Sukonnik T, Pasceri P, Yao S, Pawliuk R, Leboulch P, Ellis J: eGFP reporter genes silence LCRbeta-globin transgene expression via CpG dinucleotides. Mol Ther. 2005, 11: 591-599. 10.1016/j.ymthe.2004.11.012.
Garrick D, Fiering S, Martin DI, Whitelaw E: Repeat-induced gene silencing in mammals. Nat Genet. 1998, 18: 56-59. 10.1038/ng0198-56.
Dorer DR, Henikoff S: Transgene repeat arrays interact with distant heterochromatin and cause silencing in cis and trans. Genetics. 1997, 147: 1181-1190.
Chen CM, Choo KB, Cheng WT: Frequent deletions and sequence aberrations at the transgene junctions of transgenic mice carrying the papillomavirus regulatory and the SV40 TAg gene sequences. Transgenic Res. 1995, 4: 52-59. 10.1007/BF01976502.
Pravtcheva DD, Wise TL: A postimplantation lethal mutation induced by transgene insertion on mouse chromosome 8. Genomics. 1995, 30: 529-544. 10.1006/geno.1995.1274.
Nakanishi T, Kuroiwa A, Yamada S, Isotani A, Yamashita A, Tairaka A, Hayashi T, Takagi T, Ikawa M, Matsuda Y, et al: FISH analysis of 142 EGFP transgene integration sites into the mouse genome. Genomics. 2002, 80: 564-574. 10.1006/geno.2002.7008.
Bishop JO: Chromosomal Insertion of Foreign DNA. Transgenic Animals: Generation and Use. Edited by: Houdebine L-M. 1997, Amsterdam, the Netherlands: Harwood Academic Publishers, 219-223.
Covarrubias L, Nishida Y, Mintz B: Early postimplantation embryo lethality due to DNA rearrangements in a transgenic mouse strain. Proc Natl Acad Sci USA. 1986, 83: 6020-6024. 10.1073/pnas.83.16.6020.
Pravtcheva DD, Wise TL: Transgene instability in mice injected with an in vitro methylated Igf2 gene. Mutat Res. 2003, 529: 35-50.
Scrable H, Stambrook PJ: A genetic program for deletion of foreign DNA from the mammalian genome. Mutat Res. 1999, 429: 225-237.
Wilkie TM, Palmiter RD: Analysis of the integrant in MyK-103 transgenic mice in which males fail to transmit the integrant. Mol Cell Biol. 1987, 7: 1646-1655.
Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F: HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002, 110: 521-529. 10.1016/S0092-8674(02)00864-4.
Wu X, Li Y, Crise B, Burgess SM: Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003, 300: 1749-1751. 10.1126/science.1083413.
Pannell D, Ellis J: Silencing of gene expression: implications for design of retrovirus vectors. Rev Med Virol. 2001, 11: 205-217. 10.1002/rmv.316.
Hofmann A, Kessler B, Ewerling S, Kabermann A, Brem G, Wolf E, Pfeifer A: Epigenetic regulation of lentiviral transgene vectors in a large animal model. Mol Ther. 2006, 13: 59-66. 10.1016/j.ymthe.2005.07.685.
Brutnell TP: Transposon tagging in maize. Funct Integr Genomics. 2002, 2: 4-12. 10.1007/s10142-001-0044-0.
Hamer L, DeZwaan TM, Montenegro-Chamorro MV, Frank SA, Hamer JE: Recent advances in large-scale transposon mutagenesis. Curr Opin Chem Biol. 2001, 5: 67-73. 10.1016/S1367-5931(00)00162-9.
Parinov S, Sundaresan V: Functional genomics in Arabidopsis: large-scale insertional mutagenesis complements the genome sequencing project. Curr Opin Biotechnol. 2000, 11: 157-161. 10.1016/S0958-1669(00)00075-6.
Spradling AC, Stern DM, Kiss I, Roote J, Laverty T, Rubin GM: Gene disruptions using P transposable elements: an integral component of the Drosophila genome project. Proc Natl Acad Sci USA. 1995, 92: 10824-10830. 10.1073/pnas.92.24.10824.
Vidan S, Snyder M: Large-scale mutagenesis: yeast genetics in the genome era. Curr Opin Biotechnol. 2001, 12: 28-34. 10.1016/S0958-1669(00)00171-3.
Voelker LL, Dybvig K: Transposon mutagenesis. Methods Mol Biol. 1998, 104: 235-238.
Ivics Z, Hackett PB, Plasterk RH, Izsvak Z: Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997, 91: 501-510. 10.1016/S0092-8674(00)80436-5.
Izsvak Z, Ivics Z, Plasterk RH: Sleeping Beauty, a wide host-range transposon vector for genetic transformation in vertebrates. J Mol Biol. 2000, 302: 93-102. 10.1006/jmbi.2000.4047.
Koga A, Iida A, Kamiya M, Hayashi R, Hori H, Ishikawa Y, Tachibana A: The medaka fish Tol2 transposable element can undergo excision in human and mouse cells. J Hum Genet. 2003, 48: 231-235. 10.1007/s10038-003-0016-4.
Koga A, Suzuki M, Inagaki H, Bessho Y, Hori H: Transposable element in fish. Nature. 1996, 383: 30-10.1038/383030a0.
Fraser MJ, Ciszczon T, Elick T, Bauser C: Precise excision of TTAA-specific lepidopteran transposons piggyBac (IFP2) and tagalong (TFP3) from the baculovirus genome in cell lines from two species of Lepidoptera. Insect Mol Biol. 1996, 5: 141-151.
Wu SC, Meir YJ, Coates CJ, Handler AM, Pelczar P, Moisyadi S, Kaminski JM: piggyBac is a flexible and highly active transposon as compared to Sleeping Beauty, Tol2, and Mos1 in mammalian cells. Proc Natl Acad Sci USA. 2006, 103: 15008-15013. 10.1073/pnas.0606979103.
Miskey C, Izsvak Z, Plasterk RH, Ivics Z: The Frog Prince: a reconstructed transposon from Rana pipiens with high transpositional activity in vertebrate cells. Nucleic Acids Res. 2003, 31: 6873-6881. 10.1093/nar/gkg910.
Klinakis AG, Zagoraiou L, Vassilatis DK, Savakis C: Genome-wide insertional mutagenesis in human cells by the Drosophila mobile element Minos. EMBO Rep. 2000, 1: 416-421. 10.1093/embo-reports/kvd089.
Keravala A, Liu D, Lechman ER, Wolfe D, Nash JA, Lampe DJ, Robbins PD: Hyperactive Himar1 transposase mediates transposition in cell culture and enhances gene expression in vivo. Hum Gene Ther. 2006, 17: 1006-1018. 10.1089/hum.2006.17.1006.
Leaver MJ: A family of Tc1-like transposons from the genomes of fishes and frogs: evidence for horizontal transmission. Gene. 2001, 271: 203-214. 10.1016/S0378-1119(01)00530-3.
Wilber A, Frandsen JL, Geurts JL, Largaespada DA, Hackett PB, McIvor RS: RNA as a source of transposase for sleeping beauty-mediated gene insertion and expression in somatic cells and tissues. Mol Ther. 2006, 13: 625-630. 10.1016/j.ymthe.2005.10.014.
Davidson AE, Balciunas D, Mohn D, Shaffer J, Hermanson S, Sivasubbu S, Cliff MP, Hackett PB, Ekker SC: Efficient gene delivery and gene expression in zebrafish using the Sleeping Beauty transposon. Dev Biol. 2003, 263: 191-202. 10.1016/j.ydbio.2003.07.013.
Dupuy AJ, Clark K, Carlson CM, Fritz S, Davidson AE, Markley KM, Finley K, Fletcher CF, Ekker SC, Hackett PB, et al: Mammalian germ-line transgenesis by transposition. Proc Natl Acad Sci USA. 2002, 99: 4495-4499. 10.1073/pnas.062630599.
Plasterk RH, Izsvak Z, Ivics Z: Resident aliens: the Tc1/mariner superfamily of transposable elements. Trends Genet. 1999, 15: 326-332. 10.1016/S0168-9525(99)01777-1.
Sarkar A, Sim C, Hong YS, Hogan JR, Fraser MJ, Robertson HM, Collins FH: Molecular evolutionary analysis of the widespread piggyBac transposon family and related 'domesticated' sequences. Mol Genet Genomics. 2003, 270: 173-180. 10.1007/s00438-003-0909-0.
Kempken F, Windhofer F: The hAT family: a versatile transposon group common to plants, fungi, animals, and man. Chromosoma. 2001, 110: 1-9. 10.1007/s004120000118.
Hackett CS, Geurts AM, Wangensteen KJ, Balciunas D, Ekker SC, Hackett PB: Predicting transposon chromosomal insertion sites: implications for functional genomics and gene therapy. Genome Biol. 2007, 8 (Suppl 1): S12-
Izsvak Z, Stuwe EE, Fiedler D, Katzer A, Jeggo PA, Ivics Z: Healing the wounds inflicted by sleeping beauty transposition by double-strand break repair in mammalian somatic cells. Mol Cell. 2004, 13: 279-290. 10.1016/S1097-2765(03)00524-0.
Liu G, Aronovich EL, Cui Z, Whitley CB, Hackett PB: Excision of Sleeping Beauty transposons: parameters and applications to gene therapy. J Gene Med. 2004, 6: 574-583. 10.1002/jgm.486.
Kawakami K, Imanaka K, Itoh M, Taira M: Excision of the Tol2 transposable element of the medaka fish Oryzias latipes in Xenopus laevis and Xenopus tropicalis. Gene. 2004, 338: 93-98. 10.1016/j.gene.2004.05.013.
Kawakami K, Koga A, Hori H, Shima A: Excision of the tol2 transposable element of the medaka fish, Oryzias latipes, in zebrafish, Danio rerio. Gene. 1998, 225: 17-22. 10.1016/S0378-1119(98)00537-X.
Ding S, Wu X, Li G, Han M, Zhuang Y, Xu T: Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005, 122: 473-483. 10.1016/j.cell.2005.07.013.
Wilson MH, Coates CJ, George AL: PiggyBac transposon-mediated gene transfer in human cells. Mol Ther. 2007, 15: 139-145. 10.1038/sj.mt.6300028.
Hamlet MR, Yergeau DA, Kuliyev E, Takeda M, Taira M, Kawakami K, Mead PE: Tol2 transposon-mediated transgenesis in Xenopus tropicalis. Genesis. 2006, 44: 438-445. 10.1002/dvg.20234.
Kawakami K, Shima A, Kawakami N: Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc Natl Acad Sci USA. 2000, 97: 11403-11408. 10.1073/pnas.97.21.11403.
Dupuy AJ, Fritz S, Largaespada DA: Transposition and gene disruption in the male germline of the mouse. Genesis. 2001, 30: 82-88. 10.1002/gene.1037.
Fischer SE, Wienholds E, Plasterk RH: Regulated transposition of a fish transposon in the mouse germ line. Proc Natl Acad Sci USA. 2001, 98: 6759-6764. 10.1073/pnas.121569298.
Horie K, Kuroiwa A, Ikawa M, Okabe M, Kondoh G, Matsuda Y, Takeda J: Efficient chromosomal transposition of a Tc1/mariner-like transposon Sleeping Beauty in mice. Proc Natl Acad Sci USA. 2001, 98: 9191-9196. 10.1073/pnas.161071798.
Geurts AM, Wilber A, Carlson CM, Lobitz PD, Clark KJ, Hackett PB, McIvor RS, Largaespada DA: Conditional gene expression in the mouse using a Sleeping Beauty gene-trap transposon. BMC Biotechnol. 2006, 6: 30-10.1186/1472-6750-6-30.
Clark KJ, Geurts AM, Bell JB, Hackett PB: Transposon vectors for gene-trap insertional mutagenesis in vertebrates. Genesis. 2004, 39: 225-233. 10.1002/gene.20049.
Parinov S, Kondrichin I, Korzh V, Emelyanov A: Tol2 transposon-mediated enhancer trap to identify developmentally regulated zebrafish genes in vivo. Dev Dyn. 2004, 231: 449-459. 10.1002/dvdy.20157.
Grabher C, Henrich T, Sasado T, Arenz A, Wittbrodt J, Furutani-Seiki M: Transposon-mediated enhancer trapping in medaka. Gene. 2003, 322: 57-66. 10.1016/j.gene.2003.09.009.
Kawakami K, Takeda H, Kawakami N, Kobayashi M, Matsuda N, Mishina M: A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev Cell. 2004, 7: 133-144. 10.1016/j.devcel.2004.06.005.
Balciunas D, Davidson AE, Sivasubbu S, Hermanson SB, Welle Z, Ekker SC: Enhancer trapping in zebrafish using the Sleeping Beauty transposon. BMC Genomics. 2004, 5: 62-10.1186/1471-2164-5-62.
Horie K, Yusa K, Yae K, Odajima J, Fischer SE, Keng VW, Hayakawa T, Mizuno S, Kondoh G, Ijiri T, et al: Characterization of Sleeping Beauty transposition and its application to genetic screening in mice. Mol Cell Biol. 2003, 23: 9189-9207. 10.1128/MCB.23.24.9189-9207.2003.
Collier LS, Carlson CM, Ravimohan S, Dupuy AJ, Largaespada DA: Cancer gene discovery in solid tumours using transposon-based somatic mutagenesis in the mouse. Nature. 2005, 436: 272-276. 10.1038/nature03681.
Dupuy AJ, Akagi K, Largaespada DA, Copeland NG, Jenkins NA: Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature. 2005, 436: 221-226. 10.1038/nature03691.
Geurts AM, Yang Y, Clark KJ, Liu G, Cui Z, Dupuy AJ, Bell JB, Largaespada DA, Hackett PB: Gene transfer into genomes of human cells by the sleeping beauty transposon system. Mol Ther. 2003, 8: 108-117. 10.1016/S1525-0016(03)00099-6.
Karsi A, Moav B, Hackett P, Liu Z: Effects of insert size on transposition efficiency of the sleeping beauty transposon in mouse cells. Mar Biotechnol (NY). 2001, 3: 241-245. 10.1007/s101260000072.
Yin W, Xiang P, Li Q: Investigations of the effect of DNA size in transient transfection assay using dual luciferase system. Anal Biochem. 2005, 346: 289-294.
Yant SR, Wu X, Huang Y, Garrison B, Burgess SM, Kay MA: High-resolution genome-wide mapping of transposon integration in mammals. Mol Cell Biol. 2005, 25: 2085-2094. 10.1128/MCB.25.6.2085-2094.2005.
Branda CS, Dymecki SM: Talking about a revolution: the impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004, 6: 7-28. 10.1016/S1534-5807(03)00399-X.
Clark KJ, Carlson DF, Foster LK, Kong BW, Foster DN: Enzymatic engineering of the porcine genome with transposons and recombinases. BMC Biotechnol. 2007, 7: 42-10.1186/1472-6750-7-42.
Deachapunya C, Palmer-Densmore M, O'Grady SM: Insulin stimulates transepithelial sodium transport by activation of a protein phosphatase that increases Na-K ATPase activity in endometrial epithelial cells. J Gen Physiol. 1999, 114: 561-574. 10.1085/jgp.114.4.561.
Cui Z, Geurts AM, Liu G, Kaufman CD, Hackett PB: Structure-function analysis of the inverted terminal repeats of the sleeping beauty transposon. J Mol Biol. 2002, 318: 1221-1235. 10.1016/S0022-2836(02)00237-1.
Baus J, Liu L, Heggestad AD, Sanz S, Fletcher BS: Hyperactive transposase mutants of the Sleeping Beauty transposon. Mol Ther. 2005, 12: 1148-1156. 10.1016/j.ymthe.2005.06.484.
Yant SR, Park J, Huang Y, Mikkelsen JG, Kay MA: Mutational analysis of the N-terminal DNA-binding domain of sleeping beauty transposase: critical residues for DNA binding and hyperactivity in mammalian cells. Mol Cell Biol. 2004, 24: 9239-9247. 10.1128/MCB.24.20.9239-9247.2004.
Zayed H, Izsvak Z, Walisko O, Ivics Z: Development of hyperactive sleeping beauty transposon vectors by mutational analysis. Mol Ther. 2004, 9: 292-304. 10.1016/j.ymthe.2003.11.024.
Abuin A, Bradley A: Recycling selectable markers in mouse embryonic stem cells. Mol Cell Biol. 1996, 16: 1851-1856.
Karreman C: A new set of positive/negative selectable markers for mammalian cells. Gene. 1998, 218: 57-61. 10.1016/S0378-1119(98)00387-4.
Chen YT, Bradley A: A new positive/negative selectable marker, puDeltatk, for use in embryonic stem cells. Genesis. 2000, 28: 31-35. 10.1002/1526-968X(200009)28:1<31::AID-GENE40>3.0.CO;2-K.
Zeng L, Rahrmann E, Hu Q, Lund T, Sandquist L, Felten M, O'Brien TD, Zhang J, Verfaillie C: Multi-potent adult progenitor cells from swine bone marrow. Stem Cells. 2006, 24: 2355-2366. 10.1634/stemcells.2005-0551.
Price EM, Prather RS, Foley CM: Multipotent adult progenitor cell lines originating from the peripheral blood of green fluorescent protein transgenic Swine. Stem Cells Dev. 2006, 15: 507-522. 10.1089/scd.2006.15.507.
Bosch P, Fouletier-Dilling C, Olmsted-Davis EA, Davis AR, Stice SL: Efficient adenoviral-mediated gene delivery into porcine mesenchymal stem cells. Mol Reprod Dev. 2006, 73: 1393-1403. 10.1002/mrd.20593.
Yagi T, Nada S, Watanabe N, Tamemoto H, Kohmura N, Ikawa Y, Aizawa S: A novel negative selection for homologous recombinants using diphtheria toxin A fragment gene. Anal Biochem. 1993, 214: 77-86. 10.1006/abio.1993.1459.
Yusa K, Takeda J, Horie K: Enhancement of Sleeping Beauty transposition by CpG methylation: possible role of heterochromatin formation. Mol Cell Biol. 2004, 24: 4004-4018. 10.1128/MCB.24.9.4004-4018.2004.
Yant SR, Meuse L, Chiu W, Ivics Z, Izsvak Z, Kay MA: Somatic integration and long-term transgene expression in normal and haemophilic mice using a DNA transposon system. Nat Genet. 2000, 25: 35-41. 10.1038/75568.
Montini E, Held PK, Noll M, Morcinek N, Al-Dhalimy M, Finegold M, Yant SR, Kay MA, Grompe M: In vivo correction of murine tyrosinemia type I by DNA-mediated transposition. Mol Ther. 2002, 6: 759-769. 10.1006/mthe.2002.0812.
Yant SR, Ehrhardt A, Mikkelsen JG, Meuse L, Pham T, Kay MA: Transposition from a gutless adeno-transposon vector stabilizes transgene expression in vivo. Nat Biotechnol. 2002, 20: 999-1005. 10.1038/nbt738.
Belur LR, Frandsen JL, Dupuy AJ, Ingbar DH, Largaespada DA, Hackett PB, Scott McIvor R: Gene insertion and long-term expression in lung mediated by the Sleeping Beauty transposon system. Mol Ther. 2003, 8: 501-507. 10.1016/S1525-0016(03)00211-9.
He CX, Shi D, Wu WJ, Ding YF, Feng DM, Lu B, Chen HM, Yao JH, Shen Q, Lu DR, et al: Insulin expression in livers of diabetic mice mediated by hydrodynamics-based administration. World J Gastroenterol. 2004, 10: 567-572.
Liu L, Sanz S, Heggestad AD, Antharam V, Notterpek L, Fletcher BS: Endothelial targeting of the Sleeping Beauty transposon within lung. Mol Ther. 2004, 10: 97-105. 10.1016/j.ymthe.2004.04.006.
Ohlfest JR, Frandsen JL, Fritz S, Lobitz PD, Perkinson SG, Clark KJ, Nelsestuen G, Key NS, McIvor RS, Hackett PB, et al: Phenotypic correction and long-term expression of factor VIII in hemophilic mice by immunotolerization and nonviral gene transfer using the Sleeping Beauty transposon system. Blood. 2005, 105: 2691-2698. 10.1182/blood-2004-09-3496.
Liu L, Liu H, Visner G, Fletcher BS: Sleeping Beauty-mediated eNOS gene therapy attenuates monocrotaline-induced pulmonary hypertension in rats. FASEB J. 2006, 20: 2594-2596. 10.1096/fj.06-6254fje.
Liu L, Mah C, Fletcher BS: Sustained FVIII expression and phenotypic correction of hemophilia A in neonatal mice using an endothelial-targeted sleeping beauty transposon. Mol Ther. 2006, 13: 1006-1015. 10.1016/j.ymthe.2005.11.021.
Nabel EG, Yang ZY, Plautz G, Forough R, Zhan X, Haudenschild CC, Maciag T, Nabel GJ: Recombinant fibroblast growth factor-1 promotes intimal hyperplasia and angiogenesis in arteries in vivo. Nature. 1993, 362: 844-846. 10.1038/362844a0.
Danialou G, Comtois AS, Matecki S, Nalbantoglu J, Karpati G, Gilbert R, Geoffroy P, Gilligan S, Tanguay JF, Petrof BJ: Optimization of regional intraarterial naked DNA-mediated transgene delivery to skeletal muscles in a large animal model. Mol Ther. 2005, 11: 257-266. 10.1016/j.ymthe.2004.09.016.
Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL: Direct gene transfer into mouse muscle in vivo. Science. 1990, 247: 1465-1468. 10.1126/science.1690918.
Ulmer JB, Wahren B, Liu MA: Gene-based vaccines: recent technical and clinical advances. Trends Mol Med. 2006, 12: 216-222. 10.1016/j.molmed.2006.03.007.
Davis BS, Chang GJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, Bowen R, Bunning ML: West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001, 75: 4040-4047. 10.1128/JVI.75.9.4040-4047.2001.
Anderson ED, Mourich DV, Fahrenkrug SC, LaPatra S, Shepherd J, Leong JA: Genetic immunization of rainbow trout (Oncorhynchus mykiss) against infectious hematopoietic necrosis virus. Mol Mar Biol Biotechnol. 1996, 5: 114-122.
Aronovich EL, Bell JB, Belur LR, Gunther R, Koniar B, Erickson DC, Schachern PA, Matise I, McIvor RS, Whitley CB, Hackett PB: Prolonged expression of a lysosomal enzyme in mouse liver after Sleeping Beauty transposon-mediated gene delivery: implications for non-viral gene therapy of mucopolysaccharidoses. J Gene Med. 2007, 9: 403-415. 10.1002/jgm.1028.
Bonin CP, Mann RS: A piggyBac transposon gene trap for the analysis of gene expression and function in Drosophila. Genetics. 2004, 167: 1801-1811. 10.1534/genetics.104.027557.
Sivasubbu S, Balciunas D, Davidson AE, Pickart MA, Hermanson SB, Wangensteen KJ, Wolbrink DC, Ekker SC: Gene-breaking transposon mutagenesis reveals an essential role for histone H2afza in zebrafish larval development. Mech Dev. 2006, 123: 513-529. 10.1016/j.mod.2006.06.002.
Stryke D, Kawamoto M, Huang CC, Johns SJ, King LA, Harper CA, Meng EC, Lee RE, Yee A, L'Italien L, et al: BayGenomics: a resource of insertional mutations in mouse embryonic stem cells. Nucleic Acids Res. 2003, 31: 278-281. 10.1093/nar/gkg064.
White EB: Charlotte's Web. 1952, New York, NY: Harper Collins
Park KW, Lai L, Cheong HT, Cabot R, Sun QY, Wu G, Rucker EB, Durtschi D, Bonk A, Samuel M, et al: Mosaic gene expression in nuclear transfer-derived embryos and the production of cloned transgenic pigs from ear-derived fibroblasts. Biol Reprod. 2002, 66: 1001-1005. 10.1095/biolreprod66.4.1001.
Acknowledgements
This article has been published as part of Genome Biology Volume 8, Supplement 1, 2007: Transposons in vertebrate functional genomics. The full contents of the supplement are available online at http://genomebiology.com/supplements/8/S1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
KJC and SCF are both inventors on University of Minnesota Patents involving Sleeping Beauty Transposon technology.
Karl J Clark, Daniel F Carlson contributed equally to this work.
Rights and permissions
About this article
Cite this article
Clark, K.J., Carlson, D.F. & Fahrenkrug, S.C. Pigs taking wing with transposons and recombinases. Genome Biol 8 (Suppl 1), S13 (2007). https://doi.org/10.1186/gb-2007-8-s1-s13
Published:
DOI: https://doi.org/10.1186/gb-2007-8-s1-s13