Abstract
Introduction
Pulmonary vascular dysfunction, pulmonary hypertension (PH), and resulting right ventricular (RV) failure occur in many critical illnesses and may be associated with a worse prognosis. PH and RV failure may be difficult to manage: principles include maintenance of appropriate RV preload, augmentation of RV function, and reduction of RV afterload by lowering pulmonary vascular resistance (PVR). We therefore provide a detailed update on the management of PH and RV failure in adult critical care.
Methods
A systematic review was performed, based on a search of the literature from 1980 to 2010, by using prespecified search terms. Relevant studies were subjected to analysis based on the GRADE method.
Results
Clinical studies of intensive care management of pulmonary vascular dysfunction were identified, describing volume therapy, vasopressors, sympathetic inotropes, inodilators, levosimendan, pulmonary vasodilators, and mechanical devices. The following GRADE recommendations (evidence level) are made in patients with pulmonary vascular dysfunction: 1) A weak recommendation (very-low-quality evidence) is made that close monitoring of the RV is advised as volume loading may worsen RV performance; 2) A weak recommendation (low-quality evidence) is made that low-dose norepinephrine is an effective pressor in these patients; and that 3) low-dose vasopressin may be useful to manage patients with resistant vasodilatory shock. 4) A weak recommendation (low-moderate quality evidence) is made that low-dose dobutamine improves RV function in pulmonary vascular dysfunction. 5) A strong recommendation (moderate-quality evidence) is made that phosphodiesterase type III inhibitors reduce PVR and improve RV function, although hypotension is frequent. 6) A weak recommendation (low-quality evidence) is made that levosimendan may be useful for short-term improvements in RV performance. 7) A strong recommendation (moderate-quality evidence) is made that pulmonary vasodilators reduce PVR and improve RV function, notably in pulmonary vascular dysfunction after cardiac surgery, and that the side-effect profile is reduced by using inhaled rather than systemic agents. 8) A weak recommendation (very-low-quality evidence) is made that mechanical therapies may be useful rescue therapies in some settings of pulmonary vascular dysfunction awaiting definitive therapy.
Conclusions
This systematic review highlights that although some recommendations can be made to guide the critical care management of pulmonary vascular and right ventricular dysfunction, within the limitations of this review and the GRADE methodology, the quality of the evidence base is generally low, and further high-quality research is needed.
Similar content being viewed by others
Introduction
Pulmonary vascular dysfunction is a broad term and may be central to several disease processes in the intensive care unit (ICU). Components include pulmonary endothelial dysfunction, altered lung microvascular permeability, vasoactive mediator imbalance, abnormal hypoxic vasoconstriction, pulmonary metabolic failure, microvascular thrombosis, and later, vascular remodelling [1–3]. The resulting elevation in pulmonary vascular resistance (PVR) and pulmonary hypertension (PH) may increase the transpulmonary gradient, and the right ventricular "pressure overload" can in turn result in right ventricular (RV) dysfunction and failure [4]. RV dysfunction may also result from volume overload or a primary RV pathology reducing contractility, including RV infarction and sepsis (Table 1) [4–7].
PH is defined at right-heart catheterization in the outpatient setting, with resting mPAP exceeding 25 mm Hg, and a PVR greater than 240 dyn.s.cm-5 (3 Wood units) [8]. At echocardiography, the presence of PH is suggested by the estimated RV systolic pressure (RVSP) exceeding 35 mm Hg (being severe if >50 mm Hg) (see later) [9], and the pulmonary arterial acceleration time (PAT) may be shortened [10]. Pulmonary arterial hypertension (PAH) defines PH not due to left-heart disease, with PAOP <15 mm Hg or without echocardiographic evidence of increased left atrial pressure. The severity of PH may depend on the chronicity: the actual pulmonary artery pressure generated will increase with time as the RV hypertrophies.
RV dysfunction describes reduced RV contractility, which may be detected in several ways. At echocardiography, RV distention causes the intraventricular septum to deviate, with resulting paradoxic septal movement that impinges on LV function [11]. RV function may be difficult to assess on echocardiography, especially in ventilated patients, and measurement of the descent of the RV base toward the apex (tricuspid annular systolic excursion, TAPSE) or RV fractional shortening may useful [12, 13]. Invasive monitoring may show a CVP exceeding the PAOP, or increasing CVP and PVR with a decreasing cardiac output (and mPAP may therefore decrease), and high right ventricular end-diastolic filling pressure is characteristic. By using an RV ejection fraction (RVEF) PAC, an increase in RV end-diastolic index and a reduction in RVEF are seen [14]. We have defined RV failure to be the clinical result of RV dysfunction with the onset of hypotension or any resulting end-organ (for example, renal, liver, or gastrointestinal) dysfunction. Acute cor pulmonale (ACP) refers to acute right heart failure in the setting of acutely elevated PVR due to pulmonary disease [15, 16].
Pulmonary hypertension per se is frequently encountered in the ICU. It is commonly due to elevated pulmonary venous pressure in the setting of left-sided heart disease, or in patients with preexisting pulmonary vascular disease. It is well recognized after cardiothoracic surgery, in part related to the endothelial dysfunction seen with cardiopulmonary bypass (CPB) [17, 18]. PH is also associated with sepsis [19]; acute respiratory distress syndrome (ARDS) [20–22] (with associated acute RV failure in 10% to 25% of cases [23, 24]), and in up to 60% of patients after massive pulmonary embolism (PE) [25]. PH is important to recognize in the ICU because its presence predicts increased mortality in these conditions [19, 23, 25–31] as well as after surgical procedures [32–42]. Mortality from cardiogenic shock due to RV infarction (> 50%) exceeds that due to LV disease [5]. We therefore thought that a systematic review of the current evidence for the management of PH, resulting RV dysfunction, and failure in adult patients in the ICU, would be a useful addition to the critical care literature.
The pulmonary circulation and pathophysiology of right ventricular failure
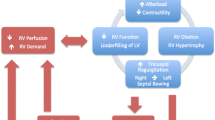
The normal pulmonary circulation is a high-flow, low-pressure system. Unlike the left ventricle (LV), the thin-walled right ventricle tolerates poorly acute increases in afterload. This may lead to acute distention (Figure 1) [4, 43], with a resulting increase in oxygen consumption and reduction in contractility [44]. The dilated RV, together with paradoxic intraventricular septal movement [45], lead to reduced LV filling [46], cardiac output (CO), and oxygen delivery [47]. The principle of ventricular interdependence is important in most settings: superficial myocardial fibers encircle both ventricles; thus they are contained within the same pericardial cavity (except maybe after cardiac surgery), as well as sharing a septum, effectively existing "in series" [48, 49]. This explains the decrease in LV output seen during positive-pressure ventilation [48, 50, 51] and why RV pressure and volume overload cause diastolic dysfunction of the LV [52]. Furthermore, because of the RV/LV interactions, the LV may markedly depend on atrial contraction for filling and may tolerate atrial fibrillation and vasodilating therapy particularly poorly [49, 53, 54].
Short-axis view of a transthoracic echocardiogram in a normal subject (a) and a patient with an acutely dilated right ventricle (RV) in the setting of high pulmonary vascular resistance (b). The intraventricular septum (IVS) is D-shaped in (b), reflecting the acute RV pressure overload in this patient, and marked enlargement of the RV in (b) compared with (a). Courtesy of Dr Susanna Price, Royal Brompton Hospital, London, UK.
In addition, perfusion of the right coronary artery is usually dependent on a pressure gradient between the aorta and the right ventricle, which, in the setting of increased RV afterload and decreased coronary blood flow, may lead to RV ischemia [55], with further severe hemodynamic decompensation [56] (Figure 2). In acute-on-chronic RV-pressure overload, the already-hypertrophied RV tolerates much higher pressures before decompensation [57, 58], although the ability of the RV to augment CO in chronic PH may be restricted by its relatively "fixed" afterload. In any setting, the most common cause of increased RV afterload is an increase in PVR (Table 2).
The gold standard for the diagnosis and management of PH and RV dysfunction in the ICU setting is considered by some to be through pulmonary artery catheterization (PAC), even though most of the information can be obtained noninvasively by echocardiography: the requirement for PAC in this population remains controversial. It must, however, be acknowledged that it provides the only direct continuous measurement of right-sided pressures and direct measurement of RV afterload, whereby, through measurement of cardiac output, pulmonary pressures and the pulmonary artery occlusion pressure (PAOP, the "wedge"), the PVR can be calculated (Figure 3). Overall outcomes are not improved when the PAC is used in general in critically ill patients; and complications do occur [59]: the use in general is therefore declining. However, no studies have been done in the "pulmonary vascular" subpopulation. Alternative invasive hemodynamic measurements, such as CVP, may be useful surrogates for volume status in RV failure, by using the diastolic component of the CVP. Importantly, when monitoring CVP in patients with significant tricuspid regurgitation (TR), the variable V wave may be misleading, as it is included in the mean CVP calculation on most automated machines, and if rising, indicates RV overdistention. In the setting of cardiac surgery, one study shows that PAC use has reduced from 100% to 9% from 1997 to 2001, thought to reflect increased use of transesophageal echocardiography (TEE) [60]. In the setting of cardiac surgery, PAC may remain indicated for patients with PH and low CO and those predicted to have a difficult postoperative course [60], when a Swan introducer sheath may be inserted preemptively, or inserted for continuous monitoring after a diagnosis of RV dysfunction made with echocardiography [61]. PAC is also a useful cardiac monitor with intraaortic balloon counterpulsation. Few data exist on PAC in other settings of pulmonary vascular dysfunction in the ICU, but one study suggests that PVR may be a poor indicator of pulmonary-circulation status in ventilated patients with ALI/ARDS [62]. The role of echocardiography, both transthoracic (TTE) and TEE, is increasingly recognized in assessing RV function in many ICU settings [63–65] and provides essential information about RV geometry and function. PA pressures may be assessed by estimating the systolic-pressure gradient across the tricuspid valve by using the modified Bernoulli equation [9, 66, 67], and although the correlation between invasive and sonographic measurement has been shown to be excellent in these studies, no studies have correlated PAC with echocardiographic measurements in the ICU population. In reality, a combination of invasive and noninvasive techniques is used. Biomarkers such as brain natriuretic peptide (BNP) are useful in monitoring chronic PAH [68], in risk-stratifying acute pulmonary embolism (see later) [69–71], and in identifying ARDS-related pulmonary vascular dysfunction [72], although their role is less clear in other ICU settings.
The diagnosis and management of acute pulmonary embolism (PE) warrants a specific mention, as it is a relatively common cause of acute RV failure in the ICU [73]. Available therapies include thrombolysis and embolectomy, reducing the clot burden and acute mortality [74, 75], as well as reducing the longer-term risk of chronic thromboembolic PH [76]. Given that more than half of related deaths occur within an hour of the onset of symptoms [77], effective supportive treatment of shock is paramount. Patients presenting with acute PE are risk stratified according to the effects of elevated RV afterload: hypotensive patients and those with elevated cardiac biomarkers or echocardiographic indices of RV strain, or both, are deemed at increased risk, and thrombolysis is indicated [78].
The management of PH and RV dysfunction in the ICU is challenging. No agreed algorithms exist, although treatment should aim to prevent pulmonary hypertensive crises and acute cor pulmonale [79]. These comprise the spectrum of acute pulmonary vascular dysfunction and may result in cardiovascular collapse due to resulting biventricular failure. Management principles include the following: 1) optimization of RV preload, 2) optimization of RV systolic function, 3) reduction of afterload by reduction of increased PVR, and 4) maintenance of aortic root pressure to ensure sufficient right coronary artery filling pressure (Table 3).
Materials and methods
Systematic review of ICU management of pulmonary vascular and RV dysfunction
We performed a systematic review of the literature over the period from 1980 to 2010, by using set search terms, and the electronic database of the US National Library of Medicine and National Institute of Health (PubMed). After initial identification, abstracts were reviewed for relevance, and appropriate studies were included in the review. Reference lists of relevant articles were hand-searched for further studies and reports. The search was limited to publications in English. Studies were deemed suitable for inclusion according to the criteria listed and where the patient population and study design was defined; and the outcomes were limited to those depending on the specific GRADE question (see Additional file 1). The breakdown of articles obtained by the systematic search is shown (Table 4). After identification, relevant studies were included and subjected to a GRADE analysis [80, 81] to see whether we could make specific management recommendations.
Results and Discussion
ICU management of pulmonary vascular and RV dysfunction
Management of PH with associated RV dysfunction in the ICU setting can be broken down into several treatment goals (Table 3). The first is to ensure adequate but not excessive RV filling or preload in the context of sufficient systemic blood pressure. The second goal is to maximize RV myocardial function, whether with inotropic support, rate or rhythm management, atrioventricular synchronization [82, 83], or by using mechanical devices. The third is to offload the right ventricle by reducing the PVR with pulmonary vasodilators as well as by ensuring adequate oxygenation, avoiding hypercapnia and acidosis, and by minimizing mechanical compression of pulmonary vessels (for example, due to excessive airway plateau pressure). The fourth is to maintain adequate aortic root pressure to allow sufficient right coronary arterial perfusion.
Management of volume and use of vasopressors
Systemic hypotension may relate to sepsis, overdiuresis, or progression of RV failure itself. Principles of volume management and vasopressor use are summarized.
Volume management
With a normal RV, RV ejection fraction is usually primarily dependent on RV preload [84]. In the setting of excessive myocardial distention (by fluids), wall tension increases according to the Frank-Starling mechanism, and muscle fiber length is increased, beyond a certain point at which ventricular function will fail. This situation may be precipitated sooner in the setting of PH and RV dysfunction, in which both hypo- and hypervolemia may reduce cardiac output [78, 85, 86]. In stable patients with PAH, high plasma volumes are associated with worse outcomes [87], but very few clinical studies have been performed in pulmonary vascular dysfunction, and the use of fluid loading remains controversial. Some animal studies show that fluids increase the cardiac index [88]; others show that they worsen shock by inducing RV ischemia or decreasing LV filling or both as the result of ventricular diastolic interdependence (due to an increase in RV volume) [89–91].
In acute cor pulmonale after massive PE, increased filling may be at least initially required [4, 92]. In observational studies in sepsis, up to 40% of patients have evidence of RV failure [93], predominantly due to primary RV dysfunction [7]. These patients have a higher CVP at baseline [94] and are unable to augment stroke volume or perfusion pressure with fluid challenges alone, and so usually also require catecholamines [93, 94].
RV volume overload is a very important principle to recognize and treat promptly in RV failure. It may be identified by a rising V wave on the CVP trace, or by increased TR due to RV overdistention seen at echocardiography. In this situation of "backwards" heart failure, no further escalation of vasoactive agents is likely to be helpful (and may even be harmful), and management involves fluid removal (by using diuresis [95] or hemofiltration [96]) and avoidance of excessive RV afterload [97]. Unmonitored fluid challenges are inadvisable in any setting of RV failure [98, 99].
GRADE RECOMMENDATION 1
Based on overall very-low-quality evidence (see Additional file 1), the following WEAK recommendation is made: Close monitoring of fluid status according to effects on RV function is recommended. Initial carefully monitored limited volume loading may be useful after acute PE, but may also worsen RV performance in some patients with pulmonary vascular dysfunction, and vasoactive agents may be required.
Vasopressors
An essential goal is to maintain systemic blood pressure above pulmonary arterial pressures, thereby preserving right coronary blood flow: unlike left coronary artery perfusion, which occurs only during diastole (as aortic pressure exceeds LV pressure only during this period), perfusion of the right coronary artery usually occurs throughout the cardiac cycle, dominating in systole. It is understood that, as PVR approaches SVR, coronary perfusion will decrease, and if PVR exceeds SVR, coronary filling will occur only in diastole. By augmenting aortic root pressure by using vasopressors in the setting of increased RV afterload, RV ischemia can therefore be reversed [55]. Vasopressors will, however, inevitably have direct effects on the pulmonary circulation as well as myocardial effects (Table 5).
Sympathomimetic pressors
These include the catecholaminergic pressor, norepinephrine, and the noncatecholaminergic pressor phenylephrine. Their complex effects on the pulmonary circulation depend on the dose-related relative α- and β-adrenoreceptor stimulation as well as the degree and nature of RV dysfunction [99, 100]. All may potentially lead to tachydysrhythmias, diastolic dysfunction, myocardial ischemia, hyperlactatemia, and hypercoagulability [101].
Norepinephrine
Norepinephrine (NE) exerts its systemic vasopressor effects through α-1 agonism [102]. Activation of these receptors also causes pulmonary vasoconstriction [102, 103], although the potential adverse effects on PVR are likely to occur only at high doses. Most evidence supporting this comes from animal studies in models of pulmonary vascular dysfunction, with NE at doses less than 0.5 μg/kg/min not increasing PVR [44]. In persistent PH of the newborn, low-dose NE (0.5 μg/kg/min) reduces the PVR/SVR ratio [104]. In adults with septic shock, higher doses of NE increase PVR/SVR, although without worsening RV performance [105]. In patients with sepsis, PH, and associated RV dysfunction, NE increases SVR and improves the RV oxygen supply/demand ratio, although it does not increase RVEF and does increase PVR [106]. Importantly, NE is positively inotropic through β-1 receptor agonism, thus improving RV/pulmonary arterial coupling, CO, and RV performance in studies of acute RV dysfunction due to PH [44, 89, 107–109], illustrated in a case report of acute PH after MVR surgery [110]. In patients with chronic PH, NE reduces the PVR/SVR ratio, although it may not improve CI [100], which may relate to the "fixed" elevation in PVR [99].
Phenylephrine
Phenylephrine (PHE) is a direct α-agonist. Its use improves right coronary perfusion in RV failure [55] without causing tachycardia, although this benefit may be offset by worsening RV function due to increased PVR [100, 108, 111].
GRADE RECOMMENDATION 2
Based on mostly low-quality evidence (see Additional file 1), the following WEAK recommendation is made: NE may be an effective systemic pressor in patients with acute RV dysfunction and RV failure, as it improves RV function both by improving SVR and by increasing CO, despite potential increases in PVR at higher doses.
Nonsympathomimetic pressors: Vasopressin
Arginine vasopressin (AVP) causes systemic vasoconstriction via the vasopressinergic (V1) receptor. Experimental studies have revealed vasodilating properties at low doses that include pulmonary vasodilatation [112] through an NO-dependent mechanism via V1 receptors [113, 114]. This property manifests clinically as a reduction in PVR and PVR/SVR ratio [105, 115, 116]. AVP has also been used as a rescue therapy in patients during PH crises [117–119], in which untreated equalization of systemic and pulmonary pressures may be rapidly fatal. At low doses (0.03-0.067 U/min), it has been used safely in sepsis [105, 120–124], as well as in patients with acute PH and RV failure with hypotension after cardiac surgery [115, 116, 125, 126] and hypotension associated with chronic PH in several settings [117, 118, 127, 128].
AVP leads to a diuretic effect in vasodilatory shock [129], reduces the heart rate [105, 121, 130–132], and induces fewer tachyarrhythmias in comparison to NE [105, 131]. However, bradycardia [133] may be encountered at high clinical doses [134, 135]. AVP may cause dose-related adverse myocardial effects at infusion rates exceeding 0.4 U/min [134, 135], or even above 0.08 U/min in cardiogenic shock [136], which probably relate to direct myocardial effects, including coronary vasoconstriction [132, 137–139].
GRADE RECOMMENDATION 3
Based on mostly low-quality evidence (see Additional file 1), the following WEAK recommendation is made: In patients with vasodilatory shock and pulmonary vascular dysfunction, low-dose AVP may be useful in difficult cases that are resistant to usual treatments, including norepinephrine.
Inotropic augmentation of RV myocardial function
The next major goal is to improve RV myocardial function by using inotropes. The use of mechanical support is discussed later. For sympathomimetic agents, desirable cardiac β1 effects at lower doses maybe offset by chronotropic effects precipitating tachyarrhythmias [140], as well as worsening pulmonary vasoconstriction at higher doses [102] through α-agonism. Systemic hypotension may result from these agents and with phosphodiesterase inhibitors, which may necessitate co-administration of vasopressors.
Inotropes
Sympathomimetic inotropes
Few clinical studies of these agents have been done in patients with PH and RV dysfunction. Dopamine increases CO, although it may cause a mild tachycardia in patients with PH [141] and increase the PVR/SVR ratio [142]. Dopamine also tends to increase the heart rate and to have less-favorable hemodynamic effects in patients with cardiomyopathy than dobutamine [143], although it does not increase PVR at doses up to 10 μg/kg/min in animals with pulmonary vascular dysfunction [144]. In patients with septic shock, PH, and RV dysfunction, dopamine improves CI without an increase in PVR [145]. In the recent large randomized controlled study comparing dopamine with norepinephrine in patients with septic shock, dopamine increased arrhythmic events and, in patients with cardiogenic shock, increased the risk of death [146]. In patients with primary RV dysfunction (without PH) due to septic shock, epinephrine improves RV contractility despite an 11% increase in mPAP [14]. In animal studies, epinephrine reduces the PVR/SVR more than does dopamine [147]. Isoproterenol has been used in RV failure primarily as a chronotrope after cardiac transplantation [148], although it may induce arrhythmias [149].
Dobutamine
At clinical doses up to 5 μg/kg/min in heart failure, dobutamine increases myocardial contractility, reduces PVR and SVR, and induces less tachycardia than does dopamine [143]. It improves RV performance in patients with PH at liver transplantation [150], after RV infarction[151], and is used in PAH exacerbations [152]. It is synergistic with NO in patients with PH [153]. Experimentally, dobutamine has favorable pulmonary vascular effects at lower doses [44, 154], although it leads to increased PVR, tachycardia, and systemic hypotension at doses exceeding 10 μg/kg/min [155]. Given the adverse effects of systemic hypotension in these patients, it is important to anticipate and treat it with vasopressors when using dobutamine.
Inodilators
An inodilator increases myocardial contractility while simultaneously causing systemic and pulmonary vasodilatation. Inodilators include the phosphodiesterase (PDE) III inhibitors and levosimendan.
PDE3 inhibitors
Several types of PDE are recognized: PDEIII usually deactivates intracellular cyclic adenosine monophosphate (cAMP), and PDE3 inhibitors therefore increase cAMP and augment myocardial contractility while dilating the vasculature [156–158]. The selective PDEIII inhibitors include enoximone, milrinone, and amrinone. They are most suited to short-term use because of tachyphylaxis [159], and mild tachycardia is common. Milrinone is most frequently used and has been shown to reduce pulmonary pressures and augment RV function in many studies in patients with pulmonary vascular dysfunction [160–164]. Enoximone improves RV function in pulmonary vascular dysfunction after cardiac surgery [165, 166] and in patients with decompensated chronic obstructive pulmonary disease (COPD) [167]. Enoximone leads to fewer postoperative myocardial infarctions than does dobutamine [168, 169], which may relate to the resulting improved gas exchange when compared with dobutamine and GTN [170]. Concerns regarding platelet aggregation with amrinone [171] do not appear to arise with enoximone [172] or milrinone after cardiac surgery [173, 174]. As with dobutamine, resulting reversible systemic hypotension means that coadministration with pressors is often necessary. Agents such as norepinephrine, phenylephrine or vasopressin are used, with the latter reducing PVR/SVR more than norepinephrine [115]. PDEIII inhibitors may also improve RV function in chronic PH [175].
Nebulized milrinone is increasingly used to manage PH crises in several settings [176–179]. Through pulmonary selectivity, it results in less systemic hypotension and less V/Q mismatch compared with intravenous use in patients with PH after mitral valve replacement surgery [177, 178]. The combination of milrinone-AVP reduces PVR/SVR and may be preferable to milrinone-NE in RV dysfunction [115].
Levosimendan
Levosimendan sensitizes troponin-C to calcium and selectively inhibits PDE III, improving diastolic function and myocardial contractility without increasing oxygen consumption [180–183]. It also acts as a vasodilator through calcium desensitization, potassium channel opening, and PDEIII inhibition [184]. Levosimendan leads to a rapid improvement in hemodynamics, including reduction in PVR in patients with decompensated heart failure [185], with significant benefit on RV efficiency [182], with effects lasting several days [186]. Levosimendan improves RV-PA coupling in experimental acute RV failure [187–189] more than dobutamine [188]. These effects have been shown clinically with improvements in RV function and reduction in PVR in ischemic RV failure [190–194], ARDS [195], and after mitral valve replacement surgery [196, 197]. In chronic PH, repetitive doses reduce mPAP and PVR from baseline and improve SvO2 [198].
GRADE RECOMMENDATION 4
Based on low-moderate-quality evidence (see Additional file 1), a WEAK recommendation can be made that low-dose dobutamine (up to 10 μg/kg/min) improves RV function and may be useful in patients with pulmonary vascular dysfunction, although it may reduce SVR. Dopamine may increase tachyarrhythmias and is not recommended in the setting of cardiogenic shock (STRONG recommendation based on high-quality evidence level).
GRADE RECOMMENDATION 5
Based on mostly moderate-quality evidence (see Additional file 1), a STRONG recommendation can be made that PDE III inhibitors improve RV performance and reduce PVR in patients with acute pulmonary vascular dysfunction, although systemic hypotension is common, usually requiring coadmininstration of pressors. Based on low-quality evidence (see Additional file 1), a WEAK recommendation can be made that inhaled milrinone may be useful to minimize systemic hypotension and V/Q mismatch in pulmonary vascular dysfunction.
GRADE RECOMMENDATION 6
Based on mostly low-quality evidence (see Additional file 1), a WEAK recommendation can be made that levosimendan may be considered for short-term improvements in RV performance in patients with biventricular heart failure.
Reduction of right ventricular afterload
Physiologic coupling between the RV and the pulmonary circulation is a vital form of autoregulation of pulmonary circulatory flow (Figure 2). The RV is even less tolerant of acute changes in afterload than the LV, presumably because of the lower myocardial muscle mass [199]. In sepsis, a reduction in PVR will increase the RV ejection fraction at no additional cost to cardiac output [47], but at levels beyond moderate PH, LV filling may be reduced, and ultimately cardiac output will decrease [199]. Measures to reduce RV afterload may be nonpharmacologic (Table 3) or pharmacologic (Table 6).
Pulmonary vasodilator therapy
Specific pulmonary vasodilators may be useful both to reduce RV afterload and to manipulate hypoxic vasoconstriction in patients with severe hypoxia. Agents are classically subdivided according to their action on the cyclic GMP, prostacyclin, or endothelin pathways [200]. In the nonacute setting, these agents also target remodeling of"resistance" pulmonary vessels and have revolutionized the care of patients with PAH [201]. Importantly, however, the management with pulmonary vasodilators in chronic PH patients differs in several ways from that with acute pulmonary vascular dysfunction, notably in terms of rapid changes in RV volume status, and potential adverse hemodynamic effects of nonselective pulmonary vasodilators in unstable patients.
Pulmonary vasodilators should be used after optimization of RV perfusion and CO. Systemic administration of pulmonary vasodilators may reduce systemic blood pressure [202], potentially reducing RV preload and worsening RV ischemia [86]. Exclusion of a fixed elevated pulmonary venous pressure is important, as increased transpulmonary flow may precipitate pulmonary edema [203, 204]. Furthermore, nonselective actions of vasodilators may result in worsening ventilation/perfusion (V/Q) matching [205]. This risk is reduced with the use of inhaled pulmonary vasodilators, with which the agent will reach vessels in only ventilated lung units [206].
Adenosine
Adenosine increases intracellular cAMP via A2 receptor agonism [207], and when administered intravenously, acts as a potent selective pulmonary vasodilator because of its rapid endothelial metabolism [208]. It has been used as a therapy for adult PH in some settings, including after cardiac surgery [209], but may elevate LV end-diastolic pressure [210] and cause bradycardia and bronchospasm [211]. It is currently therefore recommended as an alternative to NO and prostacyclin in dynamic vasoreactivity studies rather than as treatment for PH [201].
Inhaled nitric oxide
Inhaled nitric oxide (NO) is a potent pulmonary vasodilator with a short half-life due to rapid inactivation by hemoglobin. This minimizes systemic vasodilatation, although it necessitates continuous delivery into the ventilator circuit [206]. NO selectively reduces PVR and improves CO in PAH [212], secondary PH [205, 213, 214], acute PE [215, 216], ischemic RV dysfunction [217, 218], and postsurgical PH [202, 219–234]. NO also improves oxygenation [235], RVEF, and reduces vasopressor requirements in PH after cardiac surgery [236], especially in patients with higher baseline PVR [237], with no augmented effect seen at doses above 10 ppm in these patients [238]. Use of NO (or inhaled PGI2) after mitral valve replacement surgery results in easier weaning from cardiopulmonary bypass and shorter ICU stays [239, 240].
NO has been shown to reduce PVR and improve CO in several studies in patients with acute RV failure due to ARDS [79, 241–246] and to improve oxygenation at lower doses than the RV effects [247]. Administration of NO does need to be continuous for PVR reduction, and a potential exists for worsening oxygenation at excessive doses [248]. The reduction in RV afterload, however, does not correlate with clinical-outcome benefits [249–251]. Similarly, despite short-term improvements in oxygenation in ARDS [252], no studies show a survival benefit [249, 250, 253–257].
NO provides synergistic pulmonary vasodilatation with intravenous prostacyclin [258], inhaled iloprost [259], and oral sildenafil [260, 261]. Limitations include accumulation of toxic metabolites, although this is not usually a clinically significant problem [206]. Rebound PH with RV dysfunction may occur after weaning from NO [262–264], which may be reduced with PDE5 inhibitors [265–270].
Prostanoids
Prostanoids include prostaglandin-I2 (prostacyclin, PGI2) and its analogues, (iloprost) and prostaglandin-E1 (alprostadil, PGE1). An important difference between their formulations is their resulting half-life (Table 6). Prostacyclin is a potent systemic and pulmonary vasodilator, with antiplatelet [271] and antiproliferative effects [272]. In PAH, these agents reduce PVR, increase CO, and improve clinical outcomes [273–279], and are used in patients with NYHA III-IV symptoms [201].
The use of prostanoids is most commonly described in ICU after cardiac surgery or transplantation. Intravenous prostacyclin [18, 280], PGE1 [281–285], inhaled prostacyclin [223, 286–290], and iloprost [291–297] all reduce PVR and improve RV performance in these settings, with inhaled agents being most selective. Intravenous PGE1 may cause marked desaturation in patients with lung disease [205]. Inhaled prostacyclin has short-term equivalence to NO [226], and inhaled iloprost has been shown to be even more effective than NO at acutely reducing PVR and augmenting CO in PH after CPB [298] and in PAH [277]. Inhaled PGI2 also acutely improves pulmonary hemodynamics after acute massive PE [299]. Although PGI2 impairs platelet aggregation, clinical bleeding was not increased in one study [300]. The potential anticoagulant effect should be remembered, however, especially in patients after surgery and receiving concomitant heparin.
In ARDS, intravenous prostacyclin reduces PVR and improves RV function, although it may increase intrapulmonary shunt [301]. Inhaled prostacyclin [302–305] and inhaled PGE1 [306] improve oxygenation and reduce PVR in ARDS, with minimal effects on SVR. NO and intravenous PGI2 have been combined in ARDS with effective reduction of PVR without adverse effects [307].
PDE5 inhibitors
PDE5 inhibitors, including sildenafil and vardenafil, increase downstream cGMP signaling, potentiating the beneficial effects of NO (Figure 4). PDE5 inhibitors acutely reduce PVR [308, 309], and increase CO and reduce PAOP more than does NO [310]. These agents improve clinical end-points in PAH [311], where endothelial NO is reduced [312] and PDE5 expression is upregulated [313, 314]. PDE5 inhibitor may also exert milrinone-like effects through PDEIII inhibition, augmenting RV function [310, 311, 315]. Despite their relative pulmonary selectivity and rapid onset, however, adverse effects may include reduced SVR with potential effects on RV performance [316]. Oral sildenafil has been used to reduce PVR effectively in well-selected patients with PH after cardiac surgery without reducing the SVR [269, 317–319]. Even a single dose may facilitate weaning from NO [266], also without reducing SVR [266–269]. Sildenafil may also improve myocardial perfusion and reduce platelet activation [320] as well as endothelial dysfunction after CPB [321]. Oral sildenafil has been effective in patients with PH due to left ventricular systolic dysfunction, reducing PVR and increasing CO, although reducing the SVR [260]. Sildenafil has also been used in selected patients with PH due to selected cases of chronic respiratory disease without worsening oxygenation or SVR [322, 323]. A single dose of 50 mg nasogastric sildenafil has been studied in a small cohort of consecutive ARDS patients, lowering MAP, and worsening oxygenation due to increased V/Q mismatch, although RV performance did improve [324]. Intravenous sildenafil has been shown to reduce SVR and PVR in end-stage congestive heart failure patients [325], although it is not available commercially, and its use is not licensed in unstable patients (Table 6).
Increased PVR at extremes of lung volumes. This figure represents measurements made in an animal-lobe preparation in which the transmural pressure of the capillaries is held constant. It illustrates that at low lung volumes (as may occur with atelectasis), extraalveolar vessels become narrow, and smooth muscle and elastic fibers in these collapsed vessels increase PVR. At high lung volumes, as alveolar volumes are increased and walls are thinned, capillaries are stretched, reducing their caliber and also increasing PVR. (Adapted from John West's Essential Physiology, 10th edition, Philadelphia: Lippincott & Williams, with permission).
GRADE RECOMMENDATION 7
Based on mostly moderate-quality evidence (see Additional file 1), the following STRONG recommendation is made: pulmonary vasodilators reduce PVR, improve CO and oxygenation, and may be useful when PH and RV dysfunction are present, notably after cardiac surgery.
Based on mostly moderate-quality evidence (see Additional file 1), the ICU side-effect profile of intravenous pulmonary vasodilators may be less favorable than that of inhaled agents. The following STRONG recommendation is therefore made: Consideration should be given to the use of inhaled rather than systemic agents when systemic hypotension is likely, and concomitant vasopressor use should be anticipated.
Based on mostly high-quality evidence (see Additional file 1), the following STRONG recommendation is made: give consideration for the use of NO as a short-term therapy to improve oxygenation indices but not outcome in patients with ARDS. Based on low-quality evidence (see Additional file 1), a WEAK recommendation is made that pulmonary vasodilators may also be useful treat PH associated with RV dysfunction in ARDS.
Based on mostly low-quality evidence (see Additional file 1), the following WEAK recommendation is made: Oral sildenafil may reduce PVR and facilitate weaning from NO after cardiac surgery in selected patients with PH, without adverse effects on systemic blood pressure in well-selected patients.
Nonpharmacologic Management
This encompasses RV "protective" strategies to avoid factors (Table 3) that may further increase PVR. Mechanical devices are also increasingly used to give a failing RV a bridge to recovery or transplantation.
Ventilatory strategies
Important variables that may reduce pulmonary blood flow during ventilation include hypoxia, hypercapnia, and compression of the pulmonary vasculature at the extremes of lung volumes (Figure 4). Acute hypoxia leading to hypoxic pulmonary vasoconstriction is well described [326] and may be augmented by many factors, including acidosis [327]. Acute hypercapnia also leads to pulmonary vasoconstriction [328, 329], although this may be attenuated with NO [330], and, when associated with high PEEP, leads to RV dilatation and reduced cardiac output in severe ARDS [328, 329]. A reduction in pulmonary blood flow occurs both at low volumes, such as in areas of atelectasis, and at high lung volumes, such as with increased airway plateau pressure (Pplat): Increased RV afterload, reduced venous return, and acute RV dysfunction may result [331]. Both atelectasis and ventilation at high lung volumes should therefore be avoided in patients with RV dysfunction.
Before the era of protective ventilatory strategies in ARDS, the incidence of acute RV failure was 60% [332] and has since decreased to 10% to 25% [24]. This is thought to reflect the change in ventilatory practice: lower Pplat reduces the incidence of RV failure [333]. Prone ventilation may also reduce Pplat and pCO2 sufficiently to improve acute RV failure [334]. In ARDS, transition to high-frequency oscillation leads to an increase in CVP and a minor decrease in cardiac output due to preload reduction [335], and RV function may decrease during recruitment maneuvers [336]. In children after Fontan procedures, the hemodynamic effects of negative-pressure ventilation (NPV) are nicely illustrated by measuring pulmonary blood flow: after a switch from conventional intermittent positive pressure ventilation (IPPV) to NPV by using cuirass ventilation, pulmonary blood flow, stroke volume, and cardiac output increased up to 50%, and decreased to baseline when IPPV was reinstituted [337, 338].
Mechanical support
Mechanical support for the RV may be appropriate in reversible settings or as a bridge to definitive treatment. RV-assist devices (RVADs) may be used in primary RV dysfunction [339] and have been used with coexisting PH [340, 341]. There is, however, concern that pulsatile devices may cause pulmonary microcirculatory damage in PH [342, 343]. A pumpless "lung assist" device has been used in patients bridging to transplant [344]. Extracorporeal membrane oxygenation (ECMO) has been used in severe PH [345–348], as a bridge to transplant [349, 350], and after endarterectomy [351] or massive PE [352–355]. Intraaortic balloon counterpulsation (IABP) has been used for RV failure after CPB [356] and transplantation [357], thought to improve CO by augmenting left coronary flow rather than by direct RV effects [358]. Atrial septostomy creates a right-to-left shunt that improves left atrial filling and LV function while reducing RV end-diastolic pressure and improving RV contractility. It is sometimes used as a bridge to transplantation in severe PAH [359], although not in patients with very severe RV failure [360].
GRADE RECOMMENDATION 8
Based on mostly very-low-quality evidence, the following WEAK recommendation is made: Mechanical therapies including ECMO and IABP may have a role as rescue therapies in reversible pulmonary vascular dysfunction or while awaiting definitive treatment.
Conclusions
Pulmonary vascular and right ventricular dysfunction may complicate many ICU illnesses: the diagnosis may be difficult, and the acute management, challenging. Their presence is associated with a worse outcome. This review highlights that some recommendations can be made, despite limitations of the GRADE analysis. However, we do consider that "weak GRADE recommendations" could be interpreted as "management suggestions" and treated with appropriate caution. A further limitation is that several pathologies have been grouped together as one syndrome, although this relates to both the rarity of the syndrome and the lack of high-quality evidence: further research is desperately needed. In particular, only then will we learn whether PAH-targeted therapy such as use of PDE5 inhibitors or endothelin-receptor antagonists, so effective in idiopathic PAH, have a role in the ICU setting.
Key messages
-
Pulmonary hypertension (PH) and associated right ventricular (RV) failure are associated with worse outcomes in critical care, and because of nonspecific presenting symptoms and signs, may be difficult to recognize: echocardiography is a very useful initial test, and invasive monitoring may be helpful in some cases for more continuous monitoring and accurate measurement of pulmonary vascular resistance.
-
Volume loading of the right ventricle may worsen its performance: all fluid challenges should be closely monitored.
-
It is essential to maintain adequate aortic root pressure to prevent the onset of RV ischemia. Vasopressors are useful in this setting, including low-dose norepinephrine as a first-line agent. Low-dose vasopressin may also be useful in some resistant cases but has adverse myocardial effects at higher doses. Potentially useful inotropes in RV failure include dobutamine and those with additional pulmonary vasodilating effects, including PDE III inhibitors, although co-administration with pressors is often necessary. The effects of any vasoactive drug may be unpredictable in an individual and require close clinical observation of circulatory performance, potentially assisted by echocardiography.
-
Pulmonary vasodilators are useful to reduce RV afterload in several ICU settings, including PH and RV failure after cardiac surgery. Systemic administration may worsen systemic hemodynamics and oxygenation because of ventilation-perfusion mismatching.
-
The use of mechanical therapies to manage acute PH and enhance RV performance is expanding, although with evidence currently limited to case series, and may be useful in experienced centers to ameliorate RV failure while awaiting definitive therapy.
Abbreviations
- ACP:
-
acute cor pulmonale
- ARDS:
-
acute respiratory distress syndrome
- AVP:
-
arginine vasopressin
- cAMP:
-
cyclic adenosine 3',5'-cyclic monophosphate
- cGMP:
-
cyclic guanosine 3',5'-cyclic monophosphate
- CI:
-
cardiac index
- CO:
-
cardiac output
- COPD:
-
chronic obstructive pulmonary disease
- CPB:
-
cardiopulmonary bypass
- CVP:
-
central venous pressure
- ECMO:
-
extracorporeal membrane oxygenation
- ICU:
-
intensive care unit
- IABP:
-
intraaortic balloon pump
- LV:
-
left ventricle
- MVR:
-
mitral valve replacement
- NE:
-
norepinephrine
- NO:
-
nitric oxide
- PAC:
-
pulmonary artery catheter
- PAH:
-
pulmonary arterial hypertension
- PAOP:
-
pulmonary arterial occlusion pressure
- PDE:
-
phosphodiesterase
- PE:
-
pulmonary embolism
- PGE1:
-
prostaglandin E1
- PH:
-
pulmonary hypertension
- PHE:
-
phenylephrine
- PVR:
-
pulmonary vascular resistance
- RV:
-
right ventricle
- RVEF:
-
right ventricular ejection fraction
- RVF:
-
right ventricular failure
- SvO2:
-
mixed venous oxygen saturation
- SVR:
-
systemic vascular resistance
- TEE:
-
transesophageal echocardiography
- TR:
-
tricuspid regurgitation
- V/Q mismatch:
-
ventilation/perfusion mismatch.
References
Snow RL, Davies P, Pontoppidan H, Zapol WM, Reid L: Pulmonary vascular remodeling in adult respiratory distress syndrome. Am Rev Respir Dis. 1982, 126: 887-892.
Gillis CN, Pitt BR, Wiedemann HP, Hammond GL: Depressed prostaglandin E1 and 5-hydroxytryptamine removal in patients with adult respiratory distress syndrome. Am Rev Respir Dis. 1986, 134: 739-744.
Greene R, Zapol WM, Snider MT, Reid L, Snow R, O'Connell RS, Novelline RA: Early bedside detection of pulmonary vascular occlusion during acute respiratory failure. Am Rev Respir Dis. 1981, 124: 593-601.
Piazza G, Goldhaber SZ: The acutely decompensated right ventricle: pathways for diagnosis and management. Chest. 2005, 128: 1836-1852. 10.1378/chest.128.3.1836.
Jacobs AK, Leopold JA, Bates E, Mendes LA, Sleeper LA, White H, Davidoff R, Boland J, Modur S, Forman R, Hochman JS: Cardiogenic shock caused by right ventricular infarction: a report from the SHOCK registry. J Am Coll Cardiol. 2003, 41: 1273-1279. 10.1016/S0735-1097(03)00120-7.
Kimchi A, Ellrodt AG, Berman DS, Riedinger MS, Swan HJ, Murata GH: Right ventricular performance in septic shock: a combined radionuclide and hemodynamic study. J Am Coll Cardiol. 1984, 4: 945-951. 10.1016/S0735-1097(84)80055-8.
Parker MM, McCarthy KE, Ognibene FP, Parrillo JE: Right ventricular dysfunction and dilatation, similar to left ventricular changes, characterize the cardiac depression of septic shock in humans. Chest. 1990, 97: 126-131. 10.1378/chest.97.1.126.
Rubin LJ: Primary pulmonary hypertension. N Engl J Med. 1997, 336: 111-117. 10.1056/NEJM199701093360207.
Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E: Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985, 6: 359-365. 10.1016/S0735-1097(85)80172-8.
Dabestani A, Mahan G, Gardin JM, Takenaka K, Burn C, Allfie A, Henry WL: Evaluation of pulmonary artery pressure and resistance by pulsed Doppler echocardiography. Am J Cardiol. 1987, 59: 662-668. 10.1016/0002-9149(87)91189-1.
Bossone E, Bodini BD, Mazza A, Allegra L: Pulmonary arterial hypertension: the key role of echocardiography. Chest. 2005, 127: 1836-1843. 10.1378/chest.127.5.1836.
Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PM: Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006, 174: 1034-1041. 10.1164/rccm.200604-547OC.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ: Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005, 18: 1440-1463. 10.1016/j.echo.2005.10.005.
Le Tulzo Y, Seguin P, Gacouin A, Camus C, Suprin E, Jouannic I, Thomas R: Effects of epinephrine on right ventricular function in patients with severe septic shock and right ventricular failure: a preliminary descriptive study. Intensive Care Med. 1997, 23: 664-670. 10.1007/s001340050391.
Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F: Echo-Doppler demonstration of acute cor pulmonale at the bedside in the medical intensive care unit. Am J Respir Crit Care Med. 2002, 166: 1310-1319. 10.1164/rccm.200202-146CC.
Jardin F, Dubourg O, Bourdarias JP: Echocardiographic pattern of acute cor pulmonale. Chest. 1997, 111: 209-217. 10.1378/chest.111.1.209.
Fischer LG, Van Aken H, Burkle H: Management of pulmonary hypertension: physiological and pharmacological considerations for anesthesiologists. Anesth Analg. 2003, 96: 1603-1616. 10.1213/01.ANE.0000062523.67426.0B.
Ocal A, Kiris I, Erdinc M, Peker O, Yavuz T, Ibrisim E: Efficiency of prostacyclin in the treatment of protamine-mediated right ventricular failure and acute pulmonary hypertension. Tohoku J Exp Med. 2005, 207: 51-58. 10.1620/tjem.207.51.
Sibbald WJ, Paterson NA, Holliday RL, Anderson RA, Lobb TR, Duff JH: Pulmonary hypertension in sepsis: measurement by the pulmonary arterial diastolic-pulmonary wedge pressure gradient and the influence of passive and active factors. Chest. 1978, 73: 583-591. 10.1378/chest.73.5.583.
Wort SJ, Evans TW: The role of the endothelium in modulating vascular control in sepsis and related conditions. Br Med Bull. 1999, 55: 30-48. 10.1258/0007142991902286.
Albertini M, Clement MG, Hussain SN: Role of endothelin ETA receptors in sepsis-induced mortality, vascular leakage, and tissue injury in rats. Eur J Pharmacol. 2003, 474: 129-135. 10.1016/S0014-2999(03)02037-5.
Rossi P, Persson B, Boels PJ, Arner A, Weitzberg E, Oldner A: Endotoxemic pulmonary hypertension is largely mediated by endothelin-induced venous constriction. Intensive Care Med. 2008, 34: 873-880. 10.1007/s00134-007-0980-9.
Osman D, Monnet X, Castelain V, Anguel N, Warszawski J, Teboul JL, Richard C: Incidence and prognostic value of right ventricular failure in acute respiratory distress syndrome. Intensive Care Med. 2009, 35: 69-76. 10.1007/s00134-008-1307-1.
Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page B, Beauchet A, Jardin F: Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med. 2001, 29: 1551-1555. 10.1097/00003246-200108000-00009.
Vieillard-Baron A, Page B, Augarde R, Prin S, Qanadli S, Beauchet A, Dubourg O, Jardin F: Acute cor pulmonale in massive pulmonary embolism: incidence, echocardiographic pattern, clinical implications and recovery rate. Intensive Care Med. 2001, 27: 1481-1486. 10.1007/s001340101032.
Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L: Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J. 1997, 134: 479-487. 10.1016/S0002-8703(97)70085-1.
Kasper W, Konstantinides S, Geibel A, Tiede N, Krause T, Just H: Prognostic significance of right ventricular afterload stress detected by echocardiography in patients with clinically suspected pulmonary embolism. Heart. 1997, 77: 346-349.
Clowes GH, Farrington GH, Zuschneid W, Cossette GR, Saravis C: Circulating factors in the etiology of pulmonary insufficiency and right heart failure accompanying severe sepsis (peritonitis). Ann Surg. 1970, 171: 663-678. 10.1097/00000658-197005000-00005.
Monchi M, Bellenfant F, Cariou A, Joly LM, Thebert D, Laurent I, Dhainaut JF, Brunet F: Early predictive factors of survival in the acute respiratory distress syndrome: a multivariate analysis. Am J Respir Crit Care Med. 1998, 158: 1076-1081.
Squara P, Dhainaut JF, Artigas A, Carlet J: Hemodynamic profile in severe ARDS: results of the European Collaborative ARDS Study. Intensive Care Med. 1998, 24: 1018-1028. 10.1007/s001340050710.
Leeman M: Pulmonary hypertension in acute respiratory distress syndrome. Monaldi Arch Chest Dis. 1999, 54: 146-149.
Ramakrishna G, Sprung J, Ravi BS, Chandrasekaran K, McGoon MD: Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortality. J Am Coll Cardiol. 2005, 45: 1691-1699. 10.1016/j.jacc.2005.02.055.
Minai OA, Venkateshiah SB, Arroliga AC: Surgical intervention in patients with moderate to severe pulmonary arterial hypertension. Conn Med. 2006, 70: 239-243.
Lai HC, Lai HC, Wang KY, Lee WL, Ting CT, Liu TJ: Severe pulmonary hypertension complicates postoperative outcome of non-cardiac surgery. Br J Anaesth. 2007, 99: 184-190. 10.1093/bja/aem126.
Krowka MJ, Plevak DJ, Findlay JY, Rosen CB, Wiesner RH, Krom RA: Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl. 2000, 6: 443-450. 10.1053/jlts.2000.6356.
Price LC, Montani D, Jais X, Dick JR, Simonneau G, Sitbon O, Mercier FJ, Humbert M: Non-cardiothoracic non-obstetric surgery in mild-moderate pulmonary hypertension: perioperative management of 28 consecutive individual cases. Eur Respir J. 2010, 35: 1294-1302. 10.1183/09031936.00113009.
Bonnin M, Mercier FJ, Sitbon O, Roger-Christoph S, Jais X, Humbert M, Audibert F, Frydman R, Simonneau G, Benhamou D: Severe pulmonary hypertension during pregnancy: mode of delivery and anesthetic management of 15 consecutive cases. Anesthesiology. 2005, 102: 1133-1137. 10.1097/00000542-200506000-00012. discussion 1135A-1136A
Bedard E, Dimopoulos K, Gatzoulis MA: Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension?. Eur Heart J. 2009, 30: 256-265. 10.1093/eurheartj/ehn597.
Bernstein AD, Parsonnet V: Bedside estimation of risk as an aid for decision-making in cardiac surgery. Ann Thorac Surg. 2000, 69: 823-828. 10.1016/S0003-4975(99)01424-1.
Subramaniam K, Yared JP: Management of pulmonary hypertension in the operating room. Semin Cardiothorac Vasc Anesth. 2007, 11: 119-136. 10.1177/1089253207301733.
Morgan JA, John R, Lee BJ, Oz MC, Naka Y: Is severe right ventricular failure in left ventricular assist device recipients a risk factor for unsuccessful bridging to transplant and post-transplant mortality. Ann Thorac Surg. 2004, 77: 859-863. 10.1016/j.athoracsur.2003.09.048.
Price LC, Montani D, Jais X, Dick JR, Simonneau G, Sitbon O, Mercier FJ, Humbert M: Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 35: 1294-1302. 10.1183/09031936.00113009.
McIntyre KM, Sasahara AA: Determinants of right ventricular function and hemodynamics after pulmonary embolism. Chest. 1974, 65: 534-543. 10.1378/chest.65.5.534.
Kerbaul F, Rondelet B, Motte S, Fesler P, Hubloue I, Ewalenko P, Naeije R, Brimioulle S: Effects of norepinephrine and dobutamine on pressure load-induced right ventricular failure. Crit Care Med. 2004, 32: 1035-1040. 10.1097/01.CCM.0000120052.77953.07.
Nath J, Foster E, Heidenreich PA: Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004, 43: 405-409. 10.1016/j.jacc.2003.09.036.
Pinsky MR: Heart-lung interactions. Curr Opin Crit Care. 2007, 13: 528-531. 10.1097/MCC.0b013e3282efad97.
Sibbald WJ, Driedger AA: Right ventricular function in acute disease states: pathophysiologic considerations. Crit Care Med. 1983, 11: 339-345. 10.1097/00003246-198305000-00004.
Pinsky MR: Recent advances in the clinical application of heart-lung interactions. Curr Opin Crit Care. 2002, 8: 26-31. 10.1097/00075198-200202000-00005.
Stojnic BB, Brecker SJ, Xiao HB, Helmy SM, Mbaissouroum M, Gibson DG: Left ventricular filling characteristics in pulmonary hypertension: a new mode of ventricular interaction. Br Heart J. 1992, 68: 16-20. 10.1136/hrt.68.7.16.
Taylor RR, Covell JW, Sonnenblick EH, Ross J: Dependence of ventricular distensibility on filling of the opposite ventricle. Am J Physiol. 1967, 213: 711-718.
Fellahi JL, Valtier B, Beauchet A, Bourdarias JP, Jardin F: Does positive end-expiratory pressure ventilation improve left ventricular function? A comparative study by transesophageal echocardiography in cardiac and noncardiac patients. Chest. 1998, 114: 556-562. 10.1378/chest.114.2.556.
Louie EK, Lin SS, Reynertson SI, Brundage BH, Levitsky S, Rich S: Pressure and volume loading of the right ventricle have opposite effects on left ventricular ejection fraction. Circulation. 1995, 92: 819-824.
Louie EK, Rich S, Brundage BH: Doppler echocardiographic assessment of impaired left ventricular filling in patients with right ventricular pressure overload due to primary pulmonary hypertension. J Am Coll Cardiol. 1986, 8: 1298-1306. 10.1016/S0735-1097(86)80300-X.
Ricciardi MJ, Bossone E, Bach DS, Armstrong WF, Rubenfire M: Echocardiographic predictors of an adverse response to a nifedipine trial in primary pulmonary hypertension: diminished left ventricular size and leftward ventricular septal bowing. Chest. 1999, 116: 1218-1223. 10.1378/chest.116.5.1218.
Vlahakes GJ, Turley K, Hoffman JI: The pathophysiology of failure in acute right ventricular hypertension: hemodynamic and biochemical correlations. Circulation. 1981, 63: 87-95.
Weitzenblum E: Chronic cor pulmonale. Heart. 2003, 89: 225-230. 10.1136/heart.89.2.225.
Blaise G, Langleben D, Hubert B: Pulmonary arterial hypertension: pathophysiology and anesthetic approach. Anesthesiology. 2003, 99: 1415-1432. 10.1097/00000542-200312000-00027.
Chin KM, Kim NH, Rubin LJ: The right ventricle in pulmonary hypertension. Coron Artery Dis. 2005, 16: 13-18. 10.1097/00019501-200502000-00003.
Hadian M, Pinsky MR: Evidence-based review of the use of the pulmonary artery catheter impact data and complications. Crit Care. 2006, 10 Suppl 3: S8-10.1186/cc4834.
Handa F, Kyo SE, Miyao H: Reduction in the use of pulmonary artery catheter for cardiovascular surgery. Masui. 2003, 52: 420-423.
Mebazaa A, Pitsis AA, Rudiger A, Toller W, Longrois D, Ricksten SE, Bobek I, De Hert S, Wieselthaler G, Schirmer U, von Segesser LK, Sander M, Poldermans D, Ranucci M, Karpati PC, Wouters P, Seeberger M, Schmid ER, Weder W, Follath F: Clinical review: practical recommendations on the management of perioperative heart failure in cardiac surgery. Crit Care. 2010, 14: 201-10.1186/cc8153.
Zapol WM, Kobayashi K, Snider MT, Greene R, Laver MB: Vascular obstruction causes pulmonary hypertension in severe acute respiratory failure. Chest. 1977, 71: 306-307.
Vieillard-Baron A: Assessment of right ventricular function. Curr Opin Crit Care. 2009, 15: 254-260. 10.1097/MCC.0b013e32832b70c9.
Gadhinglajkar S, Sreedhar R, Jayakumar K, Misra M, Ganesh S, Panicker V: Intra-operative assessment of biventricular function using trans-esophageal echocardiography pre/post-pulmonary thromboembolectomy in patient with chronic thromboembolic pulmonary hypertension. Ann Card Anaesth. 2009, 12: 140-145. 10.4103/0971-9784.53449.
Serra E, Feltracco P, Barbieri S, Forti A, Ori C: Transesophageal echocardiography during lung transplantation. Transplant Proc. 2007, 39: 1981-1982. 10.1016/j.transproceed.2007.05.004.
Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, Reeder GS, Nishimura RA, Tajik AJ: Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985, 6: 750-756. 10.1016/S0735-1097(85)80477-0.
Yock PG, Popp RL: Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984, 70: 657-662.
Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, Kakishita M, Fukushima K, Okano Y, Nakanishi N, Miyatake K, Kangawa K: Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000, 102: 865-870.
Logeart D, Lecuyer L, Thabut G, Tabet JY, Tartiere JM, Chavelas C, Bonnin F, Stievenart JL, Solal AC: Biomarker-based strategy for screening right ventricular dysfunction in patients with non-massive pulmonary embolism. Intensive Care Med. 2007, 33: 286-292. 10.1007/s00134-006-0482-1.
Lega JC, Lacasse Y, Lakhal L, Provencher S: Natriuretic peptides and troponins in pulmonary embolism: a meta-analysis. Thorax. 2009, 64: 869-875. 10.1136/thx.2008.110965.
Mehta NJ, Jani K, Khan IA: Clinical usefulness and prognostic value of elevated cardiac troponin I levels in acute pulmonary embolism. Am Heart J. 2003, 145: 821-825. 10.1016/S0002-8703(02)94704-6.
Clark BJ, Brown NJ, Moss M, Bull TM: Increased serum BNP concentrations are associated with pulmonary vascular dysfunction in patients with acute lung injury. Am J Respir Crit Care Med. 2010, 181: A2582-
Alpert JS, Smith R, Carlson J, Ockene IS, Dexter L, Dalen JE: Mortality in patients treated for pulmonary embolism. JAMA. 1976, 236: 1477-1480. 10.1001/jama.236.13.1477.
Goldhaber SZ, Haire WD, Feldstein ML, Miller M, Toltzis R, Smith JL, Taveira da Silva AM, Come PC, Lee RT, Parker JA: Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet. 1993, 341: 507-511. 10.1016/0140-6736(93)90274-K.
Goldhaber SZ, Morpurgo M: Diagnosis, treatment, and prevention of pulmonary embolism: report of the WHO/International Society and Federation of Cardiology Task Force. JAMA. 1992, 268: 1727-1733. 10.1001/jama.268.13.1727.
Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, Albanese P, Biasiolo A, Pegoraro C, Iliceto S, Prandoni P: Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004, 350: 2257-2264. 10.1056/NEJMoa032274.
Dalen JE, Alpert JS: Natural history of pulmonary embolism. Prog Cardiovasc Dis. 1975, 17: 259-270. 10.1016/S0033-0620(75)80017-X.
Lualdi JC, Goldhaber SZ: Right ventricular dysfunction after acute pulmonary embolism: pathophysiologic factors detection, and therapeutic implications. Am Heart J. 1995, 130: 1276-1282. 10.1016/0002-8703(95)90155-8.
Bhorade S, Christenson J, O'Connor M, Lavoie A, Pohlman A, Hall JB: Response to inhaled nitric oxide in patients with acute right heart syndrome. Am J Respir Crit Care Med. 1999, 159: 571-579.
Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ: What is "quality of evidence" and why is it important to clinicians?. BMJ. 2008, 336: 995-998. 10.1136/bmj.39490.551019.BE.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008, 336: 924-926. 10.1136/bmj.39489.470347.AD.
Gan CT, Lankhaar JW, Marcus JT, Westerhof N, Marques KM, Bronzwaer JG, Boonstra A, Postmus PE, Vonk-Noordegraaf A: Impaired left ventricular filling due to right-to-left ventricular interaction in patients with pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2006, 290: H1528-H1533.
Zamanian RT, Haddad F, Doyle RL, Weinacker AB: Management strategies for patients with pulmonary hypertension in the intensive care unit. Crit Care Med. 2007, 35: 2037-2050. 10.1097/01.CCM.0000280433.74246.9E.
Reuse C, Vincent JL, Pinsky MR: Measurements of right ventricular volumes during fluid challenge. Chest. 1990, 98: 1450-1454. 10.1378/chest.98.6.1450.
Naeije R, Vachiery JL: Medical therapy of pulmonary hypertension conventional therapies. Clin Chest Med. 2001, 22: 517-527. 10.1016/S0272-5231(05)70288-4.
Layish DT, Tapson VF: Pharmacologic hemodynamic support in massive pulmonary embolism. Chest. 1997, 111: 218-224. 10.1378/chest.111.1.218.
James KB, Stelmach K, Armstrong R, Young JB, Fouad-Tarazi F: Plasma volume and outcome in pulmonary hypertension. Tex Heart Inst J. 2003, 30: 305-307.
Mathru M, Venus B, Smith RA, Shirakawa Y, Sugiura A: Treatment of low cardiac output complicating acute pulmonary hypertension in normovolemic goats. Crit Care Med. 1986, 14: 120-124. 10.1097/00003246-198602000-00008.
Molloy WD, Lee KY, Girling L, Schick U, Prewitt RM: Treatment of shock in a canine model of pulmonary embolism. Am Rev Respir Dis. 1984, 130: 870-874.
Ghignone M, Girling L, Prewitt RM: Volume expansion versus norepinephrine in treatment of a low cardiac output complicating an acute increase in right ventricular afterload in dogs. Anesthesiology. 1984, 60: 132-135. 10.1097/00000542-198402000-00009.
Belenkie I, Dani R, Smith ER, Tyberg JV: Effects of volume loading during experimental acute pulmonary embolism. Circulation. 1989, 80: 178-188.
Mercat A, Diehl JL, Meyer G, Teboul JL, Sors H: Hemodynamic effects of fluid loading in acute massive pulmonary embolism. Crit Care Med. 1999, 27: 540-544. 10.1097/00003246-199903000-00032.
Redl G, Germann P, Plattner H, Hammerle A: Right ventricular function in early septic shock states. Intensive Care Med. 1993, 19: 3-7. 10.1007/BF01709270.
Schneider AJ, Teule GJ, Groeneveld AB, Nauta J, Heidendal GA, Thijs LG: Biventricular performance during volume loading in patients with early septic shock, with emphasis on the right ventricle: a combined hemodynamic and radionuclide study. Am Heart J. 1988, 116: 103-112. 10.1016/0002-8703(88)90256-6.
Siva A, Shah AM: Moderate mitral stenosis in pregnancy: the haemodynamic impact of diuresis. Heart. 2005, 91: e3-10.1136/hrt.2004.053017.
Ducas J, Prewitt RM: Pathophysiology and therapy of right ventricular dysfunction due to pulmonary embolism. Cardiovasc Clin. 1987, 17: 191-202.
Mebazaa A, Karpati P, Renaud E, Algotsson L: Acute right ventricular failure: from pathophysiology to new treatments. Intensive Care Med. 2004, 30: 185-196. 10.1007/s00134-003-2025-3.
Goldhaber SZ: The approach to massive pulmonary embolism. Semin Respir Crit Care Med. 2000, 21: 555-561. 10.1055/s-2000-13184.
Forrest P: Anaesthesia and right ventricular failure. Anaesth Intensive Care. 2009, 37: 370-385.
Kwak YL, Lee CS, Park YH, Hong YW: The effect of phenylephrine and norepinephrine in patients with chronic pulmonary hypertension. Anaesthesia. 2002, 57: 9-14. 10.1046/j.1365-2044.2002.02324.x.
Dunser MW, Hasibeder WR: Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med. 2009, 24: 293-316. 10.1177/0885066609340519.
Bergofsky EH: Humoral control of the pulmonary circulation. Annu Rev Physiol. 1980, 42: 221-233. 10.1146/annurev.ph.42.030180.001253.
Hanson EL, O'Connor NE, Drinker PA: Hemodynamic response to controlled ventilation during hypoxia in man and animals. Surg Forum. 1972, 23: 207-209.
Tourneux P, Rakza T, Bouissou A, Krim G, Storme L: Pulmonary circulatory effects of norepinephrine in newborn infants with persistent pulmonary hypertension. J Pediatr. 2008, 153: 345-349. 10.1016/j.jpeds.2008.03.007.
Morelli A, Ertmer C, Rehberg S, Lange M, Orecchioni A, Cecchini V, Bachetoni A, D'Alessandro M, Van Aken H, Pietropaoli P, Westphal M: Continuous terlipressin versus vasopressin infusion in septic shock (TERLIVAP): a randomized, controlled pilot study. Crit Care. 2009, 13: R130-10.1186/cc7990.
Schreuder WO, Schneider AJ, Groeneveld AB, Thijs LG: Effect of dopamine vs norepinephrine on hemodynamics in septic shock: emphasis on right ventricular performance. Chest. 1989, 95: 1282-1288. 10.1378/chest.95.6.1282.
Ducas J, Duval D, Dasilva H, Boiteau P, Prewitt RM: Treatment of canine pulmonary hypertension: effects of norepinephrine and isoproterenol on pulmonary vascular pressure-flow characteristics. Circulation. 1987, 75: 235-242.
Hirsch LJ, Rooney MW, Wat SS, Kleinmann B, Mathru M: Norepinephrine and phenylephrine effects on right ventricular function in experimental canine pulmonary embolism. Chest. 1991, 100: 796-801. 10.1378/chest.100.3.796.
Martin C, Perrin G, Saux P, Papazian L, Gouin F: function Effects of norepinephrine on right ventricular in septic shock patients. Intensive Care Med. 1994, 20: 444-447. 10.1007/BF01710657.
Bertolissi M, Bassi F, Da Broi U: Norepinephrine can be useful for the treatment of right ventricular failure combined with acute pulmonary hypertension and systemic hypotension: a case report. Minerva Anestesiol. 2001, 67: 79-84.
Rich S, Gubin S, Hart K: The effects of phenylephrine on right ventricular performance in patients with pulmonary hypertension. Chest. 1990, 98: 1102-1106. 10.1378/chest.98.5.1102.
Eichinger MR, Walker BR: Enhanced pulmonary arterial dilation to arginine vasopressin in chronically hypoxic rats. Am J Physiol. 1994, 267: H2413-H2419.
Walker BR, Haynes J, Wang HL, Voelkel NF: Vasopressin-induced pulmonary vasodilation in rats. Am J Physiol. 1989, 257: H415-H422.
Evora PR, Pearson PJ, Schaff HV: Arginine vasopressin induces endothelium-dependent vasodilatation of the pulmonary artery: V1-receptor-mediated production of nitric oxide. Chest. 1993, 103: 1241-1245. 10.1378/chest.103.4.1241.
Jeon Y, Ryu JH, Lim YJ, Kim CS, Bahk JH, Yoon SZ, Choi JY: Comparative hemodynamic effects of vasopressin and norepinephrine after milrinone-induced hypotension in off-pump coronary artery bypass surgical patients. Eur J Cardiothorac Surg. 2006, 29: 952-956. 10.1016/j.ejcts.2006.02.032.
Tayama E, Ueda T, Shojima T, Akasu K, Oda T, Fukunaga S, Akashi H, Aoyagi S: Arginine vasopressin is an ideal drug after cardiac surgery for the management of low systemic vascular resistant hypotension concomitant with pulmonary hypertension. Interact Cardiovasc Thorac Surg. 2007, 6: 715-719. 10.1510/icvts.2007.159624.
Braun EB, Palin CA, Hogue CW: Vasopressin during spinal anesthesia in a patient with primary pulmonary hypertension treated with intravenous epoprostenol. Anesth Analg. 2004, 99: 36-37. 10.1213/01.ANE.0000121349.15880.DC.
Price LC, Forrest P, Sodhi V, Adamson DL, Nelson-Piercy C, Lucey M, Howard LS: Use of vasopressin after caesarean section in idiopathic pulmonary arterial hypertension. Br J Anaesth. 2007, 99: 552-555. 10.1093/bja/aem180.
Smith AM, Elliot CM, Kiely DG, Channer KS: The role of vasopressin in cardiorespiratory arrest and pulmonary hypertension. QJM. 2006, 99: 127-133. 10.1093/qjmed/hcl009.
Russell JA, Walley KR, Singer J, Gordon AC, Hebert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM, Cook DJ, Presneill JJ, Ayers D: Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008, 358: 877-887. 10.1056/NEJMoa067373.
Torgersen C, Dunser MW, Wenzel V, Jochberger S, Mayr V, Schmittinger CA, Lorenz I, Schmid S, Westphal M, Grander W, Luckner G: Comparing two different arginine vasopressin doses in advanced vasodilatory shock: a randomized, controlled, open-label trial. Intensive Care Med. 2010, 36: 57-65. 10.1007/s00134-009-1630-1.
Luckner G, Mayr VD, Jochberger S, Wenzel V, Ulmer H, Hasibeder WR, Dunser MW: Comparison of two dose regimens of arginine vasopressin in advanced vasodilatory shock. Crit Care Med. 2007, 35: 2280-2285. 10.1097/01.CCM.0000281853.50661.23.
Gold J, Cullinane S, Chen J, Seo S, Oz MC, Oliver JA, Landry DW: Vasopressin in the treatment of milrinone-induced hypotension in severe heart failure. Am J Cardiol. 2000, 85: 506-508. 10.1016/S0002-9149(99)00783-3. A511
Argenziano M, Choudhri AF, Oz MC, Rose EA, Smith CR, Landry DW: A prospective randomized trial of arginine vasopressin in the treatment of vasodilatory shock after left ventricular assist device placement. Circulation. 1997, 96: II-286-II-290.
Vida VL, Mack R, Castaneda AR: The role of vasopressin in treating systemic inflammatory syndrome complicated by right ventricular failure. Cardiol Young. 2005, 15: 88-90. 10.1017/S1047951105000193.
Scheurer MA, Bradley SM, Atz AM: Vasopressin to attenuate pulmonary hypertension and improve systemic blood pressure after correction of obstructed total anomalous pulmonary venous return. J Thorac Cardiovasc Surg. 2005, 129: 464-466. 10.1016/j.jtcvs.2004.06.043.
Wang HJ, Wong CS, Chiang CY, Yeh CC, Cherng CH, Ho ST, Wu CT: Low-dose vasopressin infusion can be an alternative in treating patients with refractory septic shock combined with chronic pulmonary hypertension: a case report. Acta Anaesthesiol Sin. 2003, 41: 77-80.
Jain RL, Horn EM: Peripheral vasodilation complicated by pulmonary hypertension: a role for vasopressin. Am J Respir Crit Care Med. 2008, 177: A919-
Holmes CL, Walley KR, Chittock DR, Lehman T, Russell JA: The effects of vasopressin on hemodynamics and renal function in severe septic shock: a case series. Intensive Care Med. 2001, 27: 1416-1421. 10.1007/s001340101014.
Dunser MW, Mayr AJ, Ulmer H, Knotzer H, Sumann G, Pajk W, Friesenecker B, Hasibeder WR: Arginine vasopressin in advanced vasodilatory shock: a prospective, randomized, controlled study. Circulation. 2003, 107: 2313-2319. 10.1161/01.CIR.0000066692.71008.BB.
Dunser MW, Mayr AJ, Stallinger A, Ulmer H, Ritsch N, Knotzer H, Pajk W, Mutz NJ, Hasibeder WR: Cardiac performance during vasopressin infusion in postcardiotomy shock. Intensive Care Med. 2002, 28: 746-751. 10.1007/s00134-002-1265-y.
Lauzier F, Levy B, Lamarre P, Lesur O: Vasopressin or norepinephrine in early hyperdynamic septic shock: a randomized clinical trial. Intensive Care Med. 2006, 32: 1782-1789. 10.1007/s00134-006-0378-0.
Varma S, Jaju BP, Bhargava KP: Mechanism of vasopressin-induced bradycardia in dogs. Circ Res. 1969, 24: 787-792.
Naeije R, Hallemans R, Mols P, Melot C, Reding P: Effect of vasopressin and somatostatin on hemodynamics and blood gases in patients with liver cirrhosis. Crit Care Med. 1982, 10: 578-582. 10.1097/00003246-198209000-00004.
Mols P, Hallemans R, Van Kuyk M, Melot C, Lejeune P, Ham H, Vertongen F, Naeije R: Hemodynamic effects of vasopressin, alone and in combination with nitroprusside, in patients with liver cirrhosis and portal hypertension. Ann Surg. 1984, 199: 176-181. 10.1097/00000658-198402000-00008.
Migotto WH, Dahi H: Effects of vasopressin on hemodynamics in cardiogenic shock. Chest. 2005, 128: 168S-
Walker BR, Childs ME, Adams EM: Direct cardiac effects of vasopressin: role of V1- and V2-vasopressinergic receptors. Am J Physiol. 1988, 255: H261-H265.
Leather HA, Segers P, Berends N, Vandermeersch E, Wouters PF: Effects of vasopressin on right ventricular function in an experimental model of acute pulmonary hypertension. Crit Care Med. 2002, 30: 2548-2552. 10.1097/00003246-200211000-00024.
Indrambarya T, Boyd JH, Wang Y, McConechy M, Walley KR: Low-dose vasopressin infusion results in increased mortality and cardiac dysfunction following ischemia-reperfusion injury in mice. Crit Care. 2009, 13: R98-10.1186/cc7930.
Tisdale JE, Patel R, Webb CR, Borzak S, Zarowitz BJ: Electrophysiologic and proarrhythmic effects of intravenous inotropic agents. Prog Cardiovasc Dis. 1995, 38: 167-180. 10.1016/S0033-0620(05)80005-2.
Holloway EL, Polumbo RA, Harrison DC: Acute circulatory effects of dopamine in patients with pulmonary hypertension. Br Heart J. 1975, 37: 482-485. 10.1136/hrt.37.5.482.
Liet JM, Boscher C, Gras-Leguen C, Gournay V, Debillon T, Roze JC: Dopamine effects on pulmonary artery pressure in hypotensive preterm infants with patent ductus arteriosus. J Pediatr. 2002, 140: 373-375. 10.1067/mpd.2002.123100.
Leier CV, Heban PT, Huss P, Bush CA, Lewis RP: Comparative systemic and regional hemodynamic effects of dopamine and dobutamine in patients with cardiomyopathic heart failure. Circulation. 1978, 58: 466-475.
Lejeune P, Naeije R, Leeman M, Melot C, Deloof T, Delcroix M: Effects of dopamine and dobutamine on hyperoxic and hypoxic pulmonary vascular tone in dogs. Am Rev Respir Dis. 1987, 136: 29-35.
Schreuder WO, Schneider AJ, Groeneveld AB, Thijs LG: The influence of catecholamines on right ventricular function in septic shock. Intensive Care Med. 1988, 14 Suppl 2: 492-495.
De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL: Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010, 362: 779-789. 10.1056/NEJMoa0907118.
Barrington KJ, Finer NN, Chan WK: A blind, randomized comparison of the circulatory effects of dopamine and epinephrine infusions in the newborn piglet during normoxia and hypoxia. Crit Care Med. 1995, 23: 740-748. 10.1097/00003246-199504000-00024.
Stobierska-Dzierzek B, Awad H, Michler RE: The evolving management of acute right-sided heart failure in cardiac transplant recipients. J Am Coll Cardiol. 2001, 38: 923-931. 10.1016/S0735-1097(01)01486-3.
Prielipp RC, McLean R, Rosenthal MH, Pearl RG: Hemodynamic profiles of prostaglandin E1, isoproterenol, prostacyclin, and nifedipine in experimental porcine pulmonary hypertension. Crit Care Med. 1991, 19: 60-67. 10.1097/00003246-199101000-00016.
Acosta F, Sansano T, Palenciano CG, Falcon L, Domenech P, Robles R, Bueno FS, Ramirez P, Parrilla P: Effects of dobutamine on right ventricular function and pulmonary circulation in pulmonary hypertension during liver transplantation. Transplant Proc. 2005, 37: 3869-3870. 10.1016/j.transproceed.2005.10.045.
Ferrario M, Poli A, Previtali M, Lanzarini L, Fetiveau R, Diotallevi P, Mussini A, Montemartini C: Hemodynamics of volume loading compared with dobutamine in severe right ventricular infarction. Am J Cardiol. 1994, 74: 329-333. 10.1016/0002-9149(94)90398-0.
Sztrymf B, Souza R, Bertoletti L, Jais X, Sitbon O, Price LC, Simonneau G, Humbert M: Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J. 2010, 35: 1286-1293. 10.1183/09031936.00070209.
Vizza CD, Rocca GD, Roma AD, Iacoboni C, Pierconti F, Venuta F, Rendina E, Schmid G, Pietropaoli P, Fedele F: Acute hemodynamic effects of inhaled nitric oxide, dobutamine and a combination of the two in patients with mild to moderate secondary pulmonary hypertension. Crit Care. 2001, 5: 355-361. 10.1186/cc1069.
Pagnamenta A, Fesler P, Vandinivit A, Brimioulle S, Naeije R: Pulmonary vascular effects of dobutamine in experimental pulmonary hypertension. Crit Care Med. 2003, 31: 1140-1146. 10.1097/01.CCM.0000060126.75746.32.
Bradford KK, Deb B, Pearl RG: Combination therapy with inhaled nitric oxide and intravenous dobutamine during pulmonary hypertension in the rabbit. J Cardiovasc Pharmacol. 2000, 36: 146-151. 10.1097/00005344-200008000-00002.
Honerjager P: Pharmacology of bipyridine phosphodiesterase III inhibitors. Am Heart J. 1991, 121: 1939-1944. 10.1016/0002-8703(91)90828-6.
Young RA, Ward A: Milrinone. A preliminary review of its pharmacological properties and therapeutic use. Drugs. 1988, 36: 158-192. 10.2165/00003495-198836020-00003.
Alousi AA, Johnson DC: Pharmacology of the bipyridines: amrinone and milrinone. Circulation. 1986, 73: III10-III24.
Farah AE, Frangakis CJ: Studies on the mechanism of action of the bipyridine milrinone on the heart. Basic Res Cardiol. 1989, 84 Suppl 1: 85-103. 10.1007/BF02650349.
Oztekin I, Yazici S, Oztekin DS, Goksel O, Issever H, Canik S: Effects of low-dose milrinone on weaning from cardiopulmonary bypass and after in patients with mitral stenosis and pulmonary hypertension. Yakugaku Zasshi. 2007, 127: 375-383. 10.1248/yakushi.127.375.
Kihara S, Kawai A, Fukuda T, Yamamoto N, Aomi S, Nishida H, Endo M, Koyanagi H: Effects of milrinone for right ventricular failure after left ventricular assist device implantation. Heart Vessels. 2002, 16: 69-71. 10.1007/s380-002-8320-z.
Fukazawa K, Poliac LC, Pretto EA: Rapid assessment and safe management of severe pulmonary hypertension with milrinone during orthotopic liver transplantation. Clin Transplant. 2010, 24: 515-519. 10.1111/j.1399-0012.2009.01119.x.
Harris MN, Daborn AK, O'Dwyer JP: Milrinone and the pulmonary vascular system. Eur J Anaesthesiol Suppl. 1992, 5: 27-30.
Eichhorn EJ, Konstam MA, Weiland DS, Roberts DJ, Martin TT, Stransky NB, Salem DN: Differential effects of milrinone and dobutamine on right ventricular preload, afterload and systolic performance in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1987, 60: 1329-1333. 10.1016/0002-9149(87)90616-3.
Boldt J, Knothe C, Zickmann B, Ballesteros M, Russ W, Dapper F, Hempelmann G: The role of enoximone in cardiac surgery. Br J Anaesth. 1992, 69: 45-50. 10.1093/bja/69.1.45.
Tarr TJ, Jeffrey RR, Kent AP, Cowen ME: Use of enoximone in weaning from cardiopulmonary bypass following mitral valve surgery. Cardiology. 1990, 77 Suppl 3: 51-57. 10.1159/000174672. discussion 62-67
Leeman M, Lejeune P, Melot C, Naeije R: Reduction in pulmonary hypertension and in airway resistances by enoximone (MDL 17,043) in decompensated COPD. Chest. 1987, 91: 662-666. 10.1378/chest.91.5.662.
Jenkins IR, Dolman J, O'Connor JP, Ansley DM: Amrinone versus dobutamine in cardiac surgical patients with severe pulmonary hypertension after cardiopulmonary bypass: a prospective, randomized double-blinded trial. Anaesth Intensive Care. 1997, 25: 245-249.
Dupuis JY, Bondy R, Cattran C, Nathan HJ, Wynands JE: Amrinone and dobutamine as primary treatment of low cardiac output syndrome following coronary artery surgery: a comparison of their effects on hemodynamics and outcome. J Cardiothorac Vasc Anesth. 1992, 6: 542-553. 10.1016/1053-0770(92)90096-P.
Hachenberg T, Mollhoff T, Holst D, Hammel D, Brussel T: Cardiopulmonary effects of enoximone or dobutamine and nitroglycerin on mitral valve regurgitation and pulmonary venous hypertension. J Cardiothorac Vasc Anesth. 1997, 11: 453-457. 10.1016/S1053-0770(97)90054-9.
Ansell J, Tiarks C, McCue J, Parrilla N, Benotti JR: Amrinone-induced thrombocytopenia. Arch Intern Med. 1984, 144: 949-952. 10.1001/archinte.144.5.949.
Boldt J, Knothe C, Zickmann B, Herold C, Dapper E, Hempelmann G: Phosphodiesterase-inhibitors enoximone and piroximone in cardiac surgery: influence on platelet count and function. Intensive Care Med. 1992, 18: 449-454. 10.1007/BF01708579.
Kikura M, Lee MK, Safon RA, Bailey JM, Levy JH: The effects of milrinone on platelets in patients undergoing cardiac surgery. Anesth Analg. 1995, 81: 44-48. 10.1097/00000539-199507000-00009.
Levy JH, Bailey JM, Deeb GM: Intravenous milrinone in cardiac surgery. Ann Thorac Surg. 2002, 73: 325-330. 10.1016/S0003-4975(01)02719-9.
Chen EP, Bittner HB, Davis RD, Van Trigt P: Milrinone improves pulmonary hemodynamics and right ventricular function in chronic pulmonary hypertension. Ann Thorac Surg. 1997, 63: 814-821. 10.1016/S0003-4975(97)00011-8.
Buckley MS, Feldman JP: Nebulized milrinone use in a pulmonary hypertensive crisis. Pharmacotherapy. 2007, 27: 1763-1766. 10.1592/phco.27.12.1763.
Wang H, Gong M, Zhou B, Dai A: Comparison of inhaled and intravenous milrinone in patients with pulmonary hypertension undergoing mitral valve surgery. Adv Ther. 2009, 26: 462-468. 10.1007/s12325-009-0019-4.
Haraldssons A, Kieler-Jensen N, Ricksten SE: The additive pulmonary vasodilatory effects of inhaled prostacyclin and inhaled milrinone in postcardiac surgical patients with pulmonary hypertension. Anesth Analg. 2001, 93: 1439-1445. 10.1097/00000539-200112000-00018. table of contents
Sablotzki A, Starzmann W, Scheubel R, Grond S, Czeslick EG: Selective pulmonary vasodilation with inhaled aerosolized milrinone in heart transplant candidates. Can J Anaesth. 2005, 52: 1076-1082. 10.1007/BF03021608.
Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L: Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet. 2002, 360: 196-202. 10.1016/S0140-6736(02)09455-2.
Barraud D, Faivre V, Damy T, Welschbillig S, Gayat E, Heymes C, Payen D, Shah AM, Mebazaa A: Levosimendan restores both systolic and diastolic cardiac performance in lipopolysaccharide-treated rabbits: comparison with dobutamine and milrinone. Crit Care Med. 2007, 35: 1376-1382. 10.1097/01.CCM.0000261889.18102.84.
Ukkonen H, Saraste M, Akkila J, Knuuti J, Karanko M, Iida H, Lehikoinen P, Nagren K, Lehtonen L, Voipio-Pulkki LM: Myocardial efficiency during levosimendan infusion in congestive heart failure. Clin Pharmacol Ther. 2000, 68: 522-531. 10.1067/mcp.2000.110972.
Ukkonen H, Saraste M, Akkila J, Knuuti MJ, Lehikoinen P, Nagren K, Lehtonen L, Voipio-Pulkki LM: Myocardial efficiency during calcium sensitization with levosimendan: a noninvasive study with positron emission tomography and echocardiography in healthy volunteers. Clin Pharmacol Ther. 1997, 61: 596-607. 10.1016/S0009-9236(97)90139-9.
Yildiz O: Vasodilating mechanisms of levosimendan: involvement of K+ channels. J Pharmacol Sci. 2007, 104: 1-5. 10.1254/jphs.CP0060010.
Slawsky MT, Colucci WS, Gottlieb SS, Greenberg BH, Haeusslein E, Hare J, Hutchins S, Leier CV, LeJemtel TH, Loh E, Nicklas J, Ogilby D, Singh BN, Smith W: Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure: study investigators. Circulation. 2000, 102: 2222-2227.
Kivikko M, Antila S, Eha J, Lehtonen L, Pentikainen PJ: Pharmacokinetics of levosimendan and its metabolites during and after a 24-hour continuous infusion in patients with severe heart failure. Int J Clin Pharmacol Ther. 2002, 40: 465-471.
Leather HA, Ver Eycken K, Segers P, Herijgers P, Vandermeersch E, Wouters PF: Effects of levosimendan on right ventricular function and ventriculovascular coupling in open chest pigs. Crit Care Med. 2003, 31: 2339-2343. 10.1097/01.CCM.0000084844.95073.C0.
Kerbaul F, Rondelet B, Demester JP, Fesler P, Huez S, Naeije R, Brimioulle S: Effects of levosimendan versus dobutamine on pressure load-induced right ventricular failure. Crit Care Med. 2006, 34: 2814-2819. 10.1097/01.CCM.0000242157.19347.50.
Missant C, Rex S, Segers P, Wouters PF: Levosimendan improves right ventriculovascular coupling in a porcine model of right ventricular dysfunction. Crit Care Med. 2007, 35: 707-715. 10.1097/01.CCM.0000257326.96342.57.
Duygu H, Ozerkan F, Zoghi M, Nalbantgil S, Yildiz A, Akilli A, Akin M, Nazli C, Ergene O: Effect of levosimendan on right ventricular systolic and diastolic functions in patients with ischaemic heart failure. Int J Clin Pract. 2008, 62: 228-233. 10.1111/j.1742-1241.2007.01510.x.
Russ MA, Prondzinsky R, Carter JM, Schlitt A, Ebelt H, Schmidt H, Lemm H, Heinroth K, Soeffker G, Winkler M, Werdan K, Buerke M: Right ventricular function in myocardial infarction complicated by cardiogenic shock: improvement with levosimendan. Crit Care Med. 2009, 37: 3017-3023. 10.1097/CCM.0b013e3181b0314a.
Yilmaz MB, Yontar C, Erdem A, Karadas F, Yalta K, Turgut OO, Yilmaz A, Tandogan I: Comparative effects of levosimendan and dobutamine on right ventricular function in patients with biventricular heart failure. Heart Vessels. 2009, 24: 16-21. 10.1007/s00380-008-1077-2.
Poelzl G, Zwick RH, Grander W, Metzler B, Jonetzko P, Frick M, Ulmer H, Pachinger O, Roithinger FX: Safety and effectiveness of levosimendan in patients with predominant right heart failure. Herz. 2008, 33: 368-373. 10.1007/s00059-008-3051-2.
Parissis JT, Paraskevaidis I, Bistola V, Farmakis D, Panou F, Kourea K, Nikolaou M, Filippatos G, Kremastinos D: Effects of levosimendan on right ventricular function in patients with advanced heart failure. Am J Cardiol. 2006, 98: 1489-1492. 10.1016/j.amjcard.2006.06.052.
Morelli A, Teboul JL, Maggiore SM, Vieillard-Baron A, Rocco M, Conti G, De Gaetano A, Picchini U, Orecchioni A, Carbone I, Tritapepe L, Pietropaoli P, Westphal M: Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: a pilot study. Crit Care Med. 2006, 34: 2287-2293. 10.1097/01.CCM.0000230244.17174.4F.
Morais RJ: Levosimendan in severe right ventricular failure following mitral valve replacement. J Cardiothorac Vasc Anesth. 2006, 20: 82-84. 10.1053/j.jvca.2005.01.039.
Cicekcioglu F, Parlar AI, Ersoy O, Yay K, Hijazi A, Katircioglu SF: Levosimendan and severe pulmonary hypertension during open heart surgery. Gen Thorac Cardiovasc Surg. 2008, 56: 563-565. 10.1007/s11748-008-0301-4.
Kleber FX, Bollmann T, Borst MM, Costard-Jackle A, Ewert R, Kivikko M, Petterson T, Pohjanjousi P, Sonntag S, Wikstrom G: Repetitive dosing of intravenous levosimendan improves pulmonary hemodynamics in patients with pulmonary hypertension: results of a pilot study. J Clin Pharmacol. 2009, 49: 109-115.
Raper R: FMM: The heart and circulation in sepsis. Balliere's Clin Anaesthesiol. 1990, 4: 333-355. 10.1016/S0950-3501(05)80290-9.
Humbert M, Sitbon O, Simonneau G: Treatment of pulmonary arterial hypertension. N Engl J Med. 2004, 351: 1425-1436. 10.1056/NEJMra040291.
Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G: Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Respir J. 2009, 34: 1219-1263. 10.1183/09031936.00139009.
Kieler-Jensen N, Lundin S, Ricksten SE: Vasodilator therapy after heart transplantation: effects of inhaled nitric oxide and intravenous prostacyclin, prostaglandin E1, and sodium nitroprusside. J Heart Lung Transplant. 1995, 14: 436-443.
Creagh-Brown BC, Griffiths MJ, Evans TW: Bench-to-bedside review: inhaled nitric oxide therapy in adults. Crit Care. 2009, 13: 221-10.1186/cc7734.
McNeil K, Dunning J, Morrell NW: The pulmonary physician in critical care, 13: the pulmonary circulation and right ventricular failure in the ITU. Thorax. 2003, 58: 157-162. 10.1136/thorax.58.2.157.
Channick RN, Hoch RC, Newhart JW, Johnson FW, Smith CM: Improvement in pulmonary hypertension and hypoxemia during nitric oxide inhalation in a patient with end-stage pulmonary fibrosis. Am J Respir Crit Care Med. 1994, 149: 811-814.
Griffiths MJ, Evans TW: Inhaled nitric oxide therapy in adults. N Engl J Med. 2005, 353: 2683-2695. 10.1056/NEJMra051884.
Aranda M, Bradford KK, Pearl RG: Combined therapy with inhaled nitric oxide and intravenous vasodilators during acute and chronic experimental pulmonary hypertension. Anesth Analg. 1999, 89: 152-158. 10.1097/00000539-199907000-00027.
Morgan JM, McCormack DG, Griffiths MJ, Morgan CJ, Barnes PJ, Evans TW: Adenosine as a vasodilator in primary pulmonary hypertension. Circulation. 1991, 84: 1145-1149.
Fullerton DA, Jones SD, Grover FL, McIntyre RC: Adenosine effectively controls pulmonary hypertension after cardiac operations. Ann Thorac Surg. 1996, 61: 1118-1123. 10.1016/0003-4975(95)01149-8. discussion 1123-1114
Haywood GA, Sneddon JF, Bashir Y, Jennison SH, Gray HH, McKenna WJ: Adenosine infusion for the reversal of pulmonary vasoconstriction in biventricular failure: a good test but a poor therapy. Circulation. 1992, 86: 896-902.
Oliveira EC, Ribeiro AL, Amaral CF: Adenosine for vasoreactivity testing in pulmonary hypertension: a head-to-head comparison with inhaled nitric oxide. Respir Med. 104: 606-611. 10.1016/j.rmed.2009.11.010.
Pepke-Zaba J, Higenbottam TW, Dinh-Xuan AT, Stone D, Wallwork J: Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet. 1991, 338: 1173-1174. 10.1016/0140-6736(91)92033-X.
King R, Esmail M, Mahon S, Dingley J, Dwyer S: Use of nitric oxide for decompensated right ventricular failure and circulatory shock after cardiac arrest. Br J Anaesth. 2000, 85: 628-631. 10.1093/bja/85.4.628.
Frostell CG, Blomqvist H, Hedenstierna G, Lundberg J, Zapol WM: Inhaled nitric oxide selectively reverses human hypoxic pulmonary vasoconstriction without causing systemic vasodilation. Anesthesiology. 1993, 78: 427-435. 10.1097/00000542-199303000-00005.
Schenk P, Mittermayer C, Ratheiser K: Inhaled nitric oxide in a patient with severe pulmonary embolism. Ann Emerg Med. 1999, 33: 710-714.
Capellier G, Jacques T, Balvay P, Blasco G, Belle E, Barale F: Inhaled nitric oxide in patients with pulmonary embolism. Intensive Care Med. 1997, 23: 1089-1092. 10.1007/s001340050461.
Inglessis I, Shin JT, Lepore JJ, Palacios IF, Zapol WM, Bloch KD, Semigran MJ: Hemodynamic effects of inhaled nitric oxide in right ventricular myocardial infarction and cardiogenic shock. J Am Coll Cardiol. 2004, 44: 793-798. 10.1016/j.jacc.2004.05.047.
Fujita Y, Nishida O, Sobue K, Ito H, Kusama N, Inagaki M, Katsuya H: Nitric oxide inhalation is useful in the management of right ventricular failure caused by myocardial infarction. Crit Care Med. 2002, 30: 1379-1381. 10.1097/00003246-200206000-00042.
Rich GF, Murphy GD, Roos CM, Johns RA: Inhaled nitric oxide: selective pulmonary vasodilation in cardiac surgical patients. Anesthesiology. 1993, 78: 1028-1035. 10.1097/00000542-199306000-00004.
Macdonald PS, Keogh A, Mundy J, Rogers P, Nicholson A, Harrison G, Jansz P, Kaan AM, Spratt P: Adjunctive use of inhaled nitric oxide during implantation of a left ventricular assist device. J Heart Lung Transplant. 1998, 17: 312-316.
Beck JR, Mongero LB, Kroslowitz RM, Choudhri AF, Chen JM, DeRose JJ, Argenziano M, Smerling AJ, Oz MC: Inhaled nitric oxide improves hemodynamics in patients with acute pulmonary hypertension after high-risk cardiac surgery. Perfusion. 1999, 14: 37-42.
Fernandez-Perez ER, Keegan MT, Harrison BA: Inhaled nitric oxide for acute right-ventricular dysfunction after extrapleural pneumonectomy. Respir Care. 2006, 51: 1172-1176.
Fattouch K, Sbraga F, Bianco G, Speziale G, Gucciardo M, Sampognaro R, Ruvolo G: Inhaled prostacyclin, nitric oxide, and nitroprusside in pulmonary hypertension after mitral valve replacement. J Card Surg. 2005, 20: 171-176. 10.1111/j.0886-0440.2005.200383w.x.
Takaba K, Aota M, Nonaka M, Sugimoto A, Konishi Y: Successful treatment of chronic thromboembolic pulmonary hypertension with inhaled nitric oxide after right ventricular thrombectomy. Jpn J Thorac Cardiovasc Surg. 2004, 52: 257-260. 10.1007/s11748-004-0120-1.
Maxey TS, Smith CD, Kern JA, Tribble CG, Jones DR, Kron IL, Crosby IK: Beneficial effects of inhaled nitric oxide in adult cardiac surgical patients. Ann Thorac Surg. 2002, 73: 529-532. 10.1016/S0003-4975(01)03398-7. discussion 532-523
Khan TA, Schnickel G, Ross D, Bastani S, Laks H, Esmailian F, Marelli D, Beygui R, Shemin R, Watson L, Vartapetian I, Ardehali A: A prospective, randomized, crossover pilot study of inhaled nitric oxide versus inhaled prostacyclin in heart transplant and lung transplant recipients. J Thorac Cardiovasc Surg. 2009, 138: 1417-1424. 10.1016/j.jtcvs.2009.04.063.
Carrier M, Blaise G, Belisle S, Perrault LP, Pellerin M, Petitclerc R, Pelletier LC: Nitric oxide inhalation in the treatment of primary graft failure following heart transplantation. J Heart Lung Transplant. 1999, 18: 664-667. 10.1016/S1053-2498(99)00025-X.
Trummer G, Berchtold-Herz M, Martin J, Beyersdorf F: Successful treatment of pulmonary hypertension with inhaled nitric oxide after pulmonary embolectomy. Ann Thorac Surg. 2002, 73: 1299-1301. 10.1016/S0003-4975(01)03265-9.
Yoshikawa T, Date H, Yamashita M, Nagahiro I, Aoe M, Shimizu N: Inhaled nitric oxide ameliorates postoperative acute graft dysfunction after living-donor lobar lung transplantation. Jpn J Thorac Cardiovasc Surg. 2000, 48: 742-745.
Girardis M, Pasqualotto A, Colo F, Dal Pos L, Sabbadini D, Pasqualucci A, Pasetto A: Severe hypoxemia and pulmonary hypertension during orthotopic liver transplantation: a successful use of inhaled nitric oxide. Intensive Care Med. 1999, 25: 638-10.1007/s001340050919.
Ralley FE: The use of nitric oxide for managing catastrophic pulmonary vasoconstriction arising from protamine administration. Anesth Analg. 1999, 88: 505-507. 10.1097/00000539-199903000-00007.
Williams TJ, Salamonsen RF, Snell G, Kaye D, Esmore DS: Preliminary experience with inhaled nitric oxide for acute pulmonary hypertension after heart transplantation. J Heart Lung Transplant. 1995, 14: 419-423.
Molmenti EP, Ramsay M, Ramsay K, Lynch K, Tillmann Hein HA, Molmenti H, Levy M, Goldstein R, Ausloos K, East C, Fasola C, Jung G, Escobar J, Klintmalm G: Epoprostenol and nitric oxide therapy for severe pulmonary hypertension in liver transplantation. Transplant Proc. 2001, 33: 1332-10.1016/S0041-1345(00)02807-4.
Snow DJ, Gray SJ, Ghosh S, Foubert L, Oduro A, Higenbottam TW, Wells FC, Latimer RD: Inhaled nitric oxide in patients with normal and increased pulmonary vascular resistance after cardiac surgery. Br J Anaesth. 1994, 72: 185-189. 10.1093/bja/72.2.185.
Bender KA, Alexander JA, Enos JM, Skimming JW: Effects of inhaled nitric oxide in patients with hypoxemia and pulmonary hypertension after cardiac surgery. Am J Crit Care. 1997, 6: 127-131.
Solina A, Papp D, Ginsberg S, Krause T, Grubb W, Scholz P, Pena LL, Cody R: A comparison of inhaled nitric oxide and milrinone for the treatment of pulmonary hypertension in adult cardiac surgery patients. J Cardiothorac Vasc Anesth. 2000, 14: 12-17. 10.1016/S1053-0770(00)90048-X.
Solina AR, Ginsberg SH, Papp D, Pantin EJ, Denny J, Ghandivel I, Krause TJ: Response to nitric oxide during adult cardiac surgery. J Invest Surg. 2002, 15: 5-14. 10.1080/08941930252807732.
Solina AR, Ginsberg SH, Papp D, Grubb WR, Scholz PM, Pantin EJ, Cody RP, Krause TJ: Dose response to nitric oxide in adult cardiac surgery patients. J Clin Anesth. 2001, 13: 281-286. 10.1016/S0952-8180(01)00270-7.
Fattouch K, Sbraga F, Sampognaro R, Bianco G, Gucciardo M, Lavalle C, Vizza CD, Fedele F, Ruvolo G: Treatment of pulmonary hypertension in patients undergoing cardiac surgery with cardiopulmonary bypass: a randomized, prospective, double-blind study. J Cardiovasc Med (Hagerstown). 2006, 7: 119-123. 10.2459/01.JCM.0000203850.97890.fe.
Healy DG, Veerasingam D, McHale J, Luke D: Successful perioperative utilisation of inhaled nitric oxide in mitral valve surgery. J Cardiovasc Surg (Torino). 2006, 47: 217-220.
Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM: Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993, 328: 399-405. 10.1056/NEJM199302113280605.
Fierobe L, Brunet F, Dhainaut JF, Monchi M, Belghith M, Mira JP, Dall'ava-Santucci J, Dinh-Xuan AT: Effect of inhaled nitric oxide on right ventricular function in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995, 151: 1414-1419.
Romberg-Camps MJ, Korsten HH, Botman CJ, Bindels AJ, Roos AN: Right ventricular failure in acute respiratory distress syndrome. Neth J Med. 2000, 57: 94-97. 10.1016/S0300-2977(00)00052-8.
Chiche JD, Canivet JL, Damas P, Joris J, Lamy M: Inhaled nitric oxide for hemodynamic support after postpneumonectomy ARDS. Intensive Care Med. 1995, 21: 675-678. 10.1007/BF01711547.
Benzing A, Mols G, Beyer U, Geiger K: Large increase in cardiac output in a patient with ARDS and acute right heart failure during inhalation of nitric oxide. Acta Anaesthesiol Scand. 1997, 41: 643-646. 10.1111/j.1399-6576.1997.tb04758.x.
Bigatello LM, Hurford WE, Kacmarek RM, Roberts JD, Zapol WM: Prolonged inhalation of low concentrations of nitric oxide in patients with severe adult respiratory distress syndrome: effects on pulmonary hemodynamics and oxygenation. Anesthesiology. 1994, 80: 761-770. 10.1097/00000542-199404000-00007.
Hsu CW, Lee DL, Lin SL, Sun SF, Chang HW: The initial response to inhaled nitric oxide treatment for intensive care unit patients with acute respiratory distress syndrome. Respiration. 2008, 75: 288-295. 10.1159/000101478.
Gerlach H, Keh D, Semmerow A, Busch T, Lewandowski K, Pappert DM, Rossaint R, Falke KJ: Dose-response characteristics during long-term inhalation of nitric oxide in patients with severe acute respiratory distress syndrome: a prospective, randomized, controlled study. Am J Respir Crit Care Med. 2003, 167: 1008-1015. 10.1164/rccm.2108121.
Dellinger RP, Zimmerman JL, Taylor RW, Straube RC, Hauser DL, Criner GJ, Davis K, Hyers TM, Papadakos P: Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial: inhaled nitric oxide in ARDS study group. Crit Care Med. 1998, 26: 15-23. 10.1097/00003246-199801000-00011.
Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K, Kelly KM, Smith TC, Small RJ: Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004, 291: 1603-1609. 10.1001/jama.291.13.1603.
Brett SJ, Hansell DM, Evans TW: Clinical correlates in acute lung injury: response to inhaled nitric oxide. Chest. 1998, 114: 1397-1404. 10.1378/chest.114.5.1397.
Manktelow C, Bigatello LM, Hess D, Hurford WE: Physiologic determinants of the response to inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology. 1997, 87: 297-307. 10.1097/00000542-199708000-00017.
Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO: Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007, 334: 779-10.1136/bmj.39139.716794.55.
Sokol J, Jacobs SE, Bohn D: Inhaled nitric oxide for acute hypoxic respiratory failure in children and adults: a meta-analysis. Anesth Analg. 2003, 97: 989-998. 10.1213/01.ANE.0000078819.48523.26.
Troncy E, Collet JP, Shapiro S, Guimond JG, Blair L, Ducruet T, Francoeur M, Charbonneau M, Blaise G: Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study. Am J Respir Crit Care Med. 1998, 157: 1483-1488.
Michael JR, Barton RG, Saffle JR, Mone M, Markewitz BA, Hillier K, Elstad MR, Campbell EJ, Troyer BE, Whatley RE, Liou TG, Samuelson WM, Carveth HJ, Hinson DM, Morris SE, Davis BL, Day RW: Inhaled nitric oxide versus conventional therapy: effect on oxygenation in ARDS. Am J Respir Crit Care Med. 1998, 157: 1372-1380.
Lundin S, Mang H, Smithies M, Stenqvist O, Frostell C: Inhalation of nitric oxide in acute lung injury: results of a European multicentre study: the European Study Group of Inhaled Nitric Oxide. Intensive Care Med. 1999, 25: 911-919. 10.1007/s001340050982.
Vater Y, Martay K, Dembo G, Bowdle TA, Weinbroum AA: Intraoperative epoprostenol and nitric oxide for severe pulmonary hypertension during orthotopic liver transplantation: a case report and review of the literature. Med Sci Monit. 2006, 12: CS115-CS118.
Flondor M, Merkel M, Hofstetter C, Irlbeck M, Frey L, Zwissler B: The effect of inhaled nitric oxide and inhaled iloprost on hypoxaemia in a patient with pulmonary hypertension after pulmonary thrombarterectomy. Anaesthesia. 2006, 61: 1200-1203. 10.1111/j.1365-2044.2006.04861.x.
Lepore JJ, Maroo A, Bigatello LM, Dec GW, Zapol WM, Bloch KD, Semigran MJ: Hemodynamic effects of sildenafil in patients with congestive heart failure and pulmonary hypertension: combined administration with inhaled nitric oxide. Chest. 2005, 127: 1647-1653. 10.1378/chest.127.5.1647.
Lepore JJ, Maroo A, Pereira NL, Ginns LC, Dec GW, Zapol WM, Bloch KD, Semigran MJ: Effect of sildenafil on the acute pulmonary vasodilator response to inhaled nitric oxide in adults with primary pulmonary hypertension. Am J Cardiol. 2002, 90: 677-680. 10.1016/S0002-9149(02)02586-9.
Lavoie A, Hall JB, Olson DM, Wylam ME: Life-threatening effects of discontinuing inhaled nitric oxide in severe respiratory failure. Am J Respir Crit Care Med. 1996, 153: 1985-1987.
Atz AM, Adatia I, Wessel DL: Rebound pulmonary hypertension after inhalation of nitric oxide. Ann Thorac Surg. 1996, 62: 1759-1764. 10.1016/S0003-4975(96)00542-5.
Christenson J, Lavoie A, O'Connor M, Bhorade S, Pohlman A, Hall JB: The incidence and pathogenesis of cardiopulmonary deterioration after abrupt withdrawal of inhaled nitric oxide. Am J Respir Crit Care Med. 2000, 161: 1443-1449.
Giacomini M, Borotto E, Bosotti L, Denkewitz T, Reali-Forster C, Carlucci P, Centanni S, Mantero A, Iapichino G: Vardenafil and weaning from inhaled nitric oxide: effect on pulmonary hypertension in ARDS. Anaesth Intensive Care. 2007, 35: 91-93.
Trachte AL, Lobato EB, Urdaneta F, Hess PJ, Klodell CT, Martin TD, Staples ED, Beaver TM: Oral sildenafil reduces pulmonary hypertension after cardiac surgery. Ann Thorac Surg. 2005, 79: 194-197. 10.1016/j.athoracsur.2004.06.086. discussion 194-197
Atz AM, Wessel DL: Sildenafil ameliorates effects of inhaled nitric oxide withdrawal. Anesthesiology. 1999, 91: 307-310. 10.1097/00000542-199907000-00041.
Namachivayam P, Theilen U, Butt WW, Cooper SM, Penny DJ, Shekerdemian LS: Sildenafil prevents rebound pulmonary hypertension after withdrawal of nitric oxide in children. Am J Respir Crit Care Med. 2006, 174: 1042-1047. 10.1164/rccm.200605-694OC.
Klodell CT, Morey TE, Lobato EB, Aranda JM, Staples ED, Schofield RS, Hess PJ, Martin TD, Beaver TM: Effect of sildenafil on pulmonary artery pressure, systemic pressure, and nitric oxide utilization in patients with left ventricular assist devices. Ann Thorac Surg. 2007, 83: 68-71. 10.1016/j.athoracsur.2006.08.051. discussion 71
Mychaskiw G, Sachdev V, Heath BJ: Sildenafil (Viagra) facilitates weaning of inhaled nitric oxide following placement of a biventricular-assist device. J Clin Anesth. 2001, 13: 218-220. 10.1016/S0952-8180(01)00252-5.
Moncada S, Gryglewski RJ, Bunting S, Vane JR: A lipid peroxide inhibits the enzyme in blood vessel microsomes that generates from prostaglandin endoperoxides the substance (prostaglandin X) which prevents platelet aggregation. Prostaglandins. 1976, 12: 715-737. 10.1016/0090-6980(76)90048-4.
Clapp LH, Finney P, Turcato S, Tran S, Rubin LJ, Tinker A: Differential effects of stable prostacyclin analogs on smooth muscle proliferation and cyclic AMP generation in human pulmonary artery. Am J Respir Cell Mol Biol. 2002, 26: 194-201.
Rubin LJ, Mendoza J, Hood M, McGoon M, Barst R, Williams WB, Diehl JH, Crow J, Long W: Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol): results of a randomized trial. Ann Intern Med. 1990, 112: 485-491.
Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH, Koerner SK, Langleben D, Keller CA, Murali S, Uretsky BF, Clayton LM, Jobsis MM, Blackburn DS, Shortino D, Crow JW: A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension: the Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996, 334: 296-302. 10.1056/NEJM199602013340504.
Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, Speich R, Hoeper MM, Behr J, Winkler J, Sitbon O, Popov W, Ghofrani HA, Manes A, Kiely DG, Ewert R, Meyer A, Corris PA, Delcroix M, Gomez-Sanchez M, Siedentop H, Seeger W: Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002, 347: 322-329. 10.1056/NEJMoa020204.
Hoeper MM, Schwarze M, Ehlerding S, Adler-Schuermeyer A, Spiekerkoetter E, Niedermeyer J, Hamm M, Fabel H: Long-term treatment of primary pulmonary hypertension with aerosolized iloprost, a prostacyclin analogue. N Engl J Med. 2000, 342: 1866-1870. 10.1056/NEJM200006223422503.
Hoeper MM, Olschewski H, Ghofrani HA, Wilkens H, Winkler J, Borst MM, Niedermeyer J, Fabel H, Seeger W: A comparison of the acute hemodynamic effects of inhaled nitric oxide and aerosolized iloprost in primary pulmonary hypertension: German PPH study group. J Am Coll Cardiol. 2000, 35: 176-182. 10.1016/S0735-1097(99)00494-5.
Olschewski H, Ghofrani HA, Walmrath D, Temmesfeld-Wollbruck B, Grimminger F, Seeger W: Recovery from circulatory shock in severe primary pulmonary hypertension (PPH) with aerosolization of iloprost. Intensive Care Med. 1998, 24: 631-634. 10.1007/s001340050628.
Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Herve P, Rainisio M, Simonneau G: Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002, 40: 780-788. 10.1016/S0735-1097(02)02012-0.
Kieler-Jensen N, Milocco I, Ricksten SE: Pulmonary vasodilation after heart transplantation: a comparison among prostacyclin, sodium nitroprusside, and nitroglycerin on right ventricular function and pulmonary selectivity. J Heart Lung Transplant. 1993, 12: 179-184.
D'Ambra MN, LaRaia PJ, Philbin DM, Watkins WD, Hilgenberg AD, Buckley MJ: Prostaglandin E1: a new therapy for refractory right heart failure and pulmonary hypertension after mitral valve replacement. J Thorac Cardiovasc Surg. 1985, 89: 567-572.
Vincent JL, Carlier E, Pinsky MR, Goldstein J, Naeije R, Lejeune P, Brimioulle S, Leclerc JL, Kahn RJ, Primo G: Prostaglandin E1 infusion for right ventricular failure after cardiac transplantation. J Thorac Cardiovasc Surg. 1992, 103: 33-39.
Radovancevic B, Vrtovec B, Thomas CD, Croitoru M, Myers TJ, Radovancevic R, Khan T, Massin EK, Frazier OH: Nitric oxide versus prostaglandin E1 for reduction of pulmonary hypertension in heart transplant candidates. J Heart Lung Transplant. 2005, 24: 690-695. 10.1016/j.healun.2004.04.016.
Schmid ER, Burki C, Engel MH, Schmidlin D, Tornic M, Seifert B: Inhaled nitric oxide versus intravenous vasodilators in severe pulmonary hypertension after cardiac surgery. Anesth Analg. 1999, 89: 1108-1115. 10.1097/00000539-199911000-00007.
Elliott CG, Palevsky HI: Treatment with epoprostenol of pulmonary arterial hypertension following mitral valve replacement for mitral stenosis. Thorax. 2004, 59: 536-537. 10.1136/thx.2003.008193.
Haraldsson A, Kieler-Jensen N, Ricksten SE: Inhaled prostacyclin for treatment of pulmonary hypertension after cardiac surgery or heart transplantation: a pharmacodynamic study. J Cardiothorac Vasc Anesth. 1996, 10: 864-868. 10.1016/S1053-0770(96)80047-4.
Hache M, Denault AY, Belisle S, Couture P, Babin D, Tetrault F, Guimond JG: Inhaled prostacyclin (PGI2) is an effective addition to the treatment of pulmonary hypertension and hypoxia in the operating room and intensive care unit. Can J Anaesth. 2001, 48: 924-929. 10.1007/BF03017361.
De Wet CJ, Affleck DG, Jacobsohn E, Avidan MS, Tymkew H, Hill LL, Zanaboni PB, Moazami N, Smith JR: Inhaled prostacyclin is safe, effective, and affordable in patients with pulmonary hypertension, right heart dysfunction, and refractory hypoxemia after cardiothoracic surgery. J Thorac Cardiovasc Surg. 2004, 127: 1058-1067. 10.1016/j.jtcvs.2003.11.035.
Schroeder RA, Wood GL, Plotkin JS, Kuo PC: Intraoperative use of inhaled PGI(2) for acute pulmonary hypertension and right ventricular failure. Anesth Analg. 2000, 91: 291-295. 10.1097/00000539-200008000-00008.
Lowson SM, Doctor A, Walsh BK, Doorley PA: Inhaled prostacyclin for the treatment of pulmonary hypertension after cardiac surgery. Crit Care Med. 2002, 30: 2762-2764. 10.1097/00003246-200212000-00023.
Kramm T, Eberle B, Guth S, Mayer E: Inhaled iloprost to control residual pulmonary hypertension following pulmonary endarterectomy. Eur J Cardiothorac Surg. 2005, 28: 882-888. 10.1016/j.ejcts.2005.09.007.
Rex S, Schaelte G, Metzelder S, Flier S, de Waal EE, Autschbach R, Rossaint R, Buhre W: Inhaled iloprost to control pulmonary artery hypertension in patients undergoing mitral valve surgery: a prospective, randomized-controlled trial. Acta Anaesthesiol Scand. 2008, 52: 65-72. 10.1111/j.1399-6576.2007.01476.x.
Yurtseven N, Karaca P, Uysal G, Ozkul V, Cimen S, Tuygun AK, Yuksek A, Canik S: A comparison of the acute hemodynamic effects of inhaled nitroglycerin and iloprost in patients with pulmonary hypertension undergoing mitral valve surgery. Ann Thorac Cardiovasc Surg. 2006, 12: 319-323.
Baysal A, Bilsel S, Bulbul OG, Kayacioglu I, Idiz M, Yekeler I: Comparison of the usage of intravenous iloprost and nitroglycerin for pulmonary hypertension during valvular heart surgery. Heart Surg Forum. 2006, 9: E536-E542. 10.1532/HSF98.20051161.
Tissieres P, Nicod L, Barazzone-Argiroffo C, Rimensberger PC, Beghetti M: Aerosolized iloprost as a bridge to lung transplantation in a patient with cystic fibrosis and pulmonary hypertension. Ann Thorac Surg. 2004, 78: e48-50. 10.1016/j.athoracsur.2003.12.096.
Sablotzki A, Hentschel T, Gruenig E, Schubert S, Friedrich I, Muhling J, Dehne MG, Czeslick E: Hemodynamic effects of inhaled aerosolized iloprost and inhaled nitric oxide in heart transplant candidates with elevated pulmonary vascular resistance. Eur J Cardiothorac Surg. 2002, 22: 746-752. 10.1016/S1010-7940(02)00488-8.
Theodoraki K, Rellia P, Thanopoulos A, Tsourelis L, Zarkalis D, Sfyrakis P, Antoniou T: Inhaled iloprost controls pulmonary hypertension after cardiopulmonary bypass. Can J Anaesth. 2002, 49: 963-967. 10.1007/BF03016884.
Winterhalter M, Simon A, Fischer S, Rahe-Meyer N, Chamtzidou N, Hecker H, Zuk J, Piepenbrock S, Struber M: Comparison of inhaled iloprost and nitric oxide in patients with pulmonary hypertension during weaning from cardiopulmonary bypass in cardiac surgery: a prospective randomized trial. J Cardiothorac Vasc Anesth. 2008, 22: 406-413. 10.1053/j.jvca.2007.10.015.
Webb SA, Stott S, van Heerden PV: The use of inhaled aerosolized prostacyclin (IAP) in the treatment of pulmonary hypertension secondary to pulmonary embolism. Intensive Care Med. 1996, 22: 353-355. 10.1007/BF01700458.
Haraldsson A, Kieler-Jensen N, Wadenvik H, Ricksten SE: Inhaled prostacyclin and platelet function after cardiac surgery and cardiopulmonary bypass. Intensive Care Med. 2000, 26: 188-194. 10.1007/s001340050044.
Radermacher P, Santak B, Wust HJ, Tarnow J, Falke KJ: Prostacyclin and right ventricular function in patients with pulmonary hypertension associated with ARDS. Intensive Care Med. 1990, 16: 227-232. 10.1007/BF01705156.
Zwissler B, Kemming G, Habler O, Kleen M, Merkel M, Haller M, Briegel J, Welte M, Peter K: Inhaled prostacyclin (PGI2) versus inhaled nitric oxide in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1996, 154: 1671-1677.
van Heerden PV, Barden A, Michalopoulos N, Bulsara MK, Roberts BL: Dose-response to inhaled aerosolized prostacyclin for hypoxemia due to ARDS. Chest. 2000, 117: 819-827. 10.1378/chest.117.3.819.
Walmrath D, Schneider T, Pilch J, Grimminger F, Seeger W: Aerosolised prostacyclin in adult respiratory distress syndrome. Lancet. 1993, 342: 961-962. 10.1016/0140-6736(93)92004-D.
Van Heerden PV, Webb SA, Hee G, Corkeron M, Thompson WR: Inhaled aerosolized prostacyclin as a selective pulmonary vasodilator for the treatment of severe hypoxaemia. Anaesth Intensive Care. 1996, 24: 87-90.
Meyer J, Theilmeier G, Van Aken H, Bone HG, Busse H, Waurick R, Hinder F, Booke M: Inhaled prostaglandin E1 for treatment of acute lung injury in severe multiple organ failure. Anesth Analg. 1998, 86: 753-758. 10.1097/00000539-199804000-00015.
Kuhlen R, Walbert E, Frankel P, Thaden S, Behrendt W, Rossaint R: Combination of inhaled nitric oxide and intravenous prostacyclin for successful treatment of severe pulmonary hypertension in a patient with acute respiratory distress syndrome. Intensive Care Med. 1999, 25: 752-754. 10.1007/s001340050941.
Prasad S, Wilkinson J, Gatzoulis MA: Sildenafil in primary pulmonary hypertension. N Engl J Med. 2000, 343: 1342-10.1056/NEJM200011023431814.
Preston IR, Klinger JR, Houtches J, Nelson D, Farber HW, Hill NS: Acute and chronic effects of sildenafil in patients with pulmonary arterial hypertension. Respir Med. 2005, 99: 1501-1510. 10.1016/j.rmed.2005.03.026.
Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S: Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation. 2002, 105: 2398-2403. 10.1161/01.CIR.0000016641.12984.DC.
Archer SL, Michelakis ED: Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. N Engl J Med. 2009, 361: 1864-1871. 10.1056/NEJMct0904473.
Giaid A, Saleh D: Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995, 333: 214-221. 10.1056/NEJM199507273330403.
Black SM, Sanchez LS, Mata-Greenwood E, Bekker JM, Steinhorn RH, Fineman JR: sGC and PDE5 are elevated in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2001, 281: L1051-L1057.
Wharton J, Strange JW, Moller GM, Growcott EJ, Ren X, Franklyn AP, Phillips SC, Wilkins MR: Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am J Respir Crit Care Med. 2005, 172: 105-113. 10.1164/rccm.200411-1587OC.
Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, Light PE, Dyck JR, Michelakis ED: Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007, 116: 238-248. 10.1161/CIRCULATIONAHA.106.655266.
Packer M: Vasodilator therapy for primary pulmonary hypertension: limitations and hazards. Ann Intern Med. 1985, 103: 258-270.
De Santo LS, Mastroianni C, Romano G, Amarelli C, Marra C, Maiello C, Galdieri N, Della Corte A, Cotrufo M, Caianiello G: Role of sildenafil in acute posttransplant right ventricular dysfunction: successful experience in 13 consecutive patients. Transplant Proc. 2008, 40: 2015-2018. 10.1016/j.transproceed.2008.05.055.
Shim JK, Choi YS, Oh YJ, Kim DH, Hong YW, Kwak YL: Effect of oral sildenafil citrate on intraoperative hemodynamics in patients with pulmonary hypertension undergoing valvular heart surgery. J Thorac Cardiovasc Surg. 2006, 132: 1420-1425. 10.1016/j.jtcvs.2006.08.035.
Madden BP, Sheth A, Ho TB, Park JE, Kanagasabay RR: Potential role for sildenafil in the management of perioperative pulmonary hypertension and right ventricular dysfunction after cardiac surgery. Br J Anaesth. 2004, 93: 155-156. 10.1093/bja/aeh571.
Fung E, Fiscus RR, Yim AP, Angelini GD, Arifi AA: The potential use of type-5 phosphodiesterase inhibitors in coronary artery bypass graft surgery. Chest. 2005, 128: 3065-3073. 10.1378/chest.128.4.3065.
Aubin MC, Laurendeau S, Mommerot A, Lamarche Y, Denault A, Carrier M, Perrault LP: Differential effects of inhaled and intravenous sildenafil in the prevention of the pulmonary endothelial dysfunction due to cardiopulmonary bypass. J Cardiovasc Pharmacol. 2008, 51: 11-17. 10.1097/FJC.0b013e3181598279.
Ghofrani HA, Wiedemann R, Rose F, Schermuly RT, Olschewski H, Weissmann N, Gunther A, Walmrath D, Seeger W, Grimminger F: Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet. 2002, 360: 895-900. 10.1016/S0140-6736(02)11024-5.
Ng J, Finney SJ, Shulman R, Bellingan GJ, Singer M, Glynne PA: Treatment of pulmonary hypertension in the general adult intensive care unit: a role for oral sildenafil?. Br J Anaesth. 2005, 94: 774-777. 10.1093/bja/aei114.
Cornet AD, Hofstra JJ, Swart EL, Girbes AR, Juffermans NP: Sildenafil attenuates pulmonary arterial pressure but does not improve oxygenation during ARDS. Intensive Care Med. 2010, 36: 758-764. 10.1007/s00134-010-1754-3.
Botha P, Parry G, Dark JH, Macgowan GA: Acute hemodynamic effects of intravenous sildenafil citrate in congestive heart failure: comparison of phosphodiesterase type-3 and -5 inhibition. J Heart Lung Transplant. 2009, 28: 676-682. 10.1016/j.healun.2009.04.013.
Moudgil R, Michelakis ED, Archer SL: Hypoxic pulmonary vasoconstriction. J Appl Physiol. 2005, 98: 390-403. 10.1152/japplphysiol.00733.2004.
Lejeune P, Brimioulle S, Leeman M, Hallemans R, Melot C, Naeije R: Enhancement of hypoxic pulmonary vasoconstriction by metabolic acidosis in dogs. Anesthesiology. 1990, 73: 256-264. 10.1097/00000542-199008000-00012.
Balanos GM, Talbot NP, Dorrington KL, Robbins PA: Human pulmonary vascular response to 4 h of hypercapnia and hypocapnia measured using Doppler echocardiography. J Appl Physiol. 2003, 94: 1543-1551.
Mekontso Dessap A, Charron C, Devaquet J, Aboab J, Jardin F, Brochard L, Vieillard-Baron A: Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med. 2009, 35: 1850-1858. 10.1007/s00134-009-1569-2.
Puybasset L, Stewart T, Rouby JJ, Cluzel P, Mourgeon E, Belin MF, Arthaud M, Landault C, Viars P: Inhaled nitric oxide reverses the increase in pulmonary vascular resistance induced by permissive hypercapnia in patients with acute respiratory distress syndrome. Anesthesiology. 1994, 80: 1254-1267. 10.1097/00000542-199406000-00013.
Biondi JW, Schulman DS, Matthay RA: Effects of mechanical ventilation on right and left ventricular function. Clin Chest Med. 1988, 9: 55-71.
Jardin F, Gueret P, Dubourg O, Farcot JC, Margairaz A, Bourdarias JP: Two-dimensional echocardiographic evaluation of right ventricular size and contractility in acute respiratory failure. Crit Care Med. 1985, 13: 952-956. 10.1097/00003246-198511000-00035.
Jardin F, Vieillard-Baron A: Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007, 33: 444-447. 10.1007/s00134-007-0552-z.
Vieillard-Baron A, Rabiller A, Chergui K, Peyrouset O, Page B, Beauchet A, Jardin F: Prone position improves mechanics and alveolar ventilation in acute respiratory distress syndrome. Intensive Care Med. 2005, 31: 220-226. 10.1007/s00134-004-2478-z.
David M, von Bardeleben RS, Weiler N, Markstaller K, Scholz A, Karmrodt J, Eberle B: Cardiac function and haemodynamics during transition to high-frequency oscillatory ventilation. Eur J Anaesthesiol. 2004, 21: 944-952.
Gernoth C, Wagner G, Pelosi P, Luecke T: Respiratory and haemodynamic changes during decremental open lung positive end-expiratory pressure titration in patients with acute respiratory distress syndrome. Crit Care. 2009, 13: R59-10.1186/cc7786.
Shekerdemian LS, Shore DF, Lincoln C, Bush A, Redington AN: Negative-pressure ventilation improves cardiac output after right heart surgery. Circulation. 1996, 94: II49-II55.
Shekerdemian LS, Bush A, Shore DF, Lincoln C, Redington AN: Cardiopulmonary interactions after Fontan operations: augmentation of cardiac output using negative pressure ventilation. Circulation. 1997, 96: 3934-3942.
Giesler GM, Gomez JS, Letsou G, Vooletich M, Smalling RW: Initial report of percutaneous right ventricular assist for right ventricular shock secondary to right ventricular infarction. Catheter Cardiovasc Interv. 2006, 68: 263-266. 10.1002/ccd.20846.
Fonger JD, Borkon AM, Baumgartner WA, Achuff SC, Augustine S, Reitz BA: Acute right ventricular failure following heart transplantation: improvement with prostaglandin E1 and right ventricular assist. J Heart Transplant. 1986, 5: 317-321.
Nagarsheth NP, Pinney S, Bassily-Marcus A, Anyanwu A, Friedman L, Beilin Y: Successful placement of a right ventricular assist device for treatment of a presumed amniotic fluid embolism. Anesth Analg. 2008, 107: 962-964. 10.1213/ane.0b013e31817f10e8.
Berman M, Tsui S, Vuylsteke A, Klein A, Jenkins DP: Life-threatening right ventricular failure in pulmonary hypertension: RVAD or ECMO?. J Heart Lung Transplant. 2008, 27: 1188-1189. 10.1016/j.healun.2008.07.017.
Keogh AM, Mayer E, Benza RL, Corris P, Dartevelle PG, Frost AE, Kim NH, Lang IM, Pepke-Zaba J, Sandoval J: Interventional and surgical modalities of treatment in pulmonary hypertension. J Am Coll Cardiol. 2009, 54: S67-S77. 10.1016/j.jacc.2009.04.016.
Strueber M, Hoeper MM, Fischer S, Cypel M, Warnecke G, Gottlieb J, Pierre A, Welte T, Haverich A, Simon AR, Keshavjee S: Bridge to thoracic organ transplantation in patients with pulmonary arterial hypertension using a pumpless lung assist device. Am J Transplant. 2009, 9: 853-857. 10.1111/j.1600-6143.2009.02549.x.
Felton TW, McCormick BA, Finfer SR, Fisher MM: Life-threatening pulmonary hypertension and right ventricular failure complicating calcium and phosphate replacement in the intensive care unit. Anaesthesia. 2006, 61: 49-53. 10.1111/j.1365-2044.2005.04381.x.
Satoh H, Masuda Y, Izuta S, Yaku H, Obara H: Pregnant patient with primary pulmonary hypertension: general anesthesia and extracorporeal membrane oxygenation support for termination of pregnancy. Anesthesiology. 2002, 97: 1638-1640. 10.1097/00000542-200212000-00045.
Chan CY, Chen YS, Ko WJ, Wang SS, Chiu IS, Lee YC, Chu SH: Extracorporeal membrane oxygenation support for single lung transplantation in a patient with primary pulmonary hypertension. J Heart Lung Transplant. 1998, 17: 325-327.
Jones RL, St Cyr JA, Tornabene SP, Lauber B, Harken AH: Reversible pulmonary hypertension secondary to mitral valvular disease as an indication for extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 1991, 5: 494-497. 10.1016/1053-0770(91)90126-E.
Gregoric ID, Chandra D, Myers TJ, Scheinin SA, Loyalka P, Kar B: Extracorporeal membrane oxygenation as a bridge to emergency heart-lung transplantation in a patient with idiopathic pulmonary arterial hypertension. J Heart Lung Transplant. 2008, 27: 466-468. 10.1016/j.healun.2008.01.016.
Hsu HH, Ko WJ, Chen JS, Lin CH, Kuo SW, Huang SC, Lee YC: Extracorporeal membrane oxygenation in pulmonary crisis and primary graft dysfunction. J Heart Lung Transplant. 2008, 27: 233-237. 10.1016/j.healun.2007.11.570.
Berman M, Tsui S, Vuylsteke A, Snell A, Colah S, Latimer R, Hall R, Arrowsmith JE, Kneeshaw J, Klein AA, Jenkins DP: Successful extracorporeal membrane oxygenation support after pulmonary thromboendarterectomy. Ann Thorac Surg. 2008, 86: 1261-1267. 10.1016/j.athoracsur.2008.06.037.
Deehring R, Kiss AB, Garrett A, Hillier AG: Extracorporeal membrane oxygenation as a bridge to surgical embolectomy in acute fulminant pulmonary embolism. Am J Emerg Med. 2006, 24: 879-880. 10.1016/j.ajem.2006.03.009.
Haller I, Kofler A, Lederer W, Chemelli A, Wiedermann FJ: Acute pulmonary artery embolism during transcatheter embolization: successful resuscitation with veno-arterial extracorporeal membrane oxygenation. Anesth Analg. 2008, 107: 945-947. 10.1213/ane.0b013e31817f91d8.
Szocik J, Rudich S, Csete M: ECMO resuscitation after massive pulmonary embolism during liver transplantation. Anesthesiology. 2002, 97: 763-764. 10.1097/00000542-200209000-00059.
Arlt M, Philipp A, Iesalnieks I, Kobuch R, Graf BM: Successful use of a new hand-held ECMO system in cardiopulmonary failure and bleeding shock after thrombolysis in massive post-partal pulmonary embolism. Perfusion. 2009, 24: 49-50. 10.1177/0267659109106295.
Gold JP, Shemin RJ, DiSesa VJ, Cohn L, Collins JJ: Balloon pump support of the failing right heart. Clin Cardiol. 1985, 8: 599-602. 10.1002/clc.4960081110.
Arafa OE, Geiran OR, Andersen K, Fosse E, Simonsen S, Svennevig JL: Intraaortic balloon pumping for predominantly right ventricular failure after heart transplantation. Ann Thorac Surg. 2000, 70: 1587-1593. 10.1016/S0003-4975(00)01864-6.
Darrah WC, Sharpe MD, Guiraudon GM, Neal A: Intraaortic balloon counterpulsation improves right ventricular failure resulting from pressure overload. Ann Thorac Surg. 1997, 64: 1718-1723. 10.1016/S0003-4975(97)01102-8. discussion 1723-1714
Rothman A, Sklansky MS, Lucas VW, Kashani IA, Shaughnessy RD, Channick RN, Auger WR, Fedullo PF, Smith CM, Kriett JM, Jamieson SW: Atrial septostomy as a bridge to lung transplantation in patients with severe pulmonary hypertension. Am J Cardiol. 1999, 84: 682-686. 10.1016/S0002-9149(99)00416-6.
Rich S, Dodin E, McLaughlin VV: Usefulness of atrial septostomy as a treatment for primary pulmonary hypertension and guidelines for its application. Am J Cardiol. 1997, 80: 369-371. 10.1016/S0002-9149(97)00370-6.
Druml W, Steltzer H, Waldhausl W, Lenz K, Hammerle A, Vierhapper H, Gasic S, Wagner OF: Endothelin-1 in adult respiratory distress syndrome. Am Rev Respir Dis. 1993, 148: 1169-1173.
Lambermont B, Ghuysen A, Kolh P, Tchana-Sato V, Segers P, Gerard P, Morimont P, Magis D, Dogne JM, Masereel B, D'Orio V: Effects of endotoxic shock on right ventricular systolic function and mechanical efficiency. Cardiovasc Res. 2003, 59: 412-418. 10.1016/S0008-6363(03)00368-7.
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA: Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002, 346: 1281-1286. 10.1056/NEJMoa012835.
Marshall BE, Marshall C, Frasch F, Hanson CW: Role of hypoxic pulmonary vasoconstriction in pulmonary gas exchange and blood flow distribution. 1. Physiologic concepts. Intensive Care Med. 1994, 20: 291-297. 10.1007/BF01708968.
Tomashefski JF, Davies P, Boggis C, Greene R, Zapol WM, Reid LM: The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983, 112: 112-126.
Acknowledgements
SJB is grateful for the support of the UK NIHR Biomedical Research Centre Scheme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
LCP has received honoraria from Encysive Pharmaceuticals. SJW has received honoraria from Actelion Pharmaceuticals. SJB has received support for clinical trials from Pfizer, Astra Zeneca, and Baxter Healthcare.
Authors' contributions
LCP and SJB conceived of the review and participated in its design. LCP and SJW carried out the literature search and drafted the initial manuscript. All authors read and approved the final manuscript.
Laura C Price, Stephen J Wort contributed equally to this work.
Electronic supplementary material
13054_2010_8659_MOESM1_ESM.DOC
Additional file 1: Population, Intervention, Comparison and Outcome (PICO) evidence tables. This file contains structured detail for all studies included in the systematic review. According to GRADE method guidelines, a series of eight study questions was devised to approach the questions posed by the systematic literature review. The PICO table then describes each study according to the study type, the population studied, the intervention applied, the nature of the comparison or control group, and the studied outcome of interest appropriate to the study question. The final column grades the evidence according to the GRADE evidence level as very low-, low-, moderate-, or high-level evidence [80, 81]. (DOC 785 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Price, L.C., Wort, S.J., Finney, S.J. et al. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care 14, R169 (2010). https://doi.org/10.1186/cc9264
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc9264