Abstract
Idiopathic pulmonary fibrosis (IPF) is a dreadful, chronic, and irreversibly progressive fibrosing disease leading to death in all patients affected, and IPF acute exacerbations constitute the most devastating complication during its clinical course. IPF exacerbations are subacute/acute, clinically significant deteriorations of unidentifiable cause that usually transform the slow and more or less steady disease decline to the unexpected appearance of acute lung injury/acute respiratory distress syndrome (ALI/ARDS) ending in death. The histological picture is that of diffuse alveolar damage (DAD), which is the tissue counterpart of ARDS, upon usual interstitial pneumonia, which is the tissue equivalent of IPF. ALI/ARDS and acute interstitial pneumonia share with IPF exacerbations the tissue damage pattern of DAD. 'Treatment' with high-dose corticosteroids with or without an immunosuppressant proved ineffective and represents the coup de grace for these patients. Provision of excellent supportive care and the search for and treatment of the 'underlying cause' remain the only options. IPF exacerbations require rapid decisions about when and whether to initiate mechanical support. Admission to an intensive care unit (ICU) is a particular clinical and ethical challenge because of the extremely poor outcome. Transplantation in the ICU setting often presents insurmountable difficulties.
Similar content being viewed by others
Introduction and definitions
Idiopathic pulmonary fibrosis (IPF) is a dreadful, chronic, and irreversibly progressive fibrosing disease leading to death in all patients affected, and IPF exacerbations constitute the most devastating complication during its course [1–6]. IPF exacerbations appear more frequently than previously thought and represent a common terminal event [7, 8]. IPF lacks effective treatment, and survival is approximately 3 years [2, 6, 9, 10]. Best supportive care constitutes the only attainable therapeutic strategy and includes a more or less effective attempt to alleviate symptoms and prevent complications and a far more efficacious interventional approach consisting of the withdrawal of corticosteroids and immunosuppressants (commonly administered by clinicians) that are ineffective and harmful [2, 9, 11]. Transplantation is the only therapeutic option [12].
IPF exacerbations represent acute and clinically significant deteriorations of unidentifiable cause, transforming the slow and more or less steady disease decline [13] to the unexpected appearance of acute lung injury/acute respiratory distress syndrome (ALI/ARDS) ending in death [6, 14]. Occasionally, IPF exacerbations may present in a previously apparently healthy or minimally symptomatic individual and might represent acute progression of an unsuspected or undiagnosed early IPF [3, 15]. Definition criteria include IPF diagnosis, unexplained worsening or development of dyspnea within 30 days, new lung infiltrates (mainly ground glass upon honeycomb), and exclusion of any identifiable or treatable cause of lung injury [6]. Surgical lung biopsy per se constitutes a risk factor for their development [16] but, when performed for the investigation of the etiology of exacerbations or in autopsies, discloses a histological picture of diffuse alveolar damage (DAD), which is the ARDS tissue counterpart, upon usual interstitial pneumonia (UIP), which is the IPF tissue equivalent [4, 8, 17–19].
In IPF, anachronic and reiterative epithelial injury and loss of the alveolar-capillary integrity constitute the initial event and 'the point of no return' that trigger aberrant repair pathways leading to inappropriate, progressive, and heterogeneous lung scarring (UIP) [20–22]. DAD upon UIP might represent massive epithelial and endothelial injury of the lung areas yet preserved from scarring [9, 23]. Putative initiators of IPF include viruses, cigarette smoke, gastroesophageal reflux, and occupational exposure to wood and metals [24, 25]. Aging, by reducing efficiency in repairing damage, represents a cofactor [26]. The development of DAD upon UIP may relate to a clinically occult infection [14, 27], aspiration, or a distinct pathobiological manifestation of IPF [6]. 'Treatment' with high-dose corticosteroids with or without an immunosuppressant proved ineffective and represents the coup de grace for these patients [8]. IPF exacerbations require rapid decisions about when and whether to initiate mechanical support. However, the consideration of admission to an intensive care unit (ICU) is a particular clinical and ethical challenge because of poor outcome [28–32]. Transplantation in this setting presents insurmountable difficulties.
Epidemiology and risk factors
The incidence of IPF exacerbations varies greatly between studies (from 8.5% to 60%) mainly because of differences in their design [3–6, 8, 14, 16, 19, 28, 29, 31–35]: (a) case series and retrospective cohorts [4, 17–19], (b) randomized controlled trials of specific treatments for IPF [3], (c) autopsy reviews [8, 16, 33], and (d) retrospective reviews of ICU admissions [28, 29, 31, 32]. Discrepancies in reported frequencies should be attributed to the difficulty in strictly respecting the definition criteria especially concerning symptom duration (less than 4 weeks) and the definite exclusion of infection [3, 6, 36]. IPF exacerbations do not appear to be linked to disease duration, functional derangement, age, gender, or smoking history [4, 29], although further studies are necessary to confirm early development as well as lack of association with immunosuppression [37]. Exacerbation mortality approaches 100%, questioning the need for ICU admission [2–6, 8, 14, 16, 19, 28, 29, 31–34].
Etiologic and pathogenetic considerations
The definition of IPF exacerbations 'after excluding identifiable causes of lung injury' implies that in 'idiopathic' pulmonary fibrosis, 'idiopathic' exacerbations occur [3, 4, 6]. However, in clinical practice, when such a patient is referred to the emergency department (ED), the attending clinician has to face one of three clinical scenarios [38] (Figure 1). The first scenario is the case in which the physical evolution of IPF comes to the final end in which spontaneous breathing becomes unsupportable [39] (Figures 1a and 2). In this scenario, the exclusion of 'identifiable-treatable causes of lung deterioration' is demanding, but the only option attainable is palliation. The second scenario refers to 'true' IPF exacerbation that brings the patient to the ED (Figures 1b and 3). In this case, after admission to the hospital ward, the patient usually becomes unable to maintain spontaneous breathing within hours or very few days, often not enough time for the extensive work-up required to identify treatable factors of deterioration, and needs ventilatory support and ICU transfer [5, 7, 8]. The third scenario refers to the admission to the hospital ward of an IPF-deteriorated patient because of reversible causes either affecting the lung or not; in this case, early identification of the precipitating factor(s) and their prompt treatment are imperative (Figures 1c and 4). Nevertheless, borders between the above scenarios are unclear in routine clinical practice since exacerbations occur as a spectrum rather than a clearly definable event.
Clinical scenarios for the idiopathic pulmonary fibrosis (IPF) patient presenting in the emergency department with subacute/acute dyspnea. (a) Progression to the final end of the disease. (b) 'True' IPF exacerbation. (c) IPF exacerbation due to treatable causes. Acute exacerbation (AE) criteria are presented in [7]. For details about laboratory tests and blood/sputum/bronchoalveolar lavage (BAL) tests, see the 'Clinical and laboratory assessment' section. Cardiac echo, cardiac echocardiography; CTPA, computed tomography pulmonary angiography; HRCT, high-resolution computed tomography; ICU, intensive care unit; PE, pulmonary embolism; PH, pulmonary hypertension; PNX, pneumothorax; proBNP, pro-brain natriuretic peptide.
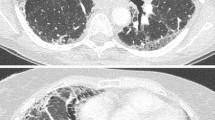
Idiopathic pulmonary fibrosis (IPF) progressing to the final end. Roentgenograms of a 57-year-old female (a) 6 years before diagnosis (normal) and (b) at the time of diagnosis of IPF. The latter reveals a bilateral reticular pattern. (c) High-resolution computed tomography shows mild reticulation. (d) Roentgenogram of the patient 24 months after diagnosis demonstrates worsening of the reticular pattern superimposed on a ground-glass pattern. The patient was admitted with severe breathlessness and productive cough. Her symptoms were severely aggravated in the last 9 months and she was hospitalized many times. She had received corticosteroids and mycophenolate mofetil, which were discontinued months prior to this roentgenogram because of lower respiratory tract infections. At the time of the roentgenogram, she was receiving only proton pump inhibitors. She deteriorated further despite best supportive care and died while on palliation treatment. Our putative diagnosis was IPF progressing to the final end.
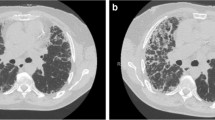
Idiopathic pulmonary fibrosis (IPF) 'idiopathic' exacerbation. (a-c) High-resolution computed tomography in an IPF patient at three different parenchymal levels shows diffuse areas of ground-glass attenuation, bronchiolectasis, and honeycombing. The patient was a 71-year-old male physician recently diagnosed with IPF. He was self-administering high-dose oral corticosteroids for months and presented severe deterioration of dyspnea and cough and developed severe acute respiratory failure. Despite empirical treatment with broad-spectrum antimicrobials, he deteriorated and was intubated. He died in the intensive care unit 4 weeks later. Extensive work-up disclosed no causative factors.
Exacerbation of idiopathic pulmonary fibrosis (IPF) due to treatable causes. (a) High-resolution computed tomography (HRCT) shows minimal evidence of apical interstitial lung disease. (b) HRCT shows, at the lung bases, ground-glass opacities upon extensive peripheral thickening of intralobular septa. The patient was a 65-year-old male with IPF and initiated treatment with high doses of corticosteroids. (c) Four months later, HRCT denotes diffuse ground-glass with irregular reticulation. Note the extensive lipomatosis of the mediastinum due to chronic steroid use. Owing to deterioration of dyspnea, he was admitted to another hospital, where bronchoalveolar lavage (BAL) was performed and the immunosuppressive treatment was intensified. A few weeks later, he was admitted to our department with respiratory failure, severe corticosteroid-related myopathy, diabetes mellitus, severe dyspnea, and purulent sputum. Clinical examination disclosed herpes simplex virus keratitis in the left eye, and BAL cultures grew positive for Pseudomonas aeruginosa. Corticosteroids were tapered, and antimicrobial and antiviral treatment was initiated. Both eye and lower respiratory tract infections subsided, and the patient was discharged home a few weeks later. (d) Eighteen months after the exacerbation, the ground-glass opacities completely resolved as did the lipomatosis of the mediastinum. The patient is still alive and at home.
However, even after the exclusion of any identifiable and treatable factor(s) inducing IPF exacerbations, the most important etiologic hypothesis remains that of a clinically occult infection that precipitates an already UIP-scarred lung into DAD [6]. For several reasons, viruses are the best etiologic candidates: (a) Epstein-Barr, cytomegalovirus, hepatitis C, herpes simplex, parvovirus B19, torque teno, and especially herpes viruses 7 and 8 have been implicated in IPF pathogenesis [40–42]; (b) flulike illness heralds the onset of exacerbations, and IPF mortality seems to peak in winter time and coincides with the peak of viral respiratory infections [43]; (c) in the mice pulmonary fibrosis experimental model, gammaherpesvirus induces exacerbations [44] as well as other viruses in vivo [45]; and (d) latent lung viral infections may reactivate under immunosuppression commonly used by clinicians [41]. Therefore, in IPF, viruses may act as both initiators and exacerbators because of their formidable ability to induce ARDS [46]. Besides viruses, microbials in traction bronchiectases/bronchiolectases are equally strong candidates. Bronchiectases are among the most common of the whole spectrum of lesions that characterize the architectural distortion in IPF. Interleukin-8, neutrophils, and alpha-defensins are increased or activated in stable or exacerbated patients with IPF [47, 48] and possibly play a role in triggering ARDS. In addition, immunosuppressive treatment certainly increases susceptibility to microbials.
Accordingly, further considerations have to be made. ALI/ARDS, acute interstitial pneumonia (AIP), and IPF exacerbations have common clinical, physiological, imaging, and histopathology features, and it is inconceivable that they do not also have common etiopathogenetic mechanisms (Figure 5). ALI/ARDS develops by different insults to the lung, and the mainstay of its treatment is provision of excellent supportive care and etiologic management of the underlying cause [46]. AIP is precisely an ARDS of 'unknown cause', and no specific clinical clues to differentiate between 'known and unknown cause' ARDS exist [49]. Criteria for the diagnosis of AIP are the same as in IPF exacerbations with the exception of the 'incubation' time (2 months instead of 4 weeks) and the prerequisite of normal chest roentgenogram. AIP, incomprehensibly, is included among the idiopathic interstitial pneumonias (IIPs) and probably should be added to the list of unknown cause ALI/ARDS, although some believe that AIP may represent a fulminant presentation of IIP secondary to imprecise autoimmune factors [1, 2]. Although there are no controlled trials of specific treatment, intensive immunosuppression has been the mainstay of treatment (usually under the coverage of several broad-spectrum antimicrobials, although this is not always stated) because of the inclusion of AIP among the IIPs [50]. However, AIP mortality approaches that of IPF exacerbations, and the provision of excellent supportive care and further search of underlying causative factors and adequate treatment seem more logical. In stable IPF (in contrast to other pneumonias), lung damage is not resolved by restitutio ad integrum. IPF exacerbations characterized by DAD upon UIP may represent the acute response of scarred and irreparably damaged lung. Epithelial cell apoptosis involves and is considered to be among the main pathogenetic mechanisms in the development of any DAD [51, 52]. Therefore, it seems incoherent that DAD, which is the common denominator of all ALI/ARDS, AIP, and IPF exacerbations and which develops upon different histology substrates (UIP in IPF exacerbations, normal lungs in AIP, and normal or diseased lungs in ARDS), presents at different time intervals (7 days for ARDS [46, 53], 4 weeks for IPF exacerbations [7], and 2 months for AIP [1]) and requires different pharmacologic approaches, which proved certainly fatal in AIP and in IPF 'true' exacerbations.
Different clinical settings characterized by diffuse alveolar damage (DAD) pathology. This non-proportional figure denotes the incoherence of the clinical significance of acute respiratory distress syndrome (ARDS), acute interstitial pneumonia (AIP), and idiopathic pulmonary fibrosis (IPF) exacerbations in which DAD, despite being the common denominator, develops upon different histology substrates (UIP in IPF exacerbations, normal lungs in AIP, and normal or diseased lungs in ARDS) and, according to current definitions, presents at different time intervals: 7 days for ARDS, 4 weeks for IPF exacerbations, and 2 months for AIP. This incoherence led also to a different pharmacologic approach, which proved to be unsuccessful at least in AIP and in IPF true exacerbations. ALI, acute lung injury.
Clinical and laboratory assessment
Early, accurate, and secure diagnosis is critical in IPF-exacerbated patients with reversible precipitating factor(s) (Figure 1) [2]. Investigation into medical history should focus on smoking habits, toxic exposures, prescribed medications, immunosuppression, and signs and symptoms of potentially undiagnosed autoimmune rheumatic disease [2, 54, 55]. Physical examination frequently reveals tachypnea, cyanosis, digital clubbing, bilateral inspiratory crackles, and lower extremity edema. In the most severe cases, the patient may be obtund or comatose because of severe hypoxemic and potentially hypercapnic respiratory failure. The presence of arrhythmias, chest pain, hemoptysis, or hemodynamic instability should guide the physician to an overlapping or alternative diagnosis such as acute coronary syndrome or pulmonary embolism.
Chest roentgenograms, including past imaging data, may help to orientate the clinician toward the identification of the causative agents of the exacerbation. Computed tomography pulmonary angiography is mandatory to exclude pulmonary embolism, and high-resolution computed tomography (HRCT) may document extension of honeycombing or other lung comorbidities (Figure 1). Echocardiography may also be useful. When early undiagnosed IPF presents with fulminant respiratory insufficiency and ARDS [3, 29], honeycombing with bibasilar and subpleural distribution on HRCT [1] can establish the diagnosis of IPF exacerbation and differentiate definitely from AIP [49]. In IPF exacerbations, HRCT reveals new bilateral ground-glass abnormalities or consolidations (or both) upon UIP pattern [6]. A ground-glass pattern, especially if extensive, is not a feature of stable IPF, and its rapid development away from areas of fibrosis heralds DAD. Akira and colleagues [18, 56] have proposed a classification of acute exacerbations of IPF on the basis of three ground-glass and consolidation computed tomography patterns that appear to have prognostic implications: (a) peripheral, (b) multifocal, and (c) diffuse, though others did not confirm a similar assumption [57].
Since no laboratory test is specific to IPF exacerbations, most tests are performed to exclude treatable causes of deterioration and to document the severity of the exacerbations. The standard laboratory work-up should include all necessary tests for the investigation of a critically ill patient with impeding ALI/ARDS of unknown etiology. ALI and ARDS criteria (arterial partial pressure of oxygen/fraction of inspired oxygen [PaO2/FiO2] of less than 300 and less than 200, respectively) should prepare the clinician for the possibility of mechanical support. Accurate diagnosis in IPF exacerbations requires bronchoalveolar lavage (BAL) to exclude infection or alternative diagnoses; BAL is best performed before mechanical support or immediately afterwards [2]. Performing lung biopsy could be justifiable when facing a disease with grave prognosis but bears an increased risk for post-surgical complications and should be individualized to each patient [2].
Current management
IPF exacerbations lack an effective treatment. Intensive immunosuppression proved harmful and fatal [2]. Patients presenting with IPF exacerbations must be managed in centers specializing in interstitial lung diseases (ILDs) with the availability of various specialties and departments such as a respiratory ward with a respiratory ICU/high-dependency unit (RICU/HDU), an ICU, and possibly a cardiothoracic transplantation center on a 24-hour basis. Lung transplantation constitutes a treatment option for IPF 'true' exacerbations [2, 38] but faces insurmountable difficulties, even in specialized centers.
Management depends on the clinical scenario (Figure 1). In case of progression to the final end (Figure 1a), palliation is more appropriate [2]. Non-invasive ventilation (NIV), by decreasing breathing work, is considered a major palliative option that, together with best supportive care, may help to reduce patient discomfort and permits management in an RICU [58, 59]. Patients with 'true' IPF exacerbations (Figure 1b), in which the diagnostic approach fails to identify a possible infective etiology, must continue to receive empirical antimicrobial therapy that takes into consideration factors such as immunosuppression, previous colonization, BAL timing, onset of mechanical support, and results of obtained cultures [2]. 'Specific' therapies for 'true' IPF exacerbations until now have consisted of high-dose intravenous corticosteroids plus an immunosuppressant [2]. However, Cochrane reviews for the efficacy of these therapies concluded that there is no evidence for any benefit of both corticosteroids and immunosuppressants in IPF [60, 61]. Besides, both progression of fibrosis on native lung in single-lung transplant patients and IPF 'true' exacerbations have been described in the heavily immunodepressed transplanted patient [10]. NIV may also help to wean the very few survivors from the IPF exacerbations and also constitutes the bridge to transplantation [62]. To promptly recognize and treat reversible precipitating factors implicated in IPF exacerbations (Figure 1c), recovery in the RICU/HDU or (in case of multiorgan failure) in the ICU is mandatory [63].
Toward the intensive care unit
An IPF patient is referred to the ICU for severe acute respiratory failure as a consequence of the clinical scenarios (mentioned above) that may lead to ventilatory support (Figure 1). Progression to the final end reaches a point at which spontaneous ventilation in no longer possible (Figure 1a). ICU admission of these patients, because of the poor outcome, should be avoided [2] (Figure 2). In 'true' IPF exacerbations (Figure 1b), ventilatory support and ICU transfer buy time and could have some influence on final outcome in specific patients. Unfortunately, in the vast majority, this does not happen, and the mortality of this patient population is high, higher even than that predicted by the usual clinical score [2, 31] (Figure 3). Admission of an IPF patient to the ICU because of reversible causes either affecting the lung or not (Figures 1c and 4) bears better prognosis, but special attention should be paid to avoid further complications. The complexity of the above scenarios underscores the importance of good communication between referring and ICU physicians.
So far, the studies of IPF patients in the ICU have had many limitations (Table 1) [4, 28, 29, 31, 32, 34, 59, 64]. These studies are usually retrospective and single-centered and include limited numbers of patients. In addition, most of these studies include all IPF patients admitted to the ICU for respiratory failure regardless of etiology, the proportion of patients with confirmed diagnosis is variable, the ventilator parameters are usually not reported, and the pharmacologic therapy demonstrates a significant diversity. The only common parameter is the conclusion: the prognosis of ventilated IPF patients is disappointing [2]. Given these results, what may be the goals of ICU support for a patient with an IPF exacerbation? Although definite conclusions cannot be drawn, there is a general feeling that mechanical ventilation and intensive support do not have a significant effect on outcome [2]. Could this be due to the disease itself, ventilator-induced lung injury, complications of intensive support (sepsis, critical care myoneuropathy, or ventilator-associated pneumonia), or a combination of the above? Only assumptions can be made, and patients (at an earlier stage) and relatives as well as physicians outside of the ICU before or at admission should become aware of the poor prognosis. This does not mean that IPF patients with acute respiratory failure should be denied admission; in many hospitals, the ICU is the right place to perform in a safe and timely fashion the necessary extended investigation to exclude reversible causes of deterioration in these patients.
Ventilating a patient with an IPF exacerbation is a difficult and demanding task, and no 'cookbook' prescriptions can make the work easier for the intensivist. The evidence for the best ventilator strategy applying to an IPF exacerbation is extremely scarce, and the effect of ventilatory management on outcome has not been systematically assessed; therefore, every suggestion is based on theoretical principles and pathologic data that are characterized mainly by extended DAD [7]. Recently, Bates and colleagues [65] introduced the concept of percolation, according to which the progression of parenchymal lung disease can suddenly reach a threshold that dramatically alters the mechanical properties of the lung. IPF exacerbations that require ventilator support could be an example of crossing this percolation threshold. Under these circumstances, mechanical ventilation could represent a second hit for the lung parenchyma, further deteriorating the mechanical properties of lung parenchyma and introducing a vicious cycle that ends in death. Mechanical ventilation with conventional volumes (8 mL/kg) in patients without lung injury can induce severe surfactant impairment, and sustained plasma cytokine production has been demonstrated in patients without ALI ventilated with conventional tidal volumes (10 mL/kg) compared with those ventilated with low tidal volumes (6 mL/kg) [66, 67]. So it should not be surprising, although it may be very difficult to prove, that the employment of traditional tidal volumes in patients with IPF exacerbations would be detrimental given that their lungs are characterized by extended parenchymal alterations, severe inhomogeneity, and decreased compliance even prior to initiation of mechanical ventilation. Especially the inhomogeneity of the lung parenchyma could cause severe overinflation of the 'healthy' lung units with higher compliance and jeopardize the 'healthy' parenchyma left. A ventilation strategy employing low tidal volumes (4 to 6 mL/kg ideal body weight), such as that used for patients with ARDS, seems prudent and is advised by many experts [68]. Positive end-expiratory pressure (PEEP) should be used moderately because of the aforementioned risk of overinflation of intact lung units. Fernández-Pérez and colleagues [64] demonstrated that high PEEP was independently associated with increased mortality in chronic ILD [64]. In the same context, there is no place for recruitment or prone position [69]. Given that intubated patients with IPF exacerbations require high-minute volume because of increased dead space, the respiratory frequency should be increased to the maximum acceptable rate and the target of a normal PaCO2 (arterial partial pressure of carbon dioxide) should be abandoned. This high respiratory rate might require the use of heavy sedation and quite often paralysis, and care should be given to avoid auto-PEEP [70]. The effect of prolonged sedation and paralysis on the neuromuscular function of these patients, who have often been administered steroids for a long time, is an unavoidable cost. The earliest possible interruption of sedation will facilitate weaning provided that gas exchange and lung mechanics have improved.
NIV has some theoretical advantages in IPF patients and has been used extensively in cases of acute respiratory failure to avoid intubation. Unfortunately, the studies about its use have the same methodological problems as those for the invasive ventilation studies mentioned previously, and no firm conclusions can be drawn. There are two things that make NIV more 'attractive' in this setting: the almost absolute mortality that invasive mechanical ventilation carries and the avoidance of intubation and ventilation risks (aspiration, ventilator- associated pneumonia, and ventilator-associated injury). The problem is that in most cases the excessive work of breathing associated with IPF exacerbation cannot be managed effectively by NIV. Extracorporeal membrane oxygenation could represent a valuable adjunct to conventional treatment for selected cases of IPF. Limited availability, high cost, complicated technology, and increased rates of complications have been the most important factors limiting its use so far [71–73]. In the setting of IPF therapeutics, it has been used mainly as a bridge to transplantation [74]. Transplantation represents the final line of defense for the IPF patient and is the only therapy with a proven survival benefit. Early referral (even at the time of diagnosis) to a lung transplant center is mandatory [75] because of the prolonged waiting-list time, which sometimes exceeds the patient's life expectancy.
Conclusions
IPF exacerbations constitute the most devastating complication of IPF. Different and hard-to-differentiate clinical scenarios may reproduce the hallmark of their definition: subacute/acute deterioration of dyspnea and bilateral chest infiltrates, corresponding in 'true' IPF exacerbations, to a tissue pattern of DAD upon UIP. Also, ALI/ARDS and AIP present DAD. Intensive immunosuppression proved ineffective and represents the coup de grace for these patients. Provision of excellent supportive care and the search for and treatment of the 'underlying cause' remain the only options. The unravelling of Ariadne's thread continues.
Abbreviations
- AIP:
-
acute interstitial pneumonia
- ALI:
-
acute lung injury
- ARDS:
-
acute respiratory distress syndrome
- BAL:
-
bronchoalveolar lavage
- DAD:
-
diffuse alveolar damage
- ED:
-
emergency department
- HDU:
-
high-dependency unit
- HRCT:
-
high-resolution computed tomography
- ICU:
-
intensive care unit
- IIP:
-
idiopathic interstitial pneumonia
- ILD:
-
interstitial lung disease
- IPF:
-
idiopathic pulmonary fibrosis
- NIV:
-
non-invasive ventilation
- PEEP:
-
positive end-expiratory pressure
- RICU:
-
respiratory intensive care unit
- UIP:
-
usual interstitial pneumonia.
References
American Thoracic Society/European Respiratory Society: International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med 2002, 165: 277-304.
Wells AU, Hirani N, on behalf of the BTS Group, a subgroup of the British Thoracic Society Standards of Care Committee, in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society: Interstitial lung disease guideline. Thorax 2008,63(Suppl 5):1-58. 10.1136/thx.2008.101691
Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE Jr, Flaherty KR, Schwartz DA, Noble PW, Raghu G, Brown KK: The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med 2005, 142: 963-967.
Kim DS, Park JH, Park BK, Nicholson AG, Colby T: Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 2006, 27: 143-150. 10.1183/09031936.06.00114004
Crowley SP, Kelly P, Egan JJ: Acute exacerbations in idiopathic pulmonary fibrosis. Ann Intern Med 2006, 144: 218-219.
Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE Jr, Lasky JA, Loyd JE, Noth I, Olman MA, Raghu G, Roman J, Ryu JH, Zisman DA, Hunninghake GW, Colby TV, Egan JJ, Hansell DM, Johkoh T, Kaminski N, Kim DS, Kondoh Y, Lynch DA, Müller-Quernheim J, Myers JL, Nicholson AG, Selman M, Toews GB, Wells AU, Martinez FJ, Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators: Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007, 176: 636-643. 10.1164/rccm.200703-463PP
Daniels CE, Yi ES, Ryu JH: Autopsy findings in 42 consecutive patients with idiopathic pulmonary fibrosis. Eur Respir J 2008, 32: 170-174. 10.1183/09031936.00176307
Rice AJ, Wells AU, Bouros D, du Bois RM, Hansell DM, Polychronopoulos V, Vassilakis D, Kerr JR, Evans TW, Nicholson AG: Terminal diff use alveolar damage in relation to interstitial pneumonias. An autopsy study. Am J Clin Pathol 2003, 119: 709-714. 10.1309/UVARMDY8FE9FJDKU
du Bois RM: Strategies for treating idiopathic pulmonary fibrosis. Nat Rev Drug Discov 2010, 9: 129-140. 10.1038/nrd2958
Elicker BM, Golden JA, Ordovas KG, Leard L, Golden TR, Hays SR: Progression of native lung fibrosis in lung transplant recipients with idiopathic pulmonary fibrosis. Respir Med 2010, 104: 426-433. 10.1016/j.rmed.2009.10.019
Walter N, Collard HR, King TE: Current perspectives on the treatment of idiopathic pulmonary fibrosis. Proc Am Thorac Soc 2006, 3: 330-338. 10.1513/pats.200602-016TK
Mason DP, Brizzio ME, Alster JM, McNeill AM, Murthy SC, Budev MM, Mehta AC, Minai OA, Pettersson GB, Blackstone EH: Lung transplantation for idiopathic pulmonary fibrosis. Ann Thorac Surg 2007, 84: 1121-1128. 10.1016/j.athoracsur.2007.04.096
Selman M, Carrillo G, Estrada A, Mejia M, Becerril C, Cisneros J, Gaxiola M, Pėrez-Padilla R, Navarro C, Richards T, Dauber J, King TE Jr, Pardo A, Kaminski N: Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS One 2007, 2: e482. 10.1371/journal.pone.0000482
Hyzy R, Huang S, Myers J, Flaherty K, Martinez F: Acute exacerbation of idiopathic pulmonary fibrosis. Chest 2007, 132: 1652-1658. 10.1378/chest.07-0299
Sakamoto K, Taniguchi H, Kondoh Y, Ono K, Hasegawa Y, Kitaichi M: Acute exacerbation of idiopathic pulmonary fibrosis as the initial presentation of the disease. Eur Respir Rev 2009, 18: 129-132. 10.1183/09059180.00000409
Kondoh Y, Taniguchi H, Kitaichi M, Yokoi T, Johkoh T, Oishi T, Kimura T, Nishiyama O, Kato K, du Bois RM: Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Respir Med 2006, 100: 1753-1759. 10.1016/j.rmed.2006.02.002
Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, Takagi K: Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest 1993, 103: 1808-1812. 10.1378/chest.103.6.1808
Akira M, Hamada H, Sakatani M, Kobayashi C, Nishioka M, Yamamoto S: CT findings during phase of accelerated deterioration in patients with idiopathic pulmonary fibrosis. AJR 1997, 168: 79-83.
Churg A, Muller NL, Silva CI, Wright JL: Acute exacerbation (acute lung injury of unknown cause) in UIP and other forms of fibrotic interstitial pneumonias. Am J Surg Pathol 2007, 31: 277-284. 10.1097/01.pas.0000213341.70852.9d
Selman M, King TE, Pardo A: Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001, 134: 136-151.
Selman M, Pardo A: Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 2006, 3: 364-372. 10.1513/pats.200601-003TK
Strieter RM, Mehrad B: New mechanisms of pulmonary fibrosis. Chest 2009, 136: 1364-1370. 10.1378/chest.09-0510
Konishi K, Gibson KF, Lindell KO, Richards TJ, Zhang Y, Dhir R, Bisceglia M, Gilbert S, Yousem SA, Song JW, Kim DS, Kaminski N: Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009, 180: 167-175. 10.1164/rccm.200810-1596OC
Kuwano K, Nomoto Y, Kunitake R, Hagimoto N, Matsuba T, Nakanishi Y, Hara N: Detection of adenovirus E1A DNA in pulmonary fibrosis using nested polymerase chain reaction. Eur Respir J 1997, 10: 1445-1449. 10.1183/09031936.97.10071445
Tobin RW, Pope CE, Pellegrini CA, Emond MJ, Sillery J, Raghu G: Increased prevalence of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998, 158: 1804-1808.
Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, Lansdorp PM, Greider CW, Loyd JE: Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 2007, 356: 1317-1326. 10.1056/NEJMoa066157
Vannella KM, Moore BB: Viruses as co-factors for the initiation of exacerbation of lung fibrosis. Fibrogenesis Tissue Repair 2008, 1: 2. 10.1186/1755-1536-1-2
Blivet S, Philit F, Sab JM, Langevin B, Paret M, Guérin C, Robert D: Outcome of patients with idiopathic pulmonary fibrosis admitted to the ICU for respiratory failure. Chest 2001, 120: 209-212. 10.1378/chest.120.1.209
Stern JB, Mal H, Groussard O, Brugiere O, Marceau A, Jebrak G, Fournier M: Prognosis of patients with advanced idiopathic pulmonary fibrosis requiring mechanical ventilation for acute respiratory failure. Chest 2001, 120: 213-219. 10.1378/chest.120.1.213
Fumeaux T, Rothmeier C, Jolliet P: Outcome of mechanical ventilation for acute respiratory failure in patients with pulmonary fibrosis. Intensive Care Med 2001, 27: 1868-1874. 10.1007/s00134-001-1150-0
Saydain G, Islam A, Afessa B, Ryu JH, Scott JP, Peters SG: Outcome of patients with idiopathic pulmonary fibrosis admitted to the intensive care unit. Am J Respir Crit Care Med 2002, 166: 839-842. 10.1164/rccm.2104038
Al-Hameed FM, Sharma S: Outcome of patients admitted to the intensive care unit for acute exacerbation of idiopathic pulmonary fibrosis. Can Respir J 2004, 11: 117-122.
Tiitto L, Bloigu R, Heiskanen U, Pääkkö P, Kinnula VL, Kaarteenaho-Wiik R: Relationship between histopathological features and the course of idiopathic pulmonary fibrosis/usual interstitial pneumonia. Thorax 2006, 61: 1091-1095. 10.1136/thx.2005.055814
Rangappa P, Moran JL: Outcomes of patients admitted to the intensive care unit with idiopathic pulmonary fibrosis. Crit Care Resusc 2009, 11: 102-109.
Fernández Pérez ER, Daniels CE, Schroeder DR, St Sauver J, Hartman TE, Bartholmai BJ, Yi ES, Ryu JH: Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis. Chest 2010, 137: 129-137. 10.1378/chest.09-1002
Kim DS, Collard HR, King TE Jr: Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006, 3: 285-292. 10.1513/pats.200601-005TK
Noth I, Martinez FJ: Recent advances in idiopathic pulmonary fibrosis. Chest 2007, 132: 637-650. 10.1378/chest.06-1927
Wuyts WA, Thomeer M, Dupont LJ, Verleden GM: An algorithm to tackle acute exacerbations in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2008, 177: 1397.
Panos RJ, Mortenson RL, Niccoli SA, King TE: Clinical deterioration in patients with idiopathic pulmonary fibrosis: causes and assessment. Am J Med 1990, 88: 396-404. 10.1016/0002-9343(90)90495-Y
Egan JJ, Stewart JP, Hasleton PS, Arrand JR, Carroll KB, Woodcock AA: Epstein-Barr virus replication within pulmonary epithelial cells in cryptogenic fibrosing alveolitis. Thorax 1995, 50: 1234-1239. 10.1136/thx.50.12.1234
Kottmann RM, Hogan CM, Phipps RP, Sime PJ: Determinants of initiation and progression of idiopathic pulmonary fibrosis. Respirology 2009, 14: 917-933. 10.1111/j.1440-1843.2009.01624.x
Vannella KM, Luckhardt TR, Wilke CA, van Dyk LF, Toews GB, Moore BB: Latent herpesvirus infection augments experimental pulmonary fibrosis. Am J Respir Crit Care Med 2010, 181: 465-477. 10.1164/rccm.200905-0798OC
Olson AL, Swigris JJ, Raghu G, Brown KK: Seasonal variation: mortality from pulmonary fibrosis is greatest in the winter. Chest 2009, 136: 16-22. 10.1378/chest.08-0703
McMillan TR, Moore BB, Weinberg JB, Vannella KM, Fields WB, Christensen PJ, van Dyk LF, Toews GB: Exacerbation of established pulmonary fibrosis in a murine model by gammaherpesvirus. Am J Respir Crit Care Med 2008, 177: 771-780. 10.1164/rccm.200708-1184OC
Malizia AP, Keating DT, Smith SM, Walls D, Doran PP, Egan JJ: Alveolar epithelial cell injury with Epstein-Barr virus upregulates TGFbeta1 expression. Am J Physiol Lung Cell Mol Physiol 2008, 295: L451-60. 10.1152/ajplung.00376.2007
Wheeler AP, Bernard GR: Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 2007, 369: 1553-1565. 10.1016/S0140-6736(07)60604-7
Ziegenhagen MW, Zabel P, Zissel G, Schlaak M, Müller-Quernheim J: Serum level of interleukin 8 is elevated in idiopathic pulmonary fibrosis and indicates disease activity. Am J Respir Crit Care Med 1998, 157: 762-768.
Konishi K, Gibson KF, Richards TJ, Lindell KO, Chensny LJ, Zhang Y, Kaminski N, Kim DS: Expression of alpha-defensins in the lungs and peripheral blood of patients with acute exacerbations of idiopathic pulmonary fibrosis [abstract]. Am J Respir Crit Care Med 2009, 179: s3020.
Swigris JJ, Brown KK: Acute interstitial pneumonia and acute exacerbations of idiopathic pulmonary fibrosis. Semin Respir Crit Care Med 2006, 27: 659-667. 10.1055/s-2006-957337
Bouros D, Nicholson AC, Polychronopoulos V, du Bois RM: Acute interstitial pneumonia. Eur Respir J 2000, 15: 412-418. 10.1034/j.1399-3003.2000.15b31.x
Martin TR, Nakamura M, Matute-Bello G: The role of apoptosis in acute lung injury. Crit Care Med 2003,31(Suppl 4):S184-S188. 10.1097/01.CCM.0000057841.33876.B1
Matute-Bello G, Martin TR: Science review: apoptosis in acute lung injury. Crit Care 2003, 7: 355-358. 10.1186/cc1861
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R: The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994, 149: 818-824.
Hamilton CD: Immunosuppression related to collagen-vascular disease or its treatment. Proc Am Thorac Soc 2005, 2: 456-460. 10.1513/pats.200508-091JS
Tzelepis GE, Toya SP, Moutsopoulos HM: Occult connective tissue diseases mimicking idiopathic interstitial pneumonias. Eur Respir J 2008, 31: 11-20. 10.1183/09031936.00060107
Akira M, Kozuka T, Yamamoto S, Sakatani M: Computed tomography findings in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2008, 178: 372-378. 10.1164/rccm.200709-1365OC
Silva CI, Müller NL, Fujimoto K, Kato S, Ichikado K, Taniguchi H, Kondoh Y, Johkoh T, Churg A: Acute exacerbation of chronic interstitial pneumonia: high-resolution computed tomography and pathologic findings. J Thorac Imaging 2007, 22: 221-229. 10.1097/01.rti.0000213588.52343.13
Bertolini G, Confalonieri M, Rossi C, Rossi G, Simini B, Gorini M, Corrado A, GiViTI (Gruppo italiano per la Valutazione degli interventi in Terapia Intensiva) Group; Aipo (Associazione Italiana Pneumologi Ospedalieri) Group: Costs of the COPD. Differences between intensive care unit and respiratory intermediate care unit. Respir Med 2005, 99: 894-900. 10.1016/j.rmed.2004.11.014
Mollica C, Paone G, Conti V, Ceccarelli D, Schmid G, Mattia P, Perrone N, Petroianni A, Sebastiani A, Cecchini L, Orsetti R, Terzano C: Mechanical ventilation in patients with end-stage idiopathic pulmonary fibrosis. Respiration 2010, 79: 209-215. 10.1159/000225932
Richeldi L, Davies HR, Ferrara G, Franco F: Corticosteroids for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev 2003, 3: CD002880.
Davies HR, Richeldi L, Walters EH: Immunomodulatory agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev 2003, 3: CD003134.
Trulock EP, Edwards LB, Taylor DO, Boucek MM, Mohacsi PJ, Keck BM, Hertz MI: The Registry of the International Society for Heart and Lung Transplantation: twentieth official adult lung and heart-lung transplant report-2003. J Heart Lung Transplant 2003, 22: 625-635. 10.1016/S1053-2498(03)00182-7
Corrado A, Roussos C, Ambrosino N, Confalonieri M, Cuvelier A, Elliott M, Ferrer M, Gorini M, Gurkan O, Muir JF, Quareni L, Robert D, Rodenstein D, Rossi A, Schoenhofer B, Simonds AK, Strom K, Torres A, Zakynthinos S, European Respiratory Society Task Force on epidemiology of respiratory intermediate care in Europe: Respiratory intermediate care units: a European survey. Eur Respir J 2002, 20: 1343-1350. 10.1183/09031936.02.00302602
Fernández-Pérez E, Yilmaz M, Jenad H, Daniels C, Ryu J, Hubmayr R, Gajic O: Ventilator settings and outcome of respiratory failure in chronic interstitial lung disease. Chest 2008, 133: 1113-1119. 10.1378/chest.07-1481
Bates JH, Davis GS, Majumdar A, Butnor KJ, Suki B: Linking parenchymal disease progression to changes in lung mechanical function by percolation. Am J Respir Crit Care Med 2007, 176: 617-623. 10.1164/rccm.200611-1739OC
Tsangaris I, Lekka ME, Kitsiouli E, Constantopoulos S, Nakos G: Bronchoalveolar lavage alterations during prolonged ventilation of patients without acute lung injury. Eur Respir J 2003, 21: 495-501. 10.1183/09031936.03.00037902
Determann RM, Royakkers A, Wolthuis EK, Vlaar AP, Choi G, Paulus F, Hofstra JJ, de Graaff MJ, Korevaar JC, Schultz MJ: Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care 2010, 14: R1. 10.1186/cc8230
The Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000, 342: 1301-1307. 10.1056/NEJM200005043421801
Nakos G, Tsangaris I, Kostanti E, Nathanail C, Lachana A, Koulouras V, Kastani D: Effect of the prone position on patients with hydrostatic pulmonary edema compared with patients with acute respiratory distress syndrome and pulmonary fibrosis. Am J Respir Crit Care Med 2000, 161: 360-368.
Patroniti N, Pesenti A: Low tidal volume, high respiratory rate and auto-PEEP: the importance of the basics. Crit Care 2003, 7: 105-106. 10.1186/cc1883
Lewandowski K: Extracorporeal membrane oxygenation for severe acute respiratory failure. Crit Care 2000, 4: 156-168. 10.1186/cc689
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D, CESAR trial collaboration: Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009, 374: 1351-1363. 10.1016/S0140-6736(09)61069-2
Zimmermann M, Bein T, Arlt M, Philipp A, Rupprecht L, Mueller T, Lubnow M, Graf BM, Schlitt HJ: Pumpless extracorporeal interventional lung assist in patients with acute respiratory distress syndrome: a prospective pilot study. Crit Care 2009, 13: R10. 10.1186/cc7703
Santambrogio L, Nosotti M, Palleschi A, Tosi D, Mendogni P, Lissoni A, Blasi F, Rosso L: Use of venovenous extracorporeal membrane oxygenation as a bridge to urgent lung transplantation in a case of acute respiratory failure. Transplant Proc 2009, 41: 1345-1346. 10.1016/j.transproceed.2009.02.065
Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, Egan T, Keshavjee S, Knoop C, Kotloff R, Martinez FJ, Nathan S, Palmer S, Patterson A, Singer L, Snell G, Studer S, Vachiery JL, Glanville AR: International guidelines for the selection of lung transplant candidates: 2006 update-a consensus report from the pulmonary scientific council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006, 25: 745-755. 10.1016/j.healun.2006.03.011
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
Papiris, S.A., Manali, E.D., Kolilekas, L. et al. Clinical review: Idiopathic pulmonary fibrosis acute exacerbations - unravelling Ariadne's thread. Crit Care 14, 246 (2010). https://doi.org/10.1186/cc9241
Published:
DOI: https://doi.org/10.1186/cc9241