Abstract
Candida is one of the most frequent pathogens in bloodstream infections, and is associated with significant morbidity and mortality. The epidemiology of species responsible for invasive candidiasis, both at local and worldwide levels, has been changing - shifting from Candida albicans to non-albicans species, which can be resistant to fluconazole (Candida krusei and Candida glabrata) or difficult to eradicate because of biofilm production (Candida parapsilosis). Numerous intensive care unit patients have multiple risk factors for developing this infection, which include prolonged hospitalisation, use of broad-spectrum antibiotics, presence of intravascular catheters, parenteral nutrition, high Acute Physiology and Chronic Health Evaluation score, and so forth. Moreover, delaying the specific therapy was shown to further increase morbidity and mortality. To minimise the impact of this infection, several management strategies have been developed - prophylaxis, empirical therapy, pre-emptive therapy and culture-based treatment. Compared with prophylaxis, empirical and pre-emptive approaches allow one to reduce the exposure to antifungals by targeting only the patients at high risk of candidemia, without delaying therapy until the moment blood Candida is identified in blood cultures. The agents recommended for initial treatment of candidemia in critically ill patients include echinocandins and lipid formulation of amphotericin B.
Similar content being viewed by others
Introduction
Fungal infections are being increasingly diagnosed in patients admitted to the intensive care unit (ICU). Advances in medical science allow patients with severe and complicated diseases to survive, and thus a population of subjects vulnerable to a range of infections is created. Candida is the most common fungal pathogen in ICU patients, and the main clinical forms are bloodstream infection, followed by peritonitis and other abdominal infection, endocarditis, and so forth. Most of the patients included in studies on epidemiology or treatment of invasive candidiasis had candidemia (approximately 68 to 90%), with or without other sites affected, while peritonitis was the second most common disease (approximately 7 to 30% of subjects) [1–3].
Candidemia is a life-threatening infection with high morbidity and mortality, especially in immunocom promised and critically ill patients [4–7]. In the ICU, this infection may represent up to 15% of nosocomial infections and the crude mortality rate has been found as high as 25 to 60%, varying according to the study design and the population - with the estimated attributable mortality as high as 47% [8–11]. Additionally, the estimated costs of each episode of invasive candidiasis in hospitalised adults are tremendous [11, 12]. Finally, nosocomial fungal infections have one of the highest rates of inappropriate therapy - consisting mostly of omission of initial empirical therapy and an inadequate dose of fluconazole - which has been associated with increased mortality [13–16].
Moreover, during the past decade, several new antifungal drugs have been developed and obtained approval for treatment of Candida infections. Among them, echinocandins are the most important from the point of view of treating candidemia in critically ill patients. Epidemiology, risk factors, diagnosis and, in particular, treatment strategies and guidelines will therefore be discussed further.
Epidemiology of invasive candidiasis
Candidemia is one of the most frequent and most serious infections in patients admitted to the ICU, being the fourth most frequent pathogen of bloodstream infections in North America [17]. Moreover, candidemia in the ICU is by far more common than in most other wards and can affect up to about 10% of all admitted patients [18, 19]. Additionally, Candida species account for approximately 3% of all surgery-related peritoneal infections, both community-acquired infections and nosocomial infections [20]. A recent French study on invasive candidiasis in the ICU revealed that approximately one-third of patients presented each of the clinical forms: isolated candidemia, invasive candidiasis with candidemia and invasive candidiasis only [1].
The epidemiology of Candida infections - both on a worldwide scale and, more importantly, on a local level - has significant implications for management of this infection. In particular, given the well-known, albeit not universal, differences in antifungal susceptibility among different Candida species, the choice of the most appropriate empirical treatment can be successfully based on epidemiological data regarding the frequency of Candida parapsilosis and fluconazole-resistant species in a given centre.
During the past two decades, most hospitals have reported a progressive shift in the species of Candida. In the past, almost all isolates responsible for bloodstream infections were Candida albicans, whereas in recent years a growing proportion of episodes of candidemia have been caused by Candida species other than C. albicans [21–25]. Even though, C. albicans remains the predominant strain in most countries [26, 27], even among critically ill patients [1, 7, 21, 28, 29], non-albicans species are increasingly common - some ICUs have reported recently that non-albicans species are responsible for over 50% of candidemia episodes in adult critically ill patients [24, 30]. The most common non-albicans species are C. parapsilosis or Candida glabrata, followed by Candida tropicalis and Candida krusei [1, 24, 31–33]. Rare Candida species reported to cause candidemia include Candida lusitaniae, Candida guilliermondii and Candida rugosa [15, 31].
Numerous studies have tried to find reasons for this shift, and several risk factors have been associated with candidemia due to different species [25, 34]. It is understandableh that the widespread use of fluconazole can predispose patients to the development of infections due to species that are resistant to azoles, either intrinsically fully resistant such as C. krusei or in dose-dependent fashion such as C. glabrata. Indeed, previous use of fluconazole has been found to be a risk factor for the presence of non-albicans fungaemia [24, 25, 35] even though some studies did not find this association [23]. Specific risk factors for candidemia due to other non albicans strains have also been reported, such as for example the presence of in-dwelling devices, hyperalimentation and being a neonate for C. parapsilosis [31]. The specific risk factors associated with different Candida species are outlined in Table 1.
Even though the overall rise in incidence of non albicans strains is alarming, from the clinical point of view there are important differences among the different species. Specifically, the main difference between C. albicans and C. kusei or C. glabrata is the resistance to the most frequently used antifungal (that is, fluconazole). The differences in susceptibility to various antifungals are partially predictable and are reported in Table 2. Species identification and knowledge of the local epidemiology of Candida strains causing candidemia are therefore of the utmost importance for guiding appropriate empirical therapy. On the contrary, in vitro susceptibility testing of clinical isolates of Candida proves extremely valuable for guiding therapy in patients who have received prior antifungal treatment or who are not responding to empirical therapy.
Risk factors for invasive candidiasis in the ICU and predictive scores
Although invasive Candida infections can affect any hospitalised patient, they are more common and have unique attributes in certain populations, including patients with cancer, haematological malignancy or other immunosuppression. The predominant source of invasive Candida infection is endogenous, from superficial mucosal and cutaneous proliferation to haematogenous dissemination [36]. Rare cases of exogenous transmission have been described due to contaminated solutions and materials or transmission from healthcare workers to patients and from patients to patients [37, 38].
The suppression of the normal bacterial flora in the gastrointestinal tract by broad-spectrum antibiotic therapy also allows the yeast to proliferate, both in neutropenic patients with haematological malignancies [39] and in non-neutropenic patients [40], and long-term and high-density colonisation has been shown to lead to candidemia. Numerous conditions frequent in ICU patients - such as parenteral nutrition, intravascular catheters, trauma, hypotension, therapy with steroids or cyclosporine, and ischaemia and reperfusion - may damage the integrity of skin or the gastrointestinal mucosa with penetration by the yeast, potentially leading to systemic infection. The factors predisposing critically ill patients to candidemia are presented in Table 3, with the presence of vascular catheters or disruption of the gut or skin barrier among the most important.
Important efforts are focused on identifying critically ill patients at high risk of developing candidemia in order to apply the most efficacious management strategy and avoid high mortality. Risk prediction scores have thus been developed and different parameters combined to predict which patients would develop candidiasis. In particular, the score by Leon and colleagues included parenteral nutrition (1 point), surgery (1 point), multifocal colonisation (1 point), and severe sepsis (2 points); subjects with score >2.5 were almost eight times more likely to develop candidiasis than those with score <2.5 [41]. The other score by Ostrosky-Zeichner and colleagues found that the combination of the systemic antibiotic treatment or central venous catheter and two or more of five other variables (parenteral nutrition, dialysis, major surgery, pancreatitis, treatment with steroids or other immunosuppressive agents) had, in their population, positive and negative predictive values of 10% and 97%, respectively [42]. Additionally, Dupont and colleagues published in 2003 a predictive score for peritoneum Candida infection in the ICU: the presence of three out of four factors (female gender, upper gastrointestinal tract origin of peritonitis, intraoperative cardiovascular failure and previous antibiotic therapy) had positive and negative predictive values of 67% and 72%, respectively [43].
Thanks to such scores specifically developed and then validated in ICU patients, the risk of invasive candidiasis can be estimated because the presence of the abovementioned risk factors is directly related to the percentage probability of developing invasive candidiasis, allowing one to judge whether risk of candidemia warrants any therapeutic measures.
Diagnosis of candidemia
Blood cultures remain the mainstay for diagnosing candidemia, but the sensitivity reported frequently is not optimal. Moreover, the time from blood sample collection and the microbiological response of growing yeast is often lengthy. Furthermore, several more days are required for species identification and susceptibility testing. New methods for diagnosis of invasive Candida infection have therefore been investigated, including serological markers (mannan and β-D-glucan) and realtime PCR; however, only the use of β-D-glucan has been included in the 2008 Infectious Diseases Society of America guidelines for diagnosing invasive fungal disease [44].
The use of β-D-glucan is currently being investigated in ICU populations. Even though the results seem promising, no large prospective studies have been performed and the main problems for the use of β-D-glucan remain its high cost and high rate of false positive results (mostly due to concomitant bacterial bloodstream infections and intensive care measures such as haemofiltration, albumin or immunoglobulin use) [45]. Traditional culture from sterile sites other than the bloodstream (for example, the peritoneum) are useful for diagnosis of invasive candidiasis. For specific details on the diagnosis of invasive candidiasis in the ICU, a recent review is available [46].
Management of candidemia in the ICU
As far as management of candidemia in the ICU is concerned, there is no single strategy that can be considered the most appropriate. In fact, different approaches can be chosen and can be judged as the best for a given clinical situation. In particular, four management options are available: prophylaxis, empirical therapy, pre-emptive therapy and treatment of a cultureproven infection. So how is the best strategy chosen?
The knowledge of epidemiological data, the abovementioned risk factors and, first of all, the analysis of local epidemiology of candidemia in a singular ICU allow one to determine whether a patient is at low, modest or high risk of developing this infection. Consequently, a choice between the most appropriate management strategies can be made - the patients with low or modest risk of infection can be monitored less frequently for Candida colonisation, while high-risk subjects may benefit from immediate diagnostic procedures (cultures of both sterile and nonsterile sites, testing for serological markers) and empirical antifungal therapy. In the case of negative results of testing for yeasts, antifungal prophylaxis might be considered. Naturally, knowing the most frequent species and susceptibility patterns of Candida isolated in a single ICU is the basis for choosing an adequate antifungal agent (Table 4).
Prophylaxis in the ICU
Prophylaxis - defined as administration of an antifungal agent to a patient with no evidence of infection - has been evaluated in several studies and meta-analyses [27, 47–50], and its main advantage is a possible reduction in the rate of candidemia.
Since morbidity and mortality rates in patients with systemic fungal infections are exceedingly high, the use of an effective antifungal prophylaxis in selected highrisk patients is very attractive and might be an option in this select population. The strategy of antifungal prophylaxis is now well established in patients with persistent neutropenia after treatment for haematological malignancies or after bone marrow transplantation, but routine use of antifungal prophylaxis in the general ICU setting is discouraged [51–53]. Nonetheless, the implementation of targeted anti fungal prophylaxis has been shown to be effective in certain ICU settings [27, 47], and three randomised placebo-controlled trails reported a clear >50% decrease in the incidence of Candida infections with fluconazole prophylaxis [54–56]. Moreover, two meta-analyses con firmed that prophylactic fluconazole administration in ICU patients reduced the rate of Candida infection, but no clear survival advantage was observed [49, 50]. In the meta-analysis by Playford and colleagues, however, only when the studies on prophylaxis with both fluconazole and ketonazole were considered was the total mortality found reduced by approximately 25% and the incidence of fungal infection by 50% [50]. The meta-analysis by Cruciani and colleagues also reported, along with a relative risk reduction in candidemia, a decrease in overall mortality with antifungal prophylaxis with various agents [48].
On the other hand, the disadvantages of fluconazole prophylaxis include possible toxicity and profound influence on local epidemiology with the emergence of fluconazole-resistant isolates [57]. From expert opinion expressed in reviews and guidelines [44, 58], therefore, antifungal prophylaxis might be warranted only for ICUs with a high rate of invasive candidiasis, as compared with the normal rates of 1 to 2%, particularly for selected patients who are at highest risk (> 10%) [42]. The approach of limiting prophylaxis to a subgroup of patients with the highest risk of candidemia may help to limit the quantity of antifungals used and delay the emergence of infections due to fluconazole-resistant Candida strains seen in immunocompromised patients. In fact, this approach is supported by the recent Infectious Diseases Society of America guidelines that recommend fluconazole prophylaxis at a dose of 400 mg (6 mg/kg) daily for high-risk adult patients hospitalised in ICUs that have a high incidence of invasive candidiasis [44].
Empirical therapy
Empirical treatment is defined as administration of antifungals in the presence of persistent and refractory fever in patients who are at high risk for fungal infection. This strategy was developed almost three decades ago for neutropenic patients, when it became evident that the lack of sensitivity of microbiological and clinical findings resulted in delayed diagnosis and increased morbidity and mortality.
Even though the first studies on empirical therapy were underpowered, the treatment being used in different clinical settings and numerous antifungals are being registered and recommended for empirical treatment of invasive candidiasis, both in neutropenic patients and in non-neutropenic patients [44]. All these efforts are aimed at reducing morbidity and mortality by starting treatment as early as possible, given the evidence that a delay in antifungal prescription increases mortality rates significantly in candidemia [13, 14]. In the ICU, however, where numerous patients have different risk factors for fungal infections, the routine use of empirical therapy in cases of persistent fever may result in significant overtreatment. The strategy of a pre-emptive approach therefore appears promising. In fact, US guidelines recommend that such an approach (although they continue to call it empirical treatment) should be considered for critically ill patients with risk factors for invasive candidiasis and no other known cause of fever, based on clinical assessment of risk factors, serologic markers for invasive candidiasis and/or culture data from nonsterile sites [44] (Table 4).
Pre-emptive therapy
The main concept of a pre-emptive strategy is to better identify patients at high risk for developing candidemia. The overall use of antifungals in the ICU can therefore be reduced, without delaying therapy in patients who need it. The recent availability of more sensitive and specific clinical and laboratory tools allows for better identification of high-risk patients, and this approach has been used success fully [59]. The question arises, however, of how to define a patient at high risk for developing candidemia. No clear predictive rule exists, but the two score systems described above for ICU patients can be of some help. In brief, multifocal colonisation by Candida and/or the presence of well-described factors outlined in Table 3 make the patient a suitable candidate for empirical therapy if any signs or symptoms of infection compare.
In particular, the efficacy of a pre-emptive strategy in ICU patients has been recently established in a single-institution study in which the use of fluconazole in patients with corrected colonisation index ≥ 0.5, described previously by Pittet and colleagues [40], has significantly decreased the incidence of invasive candidiasis [60]. Moreover, surrogate markers of invasive fungal infections have been studied extensively. In particular, β-D-glucan is a component of the cell wall of Candida and other fungi and has been investigated as a serological marker for fungal infections, including candidemia [61]. Even though false positive results have been reported and its routine use in the ICU requires further validation, persistently high serum levels of β-D-glucan in ICU patients were found indicative of fungal disease [45].
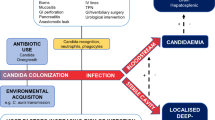
A pre-emptive approach in critically ill patients might therefore be defined as starting antifungals when the following conditions are satisfied: the presence of long ICU stay (> 96 hours), and broad-spectrum antibiotic therapy; the presence of any other risk factors, such as severe sepsis, gastrointestinal surgery or parenteral nutrition; plus microbiological evidence of Candida infection, including multifocal colonisation or a positive result for serum β-D-glucan. The proposed approach is shown in Figure 1 and, as with any new strategy, will warrant validation in prospective trails. One of the main advantages of such an approach is limiting the use of antifungals in low-risk patients, while starting treatment for candidemia without delay when symptoms appear in patients at high risk for this infection. The benefit of early therapy, in terms of morbidity and mortality, can thus be obtained, while overtreatment can be avoided (Figure 2).
Treatment of a culture-documented candidemia
For ICU patients with low/medium risk of developing candidemia, blood cultures should be performed if a clinical suspicion of systemic infection is present, even in the absence of fever. Numerous blood cultures, both from a central venous catheter and a peripheral line, remain the cornerstone for diagnosis of candidemia. As any delay before administering primary therapy can lead to a noticeable increase in mortality, antifungals should be prescribed as soon as there is growth of yeasts in blood samples.
The choice of antifungal for an unknown Candida species should be based on the knowledge of local epidemiology. In an ICU where most infections are due to C. albicans and where fluconazole resistance is low, fluconazole is the drug of choice. On the other hand, in an ICU where fluconazole-resistant species are common (for example, C. glabrata) or in patients colonised with fluconazole-resistant strains, echinocandins are the drugs of choice. Moreover, for patients in severe or moderately severe clinical conditions (for example, haemodynamically unstable patients, with suspected concomitant endocardial involvement), echinocandins are recommended because of their bactericidal activity against Candida; the side effects are less common than those reported for the other fungicidal agent - liposomal amphotericin B [44]. The antifungal treatment might be modified according to the results of susceptibility testing, and de-escalation to fluconazole has been successful for stable patients with susceptible isolates [44].
Other aspects of treating candidemia in the ICU
Once the initial therapy for candidemia is started, several clinical issues remain open. Firstly, the efficacy of the treatment should be assessed by the documentation of blood cultures returning sterile. Moreover, the date of the first negative blood culture is important, because the recommended length of treatment is 14 days after the documented clearance of Candida from the bloodstream and resolution of symptoms attributable to candidemia.
Secondly, the antifungal chosen empirically can be changed based on the results of species determination or susceptibility testing. For stable patients with C. albicans or other fluconazole-susceptible strains, fluconazole is therefore the drug of choice. Importantly, fluconazole is the preferred treatment for C. parapsilosis, since resistance to echinocandins has been reported [62].
Thirdly, patients who improved clinically and who cleared Candida from the bloodstream might be suitable for step-down oral therapy to complete the course of 14 days. The available oral antifungals are fluconazole, itraconazole, voriconazole and posaconazole. Fluconazole is an obvious choice for susceptible species, while voriconazole can be indicated as step-down therapy for C. krusei or voriconazole-susceptible C. glabrata.
Additionally, ophthalmologic fundus examination is warranted in all the patients to exclude disseminated endocular infection, and endocarditis should be excluded in case of persistently positive blood cultures, known valve pathology or any other sign or symptom suggestive of endocardial involvement. In both cases, the duration of treatment is much longer (> 4 weeks and up to lifelong suppressive therapy) and is described in detail elsewhere [44].
Last but not least, intravenous catheter removal is strongly recommended for patients with candidemia. Guidelines both on management of candidiasis and on management of catheter-related bloodstream infection state clearly that catheters should be removed, even though grade II and grade III of such statements indicate that there are no data from properly randomised, controlled trials [44, 63, 64]. Interestingly, a recent study of 842 adults included in candidemia trials did not find on multivariate analysis any benefit from early catheter removal; the expert guidelines remain the best synthesis of all available data, however, and removing the catheter should thus be attempted in all ICU patients with candidemia [64].
Biofilm production is a well-documented phenomenon for Candida species that significantly contributes to Candida pathogenicity in catheter-related bloodstream infections, resulting in recurrent or persistent infections and biofilm-mediated antifungal resistance leading to treatment failure [65]. Moreover, the mortality in patients with invasive infections due to biofilm-producing Candida species has been reported significantly higher [66]. The activity of antifungals against biofilm therefore has important clinical implications and is known to vary among different agents. In particular, fluconazole and azoles - which are static against Candida - are also not active against sessile forms, while echinocandins and amphotericin B offer both bactericidal activity and good penetration into a biofilm formed on vascular devices. However, a study performed on 43 Candida species - including 12 C. albicans, 12 C. parapsilosis, 10 C. tropicalis and nine C. glabrata isolates - found that the activity of caspofungin and micafungin against a biofilm of C. parapsilosis and C. tropicalis was significantly lower than that of amphotericin B [67]. Table 5 outlines the susceptibility of different Candida species to two antifungals that are active against biofilm-producing strains.
Antifungal agents
In recent years, numerous new antifungal drugs have been developed, studied and approved for various indications, and almost all of these new drugs are licensed to treat candidemia in different patient populations. The most appropriate antifungal drug can be chosen from the three main groups: the polyenes (amphotericin B deoxycholate, lipid complex, liposomal); the azoles (fluconazole, voriconazole, posaconazole, itraconazole, ravuconazole); and the echinocandins (caspofungin, micafungin, anidulafungin).
Most of the studies on efficacy in candidemia have not shown significant differences between various agents. The differences in drug-related toxicity are significant, however, and the possibility of drug-drug interactions - so important in critically ill patients that receive numerous medications - varies significantly among the single agents. The choice of the best antifungal therefore still poses a challenge for a clinician. The detailed description of various agents used for treating candidemia is beyond the scope of the present review, but the dosing of the main antifungals is reported in Table 6. Moreover, given that echinocandins are the most recently introduced class of antifungals and general recommendations do not usually specify which of them should be used, Table 7 outlines the differences in indication, dosing, and so forth, for three echinocandin compounds. Considering that many ICU patients have other significant comorbidities, data on the treatment with various antifungals in the case of renal or hepatic insufficiency are reported in Table 8.
Management of candidemia in the neonatal ICU
The incidence of candidemia in the neonatal ICU has been increasing, mostly due to the fact that more low-birth-weight and very-low-birth-weight newborns survive longer thanks to advances in medical technology. These newborns are more likely to develop infectious complications, and candidemia is one of the most frequent nosocomial bloodstream infections in this population. The reported risk factors for candidemia in neonates and adults are similar, and include central venous catheters and arterial lines, parenteral nutrition, mechanical ventilation, and the extended use of antibiotics. Unlike in the adult ICU, C. albicans remains the most common isolate in the neonatal ICU - although non-albicans species such as C. parapsilosis and C. tropicalis are increasingly common [68, 69]. Fortunately, these species are susceptible to fluconazole.
The recent Infectious Diseases Society of America guidelines on management of Candida infections offer recommendations for paediatric patients. In particular, the following treatments are regarded as first-line therapy for neonatal candidiasis: amphotericin B deoxycholate, or liposomal amphotericin B if urinary tract involvement is excluded, and fluconazole. The guidelines also state that echinocandins should be used with caution and are generally limited to situations in which resistance or toxicity precludes the use of fluconazole or amphotericin B. Dosing of antifungals in paediatric patients is outlined in Table 9.
Additionally for neonates, a lumbar puncture and a dilated retinal examination - preferably by an ophthalmologist - are recommended in those with sterile body fluid and/or urine cultures positive for Candida, and removal of the intravascular catheter is strongly recommended. Finally, in nurseries with high rates of invasive candidiasis, fluconazole prophylaxis may be considered in neonates with birth weight <1,000 g.
Conclusions
Candida is one of the most common causes of nosocomial bloodstream infection. Morbidity and mortality associated with candidemia are significant and the epidemiology of species has been changing, at both local and worldwide levels. Even though numerous risk factors for invasive Candida infection have been reported and several antifungals are widely available, the optimal management of candidemia remains a challenge. The agents recommended for initial treatment of candidemia in critically ill patients include echinocandins and lipid formulation of amphotericin B, but the choice between prophylactic, empirical and pre-emptive therapy is crucial. Compared with prophylaxis, empirical and pre-emptive approaches allow the clinician to reduce exposure to antifungals by targeting only patients at high risk of candidemia, without delaying therapy until yeast is identified in blood cultures. A pre-emptive strategy is based on the presence of numerous risk factors, together with micro biological documentation for the presence of Candida, such as multifocal colonisation or positive serum β-D-glucan. Further prospective studies are warranted to confirm the benefits from routine use of pre-emptive treatment of candidemia.
Abbreviations
- ICU:
-
intensive care unit
- PCR:
-
polymerase chain reaction.
References
Leroy O, Gangneux JP, Montravers P, Mira JP, Gouin F, Sollet JP, Carlet J, Reynes J, Rosenheim M, Regnier B, Lortholary O, AmarCand Study Group: Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005-2006). Crit Care Med 2009, 37: 1612-1618. 10.1097/CCM.0b013e31819efac0
Kuse ER, Chetchotisakd P, da Cunha CA, Ruhnke M, Barrios C, Raghunadharao D, Sekhon JS, Freire A, Ramasubramanian V, Demeyer I, Nucci M, Leelarasamee A, Jacobs F, Decruyenaere J, Pittet D, Ullmann AJ, Ostrosky-Zeichner L, Lortholary O, Koblinger S, Diekmann-Berndt H, Cornely OA, Micafungin Invasive Candidiasis Working Group: Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007, 369: 1519-1527. 10.1016/S0140-6736(07)60605-9
Reboli AC, Rotstein C, Pappas PG, Chapman SW, Kett DH, Kumar D, Betts R, Wible M, Goldstein BP, Schranz J, Krause DS, Walsh TJ, Anidulafungin Study Group: Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007, 356: 2472-2482. 10.1056/NEJMoa066906
Blumberg HM, Jarvis WR, Soucie JM, Edwards JE, Patterson JE, Pfaller MA, Rangel-Frausto MS, Rinaldi MG, Saiman L, Wiblin RT, Wenzel RP, National Epidemiology of Mycoses Survey(NEMIS) Study Group: Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clin Infect Dis 2001, 33: 177-186. 10.1086/321811
Jarvis WR: Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis 1995, 20: 1526-1530.
Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP: Risk factors for hospitalacquired candidemia. A matched case-control study. Arch Intern Med 1989, 149: 2349-2353. 10.1001/archinte.149.10.2349
Richards MJ, Edwards JR, Culver DH, Gaynes RP: Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol 2000, 21: 510-515. 10.1086/501795
Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D: Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis 2003, 37: 1172-1177. 10.1086/378745
Macphail GL, Taylor GD, Buchanan-Chell M, Ross C, Wilson S, Kureishi A: Epidemiology, treatment and outcome of candidemia: a five-year review at three Canadian hospitals. Mycoses 2002, 45: 141-145. 10.1046/j.1439-0507.2002.00741.x
Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C: The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 2005, 41: 1232-1239. 10.1086/496922
Morgan J, Meltzer MI, Plikaytis BD, Sofair AN, Huie-White S, Wilcox S, Harrison LH, Seaberg EC, Hajjeh RA, Teutsch SM: Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from populationbased candidemia surveillance. Infect Control Hosp Epidemiol 2005, 26: 540-547. 10.1086/502581
Morgan J, Wannemuehler KA, Marr KA, Hadley S, Kontoyiannis DP, Walsh TJ, Fridkin SK, Pappas PG, Warnock DW: Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med Mycol 2005,43(Suppl 1):S49-S58. 10.1080/13693780400020113
Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT: Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 2006, 43: 25-31. 10.1086/504810
Morrell M, Fraser VJ, Kollef MH: Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 2005, 49: 3640-3645. 10.1128/AAC.49.9.3640-3645.2005
Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM: Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 2009, 48: 1695-1703. 10.1086/599039
Parkins MD, Sabuda DM, Elsayed S, Laupland KB: Adequacy of empirical antifungal therapy and effect on outcome among patients with invasive Candida species infections. J Antimicrob Chemother 2007, 60: 613-618. 10.1093/jac/dkm212
Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB: Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004, 39: 309-317. 10.1086/421946
Eggimann P, Garbino J, Pittet D: Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis 2003, 3: 685-702. 10.1016/S1473-3099(03)00801-6
Magnason S, Kristinsson KG, Stefansson T, Erlendsdottir H, Jonsdottir K, Kristjansson M, Jonmundsson E, Baldursdottir L, Sigvaldason H, Gudmundsson S: Risk factors and outcome in ICU-acquired infections. Acta Anaesthesiol Scand 2008, 52: 1238-1245. 10.1111/j.1399-6576.2008.01763.x
Montravers P, Lepape A, Dubreuil L, Gauzit R, Pean Y, Benchimol D, Dupont H: Clinical and microbiological profiles of community-acquired and nosocomial intra-abdominal infections: results of the French prospective, observational EBIIA study. J Antimicrob Chemother 2009, 63: 785-794. 10.1093/jac/dkp005
Diekema DJ, Messer SA, Brueggemann AB, Coffman SL, Doern GV, Herwaldt LA, Pfaller MA: Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J Clin Microbiol 2002, 40: 1298-1302. 10.1128/JCM.40.4.1298-1302.2002
Passos XS, Costa CR, Araujo CR, Nascimento ES, e Souza LK, Fernandes Ode F, Sales WS, Silva Mdo R: Species distribution and antifungal susceptibility patterns of Candida spp. bloodstream isolates from a Brazilian tertiary care hospital. Mycopathologia 2007, 163: 145-151. 10.1007/s11046-007-0094-5
Shorr AF, Lazarus DR, Sherner JH, Jackson WL, Morrel M, Fraser VJ, Kollef MH: Do clinical features allow for accurate prediction of fungal pathogenesis in bloodstream infections? Potential implications of the increasing prevalence of non-albicans candidemia. Crit Care Med 2007, 35: 1077-1083. 10.1097/01.CCM.0000259379.97694.00
Bassetti M, Righi E, Costa A, Fasce R, Molinari MP, Rosso R, Pallavicini FB, Viscoli C: Epidemiological trends in nosocomial candidemia in intensive care. BMC Infect Dis 2006, 10: 21. 10.1186/1471-2334-6-21
Chow JK, Golan Y, Ruthazer R, Karchmer AW, Carmeli Y, Lichtenberg D, Chawla V, Young J, Hadley S: Factors associated with candidemia caused by nonalbicans Candida species versus Candida albicans in the intensive care unit. Clin Infect Dis 2008, 46: 1206-1213. 10.1086/529435
Klingspor L, Tornqvist E, Johansson A, Petrini B, Forsum U, Hedin G: A prospective epidemiological survey of candidaemia in Sweden. Scand J Infect Dis 2004, 36: 52-55. 10.1080/00365540310017447
Calandra T, Marchetti O: Clinical trials of antifungal prophylaxis among patients undergoing surgery. Clin Infect Dis 2004,39(Suppl 4):S185-S192. 10.1086/421955
Nolla-Salas J, Sitges-Serra A, Leon-Gil C, Martinez-Gonzalez J, Leon-Regidor MA, Ibanez-Lucia P, Torres-Rodriguez JM: Candidemia in non-neutropenic critically ill patients: analysis of prognostic factors and assessment of systemic antifungal therapy. Study Group of Fungal Infection in the ICU. Intensive Care Med 1997, 23: 23-30. 10.1007/s001340050286
Voss A, le Noble JL, Verduyn Lunel FM, Foudraine NA, Meis JF: Candidemia in intensive care unit patients: risk factors for mortality. Infection 1997, 25: 8-11. 10.1007/BF02113499
Pereira GH, Muller PR, Szeszs MW, Levin AS, Melhem MS: Five-year evaluation of bloodstream yeast infections in a tertiary hospital: the predominance of non- C. albicans Candida species. Med Mycol 2010, 48: 839-842. 10.3109/13693780903580121
Krcmery V, Barnes AJ: Non-albicans Candida spp. causing fungaemia: pathogenicity and antifungal resistance. J Hosp Infect 2002, 50: 243-260. 10.1053/jhin.2001.1151
Ruan SY, Lee LN, Jerng JS, Yu CJ, Hsueh PR: Candida glabrata fungaemia in intensive care units. Clin Microbiol Infect 2008, 14: 136-140. 10.1111/j.1469-0691.2007.01892.x
Trick WE, Fridkin SK, Edwards JR, Hajjeh RA, Gaynes RP: Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin Infect Dis 2002, 35: 627-630. 10.1086/342300
Dimopoulos G, Ntziora F, Rachiotis G, Armaganidis A, Falagas ME: Candida albicans versus non-albicans intensive care unit-acquired bloodstream infections: differences in risk factors and outcome. Anesth Analg 2008, 106: 523-529. table of contents 10.1213/ane.0b013e3181607262
Viscoli C, Girmenia C, Marinus A, Collette L, Martino P, Vandercam B, Doyen C, Lebeau B, Spence D, Krcmery V, De Pauw B, Meunier F: Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin Infect Dis 1999, 28: 1071-1079. 10.1086/514731
Pfaller MA: Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin Infect Dis 1996,22(Suppl 2):S89-S94.
Asmundsdottir LR, Erlendsdottir H, Haraldsson G, Guo H, Xu J, Gottfredsson M: Molecular epidemiology of candidemia: evidence of clusters of smoldering nosocomial infections. Clin Infect Dis 2008, 47: e17-e24. 10.1086/589298
Bliss JM, Basavegowda KP, Watson WJ, Sheikh AU, Ryan RM: Vertical and horizontal transmission of Candida albicans in very low birth weight infants using DNA fingerprinting techniques. Pediatr Infect Dis J 2008, 27: 231-235. 10.1097/INF.0b013e31815bb69d
Richet HM, Andremont A, Tancrede C, Pico JL, Jarvis WR: Risk factors for candidemia in patients with acute lymphocytic leukemia. Rev Infect Dis 1991, 13: 211-215.
Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R: Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg 1994, 220: 751-758. 10.1097/00000658-199412000-00008
Leon C, Ruiz-Santana S, Saavedra P, Almirante B, Nolla-Salas J, Alvarez-Lerma F, Garnacho-Montero J, Leon MA: A bedside scoring system ('Candida score') for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med 2006, 34: 730-737. 10.1097/01.CCM.0000202208.37364.7D
Ostrosky-Zeichner L, Sable C, Sobel J, Alexander BD, Donowitz G, Kan V, Kauffman CA, Kett D, Larsen RA, Morrison V, Nucci M, Pappas PG, Bradley ME, Major S, Zimmer L, Wallace D, Dismukes WE, Rex JH: Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis 2007, 26: 271-276. 10.1007/s10096-007-0270-z
Dupont H, Bourichon A, Paugam-Burtz C, Mantz J, Desmonts JM: Can yeast isolation in peritoneal fluid be predicted in intensive care unit patients with peritonitis? Crit Care Med 2003, 31: 752-757. 10.1097/01.CCM.0000053525.49267.77
Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD, Infectious Diseases Society of America: Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009, 48: 503-535. 10.1086/596757
Presterl E, Parschalk B, Bauer E, Lassnigg A, Hajdu S, Graninger W: Invasive fungal infections and (1,3)-β- D -glucan serum concentrations in long-term intensive care patients. Int J Infect Dis 2009, 13: 707-712. 10.1016/j.ijid.2008.10.013
Guery BP, Arendrup MC, Qzinger G, Azoulay E, Borges Sá M, Johnson EM, Müller E, Putensen C, Rotstein C, Sganga G, Venditti M, Zaragoza Crespo R, Kullberg BJ: Management of invasive candidiasis and candidemia in adult non-neutropenic intensive care unit patients: Part I. Epidemiology and diagnosis. Intensive Care Med 2009, 35: 55-62. 10.1007/s00134-008-1338-7
Lipsett PA: Clinical trials of antifungal prophylaxis among patients in surgical intensive care units: concepts and considerations. Clin Infect Dis 2004,39(Suppl 4):S193-S199. 10.1086/421956
Cruciani M, de Lalla F, Mengoli C: Prophylaxis of Candida infections in adult trauma and surgical intensive care patients: a systematic review and meta-analysis. Intensive Care Med 2005, 31: 1479-1487. 10.1007/s00134-005-2794-y
Shorr AF, Chung K, Jackson WL, Waterman PE, Kollef MH: Fluconazole prophylaxis in critically ill surgical patients: a meta-analysis. Crit Care Med 2005, 33: 1928-1935. quiz 1936 10.1097/01.CCM.0000178352.14703.49
Playford EG, Webster AC, Sorrell TC, Craig JC: Antifungal agents for preventing fungal infections in non-neutropenic critically ill and surgical patients: systematic review and meta-analysis of randomized clinical trials. J Antimicrob Chemother 2006, 57: 628-638. 10.1093/jac/dki491
Rex JH, Sobel JD: Prophylactic antifungal therapy in the intensive care unit. Clin Infect Dis 2001, 32: 1191-1200. 10.1086/319763
Edwards JE Jr, Bodey GP, Bowden RA, Büchner T, de PQw BE, Filler SG, Ghannoum MA, GlQser M, Herbrecht R, KQffman CA, Kohno S, Martino P, Meunier F, Mori T, Pfaller MA, Rex JH, Rogers TR, Rubin RH, Solomkin J, Viscoli C, Walsh TJ, White M: International Conference for the Development of a Consensus on the Management and Prevention of Severe Candidal Infections. Clin Infect Dis 1997, 25: 43-59. 10.1086/514504
Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, Feld R, Pizzo PA, Rolston KV, Shenep JL, Young LS: 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 2002, 34: 730-751. 10.1086/339215
Garbino J, Lew DP, Romand JA, Hugonnet S, Auckenthaler R, Pittet D: Prevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: a randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontamination. Intensive Care Med 2002, 28: 1708-1717. 10.1007/s00134-002-1540-y
Pelz RK, Hendrix CW, Swoboda SM, Diener-West M, Merz WG, Hammond J, Lipsett PA: Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann Surg 2001, 233: 542-548. 10.1097/00000658-200104000-00010
Eggimann P, Francioli P, Bille J, Schneider R, Wu MM, Chapuis G, Chiolero R, Pannatier A, Schilling J, Geroulanos S, Glauser MP, Calandra T: Fluconazole prophylaxis prevents intra-abdominal candidiasis in high-risk surgical patients. Crit Care Med 1999, 27: 1066-1072. 10.1097/00003246-199906000-00019
Bassetti M, Ansaldi F, Nicolini L, Malfatto E, Molinari MP, Mussap M, Rebesco B, Bobbio Pallavicini F, Icardi G, Viscoli C: Incidence of candidaemia and relationship with fluconazole use in an intensive care unit. J Antimicrob Chemother 2009, 64: 625-629. 10.1093/jac/dkp251
Ostrosky-Zeichner L: Prophylaxis and treatment of invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis 2004, 23: 739-744. 10.1007/s10096-004-1215-4
Maertens J, Theunissen K, Verhoef G, Verschakelen J, Lagrou K, Verbeken E, Wilmer A, Verhaegen J, Boogaerts M, Van Eldere J: Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin Infect Dis 2005, 41: 1242-1250. 10.1086/496927
Piarroux R, Grenouillet F, Balvay P, Tran V, Blasco G, Millon L, Boillot A: Assessment of preemptive treatment to prevent severe candidiasis in critically ill surgical patients. Crit Care Med 2004, 32: 2443-2449. 10.1097/01.CCM.0000147726.62304.7F
Ostrosky-Zeichner L, Alexander BD, Kett DH, Vazquez J, Pappas PG, Saeki F, Ketchum PA, Wingard J, Schiff R, Tamura H, Finkelman MA, Rex JH: Multicenter clinical evaluation of the (1→3) β- D -glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis 2005, 41: 654-659. 10.1086/432470
Forrest GN, Weekes E, Johnson JK: Increasing incidence of Candida parapsilosis candidemia with caspofungin usage. J Infect 2008, 56: 126-129. 10.1016/j.jinf.2007.10.014
Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Rijnders BJ, Sherertz RJ, Warren DK: Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009, 49: 1-45. 10.1086/599376
Nucci M, Anaissie E, Betts RF, Dupont BF, Wu C, Buell DN, Kovanda L, Lortholary O: Early removal of central venous catheter in patients with candidemia does not improve outcome: analysis of 842 patients from 2 randomized clinical trials. Clin Infect Dis 2010, 51: 295-303. 10.1086/653935
Lewis RE, Kontoyiannis DP, Darouiche RO, Prince RA: Antifungal activity of amphotericin B, fluconazole, and voriconazole in an in vitro model of Candida catheter-related bloodstream infection. Antimicrob Agents Chemother 2002, 46: 3499-3505. 10.1128/AAC.46.11.3499-3505.2002
Tumbarello M, Posteraro B, Trecarichi EM, Fiori B, Rossi M, Porta R, de Gaetano Donati K, La Sorda M, Spanu T, Fadda G, Cauda R, Sanguinetti M: Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J Clin Microbiol 2007, 45: 1843-1850. 10.1128/JCM.00131-07
Choi HW, Shin JH, Jung SI, Park KH, Cho D, Kee SJ, Shin MG, Suh SP, Ryang DW: Species-specific differences in the susceptibilities of biofilms formed by Candida bloodstream isolates to echinocandin antifungals. Antimicrob Agents Chemother 2007, 51: 1520-1523. 10.1128/AAC.01141-06
Filioti J, Spiroglou K, Panteliadis CP, Roilides E: Invasive candidiasis in pediatric intensive care patients: epidemiology, risk factors, management, and outcome. Intensive Care Med 2007, 33: 1272-1283. 10.1007/s00134-007-0672-5
Fridkin SK, Kaufman D, Edwards JR, Shetty S, Horan T: Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995-2004. Pediatrics 2006, 117: 1680-1687. 10.1542/peds.2005-1996
Hachem R, Hanna H, Kontoyiannis D, Jiang Y, Raad I: The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer 2008, 112: 2493-2499. 10.1002/cncr.23466
Cohen Y, Karoubi P, Adrie C, Gauzit R, Marsepoil T, Zarka D, Clec'h C: Early prediction of Candida glabrata fungemia in nonneutropenic critically ill patients. Crit Care Med 2010, 38: 826-830. 10.1097/CCM.0b013e3181cc4734
Sanchez V, Vazquez JA, Barth-Jones D, Dembry L, Sobel JD, Zervos MJ: Nosocomial acquisition of Candida parapsilosis : an epidemiologic study. Am J Med 1993, 94: 577-582. 10.1016/0002-9343(93)90207-6
Hernandez-Castro R, Arroyo-Escalante S, Carrillo-Casas EM, Moncada-Barron D, Alvarez-Verona E, Hernandez-Delgado L, Torres-Narvaez P, Lavalle-Villalobos A: Outbreak of Candida parapsilosis in a neonatal intensive care unit: a health care workers source. Eur J Pediatr 2010, 169: 783-787. 10.1007/s00431-009-1109-7
Ostrosky-Zeichner L, Rex JH, Pappas PG, Hamill RJ, Larsen RA, Horowitz HW, Powderly WG, Hyslop N, Kauffman CA, Cleary J, Mangino JE, Lee J: Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob Agents Chemother 2003, 47: 3149-3154. 10.1128/AAC.47.10.3149-3154.2003
Pfaller MA, Diekema DJ: Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 2010, 36: 1-53. 10.3109/10408410903241444
De Waele JJ, Vogelaers D, Blot S, Colardyn F: Fungal infections in patients with severe acute pancreatitis and the use of prophylactic therapy. Clin Infect Dis 2003, 37: 208-213. 10.1086/375603
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
In the past 5 years, MB has been an advisor/consultant for Gilead Scienses, Merck Sharp and Dohme, Novartis, Pfizer and Schering Plough. He has been paid for talks on behalf of Angelini, Astellas, Astra Zeneca, Aventis, Bayer, Cephalon, Glaxo SmithKline, Gilead Scienses, Jansen Cilag, Merck Sharp and Dohme, Novartis, and Pfizer. The other authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
Bassetti, M., Mikulska, M. & Viscoli, C. Bench-to-bedside review: Therapeutic management of invasive candidiasis in the intensive care unit. Crit Care 14, 244 (2010). https://doi.org/10.1186/cc9239
Published:
DOI: https://doi.org/10.1186/cc9239