Abstract
Introduction
Tissue oxygen saturation (StO2) and the vascular occlusion test (VOT) can identify tissue hypoperfusion in trauma and sepsis. However, the technique is neither standardized nor uses the same monitoring site. We hypothesized that baseline and VOT StO2 would be different in the forearm (F) and thenar eminence (TH) and that different minimal StO2 values during the VOT would result in different reoxygenation rates (ReO2).
Methods
StO2 and its change during the VOT were simultaneously measured in the F and TH, with 15 mm and 25 mm probes, using the 325 InSpectra monitor in 18 healthy, adult volunteers. Two VOTs were done to a threshold thenar StO2 of 40% interchanging the 15 mm and 25 mm probes between sites. Two additional VOTs were done to thresholds of 50% and 30%. Baseline StO2 (BaseO2), the deoxygenation rate (DeO2) and ReO2 were compared between sites, probes and (%O2/minute) thresholds. Results are presented as the median (interquartile range), P-value.
Results
BaseO2, DeO2, ReO2, area under the curve and hyperemia duration values were different when comparing TH vs. F and 15 mm vs. 25 mm probes. ReO2 was different between different thresholds for the TH and 15 mm probes. TH15 mm vs. F15 mm: BaseO2, 90.4 (85.2, 93.5) vs. 85.2 (80.7, 90.2), P = 0.031; DO2, -12.1 (-16.2, -11.3) vs. -8.5 (-10.3, -7.8), P = 0.011; ReO2, 297.2 (213.7, 328.6), P < 0.0001; 15 mm vs. 25 mm probe: BaseO2, 97.2 (89.4, 94.7) vs. 87.3 (81.7, 90.9), P = 0.016; DeO2, -18.0 (-24.1, -14.8) vs. -9.9 (-15.3, -6.5), P < 0.0001; and ReO2, 401.6 (331.7, 543.2) vs. 160.5 (132.3, 366.9), P = 0.012, respectively. TH15 mm vs. TH25 mm: BaseO2, P = 0.020; DeO2, P < 0.0001; and ReO2, P < 0.0001. Threshold StO2 values (15 mm probe only): ReO2, P = 0.003; DeO2, P = 0.60. ReO2 at 40% and 50% StO2 thresholds, P = 0.01.
Conclusions
BaseO2, DeO2 and ReO2 were different when measured in different anatomical sites (F and TH) and with different probe sizes, and ReO2 was different with differing VOT release StO2 threshold values. Thus, standardization of the site, probe and VOT challenge need to be stipulated when reporting data.
Similar content being viewed by others
Introduction
Non-invasive measurements of tissue oxygen saturation (StO2) using near-infrared spectroscopy have been studied to assess tissue hypoperfusion in different populations. The absolute StO2 has been proven instrumental in predicting outcome in trauma patients [1, 2]. These same studies, however, have shown limited discriminating capacity for the absolute StO2 value (sensitivity, 78%; specificity, 39%) and have even shown it to be at least as good as systolic blood pressure (sensitivity, 74%; specificity, 32%) [1]. The addition of a provocative test (ischaemic challenge) to the measurement of the StO2 would potentially enhance this discriminatory power between different cardiovascular states by creating emerging parameters to follow, such as the deoxygenation and reoxygenation slopes. Furthermore, the use of such provocative tests [3], specifically the vascular occlusion test (VOT), has been shown to improve and expand the predictive ability of StO2 to scenarios such as trauma, severe sepsis and septic shock [4, 5].
The VOT is a provocative test in which StO2 is measured on a distal site (such as the thenar eminence (TH)) whilst a transient rapid vascular occlusion is performed, using a sphygmomanometer, for either a defined time interval (for example, 3 minutes) or until the StO2 decreases to a defined minimal threshold. Once this threshold is reached, the vascular occlusion is released and the StO2 is allowed to recover. Several emergent parameters arise from this technique, including the rate of deoxygenation (DeO2), thought to reflect the local metabolic rate, the rate of reoxygenation (ReO2), which reflects the time required to wash out stagnant blood and is thought to be determined by local cardiovascular reserve and microcirculatory flow, and the postobstructive hyperemic response.
The VOT, however, has not been standardized. In fact, multiple studies report results using different anatomical StO2 measuring sites (TH, forearm (F), deltoid) [4, 6–8], different near-infrared spectroscopy probe sizes (15 mm vs. 25 mm) [4, 8–12], and different types and levels of deflation thresholds (StO2 of 40%, 50%) versus time thresholds (3 minutes, 4 minutes, 5 minutes) [4, 6, 11, 13]. Differences between anatomical sites may potentially affect near-infrared spectroscopy-derived parameters given the fact that fat and the muscle thickness distribution along the body is variable. Furthermore, given that increasing the sensing and illuminating probe distance augments the depth that near-infrared light travels, the use of different probes may screen different capillary beds at any given time. Finally, since the primary reason to perform the VOT is to produce a transient ischaemic challenge to note the subsequent ReO2, the level of ischaemic challenge might vary for the same ischaemic time or threshold StO2. As a consequence, caution must be taken when comparing results from the available literature on the VOT, as the test may be inadequate [14, 15].
We hypothesized that absolute StO2 values and VOT-derived StO2 values obtained by monitoring different anatomical sites or using dissimilar depth may differ, and that different minimal values during the VOT (deflation thresholds) would StO2 result in dissimilar ReO2 rates.
Materials and methods
The present study was approved by the Institutional Review Board of the University of Pittsburgh. Informed consent was obtained from every volunteer enrolled in the study prior to performing any procedure.
Near-infrared spectroscopy
StO2 and its change during the VOT were simultaneously measured in the F and TH, with 15 mm and 25 mm probes, using two 325 InSpectra® tissue spectrometers (Hutchinson Technology Inc., Minneapolis, MN, USA) on the same arm in 18 healthy, adult volunteers. Placement of the TH probe was done according to the manufacturer's recommendations as previously described [4, 9]. The F probe was placed over the Flexor digitorum profundus in the F.
Research software (InSpectra Version vBeta1.1; Hutchinson Technology Inc.) on two independent laptop computers was used for data collection.
Vascular occlusion test
The VOT was performed as previously described [9]. Briefly, the baseline arterial pressure was measured using a manual sphygmomanometer. An adult-size tourniquet cuff was then placed on the arm above both probes. After StO2 signal stabilization at rest (<2% variation in 30 seconds), the tourniquet was inflated with an automatic pump (Portable Tourniquet System, ref. 9-2100-001; Delfi Medical Innovations Inc., Vancouver, BC, Canada) to >30 mmHg above systolic pressure and was kept inflated until the StO2 decreased to 40% (deflation threshold). Inflation took approximately 3 to 4 seconds. The tourniquet was then rapidly deflated (<0.5 seconds) and the StO2 response was followed until the returned to baseline values. All StO2 repeated VOTs were performed after a minimal period of 5 minutes to allow the StO2 to recover.
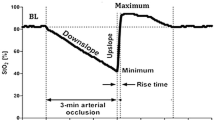
DeO2 and ReO2 slopes, the area under the curve of the hyperemic response as well as the duration of hyperemia (Figure 1) were calculated using the published methodology and a software package designed for this purpose and this specific tissue spectrometer (Version 3.03 InSpectra Analysis Program; Hutchinson Technology Inc.), as previously validated by us [9].
Protocol
Subjects were placed in a quiet environment while resting in a semirecumbent position with the studied arm resting over a cushion in the anatomical position. Probes were placed using a form-fitting plastic adhesive over the TH and volar surface of the F. The 15 mm probe was initially placed on the F and the 25 mm probe placed on the TH. A VOT was then performed. After a 5-minute resting period, the probe locations were switched (that is, the 15 mm probe to the TH, and the 25 mm probe to the F) and a second VOT was performed. Subsequently, two additional VOTs were carried out to StO2 deflation thresholds of 50% and 30% with the same probe disposition.
Healthy volunteers
Volunteers were selected according to the following inclusion and exclusion criteria: inclusion criterion, subjects older than 17 years (that is, ≥18 years); and exclusion criteria, acute or chronic cardiovascular or pulmonary disease, or subject taking any vasoactive medications.
Statistical analysis
Mean absolute StO2 values, the DeO2 and the ReO2(%O2/minute) as measured by the 15 mm and 25 mm probes between the TH and F monitoring sites were compared using the Wilcoxon signed-ranks test. Comparison of the DeO2 and ReO2 when the VOT was performed at different StO2 deflation thresholds (that is, StO2 of 30%, 40% and 50%) was performed using the Friedman test and analysis of variance for repeated measures (after running normality tests). Results are presented as the median (interquartile range), P-value unless stated otherwise. Significance reports a difference between groups corresponding to P < 0.05 unless stated otherwise.
Results
Eighteen healthy volunteers were enrolled in the present study after obtaining informed consent. Demographic characteristics of the studied population are summarized in Table 1. The mean baseline StO2, DeO2, ReO2 and their coefficients of variability for these measures for the various comparisons are summarized in Table 2.
The absolute StO2, DeO2 and ReO2 values for a deflation threshold of 40% were different between the TH and F for both the 15 mm and 25 mm probes (Figures 2 and 3), and were also different at the TH site for the 15 mm and 25 mm probes (Figure 4). Not surprisingly, the TH DeO2 was not altered by varying the deflation StO2 thresholds from 30% to 50% when measured with the 15 mm probe. This result was expected given that the DeO2 is dependent on the metabolic conditions of the tissue, independent of the intensity of the ischemic insult and given the specific calculation methodology [1]. If the metabolic rate of the sampled tissue was not varied, therefore, the DeO2 should not be significantly modified. The ReO2 was higher using the 30% threshold than either the 40% or 50% thresholds, which themselves were similar (Figure 5).
The coefficients of variability for the absolute StO2, DeO2 and ReO2 values were also different when comparing both sites and probes, being less with the TH than F measures and with the 15 mm than 25 mm probe size. We also observed a lower coefficient of variability with 40% and 50% thresholds as compared with the 30% threshold (Table 3).
The mean ± standard deviation hyperemic areas under the curve (Figure 1) as measured by TH15 mm, TH25 mm, F15 mm and F25 mm were 11.2 ± 8.8%O2/minute, 7.7 ± 5.1%O2/minute, 6.4 ± 5.7%O2/minute and 13.2 ± 9.6%O2/minute, respectively, and were all significantly different when compared among each other (Figure 6). Their coefficients of variability were 78.7%, 66.2%, 90.3% and 72.5%, respectively. The hyperemia durations (mean ± standard deviation) for the same measurements stated above were 2.3 ± 0.8 minutes, 1.8 ± 0.6 minutes, 1.6 ± 0.5 minutes and 2.8 ± 1.2 minutes, respectively. These were also statistically different from each other, as shown in Figure 6. Their coefficients of variability were 32.9%, 33.5%, 34.1% and 33.5%, respectively.
Discussion
Baseline StO2 values and their emergent parameters of the DeO2 and the ReO2, assessed by the VOT, are dependent on the site of measurement, the probe size and, to a certain extent, the ischaemic challenge. Varying any one of these components will significantly vary StO2-related measures independent of the baseline cardiovascular status. When making inferences about cardiovascular function using these measures, therefore, care must be taken in comparing or combining results from different studies that use different measurement techniques and protocols. Presently, the VOT ischaemic challenge has not been standardized. Several investigators report the DeO2 and the ReO2 from a fixed ischaemic time (for example, 3 minutes) rather than to a defined threshold StO2 value. Since the basal metabolic rate can vary amongst subjects, varying the DeO2 proportionally [3] using a fixed ischaemic time will result in differing threshold StO2 values within and among subjects, making changes in the ReO2 difficult to interpret among subjects if their metabolic rates are also different because they will reach different StO2 nadirs for a constant ischaemic time. Our data therefore support using a defined ischaemic StO2 threshold in future studies to minimize ReO2 variability.
We found that the baseline StO2, DeO2 and ReO2 values were consistently higher in the TH than F sites. Furthermore, the magnitude of the difference between the TH and F was greater for DeO2 and ReO2 values than for baseline StO2 values. There was also a trend toward a greater variability for baseline StO2 at F than at TH sites. This variability was increased for the DeO2 and ReO2. Although our study does not allow us to identify why this difference occurs, most probably it reflects the greater degree of tissue and the associated vascular variability present in the F over the TH. The F has a variable layer of subcutaneous fat, which has a lower metabolic rate and minimal microcirculatory density when compared with muscle. The TH has almost no fat and is highly consistent amongst subjects. Our data therefore support using the TH site rather then the F site when measuring StO2 and its VOT-related parameters.
We also found consistently lower baseline StO2 values with the 15 mm probe than with the 25 mm probe, and this difference increased with VOT-related parameters. Although baseline StO2 values displayed a similar variability (5.6% vs. 4.6%), VOT-derived measurements performed with the 15 mm probe showed less variability than those performed with the 25 mm probe. Presumably, by sampling a smaller amount of tissue volume, the 15 mm probe is less subject to measurement error. Our data support the routine use of the 15 mm probe over the 25 mm probe in future studies.
The hyperemic response, as quantified by the area under the curve and the duration, varied according to the anatomical site of measurement and the probe size used. Furthermore, great variability was found among healthy volunteers with regards to both parameters, as previously pointed out by our group [9]. These results suggest that the hyperemic response is not a reproducible parameter, and has been proven to be highly dependent on individual variability. Thus, it may not be a reliable parameter for clinical use.
Limitations
The present study has two primary limitations.
First, we studied only two anatomical sites, whereas StO2 can be measured anywhere on the body. Extrapolation of these findings to other anatomical sites, such as the abdominal wall, thigh and masseter muscles, should be done with caution. Since the VOT can only be carried out realistically on a peripheral site for which transient ischaemia will have no lasting deleterious effects, however, these studies reflect the present clinical practice of near-infrared spectroscopy VOT analysis.
Second, we did not study critically ill patients but healthy volunteers. Although this was the goal of the study, its application to critically ill patients might be limited. Previous studies by ourselves and others document that cardiovascular insufficiency can markedly increase intersubject variability [4, 9]. Our data demonstrated a consistently lower coefficient of variation whenever the combination of TH site and 15 mm probe was used, which suggests that this site may also yield more consistent data in clinical trials. Similarly, using a defined ischaemic threshold for the VOT, method-specific variability of the ReO2 should also be minimized.
Abbreviations
- BaseO2:
-
baseline tissue oxygen saturation
- DeO2:
-
deoxygenation rate
- F:
-
forearm
- ReO2:
-
reoxygenation rate
- StO2:
-
tissue oxygen saturation
- TH:
-
thenar eminence
- VOT:
-
vascular occlusion test.
References
Cohn SM, Nathens AB, Moore FA, Rhee P, Puyana JC, Moore EE, Beilman GJ: Tissue oxygen saturation predicts the development of organ dysfunction during traumatic shock resuscitation. J Trauma 2007, 62: 44-54. discussion 54-55 10.1097/TA.0b013e31802eb817
Crookes BA, Cohn SM, Bloch S, Amortegui J, Manning R, Li P, Proctor MS, Hallal A, Blackbourne LH, Benjamin R, Soffer D, Habib F, Schulman CI, Duncan R, Proctor KG: Can near-infrared spectroscopy identify the severity of shock in trauma patients? J Trauma 2005, 58: 806-813. discussion 813-816 10.1097/01.TA.0000158269.68409.1C
Convertino VA, Ryan KL, Rickards CA, Salinas J, McManus JG, Cooke WH, Holcomb JB: Physiological and medical monitoring for en route care of combat casualties. J Trauma 2008,64(4 Suppl):S342-S353. 10.1097/TA.0b013e31816c82f4
Creteur J, Carollo T, Soldati G, Buchele G, De Backer D, Vincent JL: The prognostic value of muscle StO 2 in septic patients. Intensive Care Med 2007, 33: 1549-1556. 10.1007/s00134-007-0739-3
Gomez H, Torres A, Polanco P, Kim H, Zenker S, Puyana J, Pinsky M: Use of non-invasive NIRS during a vascular occlusion test to assess dynamic tissue O 2 saturation response. Intensive Care Med 2008, 34: 1600-1607. 10.1007/s00134-008-1145-1
De Blasi RA, Quaglia E, Gasparetto A, Ferrari M: Muscle oxygenation by fast near infrared spectrophotometry (NIRS) in ischemic forearm. Adv Exp Med Biol 1992, 316: 163-172.
Podbregar M, Mozina H: Skeletal muscle oxygen saturation does not estimate mixed venous oxygen saturation in patients with severe left heart failure and additional severe sepsis or septic shock. Crit Care 2007, 11: R6. 10.1186/cc5153
Soller BR, Ryan KL, Rickards CA, Cooke WH, Yang Y, Soyemi OO, Crookes BA, Heard SO, Convertino VA: Oxygen saturation determined from deep muscle, not thenar tissue, is an early indicator of central hypovolemia in humans. Crit Care Med 2008, 36: 176-182. 10.1097/01.CCM.0000295586.83787.7E
Gomez H, Torres A, Polanco P, Kim HK, Zenker S, Puyana JC, Pinsky MR: Use of non-invasive NIRS during a vascular occlusion test to assess dynamic tissue O 2 saturation response. Intensive Care Med 2008, 34: 1600-1607. 10.1007/s00134-008-1145-1
Siafaka A, Angelopoulos E, Kritikos K, Poriazi M, Basios N, Gerovasili V, Andreou A, Roussos C, Nanas S: Acute effects of smoking on skeletal muscle microcirculation monitored by near-infrared spectroscopy. Chest 2007, 131: 1479-1485. 10.1378/chest.06-2017
Skarda DE, Mulier KE, Myers DE, Taylor JH, Beilman GJ: Dynamic near-infrared spectroscopy measurements in patients with severe sepsis. Shock 2007, 27: 348-353. 10.1097/01.shk.0000239779.25775.e4
Yang Y, Soyemi O, Scott PJ, Landry MR, Lee SM, Stroud L, Soller B: Quantitative measurement of muscle oxygen saturation without influence from skin and fat using continuous-wave near infrared spectroscopy. Opt Express 2007, 15: 13715-13730. 10.1364/OE.15.013715
De Blasi RA, Cope M, Elwell C, Safoue F, Ferrari M: Noninvasive measurement of human forearm oxygen consumption by near infrared spectroscopy. Eur J Appl Physiol Occup Physiol 1993, 67: 20-25. 10.1007/BF00377698
Creteur J: Muscle StO 2 in critically ill patients. Curr Opin Crit Care 2008, 14: 361-366. 10.1097/MCC.0b013e3282fad4e1
Ince C, Bezemer R, Lima A: Letters to the editor: Near infrared spectroscopy. Crit Care Med 2009, 37: 384-385. 10.1097/CCM.0b013e3181932d42
Acknowledgements
The authors want to thank the Department of Critical Care Medicine of the University of Pittsburgh, especially Dr Erick Itoman, Chief Fellow of the Multidisciplinary Critical Care Fellowship. They also want to thank Dr Alvaro Sanchez-Ortiz, MD, MPH for his contribution to the statistical analysis for the present paper. The present research was supported in part by NHLBI grants HL67181 and HL76157.
This article is part of Critical Care Volume 13 Supplement 5: Tissue oxygenation (StO2) in healthy volunteers and critically-ill patients. The full contents of the supplement are available online at http://ccforum.com/supplements/13/S5. Publication of the supplement has been supported with funding from Hutchinson Technology Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
HG, JM and HKK are co-investigators in a Hutchinson-sponsored clinical trial. CI has received honorarium for speaking at a Hutchinson Symposium, and is a co-investigator on a Hutchinson-sponsored clinical trial. MRP and JCP have received an honorarium for speaking at a Hutchinson Symposium, and are co-investigators on a Hutchinson-sponsored prospective clinical trial. PS declares that he has no competing interests.
Rights and permissions
About this article
Cite this article
Gómez, H., Mesquida, J., Simon, P. et al. Characterization of tissue oxygen saturation and the vascular occlusion test: influence of measurement sites, probe sizes and deflation thresholds. Crit Care 13 (Suppl 5), S3 (2009). https://doi.org/10.1186/cc8001
Published:
DOI: https://doi.org/10.1186/cc8001