Abstract
Introduction
Neonatal cardiac surgery is associated with a systemic inflammatory reaction that might compromise the reactivity of blood cells against an inflammatory stimulus. Our prospective study was aimed at testing this hypothesis.
Methods
We investigated 17 newborn infants with transposition of the great arteries undergoing arterial switch operation. Ex vivo production of the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α), of the regulator of the acute-phase response IL-6, and of the natural anti-inflammatory cytokine IL-10 were measured by enzyme-linked immunosorbent assay in the cell culture supernatant after whole blood stimulation by the endotoxin lipopolysaccharide before, 5 and 10 days after the operation. Results were analyzed with respect to postoperative morbidity.
Results
The ex vivo production of TNF-α and IL-6 was significantly decreased (P < 0.001 and P < 0.002, respectively), whereas ex vivo production of IL-10 tended to be lower 5 days after the operation in comparison with preoperative values (P < 0.1). Ex vivo production of all cytokines reached preoperative values 10 days after cardiac surgery. Preoperative ex vivo production of IL-6 was inversely correlated with the postoperative oxygenation index 4 hours and 24 hours after the operation (P < 0.02). In contrast, postoperative ex vivo production of cytokines did not correlate with postoperative morbidity.
Conclusion
Our results show that cardiac surgery in newborn infants is associated with a transient but significant decrease in the ex vivo production of the pro-inflammatory cytokines TNF-α and IL-6 together with a less pronounced decrease in IL-10 production. This might indicate a transient postoperative anti-inflammatory shift of the cytokine balance in this age group. Our results suggest that higher preoperative ex vivo production of IL-6 is associated with a higher risk for postoperative pulmonary dysfunction.
Similar content being viewed by others

Introduction
Cardiac surgery is associated with a systemic inflammatory reaction comprising activation of the complement system, stimulation of leukocytes, synthesis of cytokines, and increased interactions between leukocytes and endothelium [1, 2]. In children, contact activation, ischemia/reperfusion injury and endotoxin released from the gut [3, 4] are thought to be the major inductors of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and IL-6 in the cardiac surgery setting. In newborn infants, morbidity after cardiac surgery is related to the importance of the intra-operative production of pro-inflammatory cytokines such as IL-6, as we have shown previously [5].
NF-κB is the main transcription factor of many inflammatory genes, such as that encoding TNF-α [6]. TNF-α induces secondary mediators of inflammation such as IL-6, the principal regulator of the acute-phase response [7]. IL-10 is an anti-inflammatory cytokine that strongly inhibits the synthesis of pro-inflammatory cytokines at the transcriptional level by controlling the degradation of the inhibitory protein of NF-κB, IκB, and thereby the nuclear translocation of NF-κB [8]. IL-10 has a central role in the control and termination of systemic inflammation. Although IL-10 is thought to have a protective role in the early postoperative period, the maintenance of normal postoperative organ function is likely to depend on an adequate balance between the production of pro-inflammatory and anti-inflammatory cytokines [9]. It has been suggested that the overproduction of IL-10 after severe injury might be associated with a hyporesponsiveness to lipopolysaccharide (LPS) that carries a higher risk for infections [10].
The ex vivo production of cytokines by whole blood is a widely accepted method of evaluating the reactivity of immunoreactive and inflammatory cells and their potential for inflammatory responses [11]. In this study, we tested the hypothesis that neonatal cardiac surgery would influence the ex vivo production of cytokines.
Materials and methods
Patients
After approval by the Human Ethical Committee of the Aachen University Hospital as well as written consent from the parents, 17 consecutive newborn infants aged 2 to 13 days (median 8 days) were included in this study. To ensure homogeneity of the patient group, the inclusion criterion was a simple transposition of the great arteries, suitable for an arterial switch operation. All patients received prostaglandin E1 infusion (0.05 μg kg-1 min-1) before the operation, to maintain patency of the ductus arteriosus. Preoperative cardiac catheterization for balloon atrioseptostomy and angiography was performed in 13 patients.
Anesthesia, operative management and postoperative care
Conventional general anesthesia was conducted with diazepam, fentanyl sulfate and pancuronium bromide. Perioperative antibiotic prophylaxis consisted of cefotiam hydrochloride (100 mg kg-1 body weight). Dexamethasone (10 mg m-2 body surface area) was administered immediately before sternotomy.
The standardized neonatal cardiopulmonary bypass (CPB) protocol included a roller pump, a disposable membrane oxygenator and an arterial filter. All patients were operated on under deep hypothermic CPB, as described previously [5]. Epinephrine (adrenaline), dopamine and sodium nitroprusside were administered systemically for weaning the patients from CPB.
Standardized postoperative care was provided. Monitoring included continuous registration of hemodynamic variables, diuresis and blood gases. Inotropic support consisted in all cases of dopamine (5 μg kg-1 min-1) and, if necessary, epinephrine (0.05 to 0.2 μg kg-1 min-1) or dobutamine (5 to 7.5 μg kg-1 min-1) and vasodilatory treatment of sodium nitroprusside (0.5 to 2 μg kg-1 min-1). Diuretics (furosemide, single dosage of 0.1 to 1 mg kg-1) and volume substitution, which consisted of fresh-frozen plasma or human albumin 5%, were administered depending on the hemodynamic variables. Postoperative clinical endpoint variables were mean arterial blood pressure, mean central venous pressure, need for inotropic support, oxygenation index expressed as the ratio of partial arterial oxygen tension to fraction of inspired oxygen, minimal diuresis, maximal serum creatinine and maximal serum glutamate oxaloacetate transaminase values during the first 72 hours after the operation, and duration of inotropic and ventilatory support.
Blood elements
Leukocyte counts were determined by a Cell-Dyn 3700 (Abbott GmbH & Co. KG, Wiesbaden, Germany).
C-reactive protein
C-reactive protein was determined by laser nephelometry. The detection limit of this method is 5 mg dl-1.
Ex vivo stimulation
Whole blood culture was performed as described previously [12]. Blood (1 ml) was withdrawn under sterile conditions from a peripheral vein and was taken in endotoxin-free tubes (Endo tube ET; Chromogenix, Haemochrom Diagnostica GmbH, Essen, Germany) before the operation (median 5 days), as well as 5 and 10 days after operation. The timing of blood samples was dictated by the fact that ex vivo production of TNF-α was reported to be decreased up to the sixth postoperative day in adults undergoing cardiac surgery [13]. Blood was mixed in a 1:10 ratio with RPMI 1640 medium containing L-glutamine and 25 mM Hepes medium (Bio Whittaker Europe, Verviers, Belgium). Cell cultures were stimulated with LPS (LPS for cell culture, Escherichia coli, lot 026.B6:L2654; Sigma, St Louis, MO, USA) at a final concentration of 1 ng ml-1. In control samples, the LPS volume was replaced with cell culture medium. Because it has been shown that ex vivo cytokine production reaches its plateau mainly between 12 and 24 hours after stimulation [14], cell cultures were incubated for 16 hours in a humidified incubator at 37°C in an atmosphere consisting of a mixture of 5% CO2 and 95% air (Heraeus HBB 2472b; Heraeus Instruments GmbH, Hanau, Germany); the supernatant was then separated after centrifugation (2,500 r.p.m. for 3 min) and frozen at -70°C until assay.
Cytokine determination
TNF-α, IL-6 and IL-10 were determined with an immunocytometric assay (Biosource International, Camarillo, CA, USA), in accordance with the manufacturer's recommendations for cell culture supernatant. It is a solid-phase, enzyme-amplified sensitivity immunoassay performed on microtiter plates based on the oligoclonal system in which several monoclonal antibodies directed against distinct epitopes of cytokines are used, permitting a high sensitivity of the assay. The minimal detectable concentrations are 3 pg ml-1 for TNF-α, 2 pg ml-1 for IL-6, and 1 pg ml-1 for IL-10. The ranges covered by the standard curve are 0 to 1,700 pg ml-1 for TNF-α, 0 to 2,100 pg ml-1 for IL-6, and 0 to 1,750 pg ml-1 for IL-10. Samples were diluted accordingly.
Statistical analysis
Results are expressed as means ± SEM. The data were analyzed with the nonparametric paired Wilcoxon rank test. The Spearman rank correlation coefficient was assessed for correlation of independent parameters. P < 0.05 was considered significant.
Results
Clinical results
Operative data and clinical results are summarized in Table 1. Seven of the 17 newborn infants showed early postoperative complications that are summarized in Table 2. Six of the seven patients with complications had a capillary leak syndrome as previously described by our group [15]. One patient developed pneumonia. There was one postoperative death 29 days after operation in a patient having developed thrombosis of the right and of the left persistent superior caval veins.
Leukocyte count
There was no statistical difference between the counts of leukocytes, granulocytes and monocytes measured before the operation, and 5 and 10 days after it (Table 3). Leukocyte counts were not different in patients with or without complications.
C-reactive protein
C-reactive protein (CRP) increased in all patients from 7.94 ± 1.27 mg dl-1 before the operation to 15.7 ± 3.7 mg dl-1 5 days after it. At that time point, CRP values were higher in patients with complications than in those without (23.8 ± 5.5 versus 10.2 ± 4.3 mg dl-1, P = 0.001). The patient with pneumonia had a CRP value of 8 mg dl-1 before the operation and 9 mg dl-1 5 days after the operation, increasing to 50 mg dl-1 12 hours later. CRP values were still elevated in all patients 10 days after the operation (16.6 ± 4.4 mg dl-1), and at that time there was no difference between patients with and without complications. The patient with pneumonia had a CRP value of 8 mg dl-1 at that time.
Ex vivo production of cytokines after LPS stimulation before and after operation
At all time points investigated in this study there was a significant production of TNF-α, IL-6 and IL-10 after stimulation by LPS in comparison with the control sample.
Concentrations of TNF-α and IL-6 in the cell culture supernatant were significantly decreased on day 5, in comparison with preoperative levels (P < 0.001 and P < 0.002, respectively).
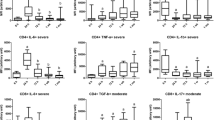
Postoperative IL-10 concentrations on day 5 were also reduced compared with the preoperative value, although not significantly (P < 0.1). On the 10th day after the operation, concentrations of TNF-α, IL-6 and IL-10 had returned to their preoperative levels (Figs 1, 2, 3).
Ex vivo production of tumor necrosis factor-α. Preoperative and postoperative (po) tumor necrosis factor-α (TNF-α) levels in whole blood culture supernatant. Values are expressed as means and SEM (error bars). TNF-α production was significantly increased after stimulation with lipopolysaccharide (LPS; white), in comparison with the unstimulated control (C; black) at all time points. In comparison with preoperative levels, TNF-α production after stimulation with LPS significantly decreased 5 days after operation (P < 0.001) but again reached preoperative levels 10 days after operation.
Ex vivo production of interleukin-6. Preoperative and postoperative (po) interleukin (IL)-6 levels in whole blood culture supernatant. Values are expressed as means and SEM (error bars). IL-6 production was significantly increased after stimulation with lipopolysaccharide (LPS; white), in comparison with the unstimulated control (C; black) at all time points. In comparison with preoperative levels, IL-6 production after stimulation with LPS significantly decreased 5 days after operation (P < 0.002) but again reached preoperative levels 10 days after operation.
Ex vivo production of interleukin-10. Preoperative and postoperative (po) IL-10 levels in whole blood culture supernatant. Values are expressed as means and SEM (error bars). IL-10 production was significantly increased after stimulation with lipopolysaccharide (LPS; white), in comparison with the control (C; black) at all time points. In comparison with preoperative levels, IL-10 production after stimulation with LPS tended to decrease 5 days after operation but again reached preoperative levels 10 days after operation.
Correlation between ex vivo production of cytokines and outcome
In all patients preoperative IL-6 production was inversely correlated with the oxygenation index, as measured 4 and 24 hours after the operation (Spearman correlation coefficient: -0.62; P < 0.02). Figure 4 shows the relationship between preoperative ex vivo IL-6 production and the oxygenation index, as measured 24 hours after the operation. There was no correlation between the ex vivo production of TNF-α and IL-10 and postoperative morbidity, respectively. In particular, the only patient with pneumonia (patient 2 in Table 2) showed ex vivo cytokine production that was in the same range as for all other patients.
Relationship between preoperative production of interleukin-6 (IL-6) and postoperative pulmonary dysfunction. Plot showing the correlation between preoperative IL-6 production after stimulation with lipopolysaccharide (LPS) and the oxygenation index 24 hours after operation (n = 14). Spearman correlation coefficient -0.62; P < 0.02.
Discussion
In previous studies we have shown that neonatal cardiac surgery induces a systemic inflammatory reaction with complement activation, leukocyte stimulation and cytokine synthesis that is associated with postoperative complications such as the capillary leak syndrome and myocardial dysfunction [2, 5, 15]. In this study we confirm the association between systemic inflammation and postoperative morbidity. Although it has been suggested that, in the setting of cardiac surgery, parenchymatous cells such as cardiomyocytes contribute to the systemic inflammatory reaction by producing cytokines, circulating blood cells, in particular leukocytes, are considered the major source of inflammatory mediators [16, 17]. This is supported by previous studies that report a clear association between uncontrolled leukocyte activation and early postoperative morbidity after cardiac surgery in newborn infants and in children [5, 15].
The systemic inflammatory reaction induced by cardiac surgery is normally controlled by a natural anti-inflammatory response. Indeed, levels of IL-10 are already increased at the end of the operation and remain substantially elevated for at least 48 hours after the operation [18].
Although the anti-inflammatory response to cardiac surgery is thought to be beneficial with regard to early postoperative organ protection [17], it remains unclear whether it could impair leukocyte reactivity and thereby decrease resistance against infections.
In this study, the reactivity of circulating cells after neonatal cardiac surgery was evaluated by the ex vivo production of pro-inflammatory and anti-inflammatory cytokines after a standardized inflammatory stimulus in a homogenous patient group.
A previous study in older children who had undergone cardiac surgery for various cardiac defects showed decreased ex vivo cytokine production on the morning of the first postoperative day. However, later time points, to document the normalization of cytokine production, were not investigated [19]. One main result of our study is that neonatal cardiac surgery is associated with a transiently decreased ex vivo production of the pro-inflammatory cytokines TNF-α and IL-6, and that this is not related to a decrease in leukocyte count. This indicates impaired reactivity of inflammatory cells. In adults this phenomenon has been reported after cardiac surgery [13], severe injury and sepsis, and defined as hyporesponsiveness to LPS [20, 21]. In adults who have undergone cardiac surgery, ex vivo TNF-α production and TNF-α mRNA in whole blood were still lower at the end of the study period, which was 6 days after surgery [13]. We also investigated the ex vivo production of cytokines at a later time point and show a return of TNF-α production to preoperative values 10 days after cardiac surgery. The reason for the transient impairment of leukocyte reactivity in our series could be ascribed to the exhaustion of circulating inflammatory cells due to the massive inflammatory stress due to cardiac surgery and also to the perioperative treatment applied. With this regard, drugs administered before, during and after the operation could have influenced hyporesponsiveness to LPS. Indeed, prostaglandin E1 has been shown to reduce the ex vivo production of TNF-α and IL-1β by adult monocytes [22]. However, in our patients who were all treated with prostaglandin-E1 infusion before the operation, preoperative levels of cytokines measured in the supernatant of the whole blood culture were similar to those after stimulation of cord blood in healthy newborn infants [12]. This suggests a minor effect of prostaglandin-E1 on the ex vivo production of cytokines in our study. In adults, anesthesia and heparin were shown not to influence the ex vivo production of TNF-α [13].
The course of ex vivo IL-10 production after cardiac surgery has so far not been followed for more than 6 hours after CPB. In a previous study, IL-10 production reached its lowest point 2 hours after cardiac surgery in adult patients and returned to preoperative values 6 hours later [10]. Our results, in contrast, show that in newborn infants the ex vivo production of IL-10 was decreased 5 days after the operation, even though not significantly in comparison with preoperative values. This reduction could be the result of negative feedback by IL-10, which inhibits not only pro-inflammatory cytokines but also its own production.
The exact mechanisms leading to hyporesponsiveness to LPS in newborn infants reported here are not yet clear. However, the anti-inflammatory cytokines IL-10 and tissue growth factor-β are thought to be important in its regulation [23]. In a clinical study, adult patients with sepsis or severe trauma showed a reduced expression of the active form of NF-κB [24]. In those who did not survive, the IL-10 plasma levels were inversely correlated to the ratio between the active and inhibitory forms of NF-κB, supporting the view that IL-10 might participate in the induction of LPS hyporesponsiveness by inhibiting cytokine synthesis at or upstream of the transcriptional level.
Newborn infants who have undergone cardiac surgery have been reported to show a higher natural production of IL-10 than older children [25]. For the reasons cited above, this natural anti-inflammatory cytokine imbalance could well have contributed to the hyporesponsiveness to LPS observed in our series. Furthermore, as reported by others in older children operated on with CPB [19], perioperative treatment with dexamethasone could also have contributed to hyporesponsiveness to LPS by inhibiting the activation of NF-κB and thereby the production of pro-inflammatory cytokines [26], as well as by stimulating the production of IL-10 [27, 28].
Although a clear association has been demonstrated between hyporesponsiveness to LPS and poor clinical outcome in sepsis [24], we were not able to confirm such an association in our series. One reason for this might be that, in the small group of patients investigated, the overall rate of complications related to inflammation or infection was low.
In contrast, we observed a clear association between the preoperative ex vivo production of IL-6 and postoperative respiratory morbidity. This suggests that a higher preoperative potential for ex vivo production of IL-6 is a risk factor for inflammation-related postoperative complications in newborn infants.
Conclusion
Our results show for the first time that cardiac surgery in newborn infants is associated with a transient but significant decrease in the ex vivo production of the pro-inflammatory cytokines TNF-α and IL-6 together with a less pronounced decrease in IL-10 production. This suggests a postoperative anti-inflammatory shift of the cytokine balance in this age group 5 days after cardiac surgery. A higher preoperative ex vivo production of IL-6 might indicate a higher risk for postoperative pulmonary dysfunction. Further studies will address the question of whether preoperative ex vivo production of IL-6 would be a suitable predictor of postoperative complications in newborn infants with congenital cardiac defects.
Key messages
-
Cardiac surgery in newborn infants decreases the reactivity of blood cells to LPS.
-
Cardiac surgery in newborn infants might lead to an anti-inflammatory shift of the cytokine balance.
-
In this series, postoperative complications related to decreased blood cell reactivity were not observed.
-
The higher the ex vivo production of IL-6 before the operation, the worse the postoperative lung function.
-
Testing the ex vivo production of IL-6 in newborn infants might help to predict postoperative pulmonary dysfunction.
Abbreviations
- CPB:
-
cardiopulmonary bypass
- CRP:
-
C-reactive protein
- IL:
-
interleukin
- LPS:
-
lipopolysaccharide
- NF-κB:
-
NF-κB = nuclear factor κB
- TNF-α:
-
TNF-α = tumor necrosis factor-α.
References
Kirklin JK, Westaby S, Blackstone EH, Kirklin JW, Chenoweth DE, Pacifico AD: Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg 1983, 86: 845-857.
Seghaye MC: The clinical implications of the systemic inflammatory reaction related to cardiac operations in children. Cardiol Young 2003, 13: 228-239.
Jansen NJG, Van Oeveren W, Gu YJ, Vanvliet MH, Eijsman L, Wildevuur CRH: Endotoxin release and tumor-necrosis-factor formation during cardiopulmonary bypass. Ann Thorac Surg 1992, 54: 744-748.
Casey WF, Hauser GJ, Hannallah RS, Midgley FM, Khan WN: Circulating endotoxin and tumor-necrosis-factor during pediatric cardiac surgery. Crit Care Med 1992, 20: 1090-1096.
Hovels-Gurich HH, Vazquez-Jimenez JF, Silvestri A, Schumacher K, Minkenberg R, Duchateau J, Messmer BJ, von Bernuth G, Seghaye MC: Production of proinflammatory cytokines and myocardial dysfunction after arterial switch operation in neonates with transposition of the great arteries. J Thorac Cardiovasc Surg 2002, 124: 811-820. 10.1067/mtc.2002.122308
Siebenlist U, Franzoso G, Brown K: Structure, regulation and function of NF-κB. Annu Rev Cell Biol 1994, 10: 405-455. 10.1146/annurev.cb.10.110194.002201
Heinrich PC, Castell JV, Andus T: Interleukin-6 and the acute phase response. Biochem J 1990, 265: 621-636.
Schottelius AJG, Mayo MW, Sartor RB, Badwin AS Jnr: Interleukin-10 signaling blocks inhibitor of kappa B kinase activity and nuclear factor kappa B DNA binding. J Biol Chem 1999, 274: 31868-31874. 10.1074/jbc.274.45.31868
Hovels-Gurich HH, Schumacher K, Vazquez-Jimenez JF, Qing M, Huffmeier U, Buding B, Messmer BJ, von Bernuth G, Seghaye MC: Cytokine balance in infants undergoing cardiac operation. Ann Thorac Surg 2002, 73: 601-608. 10.1016/S0003-4975(01)03391-4
Dehoux MS, Hernot S, Asehnoune K, Boutten A, Paquin S, Lecon-Malas V, Toueg ML, Desmonts JM, Durand G, Philip I: Cardiopulmonary bypass decreases cytokine production in lipopolysaccharide-stimulated whole blood cells: roles of interleukin-10 and the extracorporeal circuit. Crit Care Med 2000, 28: 1721-1727. 10.1097/00003246-200006000-00004
De Groote D, Zangerle PF, Gevaert Y, Fassotte MF, Beguin Y, Noizat-Pirenne F, Pirenne J, Gathy R, Lopez M, Dehart I, et al.: Direct stimulation of cytokines (IL-1-β, TNF-α, IL-6, IL-2, IFN-γ and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine 1992, 4: 239-248. 10.1016/1043-4666(92)90062-V
Seghaye MC, Heyl W, Grabitz RG, Schumacher K, von Bernuth G, Rath W, Duchateau J: The production of pro- and anti-inflammatory cytokines in neonates assessed by stimulated whole cord blood culture and by plasma levels at birth. Biol Neonate 1998, 73: 220-227. 10.1159/000013980
Borgermann J, Friedrich I, Flohe S, Spillner J, Majetschak M, Kuss O, Sablotzki A, Feldt T, Reidemeister JC, Schade FU: Tumor necrosis factor-α production in whole blood after cardiopulmonary bypass: downregulation caused by circulating cytokine-inhibitory activities. J Thorac Cardiovasc Surg 2002, 124: 608-617. 10.1067/mtc.2002.122300
Dembinski J, Behrendt D, Martini R, Heep A, Bartmann P: Modulation of pro- and anti-inflammatory cytokine production in very preterm infants. Cytokine 2003, 21: 200-206. 10.1016/S1043-4666(02)00498-2
Seghaye MC, Grabitz RG, Duchateau J, Busse S, Dabritz S, Koch D, Alzen G, Hornchen H, Messmer BJ, von Bernuth G: Inflammatory reaction and capillary leak syndrome related to cardiopulmonary bypass in neonates undergoing cardiac operations. J Thorac Cardiovasc Surg 1996, 112: 687-697.
Wan S, LeClerc JL, Vincent JL: Inflammatory response to cardiopulmonary bypass – mechanisms involved and possible therapeutic strategies. Chest 1997, 112: 676-692.
Qing M, Vazquez-Jimenez JF, Klosterhalfen B, Sigler M, Schumacher K, Duchateau J, Messmer BJ, von Bernuth G, Seghaye MC: Influence of temperature during cardiopulmonary bypass on leukocyte activation, cytokine balance, and post-operative organ damage. Shock 2001, 15: 372-377.
Seghaye MC, Duchateau J, Bruniaux J, Demontoux S, Bosson C, Serraf A, Lecronier G, Mokhfi E, Planche C: Interleukin-10 release related to cardiopulmonary bypass in infants undergoing cardiac operations. J Thorac Cardiovasc Surg 1996, 111: 545-553.
Duval EL, Kavelaars A, Veenhuizen L, van Vught AJ, van de Wal HJ, Heijnen CJ: Pro- and anti-inflammatory cytokine patterns during and after cardiac surgery in young children. Eur J Pediatr 1999, 158: 387-393. 10.1007/s004310051098
Keel M, Schregenberger N, Steckholzer U, Ungethum U, Kenney J, Trentz O, Ertel W: Endotoxin tolerance after severe injury and its regulatory mechanisms. J Trauma 1996, 41: 430-437. 10.1097/00005373-199609000-00008
Appoloni O, Vincent JL, Duchateau J: Response of tumour necrosis factor-α to delayed in vitro monocyte stimulation in patients with septic shock is related to outcome. Clin Sci (Lond) 2002, 102: 315-320. 10.1042/CS20010260
Widomski D, Fretland DJ, Gasiecki AF, Collins PW: The prostaglandin analogs, misoprostol and SC-46275, potently inhibit cytokine release from activated human monocytes. Immunopharmacol Immunotoxicol 1997, 19: 165-174.
Schroder M, Meisel C, Buhl K, Profanter N, Sievert N, Volk HD, Grutz G: Different modes of IL-10 and TGF-β to inhibit cytokine-dependent IFN-γ production: consequences for reversal of lipopolysaccharide desensitization. J Immunol 2003, 170: 5260-5267.
Adib-Conquy M, Adrie C, Moine P, Asehnoune K, Fitting C, Pinsky MR, Dhainaut JF, Cavaillon JM: NF-κB expression in mononuclear cells of patients with sepsis resembles that observed in lipopolysaccharide tolerance. Am J Respir Crit Care Med 2000, 162: 1877-1883.
Alcaraz AJ, Sancho L, Manzano L, Esquivel F, Carrillo A, Prieto A, Bernstein ED, Alvarez-Mon M: Newborn patients exhibit an unusual pattern of interleukin 10 and interferon gamma serum levels in response to cardiac surgery. J Thorac Cardiovasc Surg 2002, 123: 451-458. 10.1067/mtc.2002.120006
van Leeuwen HJ, van der Bruggen T, van Asbeck BS, Boereboom FTJ: Effect of corticosteroids on nuclear factor-κB activation and hemodynamics in late septic shock. Crit Care Med 2001, 29: 1074-1077. 10.1097/00003246-200105000-00041
Wan S, LeClerc JL, Schmartz D, Barvais L, Huynh CH, Deviere J, DeSmet JM, Vincent JL: Hepatic release of interleukin-10 during cardiopulmonary bypass in steroid-pretreated patients. Am Heart J 1997, 133: 335-339.
Tabardel Y, Duchateau J, Schmartz D, Marecaux G, Shahla M, Barvais L, LeClerc JL, Vincent JL: Corticosteroids increase blood interleukin-10 levels during cardiopulmonary bypass in men. Surgery 1996, 119: 76-80.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
KS performed whole blood cultures, ELISAs, acquisition and statistical analysis of the data, and redaction of the manuscript. SK performed ELISAs, data acquisition and analysis. JFV-J coordinated sample withdrawal and revised the manuscript. GvB drafted the manuscript and revised it critically. JD supervised the blood cultures and ELISAs, study design, data analysis and interpretation. M-CS was responsible for study conception and design, data analysis and interpretation and manuscript preparation and final revision. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Schumacher, K., Korr, S., Vazquez-Jimenez, J.F. et al. Does cardiac surgery in newborn infants compromise blood cell reactivity to endotoxin?. Crit Care 9, R549 (2005). https://doi.org/10.1186/cc3794
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc3794