Abstract
Introduction
Our aims were to assess risk factors, clinical features, management and outcomes in critically ill patients in whom Aspergillus spp. were isolated from respiratory secretions, using a database from a study designed to assess fungal infections.
Methods
A multicentre prospective study was conducted over a 9-month period in 73 intensive care units (ICUs) and included patients with an ICU stay longer than 7 days. Tracheal aspirate and urine samples, and oropharyngeal and gastric swabs were collected and cultured each week. On admission to the ICU and at the initiation of antifungal therapy, the severity of illness was evaluated using the Acute Physiology and Chronic Health Evaluation II score. Retrospectively, isolation of Aspergillus spp. was considered to reflect colonization if the patient did not fulfil criteria for pneumonia, and infection if the patient met criteria for pulmonary infection and if the clinician in charge considered the isolation to be clinically valuable. Risk factors, antifungal use and duration of therapy were noted.
Results
Out of a total of 1756 patients, Aspergillus spp. were recovered in 36. Treatment with steroids (odds ratio = 4.5) and chronic obstructive pulmonary disease (odds ratio = 2.9) were significantly associated with Aspergillus spp. isolation in multivariate analysis. In 14 patients isolation of Aspergillus spp. was interpreted as colonization, in 20 it was interpreted as invasive aspergillosis, and two cases were not classified. The mortality rates were 50% in the colonization group and 80% in the invasive infection group. Autopsy was performed in five patients with clinically suspected infection and confirmed the diagnosis in all of these cases.
Conclusion
In critically ill patients, treatment should be considered if features of pulmonary infection are present and Aspergillus spp. are isolated from respiratory secretions.
Similar content being viewed by others
Introduction
Aspergillus is a genus of mitosporic fungi, some species of which are known to cause infections in humans, particularly Aspergillus fumigatus (85% of cases) followed by A flavus and A niger [1]. Aspergillus spp. are responsible for a broad spectrum of illnesses, from saprophytic colonization of the bronchial tree to rapidly invasive and disseminated diseases. Invasive aspergillosis remains a major cause of morbidity and mortality in immunosuppressed patients with profound granulocytopenia secondary to haematological malignancies, or solid organ and bone marrow transplantation. Outbreaks of aspergillosis in patients admitted to intensive care units (ICUs) have been reported [2]. Aspergillus spp. can also cause pneumonia in ICU patients without classical predisposing factors, as well as community-acquired pneumonia in otherwise immunocompetent healthy individuals [3, 4].
Because the mortality rate with invasive aspergillosis remains high, even in the face of therapy, the work up must be prompt and aggressive. The diagnosis of invasive pulmonary aspergillosis is difficult because definitive diagnosis is based on histological documentation of typical hyphae and a culture positive for an Aspergillus sp. Uncertainty in disease definition is a key contributor to the controversy regarding the optimal method for establishing the diagnosis of invasive infection.
Standard definitions of opportunistic fungal infections in immunocompromised patients with cancer and haematopoietic stem cell transplants were recently proposed [5]. 'Proven' aspergillosis requires histopathological or cytopathological examination showing hyphae with evidence of associated tissue damage, or a positive culture result from a sample obtained using sterile technique along with suggestive clinical or radiological evidence of infection. In addition, 'probable' aspergillosis requires the presence of risk factors in the host, isolation of an Aspergillus sp. and suggestive clinical or radiological findings; 'possible' aspergillosis requires the presence of risk factors in the host and isolation of an Aspergillus sp., or suggestive clinical and radiological findings [5]. Serology is not useful in the diagnosis of aspergillosis, and data regarding the clinical utility of detection of Aspergillus antigenaemia is limited to patients with neutropenia [6].
Treatment is mandatory in severely immunocompromised patients (those with neutropenia or prolonged use of immunosuppressants) with suggestive clinical manifestations or isolation of Aspergillus spp. in respiratory secretions. However, the therapeutic approach is not well defined in critically ill patients without neutropenia or transplantation in whom Aspergillus spp. are cultured in bronchial secretions [7]. Therefore, using data from a large multicentre study designed to assess risk factors and the impact of isolation of fungi in ICU patients, the present study was performed with the following objectives: to determine risk factors for respiratory isolation of Aspergillus spp.; to assess clinical features, treatment and outcomes in patients with Aspergillus spp. recovered from respiratory secretions; and to evaluate the correlation between isolation of Aspergillus spp. in respiratory samples and histopathological findings.
Materials and methods
Study population
A total of 1765 patients older than 18 years of age who were admitted for at last 7 days to 73 medical/surgical ICUs in certain Spanish hospitals between May 1998 and January 1999 were included in the study. The institutional review board of each hospital approved the protocol and waived the need for informed consent.
Design
This was a prospective, cohort, observational, multicentre study. Based on diagnosis at the time of ICU admission, patients were classified as medical, surgical, or trauma. The severity of illness on ICU admission was calculated using the Acute Physiology and Chronic Health Evaluation (APACHE) II scoring system [8]. The definitions of severe sepsis and septic shock used were those of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference [9].
In all patients, samples obtained from sputum, tracheal aspirates (intubated patients), urine, pharyngeal exudates and gastric aspirates were cultured for fungi each week. The initial samples were obtained 8 days after admission to the ICU and once a week thereafter. Other samples of peripheral blood or from other infectious foci were obtained at the discretion of the attending physician. Samples were processed by the various reference clinical microbiology laboratories of the participating hospitals using standard procedures, including Sabouraud agar culture and BACTEC method (Becton Dickinson Diagnostic Instrument Systems, Paramus, NJ, USA), for the isolation of fungal species. The A20C system (Byomerieux, Lyon, France) was used for species identification. Candida infection was defined as recovery of Candida spp. from blood samples (in one or more culture bottles), or evidence of endophthalmitis, or a positive culture of tissue specimens or peritoneal fluid culture, or obstruction of the urinary tract by fungal balls.
Risk factors
Various risk factors before ICU admission and during the ICU stay were recorded. These are summarized in Table 1.
Clinical features
Patients with Aspergillus spp. isolated from respiratory samples were retrospectively evaluated. The clinical significance of recovery of Aspergillus spp. was determined individually by the physician in charge, who established whether isolation of Aspergillus spp. represented a case of colonization or infection. Colonization was deemed to be present when the patient did not fulfil criteria for pneumonia; if the patient fulfilled criteria for pneumonia and the clinician in charge considered the isolation of Aspergillus spp. to be clinically valuable, then the patient was considered to be infected. Specific recommendations regarding therapeutic approach when fungi were isolated from culture were not given, and so the decision regarding antifungal treatment was made on an individual basis by the physician in charge. In patients treated with antifungal drugs, adverse events, clinical cure and microbiological eradication (weekly cultures becoming negative) were recorded. For each patient in whom an Aspergillus sp. was detected, clinical data as well as radiographic and computed tomography findings were retrospectively recorded by means of a questionnaire completed by the clinician in charge. Radiographic findings included normal chest radiograph, lobar consolidation, unilateral consolidation, bilateral consolidation and ill-defined nodules [10].
Patients were followed until discharge from the hospital or death during the hospital stay. In patients who died with proven fungal infection or with high suspicion of fungal infection, an autopsy examination was sought.
Statistical analysis
Qualitative variables are expressed as the percentage distribution in each category, and quantitative variables are expressed as mean ± standard deviation in normally distributed variables or median (range) when the distribution was not normal. The Student's t-test or the Mann–Whitney U-test was used for the comparison of categorical and normally distributed and non-normally distributed variables, respectively. Analysis of variance or the Kruskal–Wallis test was used in the comparison of three groups. The χ2 test or the Fisher's exact test was used in the comparison of categorical variables. A comparison of risk factors for the isolation of Aspergillus spp. between groups of patients with Aspergillus spp., patients with Candida spp. infection, and noncolonized, uninfected patients was conducted. For this purpose, a binary logistic regression analysis prior to the bivariate analyses was performed. Variables were included in the model if P ≤ 0.05. Results are expressed as odds ratio (OR), 95% confidence interval (CI). P < 0.05 was considered statistically significant. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) for Windows (version 11.5; SPSS Inc., Chicago, IL, USA).
Results
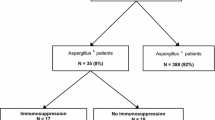
The study population included 1765 patients (1178 [67%] men; mean [± standard deviation] age 57.8 ± 17.3 years). Underlying diseases were classified as medical in 44% of patients, surgical in 47% and trauma in 9%. A total of 1045 patients were classified as colonized or infected with fungi, and 720 were classified as noncolonized, uninfected patients. Colonization with Candida spp. was diagnosed in 880 (49.8%) patients, Candida spp. infection in 105 (5.9%), and infection with fungi other than Candida spp. in 60 (3.4%). In this group of 60 patients, in whom fungi other than Candida spp. were isolated, Aspergillus spp. were recovered in 38 (63.3%). An Aspergillus sp. was isolated from respiratory secretions in 36 patients (tracheal aspirate 35, sputum 1). A fumigatus was isolated in 35 patients and A niger in one. The length of ICU stay was similar between patients infected with Aspergillus spp. and those infected with Candida spp. (32.1 ± 21.4 days versus 32.8 ± 22.6 days), but it was significantly longer than in noncolonized, uninfected patients (18.4 ± 14.1 days; P < 0.001; Table 2).
Compared with noncolonized, uninfected patients, patients with Aspergillus spp. infection had significantly greater in-hospital mortality (69.4% versus 33%; P < 0.001) and ICU mortality (52.8% versus 24.7%; P < 0.001) rates. Patients with Candida spp. infection also had significantly greater in-hospital mortality (60.9% versus 33%; P < 0.001) and ICU mortality (53.3% versus 24.7%; P < 0.001) rates than did noncolonized, uninfected patients.
Risk factors
The frequency of risk factors for fungal infection before ICU admission were similar in the three groups of patients (Table 2), except for significantly higher rates of chronic obstructive pulmonary disease (COPD), immunosuppression and transplantation in the patients with Aspergillus infection, and a greater prevalence of solid neoplasms in the patients with Candida infection. With regard to risk factors present during the ICU stay, neutropenia and treatment with steroids were significantly more frequent in the Aspergillus group, and total parenteral nutrition was significantly more common in the Candida group (Table 2). Duration of steroid administration was also significantly longer in the Aspergillus group (Table 3). In multivariate analysis, independent factors significantly associated with recovery of Aspergillus spp. compared with noncolonized, uninfected patients were treatment with steroids (OR = 4.5, 95% CI = 1.73–11; P = 0.002) and COPD (OR = 2.9, 95% CI = 1.06–8.08; P = 0.03). When comparisons with patients with Candida infection were performed, immunosuppression (OR = 12.9, 95% CI = 1.34–25; P = 0.001), neutropenia (OR = 9.4, 95% CI = 1.9–19.9; P = 0.02) and COPD (OR = 9.2, 95% CI 1.36–62.5; P = 0.02) emerged as independent factors significantly associated with isolation of Aspergillus spp.
Clinical characteristics
Aspergillus spp. were isolated from respiratory samples in severely ill patients, with a mean APACHE II score on ICU admission of 21.6 ± 6.9 and a mean age of 58.7 ± 16.6 years. Apart for eight patients with Aspergillus infection, the remaining 28 patients had debilitating underlying disorders, with COPD (n = 16), immunosuppression (n = 20) and chronic renal failure (n = 10) being the most common. During their stay in the ICU, 25 patients received steroids and all but one were mechanically ventilated. The mean length of ICU stay before isolation of Aspergillus spp. was 32.1 ± 21.4 days. Previous use of fluconazole was recorded in eight of the 36 patients (22.2%) with isolation of Aspergillus spp., and in 41 of the 105 patients (39%) with invasive candidiasis.
In 14 patients without clinical symptoms of pneumonia, isolation of Aspergillus spp. was interpreted by the clinician in charge as colonization. In two patients Aspergillus spp. were recovered 24 hours before the patient's death, and so the clinical manifestations could not be evaluated. The remaining 20 patients had signs of severe sepsis or septic shock unresponsive to broad-spectrum antibiotics in association with clinical manifestations suggestive of pneumonia. In these cases, isolation of Aspergillus spp. was interpreted to represent infection, and treatment with antifungal agents was started. In seven of these patients, however, bacteria in association with Aspergillus spp. were isolated from the tracheal aspirates, including Pseudomonas aeruginosa (n = 2), Klebsiella pneumoniae (n = 1), Acitenobacter baumannii (n = 1), Stenotrophomonas maltophilia (n = 1), coagulase-negative Staphylococcus spp. and Haemophilus spp. (n = 1). The most frequent radiographic findings were unilateral consolidation and bilateral consolidation.
Treatment and outcome
In the group of 14 patients with Aspergillus colonization, the in-hospital mortality rate was 50% (three patients died in the ICU). Eleven patients were not treated with antifungal drugs, although risk factors were present in seven. Liposomal amphotericin B was prescribed to three patients (one of these patients with predisposing risk factors died in the ICU). The mean cumulative dose of amphotericin B lipid formulation was 3100 mg and the mean duration of treatment was 9 days.
Of the 20 patients with Aspergillus spp. infection 16 died, yielding an in-hospital mortality rate of 80%. All patients were given amphotericin B except one patient, who was treated with intraconazole. Details of treatment are shown in Table 4. The mean APACHE II score at the beginning of antifungal treatment was 22.7 ± 8, as compared with 14.3 ± 2.3 in treated patients colonized with Aspergillus spp. The first choice antifungals were amphotericin B deoxycholate (administered to eight patients), liposomal amphotericin B (eight patients) and amphotericin B lipid complex (three patients). Two patients treated with amphotericin B deoxycholate developed renal failure and treatment was changed to liposomal amphotericin B in one and amphotericin B lipid complex in the other. One patient initially treated with amphotericin B lipid complex was switched to liposomal amphotericin B because of persistence of infection, with positive cultures, after 2 weeks of treatment. After 3 weeks of treatment with liposomal amphotericin B, cultures were negative. Eleven patients died, and in the remaining nine patients treatment was discontinued after clinical cure. Mean duration of treatment in these nine patients was 18 days (range 8–35 days). Clinical resolution of symptoms was achieved with amphotericin B deoxycholate only in one patient and with the lipid formulation in eight (P < 0.05).
Autopsy was performed in five patients with Aspergillus spp. infection. In all cases the examination revealed characteristic hyphae elements within the lung parenchyma with vascular invasion, which is compatible with the diagnosis of invasive aspergillosis. All were COPD patients and had been treated with corticosteroids in the ICU. One patient had a lung cancer. None of these five patients had neutropenia or haematological malignancy.
Discussion
This is the largest study to date in which Aspergillus spp. were isolated from respiratory secretions in a cohort of critically ill patients, including a large number of immunocompetent patients. In this group, isolation of Aspergillus spp. mostly occurred in those with COPD who were treated with steroids during their ICU stay. However, only 13.8% of patients had neutropenia – a classic risk factor for Aspergillus infection.
Various small series and case reports have shown that invasive aspergillosis commonly occurs in critically ill patients admitted to the ICU because of acute exacerbation of COPD and treated with intravenous corticosteroids [11–14]. In those patients steroids were given for a short period (1 week), whereas in our patients treatment was prolonged (3 weeks). In contrast, in a recent study of 250 patients with COPD admitted to the ICU because of acute respiratory failure [15], which did not report on the use of corticosteroids, Aspergillus spp. were not isolated in any respiratory sample. On the other hand, prior treatment with fluconazole was not associated with a higher rate of isolation of Aspergillus spp., as was previously reported in patients with neutropenia [16].
In one-third of cases in the present study recovery of Aspergillus spp. in respiratory secretions, in the absence of signs of pneumonia, was considered to represent colonization. However, three of these patients were given antifungal treatment because of underlying risk factors. An important finding of the study is that systemic antifungal agents were employed in patients with Aspergillus spp. colonization with clinical signs of respiratory infection, despite the fact that associated bacterial pathogens were cultured in almost one-third of cases. Although autopsies were performed in only five patients with Aspergillus infection, histopathological findings confirmed the clinical diagnosis in each case. Our findings are in agreement with those of a recent autopsy study [17] that confirmed the diagnostic value of Aspergillus spp. in respiratory secretions of COPD patients admitted to the ICU and treated with corticosteroids. In contrast, in a study conducted Petri and coworkers [18] in 435 non-neutropenic ICU patients, fungal colonization with Aspergillus spp. was found in 4% of cases, but in none of the patients was a diagnosis of invasive aspergillosis made.
In one study [19], because of the lack of reliable diagnostic tools, up to 60% of patients with invasive aspergillosis diagnosed at autopsy had not received antifungal treatment. Isolation of Aspergillus spp. from respiratory secretions has been regarded as being of limited usefulness in the antemortem diagnosis of invasive aspergillosis. In a study conducted in the 1980 s, Yu and coworkers [20] evaluated 108 patients in whom Aspergillus spp. were isolated from respiratory secretions, but invasive aspergillosis was not demonstrated in non-immunosuppressed patients. In a recent study [21], however, it was shown that malnutrition, diabetes mellitus, pulmonary disorder, or corticosteroid use were underlying risk factors for invasive aspergillosis in patients in whom Aspergillus spp. were isolated from respiratory secretions. On the other hand, invasive aspergillosis does not only occur in immunocompromised patients [3]. In a cohort of 439 non-ICU patients with invasive aspergillosis [22], nine had no apparent underlying conditions before diagnosis. Likewise, acute community-acquired pneumonia due to Aspergillus spp. – a rare infection – has been reported in 12 immunocompetent hosts [4].
It is well known that neutropenia is the main risk factor for aspergillosis because polymorphonuclear neutrophils and macrophages are the first immunological line of defence against Aspergillus spp. [6]. However, T-cell mediated, acquired immunity also plays a role in protecting against fungal infection [23]. Critically ill patients with prolonged stays in the ICU exhibit a complex decrease in immune function, with deactivation of macrophages and altered cellular response [24]. In addition, the immune function of peripheral neutrophils is influenced by acute hyperglycaemia [25]. Furthermore, it has been shown that corticosteroids suppress neutrophil action against Aspergillus hyphae [26]. These mechanisms may explain why Aspergillus infection occurs in ICU patients with a compensatory anti-inflammatory response syndrome or immunoparalysis during multiorgan failure but without any predisposing factors [27, 28]; they may also account for the association between corticosteroid use and this invasive fungal infection.
Invasive aspergillosis in ICU patients carries a very high mortality [4, 28, 29], with an attributable mortality of 18.9% after adjusting for confounding factors [30]. In non-immunocompromised patients, the success of antifungal treatment depends on early diagnosis. However, because delayed diagnosis is the rule, if therapy is not promptly initiated then patients may die from the disease. Amphotericin B deoxycholate was the only therapeutic option in the past and was the antifungal agent used in series with a reported mortality of as high as 100%. In the present study, although there were no differences in in-hospital mortality according to antifungal drug used, clinical cure rates were higher in patients treated with amphotericin B lipid formulations. In two patients amphotericin B deoxycholate was withdrawn because of nephrotoxicity, which increases mortality significantly [31]. Although greater efficacy of amphotericin B lipid formulations compared with amphotericin B deoxycholate in the treatment of invasive aspergillosis has not been demonstrated [7], the use of the lipid formulations appears preferable, especially in critically ill patients, because of better tolerance [32]. New antifungal agents with good activity against Aspergillus spp. have recently become available. Initial treatment of invasive aspergillosis with voriconazole led to better response and improved survival than with the standard approach of initial therapy with amphotericin B [33]. Caspofungine was also effective as salvage therapy in invasive pulmonary aspergillosis, as compared with standard therapy [34].
One of the main limitations of the present study was the retrospective design, in which diagnostic and treatment approaches were not standardized. Also, there were few cases in which the clinical diagnosis of invasive pulmonary aspergillosis was confirmed by histopathological evaluation. Third, mortality rates may be biased by differences in antifungal treatments used at each centre. Nevertheless, the present data add valuable information regarding the significance of isolation of Aspergillus spp. from respiratory samples in critically ill patients.
Conclusion
In summary, COPD and treatment with corticosteroids are major predisposing factors for Aspergillus spp. colonization/infection in critically ill patients. For this reason, in ICU patients with these risk factors, antifungal treatment should be considered in the presence of clinical features of pneumonia and isolation of Aspergillus spp. from respiratory secretions. In contrast, antifungal treatment should not be initiated when Aspergillus spp. are recovered from bronchial aspirates of critically ill patients without predisposing risk factors and in the absence of clinical and radiological signs of pneumonia. In these cases, isolation of Aspergillus spp. should be interpreted as colonization.
Key messages
-
COPD and treatment with corticosteroids, and neutropenia are major predisposing factors for respiratory colonization/infection with Aspergillus spp. in critically ill patients.
-
In ICU patients with these risk factors, antifungal treatment should be considered in the presence of clinical features of pneumonia and isolation of Aspergillus spp. from respiratory secretions.
-
The crude mortality associated with this entity is still very high.
Abbreviations
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- CI:
-
confidence interval
- COPD:
-
chronic obstructive pulmonary disease
- ICU:
-
intensive care unit
- OR:
-
odds ratio.
References
Denning DW: Aspergillus species. In Principles and Practice of Infectious Disease. Edited by: Mandell G, Douglas J, Bennett D. Philadelphia: Churchill Livingstone; 2000:2675-2685.
Humphreys H, Johnson EM, Warnock DW, Willatts SM, Winter RJ, Speller DC: An outbreak of aspergillosis in a general ITU. J Hosp Infect 1991, 18: 167-177. 10.1016/0195-6701(91)90141-T
Chen KY, Ko SC, Hsueh PR, Luh KT, Yang PC: Pulmonary fungal infection: emphasis on microbiological spectra, patient outcome, and prognostic factors. Chest 2001, 120: 177-184. 10.1378/chest.120.1.177
Clancy CJ, Nguyen MH: Acute community-acquired pneumonia due to Aspergillus in presumably immunocopetent host. Clues for recognition of a rare but fatal disease. Chest 1998, 114: 629-634.
Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, et al.: Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplant: an international consensus. Clin Infect Dis 2002, 34: 7-14. 10.1086/323335
Denning DW: Invasive aspergillosis. Clin Infect Dis 1998, 26: 781-805.
Stevens DA, Kan VL, Judson MA, Morrison WA, Dummer S, Denning DW, Bennett JE, Walsh TJ, Patterson TF, Pankey GA: Practice guidelines for diseases caused by Aspergillus . Clin Infect Dis 2000, 30: 696-709. 10.1086/313756
Knaus WA, Draper EA, Wagner DP, Zimmerman JE: APACHE II: a severity of disease classification system. Crit Care Med 1985, 13: 818-829.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101: 1644-1655.
Logan PM, Primack SL, Miller RR, Müller NL: Invasive aspergillosis of the airways: radiographic, CT, and pathologic findings. Radiology 1994, 193: 383-388.
Crean JM, Niederman MS, Fein AM, Feinsilver SH: Rapidly progressive respiratory failure due to Aspergillus pneumonia: a complication of short-term corticosteroid therapy. Crit Care Med 1992, 20: 148-150.
Rello J, Esandi ME, Mariscal D, Gallego M, Domingo C, Vallés J: Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: report of eight cases and review. Clin Infect Dis 1998, 26: 1473-1475.
Pittet D, Huguenin T, Dharan S, Sztajzel-Boissard J, Ducel G, Thorens JB, Auckenthaler R, Chevrolet JC: Unusual cause of lethal pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Am J Resp Crit Care Med 1996, 154: 541-544.
Bulpa PA, Dive AM, Garrino MG, Delos MA, Gonzalez MR, Evrard PA, Glupczynski Y, Installe EJ: Chronic obstructive pulmonary disease patients with invasive pulmonary aspergillosis: benefits of intensive care? Intensive Care Med 2001, 27: 59-67. 10.1007/s001340000768
Afessa B, Morales IJ, Scanlon PD, Peters SG: Prognostic factors, clinical course, and hospital outcome of patients with chronic obstructive pulmonary disease admitted to an intensive care unit for acute respiratory failure. Crit Care Med 2002, 30: 1610-1615. 10.1097/00003246-200207000-00035
Meis JF, Donnelly JP, Hoogkamp-Korstanje JA, De Pauw BE: Aspergillus fumigatus pneumonia in neutropenic patients during therapy with fluconazole for infection due to Candida spp. Clin Infect Dis 1993, 16: 734-735.
Dimopopulos G, Piagnerelli M, Berre J, Eddafali B, Salmon I, Vincet JL: Disseminated aspergillosis in intensive care unit patients: an autopsy study. J Chemother 2003, 15: 71-75. 10.1159/000070766
Petri MG, Konig J, Moecke HP, Gramm HJ, Barkow H, Kujath P, Dennhart R, Schafer H, Meyer N, Kalmar P, et al.: Epidemiology of invasive mycosis in ICU patients: a prospective multicenter study in 435 non-neutropenic patients. Intensive Care Med 1997, 23: 317-325. 10.1007/s001340050334
Groll AH, Shah PM, Mentzel C, Schneider M, Just-Nuebling G, Hubner K: Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect 1996, 33: 23-32. 10.1016/S0163-4453(96)92700-0
Yu VL, Muder RR, Poorsttar A: Significance of isolation of Aspergillus from the respiratory tract in diagnosis of invasive pulmonary aspergillosis. Results from a three-year prospective study. Am J Med 1986, 81: 249-254. 10.1016/0002-9343(86)90259-7
Perfect JR, Cox GM, Lee JY, Kauffman CA, de Repentigny L, Chapman SW, Morrison VA, Pappas P, Hiemenz JW, Stevens DA, Mycoses Study Group: The impact of culture isolation of Aspergillus species: a hospital-based survey of aspergillosis. Clin Infect Dis 2001, 33: 1824-1833. 10.1086/323900
Patterson TF, Kirkpatrick WR, White M, Hiemenz JW, Wingard JR, Dupont B, Rinaldi MG, Stevens DA, Graybill JR: Invasive aspergillosis. Disease spectrum, treatment practices, and outcome. Medicine 2000, 79: 250-260. 10.1097/00005792-200007000-00006
Latge JP: Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 1999, 12: 310-350.
Lederer JA, Rodrick ML, Mannick JA: The effects of injury on the adaptive immune response. Shock 1999, 11: 153-159.
Kwoun MO, Ling PR, Lydon E, Imrich A, Qu Z, Palombo J, Bistrian BR: Immunologic effects of acute hyperglycemia in nondiabetic rats. JPEN J Parenter Enteral Nutr 1997, 21: 91-95.
Roilides E, Uhlig K, Venzon D, Pizzo PA, Walsh TJ: Prevention of corticoid-induced suppression of human polymorphonuclear leukocyte-induced damage of Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun 1993, 61: 4870-4877.
Hartemink KJ, Paul MA, Spijkstra JJ, Girbes AR, Polderman KH: Immunoparalysis as a cause for invasive aspergillosis? Intensive Care Med 2003, 29: 2068-2071. 10.1007/s00134-003-1778-z
Meersseman W, Vandeecasteele SJ, Wilmer A, Verbeken E, Peetermans WE, Wijngaerden EV: Invasive aspergillosis in critically ill patients without malignancy. Am J Resp Crit Care Med 2004, 170: 621-625. 10.1164/rccm.200401-093OC
Jannsen JJWM, Strack van Schijndel , van der Poest Clement EH, Ossenkoppele GJ, Thijs LG, Huijgens PC: Outcome of ICU treatment in invasive aspergillosis. Intensive Care Med 1996, 22: 1315-1322. 10.1007/s001340050257
Vandewoude KH, Blot SI, Benoit D, Colardyn F, Vogelaers D: Invasive aspergillosis in critically ill patients: attributable mortality and excess in length of ICU stay and ventilator dependence. J Hosp Infect 2004, 56: 269-276. 10.1016/j.jhin.2004.01.006
Wingard JR, Kubilis P, Lee L, Yee G, White M, Walshe L, Bowden R, Anaissie E, Hiemenz J, Lister J: Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin Infect Dis 1999, 29: 1402-1407. 10.1086/313498
Gottfredson M, Perfect JR: Use of antifungal agents in the intensive care unit. Curr Opin Crit Care 1999, 5: 381-390. 10.1097/00075198-199910000-00008
Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, et al.: Voriconazole versus amphotericin B for primary therapy if invasive aspergillosis. N Engl J Med 2002, 347: 408-415. 10.1056/NEJMoa020191
Maertens J, Raad I, Petrikkos G, Boogaerts M, Selleslag D, Petersen FB, for the Caspofungin Salvage Aspergillosis Study Group: Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis 2004, 39: 1563-1571. 10.1086/423381
Acknowledgements
We thank Gilead Sciences, SL, for financial support in logistic aspects of the study and Marta Pulido, MD, for editing the manuscript and editorial assistance.
This study was supported by an unrestricted grant from Gilead.
This study was carried out with the EPCAN Study Group: J Nolla, F Álvarez-Lerma and M Salvadó (Hospital del Mar, Barcelona); N Carrasco and A Bueno (Hospital de la Princesa, Madrid); F Bobillo and P Ucio (Hospital Clínico, Valladolid); MA León, M Nolla and RA Díaz (Hospital General de Cataluña, Barcelona); JR Iruretagoyena, K Esnaola and I Andetxaga (Hospital de Cruces, Bilbao); A Blanco, F Taboada and R Fernández (Hospital Nuestra Señora de Covadonga, Oviedo); M Nieto, R Diego and F Ortuño (Hospital Clínico San Carlos, Madrid); P Marcos and E Mesalles (Hospital Germans Trias i Pujol, Badalona, Barcelona); A Martínez, M Fernández and F Jaime (Hospital Virgen de la Arrixaca, Murcia); H Sancho and N Izquierdo (Hospital Reina Sofía, Córdoba); M Ulibarrena and F Labayen (Hospital Santiago Apóstol, Vitoria); F Barcenilla, MJ Gil and B Balsera (Hospital Arnau de Villanova, Lleida); R Jordá, M Jurado and J Pérez (Hospital Son Dureta, Palma de Mallorca); E Zavala, A Alcón and N Fabregues (Hospital Clínic i Provincial, Barcelona); MV de la Torre, MA Estecha and A Soler (Hospital Virgen de la Victoria, Málaga); M Bodí and D Castander (Hospital Joan XXIII, Tarragona); A Mendía, J Artaetxebarría and C Reviejo (Hospital Nuestra Señora de Aránzazu, San Sebastián); M Sánchez, A Casamitjana and C Pérez (Hospital Insular, Las Palmas de Gran Canaria); MJ López and E Robles (Hospital General de Segovia, Segovia); Y Insausti and JA Tihistsa (Hospital de Navarra, Pamplona); C García and JM Rubio (Hospital 12 de Octubre, Madrid); R Oltra and O Rodríguez (Hospital Clínico Universitario, Valencia); P Olaechea and R de Celís (Hospital de Galdakao, Bizkaia); JM Soto and J Pomares (Hospital San Cecilio, Granada); J Luna and G Masdeu (Hospital Virgen de la Cinta, Tarragona); R Sierra and A Gordillo (Hospital Puerta del Mar, Cádiz); R Rodríguez and J Fajardo (Hospital Virgen de la Macarena, Sevilla); MA Herranz and JI Gómez (Hospital Río Hortega, Valladolid); RM García and MJ Espina (Hospital de Cabueñes, Gijón); J Garnacho and C Ortiz (Hospital Virgen del Rocío, Sevilla); M Palomar and J Montero J (Hospital Vall d'Hebron, Barcelona); C Cisneros and A Sandiumenje (UCI de Traumatología, Hospital 12 de Octubre, Madrid); M Sánchez and M Álvarez (Hospital Príncipe de Asturias, Madrid); V López and R Julve (Hospital de Sagunto, Valencia); J Solé and M Valerón (Hospital Nuestra Señora del Pino, Las Palmas de Gran Canaria); MA Blasco and S Borrás (Hospital Dr Peset, Valencia); E Maraví and JM Urtasun (Hospital Virgen del Camino, Pamplona); C Sánchez-Díaz (Hospital San Pedro de Alcántara, Cáceres); LM Tamayo (Hospital Río Carrión, Palencia); J Blanco (Complexo Hospitalario Xeral-Calde, Lugo); P Galdós (Hospital General de Móstoles, Madrid); F Barredo (Hospital de Torrecárdenas, Almería); A Rodríguez (Hospital Santa María del Rosell, Cartagena); J Castaño (Hospital Virgen de las Nieves, Granada); A Bonet (Hospital Josep Trueta, Girona); M Cerdá (Hospital de la Creu Roja, L'Hospitalet de Llobregat, Barcelona); A Torres (UVIR, Hospital Clínic i Provincial, Barcelona); F Pérez F (Fundación Jiménez Díaz, Madrid); JM Flores (UCI Traumatología, Hospital Virgen del Rocío, Sevilla); R Diego (Hospital General Universitario, Valencia); C Fernández (Complejo Hospitalario Insalud, León); A Mas (Centre Hospitalari i Cardiologic, Manresa, Barcelona); F Ruiz (Hospital Ciudad de Jaén, Jaén); C León (Hospital Nuestra Señora de Valme, Sevilla); M Casanovas (Hospital de Igualada, Igualada, Barcelona); EA Sanz (Hospital Santa Ana, Motril, Granada); JA Artola (Hospital Naval de San Carlos, Cádiz); MP Luque (UCI de Traumatología, Hospital Clínico Univresitario, Zaragoza); C Palazón (Hospital General Universitario, Murcia); C Sotillo (Hospital Gregorio Marañón, Madrid); A Bisbal (Policlínica Miramar, Palma de Mallorca); MJ Huertos (Hospital de Puerto Real, Cádiz); F Esteban (Hospital Sant Joan de Reus, Reus, Tarragona); P Ugarte (Hospital Marqués de Valdecilla, Santander); R Giral (Hospital General Yagüe, Burgos); V González (Hospital Miguel Servet, Zaragoza); MJ Serralta (Hospital San Juan, Alicante); A Cercas (Hospital de Jerez, Cádiz); A Nebra (Hospital Clínico Universitario, Zaragoza); C Castillo (Hospital Txagorritxu, Vitoria-Gasteiz); A Cercas (Hospital de Jerez, Cádiz); A Nebra (Hospital Clínico Universitario, Zaragoza); C Castillo (Hospital Txagorritxu, Vitoria), A Tejada (UCI Traumatología, Hospital Miguel Servet, Zaragoza); and JI Gómez (REA, Hospital Río Ortega, Valladolid), Spain.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
All of the authors were involved in designing the study and collecting data. JGM and RAV were involved in the statistical analysis. CL obtained funding. JGM drafted the manuscript, which was revised and approved by all of the authors.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Garnacho-Montero, J., Amaya-Villar, R., Ortiz-Leyba, C. et al. Isolation of Aspergillus spp. from the respiratory tract in critically ill patients: risk factors, clinical presentation and outcome. Crit Care 9, R191 (2005). https://doi.org/10.1186/cc3488
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc3488