Abstract
Objectives
To determine the degree of interinstitutional transfusion practice variation and reasons why red cells are administered in critically ill patients.
Study design
Multicentre cohort study combined with a cross-sectional survey of physicians requesting red cell transfusions for patients in the cohort.
Study population
The cohort included 5298 consecutive patients admitted to six tertiary level intensive care units in addition to administering a survey to 223 physicians requesting red cell transfusions in these units.
Measurements
Haemoglobin concentrations were collected, along with the number and reasons for red cell transfusions plus demographic, diagnostic, disease severity (APACHE II score), intensive care unit (ICU) mortality and lengths of stay in the ICU.
Results
Twenty five per cent of the critically ill patients in the cohort study received red cell transfusions. The overall number of transfusions per patient-day in the ICU averaged 0.95 ± 1.39 and ranged from 0.82 ± 1.69 to 1.08 ± 1.27 between institutions (P < 0.001). Independent predictors of transfusion thresholds (pre-transfusion haemoglobin concentrations) included patient age, admission APACHE II score and the institution (P < 0.0001). A very significant institution effect (P < 0.0001) persisted even after multivariate adjustments for age, APACHE II score and within four diagnostic categories (cardiovascular disease, respiratory failure, major surgery and trauma) (P < 0.0001). The evaluation of transfusion practice using the bedside survey documented that 35% (202 of 576) of pre-transfusion haemoglobin concentrations were in the range of 95-105 g/l and 80% of the orders were for two packed cell units. The most frequent reasons for administering red cells were acute bleeding (35%) and the augmentation of O2 delivery (25%).
Conclusions
There is significant institutional variation in critical care transfusion practice, many intensivists adhering to a 100g/l threshold, and opting to administer multiple units despite published guidelines to the contrary. There is a need for prospective studies to define optimal practice in the critically ill.
Similar content being viewed by others
Introduction
Physicians commonly used a threshold of 100g/l (haematocrit of 30%) as the level for transfusion of allogeneic red cells. Adams and Lundy [1], in 1941, recommended the administration of red cells for haemoglobin concentrations ranging from 80 to 100 g/l in the perioperative period. Although scientific evidence supporting this approach has been advanced, one of the most important reasons for the selection of 100 g/l as a threshold may be that it is an easily remembered figure [2,3]. Prompted by concerns over transfusion-related infections, recent guidelines emphasize that the decision to transfuse should not be determined by a single haemoglobin concentration [4,5,6]. However, surveys of transfusion practices have repeatedly documented the importance attributed to haemoglobin triggers. In 1982, 88% of anaesthesiologists surveyed believed preoperative haemoglobin levels of 90 g/l to be mandatory [7].
The decision to transfuse a critically ill patient is complex and may be influenced by factors such as age, medications, disease severity and specific diagnoses such as acute coronary ischaemia. Neither the importance of a specific transfusion threshold nor the clinical characteristics that influence transfusion practice have been documented in this high-risk patient population. This study was therefore designed to characterize actual transfusion practice, to determine whether there is any significant institutional practice variation and reasons why red cells are administered in critically ill patients.
Methods
Study design
We implemented two concurrent and complementary data gathering approaches. First, patients admitted to one of six Canadian tertiary level intensive care units (ICUs) during 1993 were enrolled in a combined retrospective and prospective cohort study and, second, a bedside questionnaire was completed by physicians requesting blood transfusions during the prospective phase of the cohort study.
Study population and data collection
The cohort study included all patients admitted to one of the six participating ICUs during the 1993 calendar year. Patients who were less than 16 years of age or who were considered brain dead within 24 h of admission were excluded. We collected demographic and transfusion-related information as well as data on patient outcomes and disease severity. The lowest overall haemoglobin concentration in patients who were not transfused or the haemoglobin concentration recorded prior to the administration of red cells in patients receiving blood were labelled pre-transfusion haemoglobin and were used as the primary outcome in the study.
In the prospective phase of the cohort, a bedside questionnaire was administered to all physicians requesting red cell transfusions. To identify potential respondents, physician order forms from patient charts were screened daily. Physicians who wrote transfusion orders were asked if they initiated the request or if another physician requested the administration of red cells. Physicians requesting the transfusion were then asked to identify the most important reason for the administration of red cells from a list of nine possible choices: age, disease severity, acute bleeding or ongoing blood loss, haemodynamic instability, severe hypoxaemia, improvement in wound healing and well being, augmentation of O2 delivery, coronary ischaemia, and others. The predominantly physiological choices were identified. Each bedside questionnaire was administered within 24 h of the request for a transfusion. For patients receiving multiple transfusion episodes in a 24-h period only the first request was analysed. In addition to the questionnaire responses and information from the cohort study, we recorded the pre- and post-transfusion haemoglobin concentrations and the level of training of respondents.
Sample size considerations
The size of the cohort study was based on pre-transfusion haemoglobin concentrations as an outcome. An analysis of variance (ANOVA) was used to test the equality of mean pre-transfusion haemoglobin concentrations in four age ranges, two APACHE II ranges, transfusion status (received or did not receive red cells) and six hospitals. Using an F-test for the comparison of these four variables, a level of significance α =0.05, a power of 80%, a number of multiple comparisons and subgroup analyses, we estimated that a total sample size of 4500 patients would be required. Based on previous ICU admission rates, one year of admissions in six critical care units was expected to identify approximately 5000 patients.
Statistical analysis
Descriptive statistical analyses were performed on all variables in each component of this study. In the cohort study, categorical variables including age ranges (< 30 years, 30-49 years, 50-69 years, ≥ 70 years), gender, APACHE II categories (15 or less, greater than 15), diagnostic categories, the number of red cell units administered per patient (0 units, 1-3 units, 4-6 units, 7-9 units, 10 or more units) and mortality rates were initially compared amongst institutions using chi-square test procedures. Pre-transfusion haemoglobin concentrations in each patient, the number of red cell units transfused per patient-day and lengths of stay between institutions were compared using a one-way ANOVA. A preliminary analysis of the influence of diagnostic categories on the administration of red cells was evaluated using a chi-square statistic.
Pre-transfusion haemoglobin was used as the primary outcome in the multivariate analysis. To determine how transfusion status (received or did not receive red cells), the institution as well as previously defined age ranges and APACHE II categories influenced pre-transfusion haemoglobin concentrations, we performed a four-way ANOVA. A similar ANOVA was performed for specific disease categories including multiple trauma, respiratory diseases, cardiovascular diseases and postoperative patients in order to control for the influence of disease categories on transfusion thresholds.
For the bedside questionnaire, we determined the response rate at each institution by cross checking recorded transfusions in the cohort study with the completed questionnaires. The term 'transfusion threshold' was defined as the pre-transfusion haemoglobin concentration recorded in the bedside questionnaire. Chi-square procedures were employed to test relationships between the nine clinical factors and other variables such as transfusion thresholds and diagnostic categories as well as the level of training of physicians responding to the questionnaire. In this study, no adjustments were made for multiple comparisons. Data are reported as means ± standard deviations (SD) unless otherwise stated.
Results
Cohort study
We enrolled 5298 consecutive patients from six tertiary level ICUs in the cohort study; 3079 patients were identified by a retrospective review of health records and 2219 patients were prospectively enrolled at the time of ICU admission. The number of patients from each institution ranged from 672 to 1355 (Table 1). Age, diagnostic categories and gender were comparable from institution to institution (P > 0.53); however, disease severity as indicated by APACHE II scores, ICU length of stay and mortality rates were significantly different between institutions (P < 0.001).
Overall, 1650 patients (25% ranging from 12% to 35% among institutions) of 5032 critically ill patients received red cell transfusions. There were significant differences in the proportion of patients transfused in the different centres using both an unadjusted chi-square statistic (P = 0.001) and a Mantel-Haenszel chi-square procedure stratified for high and low APACHE II scores (P <0.001). The total number of transfusions per patient-day in the ICU ranged from 0.82 ± 1.69 to 1.08 ± 1.27 among institutions (P < 0.001) (Table 2).
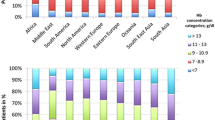
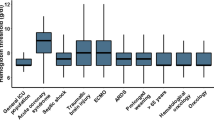
Average pre-transfusion haemoglobin concentrations up until discharge or the first 10 days in ICU (Fig 1) also differed significantly from institution to institution. ranging from 87g/l to 95g/l (P = 0.0001). Independent predictors of average pre-transfusion haemoglobin and the number of transfusions per patient-day included age, APACHE II score, transfusion status and the institution (P < 0.0001). The influence of the institution remained significant (P < 0.0001) even after performing multivariate adjustments for age ranges, transfusion status and APACHE II categories. We observed a series of significant second- and third-order interactions from the overall multivariate analysis examining pre-transfusion haemoglobin concentrations (P < 0.05). The most complex interaction noted was between transfusion status, APACHE II score and the institution (Fig 2). Significant variations in pre-transfusion haemoglobin concentrations were observed in both APACHE II categories (P < 0.0001) as well as in the transfused and non-transfused patients across all institutions (P < 0.0001). Institutions 3 and 6, with the lowest overall pre-transfusion haemoglobin concentrations, also had the least amount of change in these concentrations between the high and the low APACHE II categories.
Significant institution (P < 0.0001) and transfusion status (P < 0.0001) effects were also observed in all four diagnostic categories following similar multivariate statistical procedures. APACHE II groups in patients admitted with respiratory failure (P < 0.0001), following a cardiac event (P < 0.0001) and following multiple trauma (P = 0.055) predicted pre-transfusion haemoglobin values. Age (P = 0.009) but not APACHE II (P = 0.84) groupings were predictive in postoperative patients. Second-order interactions included 'transfusion by institution' effects in trauma (P = 0.021) and postoperative (P = 0.0005) patients. There were no significant third- or fourth-order interactions.
Average pre-transfusion haemoglobin values over time in participating institutions. This figure illustrates all haemoglobin concentrations during the first 10 days of intensive care unit (ICU) stay. There was an average decrease of 16 g/l in haemoglobin concentrations in all patients admitted to the ICU over the 10-day monitoring interval. Institution 1 had the highest values over time while institution 3 recorded the lowest concentrations during the 10 days. The solid thick line illustrates overall concentrations.
Average pre-transfusion haemoglobin concentrations stratified by institution, APACHE II categories and transfusion status. Average pre-transfusion hemoglobin concentrations stratified by transfusion status, institution and high (> 15) versus low (≤ 15) APACHE II score. Institution 3 and 6 had the lowest concentrations overall and patients who received red cells. The influence of APACHE II scores appeared least important in centres (3 and 6) with a conservative approach to the administration of red cells. This graph also illustrates significant variations in haemoglobin concentrations in both APACHE II groups and in transfused and non-transfused patients.
Bedside questionnaire
The bedside questionnaire was administered following 758 of 1459 (52%) consecutive transfusion orders written by 223 physicians for 386 patients. ICU staff as opposed to consultant staff requested over 90% of the red cell transfusions. Most of the transfusions were requested by junior (26%) or senior (46%) residents. Thirty-five per cent of pre-transfusion haemoglobin values were in the range 95-105 g/l and 80% of the orders were for two packed cell units. Post-transfusion, 30% of haemoglobin concentrations were greater than 110 g/l. The commonly stated reasons for requesting red cells by ICU physicians were: acute bleeding (35%), augmentation of O2 delivery (25%), haemodynamic instability (12%) and coronary ischaemia (3%). Acute bleeding was most often cited when patients' haemoglobin concentrations were less than 80 g/l while augmentation of O2 delivery was most often associated with pre-transfusion haemoglobin concentrations greater than 80g/l (P < 0.001).
Discussion
In this study, we documented a significant interinstitutional variation in pre-transfusion haemoglobin concentrations and the average number of transfusions per patient-days. Despite the widely disseminated American College of Physicians transfusion guidelines explicitly recommending that red cells should be administered on a unit-by-unit basis and according to clinical judgement (not a pre-defined threshold value), a significant proportion (40%) of critical care physicians still administer red cells at a threshold haemoglobin concentration of 100g/l and two units at a time.
In the multicentre cohort of critically ill patients, the institution in which patients were treated was the most powerful predictor of haemoglobin concentrations prior to transfusion. A number of other investigators have observed inter-hospital variations in the perioperative use of red cells by examining large databases [8,9,10,11] and hospital audits [12,13,14,15,16,17]. Palermo and colleagues [10] documented a six-fold difference among institutions in Connecticut. Others [2] have criticized these authors for not attempting to adjust for differences in case mix between institutions. Subsequently, other studies have documented significant practice variation within specific disease categories [14,17,18] and clinical settings [18]. In the SANGUIS study [19], transfusion rates were found to depend more on physicians than the patient population or type of procedure or hospital. Wide variation was found among 43 hospitals in 10 European countries [20,21] and between hospitals within the same country [22]. Some factors found to influence this variation were age, gender, preoperative haematocrit and blood loss. In addition, Hébert et al [23] documented the impact of numerous clinical factors (eg blood loss, preoperative status, hypoxaemia, shock, lactic acidosis) on physicians' decisions to transfuse their critically ill patients. There is, therefore, a substantial body of evidence indicating that transfusion practice varies in the perioperative period but there are few data pertaining to the critical care setting.
After controlling for the influence of all diagnostic groupings, age and disease severity, a significant variation in pre-transfusion haemoglobin concentrations from institution to institution remained. In all patients, we observed significant interactions between APACHE II score, the institution and transfusion status, suggesting a complex relationship among these variables. It appeared that the influence of APACHE II score was less pronounced in institutions that had lower pre-transfusion haemoglobin concentrations. By performing the same analysis in four representative diagnostic groupings, significant associations between these variables and pre-transfusion haemoglobin concentrations persisted without more complex interactions. Indeed, a strong institutional effect was noted in the four diagnostic groupings. APACHE II scores were also associated with pre-transfusion haemoglobin concentrations in trauma, respiratory failure and in cardiac patients.
Optimal haemoglobin concentrations in many patient populations have been proposed by a number of authors [21,24,25,26,27] and organizations [4,5,6]. Unfortunately, these recommendations are based on clinical physiology, observational or poorly controlled clinical studies, historical context or a belief that a particular consequence of anaemia or transfusion is more important than another, rather than well controlled randomized clinical trials (RCT) [28,29]. Investigators have advocated elevated haemoglobin levels in critically ill patients [21,30,31] based on several studies [24,25] that advocate augmenting systemic O2 delivery and that describe the negative consequences of anaemia in critically ill patients with cardiac disease [32,33] to decrease mortality in critically ill patients. Alternatively, a lower transfusion threshold is supported by evidence from the literature examining the role of transfused red cells in immune modulation [34,35] and in microcirculatory alteration [36,37,38]. From these studies, the liberal administration of red cells may result in increased rates of clinically significant infections as well as organ failure and mortality. We believe that the conflicting evidence may be one of the many possible factors contributing to practice variation. Recently, a large randomised controlled clinical trial in 838 critically ill patients concluded that a more restrictive transfusion strategy was at least as safe and possibly superior to a liberal strategy. Although not available at the time of this study, data from the Transfusion Requirements in Critical Care trial may substantially modify transfusion practive and possibly decrease institutional and physician transfusion practices. [39].
In this multicentre cohort, the major concern was the diversity and complexity of patients. Unknown confounders may have accounted for the persistent institutional effect noted in this study despite the use of multivariate statistical techniques that controlled for differences in patient characteristics.
In summary, we demonstrated a significant institutional transfusion practice variation amongst Canadian tertiary centres. Academic practitioners appear to have implemented, only partially, well publicized transfusion guidelines primarily developed to address perioperative red cell utilization. The significant variation in transfusion practice was more pronounced in sicker patients suggesting that both the available evidence and the derived practice guidelines were limited for high-risk patients. We believe that clinical trials evaluating different transfusion strategies in the critically ill are required prior to the development and dissemination of further practice guidelines in high-risk patient populations.
References
Adams RC, Lundy JS: Anesthesia in cases of poor surgical risk: some suggestions for decreasing the risk. Surg Gynecol Obstet 1941, 71: 1011-1014.
Welch HG, Meehan KR, Goodnough LT: Prudent strategies for elective red blood cell transfusion. Ann Intern Med 1992, 116: 393-402.
Zauder HL: Preoperative hemoglobin requirements. Anesth Clin N Am 1990, 8: 471-480.
American College of Physicians: Practice strategies for elective red blood cell transfusion. Ann Intern Med 1992, 116: 403-406.
Silberstein LE, Kruskall MS, Stehling LC, et al.: Strategies for the review of transfusion practices. JAMA 1989, 262: 1993-1997. 10.1001/jama.262.14.1993
Consensus Conference (National Institutes of Health): Perioperative red blood cell transfusion. JAMA 1988, 260: 2700-2703.
Stehling LC, Ellison N, Faust RJ, Grotta AW, Moyers JR: A survey of transfusion practices among anesthesiologists. Vox Sang 1987, 52: 60-62.
Friedman BA, Burns TL, Schork MA: An analysis of blood transfusion of surgical patients by sex: a quest for the transfusion trigger. Transfusion 1980, 20: 179-188.
Reece RL, Beckett RS: Epidemiology of single-unit transfusion: a one-year experience in a community hospital. JAMA 1966, 195: 113-118.
Palermo G, Bove JR, Katz AJ: Patterns of blood use in Connecticut. Transfusion 1980, 20: 704-710.
Surgenor DM, Wallace EL, Hale SG, Gilpatrick MW: Changing patterns of blood transfusions in four sets of United States hospitals, 1980 to 1985. Transfusion 1988, 28: 513-518. 10.1046/j.1537-2995.1988.28689059022.x
Alter HJ, Epstein JS, Swenson SG, et al.: Prevalence of human immunodeficiency virus type 1 p24 antigen in U.S. blood donors: an assessment of efficacy of testing in donor screening. N Engl JMed 1990, 323: 1312-1317.
Mozes B, Epstein M, Ben-Bassat I, Modan B, Halkin H: Evaluation of the appropriateness of blood and blood product transfusion using preset criteria. Transfusion 1989, 29: 473-476. 10.1046/j.1537-2995.1989.29689318442.x
Goodnough LT, Johnston MFM: The variability of transfusion practice in coronary artery bypass surgery. JAMA 1991, 265: 86-90. 10.1001/jama.265.1.86
Coffin C, Matz K, Rich E: Algorithms for evaluating the appropriateness of blood transfusion. Transfusion 1989, 29: 298-303. 10.1046/j.1537-2995.1989.29489242793.x
Chang RWS, Jacobs S, Lee B: Predicting outcome among intensive care unit patients using computerised trend analysis of daily Apache II scores corrected for organ system failure. Intensive Care Med 1988, 14: 558-566.
Surgenor DM, Wallace EL, Churchill WH, Hao S, Hale WB, Schnitzer J: Utility of DRG and ICD-9-CM classification codes for study of transfusion issues. Transfusions in patients with digestive diseases. Transfusion 1989, 29: 761-767. 10.1046/j.1537-2995.1989.29990070178.x
Hasley PB, Lave JR, Hanusa BH, et al.: Variation in the use of red blood cell transfusions: a study of four common medical and surgical conditions. Med Care 1995, 33: 1145-1160.
Baele PL, De Bruyère M, Deneys V, et al.: Results of the SANGUIS study in Belgium. A concerted action of the Commission of the European Communities IVth Medical and Health Research Programme. Acta Chir Belg 1994, 94: 5-61.
Mead JH, Anthony CD, Sattler M: Hemotherapy in elective surgery. An incidence report, review of the literature and alternatives for guideline appraisal. Am J Clin Pathol 1980, 74: 223-227.
Czer LSC, Shoemaker WC: Optimal hematocrit value in critically ill postoperative patients. Surg Gynecol Obstet 1978, 147: 363-368.
Baele PL, De Bruyère M, Deneys V, et al.: The SAnGUIS study in Belgium: an overview of methods and results. Acta Chir Belg 1994, 94: 69-74.
Hébert PC, Wells G, Martin C, et al.: A Canadian survey of transfusion practices in critically ill patients. Crit Care Med 1998, 26: 482-487. 10.1097/00003246-199803000-00019
Boyd O, Ground M, Bennett D: A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA 1993, 270: 2699-2707. 10.1001/jama.270.22.2699
Tuchschmidt J, Fried J, Astiz ME, Rackow E: Elevation of cardiac output and oxygen delivery improves outcome in septic shock. Chest 1992, 102: 216-220.
Crosby ET: Perioperative haemotherapy: II. Risks and complications of blood transfusion. Can J Anesth 1992, 39: 822-837.
Cane RD: Hemoglobin: how much is enough? Crit Care Med 1990, 18: 1046-1047.
Calder L, Hebert PC, Carter AO, Graham ID: Review of published recommendations and guidelines for the transfusion of allogeneic red blood cells and plasma. Can Med Assoc J 1997, 156: S1-S8.
Hebert PC, Schweitzer I, Calder L, Blajchman M, Giulivi A: Review of the clinical practice literature on allogeneic red blood cell transfusion. Can Med Assoc J 1997, 156: S9-S26.
Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee T-S: Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest 1988, 94: 1176-1186.
Czer LSC, Shoemaker WC: Myocardial performance in critically ill patients: response to whole blood transfusion as a prognostic measure. Crit Care Med 1980, 8: 710-715.
Carson JL, Duff A, Poses RM, et al.: Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet 1996, 348: 1055-1060. 10.1016/S0140-6736(96)04330-9
Hebert PC, Wells G, Tweeddale M, et al.: Does transfusion practice affect mortality in critically ill patients? Am J Respir Crit Care Med 1997, 155: A20.
Nichols RL, Smith JW, Klein DB, et al.: Risk of infection after penetrating abdominal trauma. N Engl J Med 1984, 311: 1065-1070.
Bordin JO, Heddle NM, Blajchman MA: Biologic effects of leukocytes present in transfused cellular blood products. Blood 1994, 84: 1703-1721.
Marik PE, Sibbald WJ: Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA 1993, 269: 3024-3029. 10.1001/jama.269.23.3024
Messmer K: Hemodilution. Surg Clin North Am 1975, 55: 659-678.
Messmer K, Sunder-Plassmann L, Jesch F, Gornandt L, Sinagowitz E, Kessler M: Oxygen supply to the tissues during limited normovolemic hemodilution. Res Exp Med 1973, 159: 152-166.
Hébert PC, Wells G, Blajchman MA, et al.: Transfusion requirements in critical care: a multicentre randomized controlled clinical trial. N Engl J Med 1999, 340: 409-417. 10.1056/NEJM199902113400601
Acknowledgments
The authors wish to thank Diane Ferland, Merrilee Loewen, Debrah Foster, Denise Foster, Linda Knox and XiangRu Lu for their help in completing this project. We are also indebted to the nursing staff and all other health professionals who contribute to the care of our patients and for actively supporting this research initiative. We also wish to acknowledge Fiona Daigle, My-Linh Tran, Di Wang and the data management team at the University of Ottawa, Clinical Epidemiology Unit. This work was made possible through the support of the Canadian Critical Care Trials Group, in particular Drs Tom Todd and Deborah Cook. We also would like to express a sincere thank you to the TRICC trial investigators: Ottawa General Hospital: Paul C Hébert; Toronto Hospital, General Division: John Marshall; Vancouver General Hospital: Martin Tweeddale; Victoria General Hospital, Halifax: Richard Hall; Royal Victoria Hospital, Montreal: Sheldon Magder; St Michael's Hospital, Toronto: David Mazer; Wellesley Hospital: Thomas Stewart; Hamilton General Hospital: Thomas Hillers; Foothills Hospital, Calgary: Dean Sandham; St Paul's Hospital, Vancouver: James A Russell; Hôpital Maisonneuve-Rosemont, Montreal: Yoanna Skrobik; Hôtel Dieu-Grace Hospital, Windsor: John Muscedere; Calgary General Hospital/Peter Lougheed Centre: Sidney Viner; Ottawa Civic Hospital: Giuseppe Pagliarello; Victoria Hospital, London: Claudio Martin; Health Science Centre, St John's: Sharon Peters; Montreal General Hospital: David Fleiszer; Jewish General Hospital, Montreal: Alan Spanier; Toronto Hospital, Western Division: Patricia Houston; Saint Joseph's Hospital, London: Ann Kirby; Royal University Hospital, Saskatoon: Jaime Pinilla; University Hospital, Edmonton: Mary van Wijngaarden; Kingston General Hospital: Gordon Wood and Daren Heyland; Everett Chalmers Hospital, Fredericton: Navdeep Mehta; St John Regional Hospital: Michael Jacka.
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Hébert, P.C., Wells, G., Martin, C. et al. Variation in red cell transfusion practice in the intensive care unit: a multicentre cohort study. Crit Care 3, 57 (1999). https://doi.org/10.1186/cc310
Received:
Revised:
Published:
DOI: https://doi.org/10.1186/cc310