Abstract
For many patients optimal perioperative care may require little or no additional medical management beyond that given by the anaesthetist and surgeon. However, the continued existence of a group of surgical patients at high risk for morbidity and mortality indicates an ongoing need to identify such patients and deliver optimal care throughout the perioperative period. A group of patients exists in whom the risk for death and serious complications after major surgery is in excess of 20%. The risk is related mainly to the patient's preoperative physiological condition and, in particular, the cardiovascular and respiratory reserves. Cardiovascular management of the high-risk surgical patient is of particular importance. Once the medical management of underlying disease has been optimized, two principal areas remain: the use of haemodynamic goals to guide fluid and inotropic therapy, and perioperative β blockade. A number of studies have shown that the use of goal-directed haemodynamic therapy during the perioperative period can result in large reductions in morbidity and mortality. Some patients may also benefit from perioperative β blockade, which in selected patients has also been shown to result in significant mortality reductions. In this review a pragmatic approach to perioperative management is described, giving guidance on the identification of the high-risk patient and on the use of goal-directed haemodynamic therapy and β blockade.
Similar content being viewed by others
Introduction
For many patients optimal perioperative care may require little or no additional medical management beyond that given by the anaesthetist and surgeon. However, the continued existence of a group of surgical patients at high risk of morbidity and mortality indicates an ongoing need to identify such patients and deliver optimal care throughout the perioperative period.
The cardiovascular management of the high-risk surgical patient is of particular importance. A large body of evidence now exists to guide the clinician in delivering optimal care. Once the medical management of underlying disease has been optimized, two principal areas remain: the use of haemodynamic goals to guide fluid and inotropic therapy, and perioperative β blockade.
This review describes a practical approach to the application of evidence for these therapies. The relevant clinical trials are at times inconsistent; the overall strategy described is therefore a pragmatic approach to best practice in this area.
Goal-directed therapy
Perioperative management of the cardiovascular system will always involve predefined treatment limits or targets. These targets may be very basic, such as heart rate and blood pressure, or they may be more sophisticated, for example cardiac output monitoring. Regardless of the choice of targets, some form of goal-directed therapy (GDT) is necessary to achieve them.
Shoemaker [1] provided the first observational evidence correlating various cardiovascular parameters with outcome in patients at high-risk for death or complications after surgery, and proposed the development of tissue hypoxia as a likely mechanism. Previous evidence had also suggested that when routine parameters, such as blood pressure and urine output, were stabilized in all patients, survivors had consistently higher cardiac output, oxygen flux and oxygen consumption than did those who subsequently died [2]. Emphasis was placed not on the cardiovascular parameters of a normal individual at rest but on the median levels attained by surviving patients once stabilized following surgery. The most important parameters were cardiac index (> 4.5 l/min per m2), oxygen consumption (> 170 ml/min per m2) and oxygen delivery (> 600 ml/min per m2).
Shoemaker and coworkers [3] conducted the first major outcome trial of GDT. Surgical patients considered at high perioperative risk were administered fluid, inotropes and oxygen therapy to achieve therapeutic goals. In a complex study involving two separate series of patients, an impressive reduction in mortality from 28% to 4% (P < 0.02) was reported. Although this remains a landmark report, there are some concerns regarding the methodology of the study. Individual sample groups were small and treatment regimens were not clearly reported. There is no evidence of standardized treatment in either control group, and the study was not blinded or randomized.
A subsequent trial addressed these concerns [4]. Clear protocols defined the management of both intervention and control groups. All patients were admitted to intensive care and received a pulmonary artery catheter (PAC). A substantial mortality reduction was shown in the intervention group (22.2% versus 5.7%; P = 0.015). There were no deaths in the intervention group patients who underwent abdominal surgery, although the effect was not so strong for patients undergoing vascular surgery.
Wilson and colleagues [5] then modified the ideas of previous investigators. They randomly assigned 138 patients undergoing major elective surgery to receive conventional treatment or perioperative GDT and achieved very similar results to those of both previous studies. It is important to note, however, that conventional treatment involved one-third of patients in the control group being managed on a general ward, whereas all intervention group patients remained in the intensive care unit.
Mortality following cardiac surgery is low, and studies looking at GDT after cardiac surgery have therefore failed to achieve statistically significant mortality reductions [6, 7]. They have, however, demonstrated significant reductions in morbidity and length of hospital stay. Meanwhile, several studies have failed to demonstrate mortality reduction in vascular surgery [8–11].
However, the largest trial to date, a multicentre randomized controlled study conducted by Sandham and coworkers [12] in a mixed group of surgical patients, failed to show benefit. The trial compared PAC-guided postoperative care with standard care as deemed appropriate. Hospital mortality was 7.8% in the PAC group and 7.7% in the control group (P = 0.93). There was a slightly higher incidence of pulmonary embolism in the PAC group (P = 0.004) but a lower incidence of renal failure, which was not statistically significant. The low mortality in the control group suggests that a significant mortality reduction would be difficult to achieve. Haemodynamic goals set for the PAC group were often not achieved until an unspecified point in the postoperative period, and many centres enrolled only a small number of patients.
It is often difficult to distinguish complications arising from the use of GDT from those of PAC insertion. Although failure to apply management protocols correctly precludes the use of the study by Sandham and coworkers [12] to draw conclusions regarding the efficacy of GDT, useful evidence of the safety of PAC insertion is provided.
Role of peroperative goal-directed fluid resuscitation
Sinclair and coworkers [13] used parameters derived from oesophageal Doppler cardiac output measurements as haemodynamic end-points for intraoperative fluid administration during proximal femoral fracture repair. Both patient groups received intravenous crystalloid, colloid and blood products to replace estimated losses and maintain pulse rate and blood pressure. In addition, protocol patients received fluid challenges guided by data derived using oesophageal Doppler. Median cardiac output rose by 1.2 l/min in the intervention group and fell by 0.4 l/min in the control group (P < 0.05). The study was small in size but demonstrated a reduction in length of hospital stay from 20 days to 12 days (P < 0.05). That study was repeated by Venn and colleagues [14], comparing traditional fluid management with fluid therapy guided by either central venous pressure or oesophageal Doppler readings. There was a similar reduction in time to being declared medically fit for discharge in both central venous pressure and Doppler groups in comparison with the control patients.
In cardiac surgical patients, Doppler-guided colloid challenges resulted in a lower rate of serious complications and shorter length of hospital stay. Measurement of gastric intramucosal pH suggested a reduction in gastric hypoperfusion [7]. In a mixed group of general, gynaecology and urology patients, Doppler-guided fluid therapy resulted in improvement in cardiac index, reduced length of hospital stay and an earlier return to enteral feeding, suggesting a reduction in postoperative ileus [15].
Although a mortality reduction has not been demonstrated using fluid alone, none of the studies performed to date have had adequate statistical power to answer this question. It would seem that goal-directed intravenous fluid therapy does confer an advantage, but in a select group of the patients further benefit may be accrued with the additional use of vasoactive therapy.
Use of vasoactive agents in fixed doses
Two studies have investigated the resuscitation of surgical patients following fluid resuscitation with a fixed dose of dopexamine [16, 17]. Neither demonstrated a significant improvement in terms of outcome. The value of GDT is likely to be related to the fact that management is individually tailored to the requirements of each patient. The use of a fixed dose will result in the unnecessary use of dopexamine in some patients, who are therefore exposed to risk for complications with no potential for improvement in outcome.
Reduction of perioperative cardiac ischaemia
A number of studies have considered the prophylactic use of nitrates, calcium channel blockers and β blockers for patients who are at risk for perioperative myocardial ischaemia. With the exception of β blockade, none of these therapies has resulted in an improvement in outcome. Mangano and colleagues [18] showed an improvement in outcome with prophylactic use of atenolol in patients undergoing vascular surgery. At 6 months there were no deaths in the atenolol group, as compared with 8% mortality in the control group. The beneficial effect was maintained at 2 years, with 10% of the atenolol group and 21% of the control group dying.
Further work in vascular surgery showed a mortality reduction from 17% to 3.4% with the perioperative use of bisoprolol in patients with evidence of myocardial ischaemia on dobutamine stress echocardiography [19]. Interestingly, a large proportion of patients screened also fulfilled selection criteria for preoperative GDT used in two important trials [3, 4].
Because of the much larger number of positive outcome studies, the evidence for the beneficial effect of perioperative GDT is much stronger than that for β blockade. However, it is entirely possible that both forms of treatment are beneficial and not mutually exclusive. GDT has proved most successful when applied for short periods during resuscitation of hypovolaemia [20] and least successful when applied to patients with established critical illness [21, 22]. This would suggest that much of the beneficial effect conferred by GDT as a resuscitation technique is not due to increases in cardiac output and oxygen delivery per se. The use of prophylactic β blockade in patients considered at high risk for perioperative myocardial ischaemia will not negate the requirement for fluid resuscitation during periods of hypovolaemia. The use of GDT in such patients to facilitate optimal fluid therapy is logical.
A practical approach to perioperative care for the high-risk patient
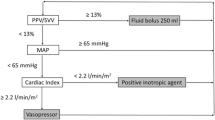
The first step in the care of such patients is to identify the individual at risk (Fig. 1). It is important to recognize the existence of a specific and easily identifiable group of patients undergoing major surgery with a predicted mortality rate that may exceed 20%. A typical district general hospital in the UK will care for approximately 500 patients a year who are at high risk for postoperative death or major complications. This group represents only 7.5% of patients undergoing major surgery but accounts for more than 80% of all postsurgical deaths [23]. Examples typically include frail elderly patients with significant cardiac or respiratory disease who are undergoing major abdominal surgery. In our practice, the selection criteria used in most interventional studies [3, 4], anaerobic threshold testing [24] and a predicted mortality of at least 5% using the P-POSSUM scoring system (with the use of anticipated operative severity) [23] are all effective tools in the identification of such patients. Once again it should be emphasized that no large randomized trials have demonstrated a benefit for this overall strategy. However, intelligent clinical application of inconsistent evidence requires a pragmatic approach.
Once the patient has been identified as being at risk, systematic investigation is required. This should follow the American College of Cardiology/American Heart Association Task Force guidelines [25], which stratify patients according to metabolic reserve. An important aspect of these guidelines is the use of dobutamine stress echocardiography to identify patients at high risk for perioperative myocardial ischaemia. This process of evaluation may indicate a patient in whom the risks for surgery are not justified by the potential benefits. In such situations the patient should be provided with sufficient information to make an informed choice from the options available.
The medical management of all coexisting disease processes should then be reviewed to ensure that current standards of best practice are adhered to. Various aspects of perioperative management should then be given consideration. It is recommended that all such patients be admitted to a critical care area ideally before surgery. There is evidence to suggest that this approach results in an overall reduction in consumption of resources [7, 13, 26].
The two specific approaches considered here are the use of perioperative GDT and perioperative β blockade. Dobutamine stress echocardiography will identify those patients in whom β blockade is indicated as a result of a high probability of perioperative myocardial ischaemia. This usually accounts for between 10% and 20% of the total population of high-risk patients. Ideally, this form of management should be commenced before surgery and continued for a minimum of 8 hours postoperatively. A number of methods of cardiac output measurement are now available, and the use of GDT no longer necessitates pulmonary artery catheterization.
Those patients in whom β blockade is not indicated should receive perioperative goal-directed haemodynamic therapy. This generally involves the use of intravenous fluid and inotropic therapies aiming to achieve an oxygen delivery index of 600 ml/min per m2 wherever possible, without causing tachycardia or myocardial ischaemia. Central venous oxygen saturation has been shown to be a valid haemodynamic goal in severe sepsis [20] and may also prove to be useful in high-risk surgery [27]. The use of inodilator agents, for example dopexamine, with modest maximum infusion rates may minimize the risk for complications of inotrope use. These agents are thought to achieve both improved global oxygen delivery as well as tissue perfusion.
The remaining subgroup of high-risk patients are those identified as being at particular risk of perioperative myocardial ischaemia. Perioperative β blockade is indicated in this group. However, optimal fluid management is still required in such patients. Improvements in outcome have been shown in cardiac [7], orthopaedic [13, 14] and general surgical patients [15] by the use of peroperative oesophageal Doppler to guide fluid administration. The use of perioperative, peroperative or postoperative cardiac output monitoring is therefore still recommended in this subgroup in order to ensure optimal fluid management.
Conclusion
There is a select group of patients in whom the risk for death and serious complications following major surgery is in excess of 20%. The risk is not related to the surgery per se but mainly to the patient's own preoperative physiological condition. In particular, it is related to the presence of poor cardiovascular and respiratory reserves. There are now a number of well conducted studies that show that the use of perioperative GDT may improve outcome. Many studies demonstrate significant reductions in morbidity and mortality [3–5, 28, 29], although some smaller studies failed to demonstrate an improvement in outcome [8, 9].
A proportion of such patients may also benefit from perioperative β blockade, which in selected patients has also been shown to result in significant mortality reductions [18, 19].
Once the at-risk patient has been identified and assessed, medical management of coexisting disease should be reviewed. A pragmatic approach to perioperative management is to administer perioperative GDT to the majority not considered to be especially at risk for myocardial ischaemia. The remainder should receive a β blocker but also receive perioperative fluid therapy guided by cardiac output monitoring technology. All patients should be admitted to a critical care area for the perioperative period.
Abbreviations
- GDT:
-
goal-directed therapy
- PAC:
-
pulmonary artery catheter.
References
Shoemaker WC: Cardiorespiratory patterns of surviving and nonsurviving postoperative patients. Surg Gynecol Obstet 1972, 134: 810-814.
Clowes GH, Del Guercio LR: Circulatory response to trauma of surgical operations. Metabolism 1960, 9: 67-81.
Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS: Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest 1988, 94: 1176-1186.
Boyd O, Grounds RM, Bennett ED: A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA 1993, 270: 2699-2707. 10.1001/jama.270.22.2699
Wilson J, Woods I, Fawcett J, Whall R, Dibb W, Morris C, McManus E: Reducing the risk of major elective surgery: randomised controlled trial of preoperative optimisation of oxygen delivery. BMJ 1999, 318: 1099-1103.
Polonen P, Ruokonen E, Hippelainen M, Poyhonen M, Takala J: A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg 2000, 90: 1052-1059.
Mythen MG, Webb AR: Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg 1995, 130: 423-429.
Ziegler DW, Wright JG, Choban PS, Flancbaum L: A prospective randomized trial of preoperative 'optimization' of cardiac function in patients undergoing elective peripheral vascular surgery. Surgery 1997, 122: 584-592. 10.1016/S0039-6060(97)90132-X
Valentine RJ, Duke ML, Inman MH, Grayburn PA, Hagino RT, Kakish HB, Clagett GP: Effectiveness of pulmonary artery catheters in aortic surgery: a randomized trial. J Vasc Surg 1998, 27: 203-211. discussion 211–212
Bender JS, Smith-Meek MA, Jones CE: Routine pulmonary artery catheterization does not reduce morbidity and mortality of elective vascular surgery: results of a prospective, randomized trial. Ann Surg 1997, 226: 229-236. discussion 236–237 10.1097/00000658-199709000-00002
Berlauk JF, Abrams JH, Gilmour IJ, O'Connor SR, Knighton DR, Cerra FB: Preoperative optimization of cardiovascular hemodynamics improves outcome in peripheral vascular surgery. A prospective, randomized clinical trial. Ann Surg 1991, 214: 289-297. discussion 298–299
Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, Laporta DP, Viner S, Passerini L, Devitt H, et al.: A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med 2003, 348: 5-14. 10.1056/NEJMoa021108
Sinclair S, James S, Singer M: Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: randomised controlled trial. BMJ 1997, 315: 909-912.
Venn R, Steele A, Richardson P, Poloniecki J, Grounds M, Newman P: Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. Br J Anaesth 2002, 88: 65-71. 10.1093/bja/88.1.65
Gan TJ, Soppitt A, Maroof M, el-Moalem H, Robertson KM, Moretti E, Dwane P, Glass PS: Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 2002, 97: 820-826. 10.1097/00000542-200210000-00012
Stone MD, Wilson RJ, Cross J, Williams BT: Effect of adding dopexamine to intraoperative volume expansion in patients undergoing major elective abdominal surgery. Br J Anaesth 2003, 91: 619-624. 10.1093/bja/aeg245
Takala J, Meier-Hellmann A, Eddleston J, Hulstaert P, Sramek V: Effect of dopexamine on outcome after major abdominal surgery: a prospective, randomized, controlled multicenter study. European Multicenter Study Group on Dopexamine in Major Abdominal Surgery. Crit Care Med 2000, 28: 3417-3423. 10.1097/00003246-200010000-00007
Mangano DT, Layug EL, Wallace A, Tateo I: Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996, 335: 1713-1720. (Published erratum appears in N Engl J Med 1997, 336: 1039.) 10.1056/NEJM199612053352301
Poldermans D, Boersma E, Bax JJ, Thomson IR, van de Ven LL, Blankensteijn JD, Baars HF, Yo TI, Trocino G, Vigna C, et al.: The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999, 341: 1789-1794. 10.1056/NEJM199912093412402
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative Group: Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001, 345: 1368-1377. 10.1056/NEJMoa010307
Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R: A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO 2 Collaborative Group. N Engl J Med 1995, 333: 1025-1032. 10.1056/NEJM199510193331601
Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D: Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med 1994, 330: 1717-1722. 10.1056/NEJM199406163302404
Copeland GP, Jones D, Walters M: POSSUM: a scoring system for surgical audit. Br J Surg 1991, 78: 355-360.
Older P, Smith R, Courtney P, Hone R: Preoperative evaluation of cardiac failure and ischemia in elderly patients by cardiopulmonary exercise testing. Chest 1993, 104: 701-704.
Eagle KA, Brundage BH, Chaitman BR, Ewy GA, Fleisher LA, Hertzer NR, Leppo JA, Ryan T, Schlant RC, Spencer WH III, et al.: Guidelines for perioperative cardiovascular evaluation for noncardiac surgery. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Committee on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. Circulation 1996, 93: 1278-1317.
Guest JF, Boyd O, Hart WM, Grounds RM, Bennett ED: A cost analysis of a treatment policy of a deliberate perioperative increase in oxygen delivery in high risk surgical patients. Intensive Care Med 1997, 23: 85-90. 10.1007/s001340050295
Pearse RM, Dawson D, Rhodes A, Grounds RM, Bennett ED: Low central venous saturation predicts post-operative mortality. Intensive Care Med 2003, 29: S15.
Bishop MH, Shoemaker WC, Appel PL, Meade P, Ordog GJ, Wasserberger J, Wo CJ, Rimle DA, Kram HB, Umali R, et al.: Prospective, randomized trial of survivor values of cardiac index, oxygen delivery, and oxygen consumption as resuscitation endpoints in severe trauma. J Trauma 1995, 38: 780-787.
Lobo SM, Salgado PF, Castillo VG, Borim AA, Polachini CA, Palchetti JC, Brienzi SL, de Oliveira GG: Effects of maximizing oxygen delivery on morbidity and mortality in high-risk surgical patients. Crit Care Med 2000, 28: 3396-3404. 10.1097/00003246-200010000-00003
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
Pearse, R.M., Rhodes, A. & Grounds, R. Clinical review: How to optimize management of high-risk surgical patients. Crit Care 8, 503 (2004). https://doi.org/10.1186/cc2922
Published:
DOI: https://doi.org/10.1186/cc2922