Abstract
Introduction
Severe sepsis is a dreaded consequence of infection and necessitates intensive care treatment. Severe sepsis has a profound impact on mortality and on hospital costs, but recent incidence data from The Netherlands are not available. The purpose of the present study was to determine the prevalence and incidence of severe sepsis occurring during the first 24 hours of admission in Dutch intensive care units (ICUs).
Methods
Forty-seven ICUs in The Netherlands participated in a point prevalence survey and included patients with infection at the time of ICU admission. Clinical symptoms of severe sepsis during the first 24 hours of each patient's ICU stay were recorded and the prevalence of severe sepsis was calculated. Then, the annual incidence of severe sepsis in The Netherlands was estimated, based on the prevalence, the estimated length of stay, and the capacity of the participating ICUs relative to the national intensive care capacity.
Results
The participating ICUs had 442 beds available for admissions, which was estimated to be 42% of the national ICU capacity. At the time of the survey, 455 patients were currently admitted and 151 were included in the analysis; 134 (29.5%) patients met criteria for severe sepsis. The most common failing organ system was the respiratory system (90%), and most patients were admitted following surgery (37%) and were admitted because of acute infection (62%). The most prevalent source of infection was the lung (47%). The estimated duration of ICU stay for severe sepsis patients was 13.3 ± 1.1 days.
Conclusion
The annual number of admissions for severe sepsis in Dutch ICUs was calculated at 8643 ± 929 cases/year, which is 0.054% of the population, 0.61% of hospital admissions and 11% of ICU admissions.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Severe sepsis is a major complication of infection, and is triggered by a systemic inflammatory and coagulation reaction. The mortality rate from severe sepsis is high. In a recent update on the epidemiology of sepsis, Angus and Wax [1] cited several studies that reported mortality rates of 20–52%. A multicentre study conducted in 170 intensive care units (ICUs) in France [2] found 28-day mortality rates of 56% and 60% in patients with severe sepsis and culture-negative severe sepsis, respectively. It has also been estimated that the number of people who died after severe sepsis in 1995 in the USA was equal to the number of deaths after acute myocardial infarction [3].
Patients suffering from severe sepsis require intensive care and account for a large part of ICU resource consumption. It was demonstrated by Bakker and coworkers [4, 5] that 52–59% of an ICU budget was allocated to sepsis and septic shock patients, but that those patients constituted only 24% of the ICU population. Moreover, new therapeutic strategies for the treatment of severe sepsis may carry considerable acquisition costs and further increase treatment expenses. In the narrow margins of current health care budgets, economical considerations are likely to be taken into account when medical decisions are made. Data on incidence and prevalence are therefore essential if we are to appreciate the scope of a health problem, both medically and macroeconomically.
The incidence of severe sepsis in ICUs in The Netherlands has been estimated in a number of studies. In 1986 Verbrugh and coworkers [6] identified 5.4 cases of bacteraemia with clinical symptoms for every 1000 hospital admissions in two general hospitals, and in 1993 Kieft and colleagues [7] found an incidence of sepsis syndrome in a Dutch university hospital of 1.36% of all hospital admissions. In 1994 the National Institute for Public Health and the Environment reported 4.8 cases of sepsis registered as first or second diagnoses for every 1000 hospital admissions on the basis of National Medical Registration in The Netherlands [8]. However, the Institute noted that this number was most likely an underestimation because sepsis may go unregistered.
More recent studies on the incidence of severe sepsis in The Netherlands are lacking. Therefore, the present cross-sectional prevalence survey was conducted in order to determine the prevalence and extrapolate the incidence of severe sepsis in Dutch ICUs.
Methods
General study design
The design of the survey was developed within the context of the development of clinical guidelines for the application of activated protein C [9]. The aim of the study was to determine the number of severe sepsis cases that one can annually expect to be eligible for therapies specifically indicated for severe sepsis, other than organ replacement or life-sustaining therapies. Some of the choices made in the study design (e.g. inclusion of patients with microbiologically proven as well as clinically suspected infections rather than proven infections only, and the restriction of the survey to intensive care wards only) are a consequence of this clinical practice orientated study objective.
All heads of Dutch ICUs were invited by letter to participate in the survey. All patients present on the ICU or admitted for any length of time during a 24-hour period – between 08:00 on Tuesday 11 December 2001 and 08:00 on Wednesday 12 December 2001 – were considered for inclusion. A case report form (CRF) was completed for every patient with a confirmed or strongly suspected infection at the time they were admitted to the ICU (Table 1), as determined by the first question in the CRF (Appendix 2). The CRF only inquired about clinical symptoms within the first 24 hours of a patient's ICU stay, thereby selectively aiming to determine only the prevalence of community-acquired and hospital-acquired severe sepsis. Including ICU-acquired severe sepsis in this cross-sectional prevalence study would have required a more complex study design, more intensive training, and greater investments of time from the participating ICUs, which were beyond the scope of the survey.
Both patients who were already present at the start of the study as well as newly admitted patients during the study period were considered for inclusion. The reason for this was that severe sepsis is difficult to measure in a prevalence survey because it is a syndrome; it is not a condition that a patient have or not have. When a patient is admitted to the ICU with severe sepsis, it may well be that all criteria defining severe sepsis are no longer present by the next day, but the patient may stay for another fortnight until they are sufficiently recovered to be transferred to a general ward or discharged. During that time, the patient does not meet the criteria for severe sepsis but is still present on the ICU for treatment of resulting pathologies. In order to ensure that these patients would be captured in the survey, the patient CRF explicitly asked the person responsible for its completion to answer the questions based on the first 24 hours of admission only.
A brief questionnaire was developed to capture several relevant ICU characteristics. The CRFs and questionnaires sent to the participating ICUs were collected and evaluated. The definitions of sepsis, severe sepsis and septic shock, which represent increasingly severe stages in the sepsis cascade, were derived from the American College of Chest Physicians/Society of Critical Care Medicine guidelines (Table 1) [10]. In this report we treated these stages as successive subgroups of the initial population, which means that, for example, septic shock patients form a subset of the severe sepsis population rather than a separate population.
Questionnaires and case report forms
Intensive care unit questionnaire
The ICU questionnaire consisted of two parts: the first questioned the total number of ICU beds in the unit and the number of beds that were closed because of understaffing or for other reasons (Appendix 1). The theoretical availability of beds in every ICU was calculated by subtracting the number of closed beds from the total number of beds. The second part of the questionnaire recorded the total number of patients present in the ICU during the 24-hour study period, thus representing the number of patient-days covered by the survey.
Case report form
Only patients with a confirmed or strongly suspected infection at the time of their admission were included in the present study. The CRF consisted of 30 closed questions, divided into four subsets (Appendix 2). The first referred to the status of the patient at the time of ICU admission and collected age, sex, date and time of ICU admission, previous location (i.e. to determine whether they had been transferred from another department/hospital or whether their admission to the ICU was primary), reason for ICU admission and source of infection. The second part collected information regarding the presence of comorbidities (including chronic organ failure). In the third part the four systemic inflammatory response syndrome (SIRS) criteria were scored for the first 24 hours of ICU admission, and the fourth part collected data regarding acute organ failure during the first 24 hours of ICU stay. Criteria for the latter were adopted from the PROWESS (Human Activated Protein C Worldwide Evaluation in Severe Sepsis) study and were described on the CRF (Table 1) [11]. The CRF was straightforward and the items pertained to standard and objectively measurable parameters (e.g. body temperature, blood concentrations and heart rate). Therefore, no problems with the comprehension of the questionnaire or interobserver reliability were expected, and no pilot study was conducted.
Participation
We invited 103 ICUs in The Netherlands to participate in the prevalence survey after telephone contact to identify a contact person. The request was accompanied by a description of the study design. Fifty-six ICUs agreed to participate. Forty-seven participating ICUs returned the completed CRFs and questionnaires. The reported data were entered into a Microsoft Excel database, and double entry was performed to certify the data.
Method of incidence calculation
A prevalence survey offers a static picture of a situation, such as the number of patients in an ICU because of severe sepsis. However, we were also interested in severe sepsis patients who are admitted to the ICU annually, and therefore we extrapolated the information from our prevalence series of cases to annual incidence estimates.
Prevalence, incidence and duration
A dependent relationship exists between prevalence, incidence and duration of a disease [12]:
Pn = Ij × Ei(D) Equation 1
where Pn is the prevalence unit in numbers of persons, Ij is the incidence rate in persons (new cases)/time, and Ei(D) is the expected value of the duration of the condition in a distribution of durations obtained from an incidence (i) series of cases. Ei(D) can also be interpreted as the sample mean duration Di from an incidence series.
In the equation, Ij is the unknown factor that we were interested in. Prevalence (Pn) and duration (Ei[D]) were retrieved from the survey results in the following manner.
The prevalence of severe sepsis patients in the survey was defined as the total number of patients present on the ICU during the study time interval that had all symptoms of severe sepsis during the first 24 hours of their stay. These first 24 hours of stay may have been in the past (i.e. outside the study period) but it was assumed that the reason these patients were still present on the ICU was the episode of severe sepsis in the past. These patients were therefore counted as prevalent severe sepsis patients.
Because the objective of the investigation was to determine the number of patients annually admitted to the ICU with severe sepsis, Pn in Eqn 1 refers to the prevalent number of patients occupying an ICU bed, and Di actually refers to the duration of ICU stay associated with severe sepsis rather than the duration of severe sepsis itself. Pn in Eqn 1 was calculated by extrapolating the prevalent number of patients in the survey (Ps) to the whole country, using the number of ICU beds as a distributive key (R = number of ICU beds in survey/number of ICU beds in The Netherlands). The annual incidence (Ij) was therefore calculated from the survey results as follows:
Ij = Pn/(Ei[D]) = (Ps/R)/Di Equation 2
It was not possible to derive the duration of stay (Di) directly from the prevalence study, because one only knows how long the patient has been in the ICU until the point prevalence survey (the 'duration to date') and not how much longer the patient will stay. The duration of stay can, however, be derived from the duration to-date. Freeman and Hutchison [12] described how both duration of stay from an incidence series of cases and duration to date from a prevalence series of cases of a similar patient population are, in theory, geometrically distributed with similar means. Therefore, we inserted the observed duration to date into the software @RISK (for Windows) in order to check whether the distribution was geometrical, and calculated the mean, which was subsequently entered into Eqn 1 as Di.
From the point prevalence survey, not only the number of prevalent but also the number of incident patients was known (i.e. the patients who were newly admitted during the study period and developed symptoms of severe sepsis in the next 24 hours). This survey incidence (Ids), however small, was used for a second calculation of the annual incidence according to Eqn 3, extrapolating the 1-day incidence in the survey to a national estimate through the distributive key:
Ij = (Ids/R) × 365 Equation 3
Statistics
Descriptive outcomes are presented in terms of means and standard deviations. When estimating the incidence, prevalence and length of stay, means are surrounded by standard errors.
Results
Questionnaire and case report form
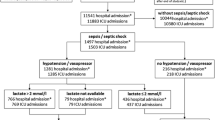
Four departments from university hospitals and 43 departments from general hospitals returned the completed questionnaires and CRFs with information on 152 patients for whom the CRF had been completed (Table 2). Data from one patient were excluded from further analyses because of missing information.
The ICUs had a joint capacity of 510 beds, of which 442 (87%) were available for admission. The remaining beds were mainly closed because of understaffing of the department. Three ICU departments specified that their ICU was a combined ICU/cardiac care unit (CCU) department. The beds and patients that could be attributed to the CCU were excluded from the calculation of the total number of beds and patients in this survey. During the prevalence study period, 455 patients were present in the participating ICUs (Table 2).
Evaluation of the 151 CRFs according to the definitions of sepsis (Table 1) resulted in the estimate that 143 patients in the prevalence series had sepsis in the first 24 hours of admission, representing 31% of all ICU patients. In 134 (93%) cases the sepsis was severe and in 53 cases (37%) the sepsis was complicated by cardiovascular and metabolic organ failure meeting the definition of septic shock. The remaining eight patients had an infection but fulfilled no more than one SIRS criterion. If we considered only patients newly admitted to the ICU during the 24-hour period (the incidence series) we identified 24 patients with sepsis, 18 (75%) of whom developed severe sepsis and seven (29%) septic shock.
On average, patients with severe sepsis were 64 ± 15 years old. Of these patients, 70% were older than 60 years and 44% were older than 70 years. The ratio of males to females was 1.7. In 121 (90%) of the prevalent patients with severe sepsis, more than one organ system failed (without being due to chronic organ failure), with an average of 3.6 ± 1.6 failing organ systems (Fig. 1). In 121 out of 134 prevalent patients the respiratory system failed (90%), followed by the cardiovascular system in 97 (72%) and the renal system in 71 patients (53%: Table 3). Patients with septic shock had on average 4.9 ± 1.2 failing organ systems, including the cardiovascular and metabolic organ systems (Fig. 1). Furthermore, the respiratory system failed in 49 (92%), the liver in 34 (64%) and the renal system in 33 (62%) patients (Table 3). Most patients with severe sepsis in the prevalence series (50 [37%]) were admitted to the ICU from the Department of Surgery, 35 (26%) patients came from the emergency room, and 20 (15%) patients were admitted from the Department of Internal Medicine (Table 4). The major cause for ICU admission for severe sepsis was acute infection in 83 (62%) patients, followed by acute surgery in 41 (31%).
Failing organ systems. Histogram of the number of failing organ systems in prevalent patients with severe sepsis (n = 134; light grey) and septic shock (n = 53; dark grey). Numbers within bars represent the absolute numbers of patients. On average, 3.6 ± 1.6 organ systems failed in patients with severe sepsis (mean ± standard deviation) and 4.9 ± 1.2 in patients with septic shock.
The most common sites of infection were the lungs (47%) and the abdomen (34%). In 35 (26%) of the prevalent severe sepsis patients respiratory comorbidity was present on admission, and 32 patients (24%) had a malignancy. Diabetes, chronic heart failure, a history of cerebrovascular accidents and chronic kidney failure were seen in many patients. The distribution of comorbidities in the prevalent patients with septic shock was similar to that of patients with severe sepsis.
Calculation of annual incidence of severe sepsis
Participation
The exact number of ICU beds in The Netherlands is unknown because the definition and capacity of hospital beds is not fixed. A hospital bed can be turned into an ICU bed and back again, depending on the hospital situation. A recent survey of the Hospital Building Board conducted among all hospitals in The Netherlands reported 108 ICU departments in 109 general hospitals and 23 ICUs in nine university hospitals, with a total capacity of 1041 ICU beds that were available for admission [13]. Another 148 beds were closed because of understaffing. The representation of ICUs in our survey was therefore calculated at 42% (442/1041). This percentage was used for all further calculations of national incidence and prevalence numbers.
Annual incidence based on the prevalence series of cases
The distribution of 'duration to date' in severe sepsis patients was geometric, with a mean of 13.3 ± 1.1 days (Table 5). The P value of the fit (observed significance level) was 0.9821. The P value can take values between 0 and 1, and as the P value approaches 1 there is increasing certainty that the fitted distribution actually generated the data set. As the observed distribution of duration to date fitted a geometric distribution well, the parameters of the distribution were used to estimate the duration of stay in the study population. The analogously estimated duration of stay for sepsis patients was 12.7 ± 1.0 days and for shock patients it was 11.6 ± 1.5 days.
Calculation according to Eqn 2 yielded an incidence rate of 9726 ± 1008 patients with sepsis in the ICUs per year, with 8643 ± 929 (89%) of them suffering from severe sepsis and 3932 ± 710 (37%) of them suffering shock (Table 5). The corresponding incidence numbers for the Dutch population, which was 16.1 million in December 2001, are 0.60 ± 0.06 cases of sepsis/1000 inhabitants, 0.54 ± 0.06 cases of severe sepsis/1000 inhabitants, and 0.24 ± 0.04 cases of septic shock/1000 inhabitants [14].
The total number of clinical hospital admissions and ICU admissions in The Netherlands in 2000 were reported to be 1,465,000 and 77,000, respectively, which yields an estimated incidence of severe sepsis in 0.61% of all hospital admissions and 11% in all ICU admissions [15].
Annual incidence based on the incidence series of cases
A second estimate of the annual incidence was calculated using Eqn 3 and the number of incident patients in the survey. The resulting daily incidence was calculated to be 57 ± 10, 42 ± 9 and 16 ± 6 for sepsis, severe sepsis and shock, respectively. The corresponding estimates for the annual incidence were 20,632 ± 3681 for sepsis, 15,474 ± 3309 for severe sepsis and 6018 ± 2195 for septic shock. Consequently, according to this calculation 1.1% of all hospital admissions and 20% of all ICU admissions are of patients with severe sepsis.
By means of an internal check, we combined Eqn 1 with Eqn 3 and substituted Pn = Ps/R, which yielded an equation that we could apply to the results of the survey:
Ps = 365 × Ids × Di Equation 4
Substituting the duration of stay of 13.3 days (expressed in years), as was estimated from the geometrical mean duration to date, told us that the expected ratio of prevalent and incident patients (Ps/Ids) in a 1-day survey is 13.3. In the actual survey we found a ratio of 134/18 = 7.4, suggesting that the number of incident patients in our survey was relatively high and/or the number of prevalent patients relatively low.
Discussion
This survey was conducted to determine the annual incidence of severe sepsis during the first 24 hours of ICU admission in The Netherlands. The number of severe sepsis patients in 47 ICUs (42% of total national ICU capacity) was captured in the 24-hour period between 08:00 on 11 December 2001 and 08:00 on 12 December 2001; 134 patients were present in the ICUs during the study period for treatment of severe sepsis, and 18 of these arrived during the study period. We calculated that the annual number of patients who need ICU treatment for severe sepsis in The Netherlands is 8643 ± 929, which was estimated to represent 0.61% of hospital admissions and 11% of ICU admissions. Some considerations regarding the interpretation of this number are addressed here.
In 1986, Verbrugh and coworkers [6] found that 0.54% of all hospital admissions in two general hospitals resulted in sepsis (defined as bacteraemia with clinical symptoms), and Kieft and coworkers [7] concluded, from a prospective study conducted in a university hospital, that sepsis syndrome accounted for 1.36% of all hospital admissions. The incidences of sepsis and severe sepsis per hospital admission, as calculated from our survey, were 0.65% and 0.61%, respectively, based on the prevalence series. These percentages are in the region of the findings reported by Verbrugh and coworkers, but they are lower than the incidence found by Kieft and colleagues, which may be because the latter group used a different definition of sepsis and admissions in a single university hospital only. The incidence of severe sepsis, at 11% of ICU admissions, was similar to the rate reported by Alberti and coworkers [16], who surveyed 14,364 ICU patients for more than 1 year in ICUs in several European countries, Canada and Israel. They identified 1634 patients with either severe sepsis or septic shock at admission. Finfer and coworkers [17] found that 571 ICU admissions were due to severe sepsis in 5878 consecutive ICU patients admitted to Australian and New Zealand ICUs. Padkin and coworkers [18] reported a much higher incidence (27.1%) within the first 24 hours of ICU admission in England, Wales and Northern Ireland from a retrospective database analysis, but at a population level the incidence was similar (51 per 100,000 inhabitants). This inconsistency suggests that ICU admission policy is an important issue to consider when comparing findings between different institutions or countries. The median ICU stay in their survey was 3.6 days, which is low compared with our and previously reported findings [3, 19]. Two recent retrospective analyses of discharge databases in the USA [3, 20] also found higher incidence rates by population (240–300 per 100,000) and by hospital admission (2.6%), but in these studies severe sepsis patients were not defined by American College of Chest Physicians/Society of Critical Care Medicine guidelines criteria but by ICD-9-CM (International Classification of Diseases, Ninth revision, Clinical Modification) codes, and all hospital patients were considered, rather than just ICU patients.
The protocol of the present survey specifically asked participating ICUs to include only those patients admitted to the ICU with an infection, which implied that patients with ICU-acquired severe sepsis were excluded. Although a true estimate of severe sepsis should have included these patients, the diagnosis of severe sepsis in patients admitted to the ICU with problems other than infection is very difficult. Current parameters of severe sepsis are probably not sufficiently accurate. In addition, this would have required consensus about new infections and newly developed organ dysfunction or worsening organ dysfunction in all ICUs, which was not possible for the present study. From the results of the EPIC (European Study of Prevalence of Infection during Intensive Care) study [21], it appeared that in 78 Dutch ICUs 17% of the patients had an ICU infection but only 0.4% of the patients had sepsis resulting from an ICU infection. A Dutch surveillance study conducted among 16 ICUs [22], however, found that 8% of patients who were admitted to the ICU (with or without prior infection) developed sepsis due to an ICU-acquired infection. Also, Alberti and coworkers [16] reported that 9.2% of ICU patients developed ICU-acquired sepsis, severe sepsis, or septic shock, whereas the rate for community-acquired and hospital-acquired sepsis, severe sepsis, or septic shock was 17.4%. However, that study did not report the exact methodology for determining organ failure developed in the ICU, or the time frame within which the symptoms of severe sepsis occurred. Nevertheless, by ignoring sepsis due to ICU-acquired infections, the present survey probably underestimates the national incidence of cases of severe sepsis.
Moreover, severe sepsis can occur outside ICUs; such cases were not picked up in the present survey. Angus and coworkers [3] concluded from their retrospective analysis that only 51.1% of severe sepsis patients (defined using ICD-9-CM codes) received ICU care in the USA, although an additional 17.3% received organ replacement therapy in CCUs or intermediate care units. Because severe sepsis is defined by the presence of organ failure, ICU admission is almost always required for organ-replacing therapeutic intervention and specific treatment for severe sepsis.
Several risk factors for severe sepsis have been identified in previous studies, including age, sex, comorbidities, and causative pathogen [3, 16]. In extrapolating data from a cross-sectional prevalence survey such as the present one, consideration of the representativeness of the ICU patients in the participating ICUs relative to the national case mix of ICU patients, especially in terms of these risk factors, is important. Unfortunately, demographic and clinical information was only collected for the included patients, rather than for all ICU patients present during the study period. It was therefore not possible to determine how representative the case mix of ICU patients in our survey was. On the other hand, the participating ICUs were estimated to be representative of The Netherlands. Participating ICUs were distributed evenly over The Netherlands, and the representation of both university and general hospitals was similar (4/9 university hospitals [44%] and 43/109 general hospitals [39%]). ICUs in Dutch general hospitals are almost always mixed; only some university hospitals have separate ICU wards for surgical and medical patients. Therefore, no bias is expected to have occurred by, for example, including a disproportionate number of medical ICUs. We knew the number of ICU beds in our survey and based the estimate of national representation on this number; there was no national information on the number of beds in general and university hospitals separately, and therefore we could not reliably derive a specified incidence of severe sepsis by type of hospital.
Although this prevalence survey did not address the length of stay in the ICU directly, we estimated this duration on the basis of the duration to date by means of fitting the data to a geometrical distribution, as described by Freeman and Hutchison [12]. We found a length of stay of 13.3 ± 1.1 days for severe sepsis patients. This result is consistent with the literature.
Angus and coworkers [3] found a duration of ICU stay of 13.8 ± 20.0 days in teaching hospitals and 10.0 ± 13.8 days in nonteaching hospitals. Edbrooke and coworkers [19] found a median ICU stay of 16.5 days for patients who developed sepsis in the ICU of a university hospital in the UK. We expected to find increasing lengths of stay with increasing severity of sepsis, but this was only true for severe sepsis versus sepsis (13.3 versus 12.7 days). For shock patients, however, we found a duration (Di) of 11.6 days, but the prevalent population of shock patients was rather small to make a powerful fit of the duration to date (P = 0.69). The method of Freeman and Hutchison [12] is an indirect method with which to estimate duration of stay, and data on length of stay should ideally be extracted from prospective studies.
From the relationship between prevalence and incidence, as expressed in Eqn 4, we concluded that the ratio of incident and prevalent patients deviated from theoretical expectation. An explanation for this trend could be associated with the study design. In order to identify prevalent patients, the participating ICUs were required to go through records for all patients on their ward to determine whether they had an infection at the time of their admission, and to search the registration for the presence of SIRS criteria and organ failure during the first 24 hours of admission. This might have been too time consuming in some cases, or the specific data may not have been available. This introduces a negative bias in the number of prevalent patients found in the survey. On the other hand, newly admitted patients are likely to be monitored more closely for infection, SIRS criteria and organ failure when members of the medical staff are aware that a survey for severe sepsis is being conducted that day. This makes it conceivable that the number of incident severe sepsis patients is an overestimation of the actual daily incidence. Moreover, the number of incident patients in the survey (i.e. 18) is small and very sensitive to daily variation, which is also expressed by a large confidence interval for the estimated daily and annual incidences. Because we only measured prevalence and incidence over 1 day, there is a good chance that the relatively high number of incident patients is attributable to coincidence. The annual incidence of severe sepsis based on the prevalence series of cases therefore forms a more reliable estimate for future reference.
The results of the survey were not corrected for any influence of time, such as the day of the week or the month of the year on which the survey was conducted. The main reason for this was the fact that severe sepsis patients are usually acute cases, and the majority of the population came to the hospital for acute surgery or acute infection (Table 4). It is not likely that these acute events differ from weekdays to holidays or weekends. Moreover, data on the admission rates for severe sepsis patients are unavailable, and any correction of the results in the survey would be as arbitrary as not correcting at all.
The present point prevalence survey was conducted to gain insight into the current incidence of severe sepsis occurring within the first 24 hours of ICU admission in The Netherlands. The results indicate that the national incidence of severe sepsis is in the range of 9000 patients per year. This number is lower (by 50–70%) than the annual incidence of diseases such as coronary heart disease and cerebrovascular accident, but it is comparable with the incidence of breast cancer, lung cancer and Parkinson's disease in The Netherlands. This demonstrates the importance of severe sepsis in terms of public health, resource allocation in intensive care and, because of the high overall mortality, causes of death in The Netherlands [23].
Key messages
-
The cross-sectional prevalence of severe sepsis patients in Dutch Intensive Care Units was 134 out of 455 patients (29.5%).
-
The most severe sepsis patients were admitted to the ICU due to acute infection (62%) or after acute surgery.
-
Severe sepsis mostly originated from a respiratory (47%) or abdominal infection (34%).
-
The estimated incidence of 'patients admitted to ICU with severe sepsis' in The Netherlands was 8,643 per year.
Abbreviations
- CCU:
-
cardiac care unit
- CRF:
-
case report form
- ICU:
-
intensive care unit
- SIRS:
-
systemic inflammatory response syndrome.
References
Angus DC, Wax RS: Epidemiology of sepsis: an update. Crit Care Med. 2001, Suppl: S109-S115. 10.1097/00003246-200107001-00035.
Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercier JC, Offenstadt G, Regnier B: Incidence, risk factors and outcome of severe sepsis and septic shock in adults; a multicenter prospective study in Intensive Care Units. JAMA. 1995, 274: 968-974. 10.1001/jama.274.12.968.
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR: Epidemiology of severe sepsis in the United States: Analysis of incidence. outcome, and associated costs of care. Crit Care Med. 2001, 29: 1303-1310. 10.1097/00003246-200107000-00002.
Bakker J, Rommes JH: Costs of ICU treatment in a general Dutch intensive care unit. Intensive Care Med. 1996, 22: S302-
Bakker J, de Munck P, Rommes H, Van Bussel H: Costs of severe sepsis in a multidisciplinary intensive care unit. Crit Care Med. 1998, 26 (1): A131-
Verbrugh HA, Mintjes-De Groot AJ, Broers DA: Bacteremia in 2 general hospitals: the tip of the iceberg of hospital infections. Ned Tijdschr Geneeskd. 1986, 130: 441-445.
Kieft H, Hoepelman AIM, Zhou W, Rozenberg-Arska M, Struyvenberg A, Verhoef J: The sepsis syndrome in a Dutch University Hospital. Arch Intern Med. 1993, 153: 2241-2247. 10.1001/archinte.153.19.2241.
RIVM: Nationaal Kompas Volksgezondheid, versie 1.4; Sepsis (National Compass of Public Health, version 1.4; Sepsis). in Dutch, [http://www.rivm.nl]
Girbes ARJ, Bakker J, Van Deuren M, van Hout BA, Van Leeuwen SJ, Levi MM: NVIC conceptrichtlijnen: Toepassing drotrecogin alpha, geactiveerd proteïne C (aPC) bij de behandeling van ernstige sepsis (NVIC guideline: application of drotrecogin alpha, activated protien C (aPC) in the treatment of severe sepsis) [in Dutch]. Netherlands J Crit Care. 2002, 6: 41-45.
Bone R: American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992, 20: 864-874.
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ, Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group: Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001, 344: 699-709. 10.1056/NEJM200103083441001.
Freeman J, Hutchison GB: Prevalence, incidence and duration. Am J Epidemiol. 1980, 112: 707-723.
College Bouw Ziekenhuisvoorzieningen: Onderzoek Intensive Care. Deel I: Aanbod en Gebruik in de Huidige Situatie. Uitkomsten van de Enquête van de NVIC en het Bouwcollege (Neatherlands Board for Hospital Facilities: Intensive Care survey. Part I: avalibility and use in current situation. Results of the NVIC and Netherlands board for Hospital Facilities survey). Utrecht, College Bouw Ziekenhuisvoorzieningen. 2002, in Dutch
Centraal Bureau voor de Statistiek: Bevolking Maand-en Jaarcijfers (Central Office for Statistics. Population: monthly and annual figures). in Dutch, [http://www.cbs.nl]
Lorsheyd JJG: Gebruik Ziekenhuisvoorzieningen 2000 (Use of hospital facilities 2000). Utrecht: Prismant. 2001, in Dutch
Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, Sicignano A, Palazzo M, Moreno R, Boulme R, Lepage E, Le Gall R: Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002, 28: 108-121. 10.1007/s00134-001-1143-z.
Finfer S, Bellomo R, Lipman J, French C, Dobb G, Myburgh J: Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Med. 2004, 30: 589-596. 10.1007/s00134-004-2263-z.
Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K: Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales and Northern Ireland. Crit Care Med. 2003, 31: 2332-2338. 10.1097/01.CCM.0000085141.75513.2B.
Edbrooke DL, Hibbert CL, Kingsley JM, Smith S, Bright NM, Quinn JM: The patient-related costs of care for sepsis patients in a United Kingdom adult general intensive care unit. Crit Care Med. 1999, 27: 1760-1767. 10.1097/00003246-199909000-00010.
Martin GS, Mannino DM, Eaton S, Moss M: The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003, 348: 1546-1554. 10.1056/NEJMoa022139.
Ibelings MS, Bruining HA: Dutch results of the European Study of Prevalence of Infection during Intensive Care (EPIIC). II. Nature of the infections [in Dutch]. Ned Tijdschr Geneeskd. 1994, 138: 2244-2247.
Groot AJ, Geubbels EL, Beaumont MT, Wille JC, de Boer AS: Hospital infections and risk factors in the intensive care units of 16 Dutch hospitals, results of surveillance of quality assurance indicators. Ned Tijdschr Geneeskd. 2001, 145: 1249-1254.
RIVM: Nationaal Kompas Volksgezondheid, Versie 1.4: Welke Ziekte heeft de Hoogste Incidentie? (RIVM: National Compass of Public Health, version 1.4; Which disease has the highest incidence?). in Dutch, [http://www.rivm.nl]
Acknowledgements
We kindly thank all ICUs that participated in this point prevalence survey of severe sepsis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
AvG, JB and BAvH received an educational grant for this study from Lilly Netherlands. CPWMV is an employee of Lilly Netherlands and owns stock options in Eli Lilly and Company.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
van Gestel, A., Bakker, J., Veraart, C.P. et al. Prevalence and incidence of severe sepsis in Dutch intensive care units. Crit Care 8, R153 (2004). https://doi.org/10.1186/cc2858
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc2858