Abstract
Objectives
To measure the mass transfer and clearance of procalcitonin (PCT) in patients with septic shock during continuous venovenous hemofiltration (CVVH), and to assess the mechanisms of elimination of PCT.
Setting
The medical department of intensive care.
Design
A prospective, observational study.
Patients
Thirteen critically ill patients with septic shock and oliguric acute renal failure requiring continuous venovenous postdilution hemofiltration with a high-flux membrane (AN69 or polyamide) and a 'conventional' substitution volume (< 2.5 l/hour).
Measurements and main results
PCT was measured with the Lumitest PCT Brahms® in the prefilter and postfilter plasma, in the ultrafiltrate at the beginning of CVVH (T0) and 15 min (T15'), 60 min (T60') and 6 hours (T6h) after setup of CVVH, and in the prefilter every 24 hours during 4 days. Mass transfer was determined and the clearance and the sieving coefficient were calculated according to the mass conservation principle. Plasma and ultrafiltrate clearances, respectively, at T15', T60' and T6h were 37 ± 8.6 ml/min (not significant) and 1.8 ± 1.7 ml/min (P < 0.01), 34.7 ± 4.1 ml/min (not significant) and 2.3 ± 1.8 ml/min (P < 0.01), and 31.5 ± 7 ml/min (not significant) and 5 ± 2.3 ml/min (P < 0.01). The sieving coefficient significantly increased from 0.07 at T15' to 0.19 at T6h, with no difference according to the nature of the membrane. PCT plasma levels were not significantly modified during the course of CCVH.
Conclusions
We conclude that PCT is removed from the plasma of patients with septic shock during CCVH. Most of the mass is eliminated by convective flow, but adsorption also contributes to elimination during the first hours of CVVH. The effect of PCT removal with a conventional CVVH substitution fluid rate (<2.5 l/hour) on PCT plasma concentration seems to be limited, and PCT remains a useful diagnostic marker in these septic patients. The impact of high-volume hemofiltration on the PCT clearance, the mass transfer and the plasma concentration should be evaluated in further studies.
Similar content being viewed by others
Introduction
Procalcitonin (PCT) is induced in the plasma of patients with sepsis and septic shock, and is a very useful marker to monitor treatment in critically ill patients [1]. This polypeptide of 166 amino acids is specifically increased in generalized bacterial or fungal infections, whereas neither local bacterial or viral infection colonization only leads to a small elevation or no elevation of PCT.
Since the first report of Assicot and colleagues in 1993, numerous studies have confirmed PCT as a very strong marker of inflammation in the fields of infectious diseases, pediatric care and critical care [2]. Measurements of PCT during multiple organ dysfunction syndrome also provide information about the severity and the course of the disease, with an association between PCT concentration and Sepsis-related Organ Failure Assessment (SOFA) or Acute Physiology, Age, Chronic Health Evaluation (APACHE) II scores [3]. An early decline of PCT is observed in patients who recovered and survived, and PCT can also be used as an adequate treatment indicator. This marker of inflammation homeostasis seems more specific and sensitive to monitor septic patients as compared with C-reactive protein, or even cytokines that are not so easy to routinely measure.
The main site of production and the distribution rate of PCT remain unknown, but there is evidence that it may be the leukocytes or neuroendocrine cells in the bronchial epithelium and the liver. Experimental kinetics have demonstrated that the secretion of PCT occurs within less than 4 hours after initiation of sepsis and that PCT is probably stimulated by tumor necrosis factor or IL-6 secretion since these cytokines peak before the appearance of PCT in plasma [4]. After the injection of bacterial endotoxin in healthy subjects, PCT increased by approximately 0.5 ng/ml per hour after a latency of about 2–3 hours, reaching a plateau after 6–12 hours and falling to their baselines values within the following 2 days.
Hoffman and colleagues recently demonstrated that PCT amplifies nitric oxide synthase gene expression and nitric oxide production, which is an explanation for the observed correlation between PCT concentration and the fatal outcome in multiple organ dysfunction syndrome and septic shock [5]. Elimination of PCT is not well known. Like other plasma proteins, PCT is probably degraded by proteolysis. Renal excretion of PCT plays a minor role and there is no accumulation of PCT in cases of patients with severe renal failure. We recently demonstrated in hemodialysis patients that PCT is positively correlated with currently used markers of inflammation such as C-reactive protein and fibrinogen, and it is negatively correlated with markers of nutritional status such as albumin. This relationship remains stable even in patients without infection, with low values of PCT concentration (< 1 ng/ml), suggesting a relationship between inflammation, nutritional status, atherosclerosis and cardiovascular mortality in chronic renal failure [6]. Nevertheless, there is no relation between serum creatinin and plasma PCT concentrations [7].
In critically ill patients, renal failure is an early and frequent complication, occurring in 19% of sepsis cases, 23% of severe sepsis cases and 51% of septic shock cases [8], with renal replacement therapy in 63–75% of the patients [9]. Continuous venovenous hemofiltration (CVVH) is actually the method of choice for renal replacement therapy in critically ill and hemodynamic instable patients. Clinical beneficial effects in septic patients (improved PaO2/FiO2, decreased vasopressor requirements, increased cardiac index) directed the clinicians to a new paradigm in a nonrenal indication and to adjunctive treatment of CVVH in sepsis, leading to more interest in cytokine removal [10]. Little is known about PCT in patients treated with CVVH. This protein of molecular weight (MW) 13,000 Da could be removed from the plasma by convection or adsorption.
The reduction of PCT is known to be associated with a better prognosis. In experiments designed for the immunomodulation hypothesis, Nylen and colleagues observed an increased mortality rate in an animal model of sepsis following intravenous injection of PCT. This was avoided when the animals were pretreated with PCT antiserum [11]. In a septic patient treated with CVVH, therefore, the ideal marker should be obtained with a minimally invasive technique as routine, should reflect the inflammatory status, should distinguish infectious diseases from noninfectious inflammatory diseases, should be without any relation to the acute phase response and biocompatibility of the membrane, and should be correlated to the severity score and prognosis. PCT seems to be this marker. Nevertheless, in the case of alteration of the PCT concentration during renal replacement therapy, diagnostic and therapeutic decisions might be influenced.
The aims of this study were to measure the mass transfer and clearance of PCT during 'conventional' CVVH (substitution < 2.5 l/hour) with a hypermeable membrane in patients with septic shock, and to determine the mechanism of elimination and its impact on the plasma concentration in the course of convective therapy.
Materials and methods
In this prospective study from January 2000 to June 2001, informed written consent was obtained from the relatives of the patients. The inclusion criterion were age >18 years old, septic shock (American College of Chief Physicians [ACCP]/Society of Critical Care Medicine [SCCM] conference consensus) [12], and anuric acute renal failure with or without multiple organ dysfunction syndrome. All the patients were monitored with a Swann–Ganz catheter to optimize the inotropic support and fluid expansion. Bacteriological data were obtained in 72% of the patients in order to prescribe an adequate antibiotherapy in the different etiologies in sepsis (pneumonia, peritonitis, cutaneous infection, intra-abdominal infection or urinary tract infection). All the patients were mechanically ventilated, 60% of them with acute respiratory distress syndrome (PaO2/FiO2 = 137 ± 20 mmHg). No patients were treated with corticosteroids or drotrecogin alpha.
Technique
Blood samples were taken from the prefilter (inlet filter plasma concentration [Ci]) and postfilter (outlet filter plasma concentration [Co]) sites of the extracorporeal circulation. The ultrafiltrate was collected directly from the outlet of the hemofilter (ultrafiltrate concentration [Cuf]). PCT was measured with the Lumitest PCT Kit (Brahms®Diagnostica, Berlin, Germany), an immunoluminometric assay with two specific monoclonal antibodies bound to PCT at two different sites (katacalcin and calcitonin segments). One of the antibodies is luminescence labeled. Immediately after centrifugation, and after 90 min incubation, luminescence was measured in the blood and ultrafiltrate samples. The samples were taken at the beginning of CVVH (T0) and at the following times, according to the kinetics of PCT and to the lowest degree of clotting formation on the dialyzer membrane during these intervals of sampling: T0, Ci; after 15 min (T15'), after 60 min (T60') and after 6 hours (T6h) of CVVH, Ci, Co and Cuf; and after 12 hours, after 24 hours and every 24 hours during 4 days (J1–J4) of CVVH.
In a preliminary study we determined the intra-assay and interassay variation, from 5% to 2.5% for PCT values from 1.3 ng/ml to 66 ng/ml, respectively. The reproducibility was guaranteed for blood samples and also for ultrafiltrate samples. There is no interaction between the PCT dosage and heparin treatment, antibiotics, vasoactive drugs or sedative drugs. The hematocrit before and after the hemofilter was measured to exclude an alteration of the PCT measurement due to the hemoconcentration.
CVVH procedure
Venous access was achieved with a 11–14 Fr double lumen catheter into the internal jugular or femoral vein. The pump-assisted circuit was the Prisma® or the BSM 22 (Hospal SA, Lyon France–Gambro SA, Colombes, France). Postdilutional bicarbonate buffered substitution fluid was used with an ultrafiltration rate of 1.5–2 l/hour, and the net ultrafiltration rate was 100 ml/hour. Blood flow of 150 ml/min was adapted with pressure monitoring. Two high-flux synthetic membranes were used: AN69 M100 (Hospal SA), 0.9 m2 Kuf, 37 ± 7 ml/hour per mmHg; or polyamide Polyflux 14S (Gambro SA), 1.4 m2 Kuf, 50 ml/ hour per mmHg. Nonfractional heparin was used with an initial dose of 400–1000 IU/hour with adaptation of the infusion to the patient and the clotting time.
Calculation
The following formulae were used, according to the mass conservation principle. All flows and clearances are expressed in milliliters per minute.
Total inlet mass (Mi) = inlet plasma flow rate (Qi) × inlet filter plasma concentration (Ci)
Total outlet mass (Mo) = outlet plasma flow rate (Qo) × outlet filter plasma concentration (Co)
Total mass transfer (plasma) (MTp) = Mi – Mo
Total mass transfer (ultrafiltrate) (MTuf) = ultrafiltrate flow rate (Quf) × ultrafiltrate concentration (Cuf)
Ultrafiltrate concentration (Cuf) = Ci × sieving coefficient (SC)
Absorbed mass (Mab) = MTp – MTuf
Plasma clearance (CLp) = MTp / Ci
Ultrafiltrate clearance (CLuf) = MTuf / Ci (equation 1) = Quf × SC (equation 2)
Sieving coefficient (SC) = 2Cuf / [Ci + Co]
Inlet plasma flow rate (Qi) = inlet blood flow (Qb) × (1 – prefilter hematocrit [Ht])
Outlet plasma flow rate (Qo) = Qi – Quf
Statistical analysis
Results are presented as the mean ± standard deviation. Comparisons of measured and calculated data were performed using analysis of variance or the Mann–Witney and Wilcoxon range test as appropriate to their distribution (Statview 5.0®; SAS Institute, Berkeley, CA, USA). The relationships between various parameters were studied by regression analysis with Pearson's correlation matrix, and were calculated using Fischer's z test. The level of significance was given for P < 0.05.
Results
Thirteen patients (nine male, four female) were included in this study. Clinical characteristics and biological data at T0 are reported in Table 1. All patients were anuric. CVVH technical data were an inlet blood flow of 160 ± 46 ml/min, an ultrafiltrate flow rate of 28 ± 2 ml/min and a net ultrafiltration rate of 111 ± 42 ml/hour, with no change for each patient during the course of CVVH. Eight patients were treated with AN69 membrane and five patients with polyamide membrane. Ten patients died, with a significant difference in the Simplified Acute Physiology Score (SAPS) II compared with survivors (63 ± 14 versus 31 ± 29, P < 0.05). The patients selected were critically ill and justly explained this mortality with a high median multiple organ failure syndrome. During the first 24 hours of CVVH, there was no significant change in arterial mean pressure, norepinephrine and dobutamine requirement for all the patients. From J1 to J4, we observed a significant decrease of norepinephrine requirement (1.02 ± 0.47 γ/kg per min versus 0.86 ± 0.47 γ/kg per min, P < 0.05) and a significant increase of mean arterial pressure (76 ± 12 mmHg versus 82 ± 11 mmHg, P < 0.05), but with no relation and change with PCT plasma clearance.
During the follow-up period from T0 to T6h, PCT was detected in the ultrafiltrate in all patients. The plasma clearance was 37.4 ± 8.7 ml/min at T15', with a very low variability from patient to patient and from the PCT plasma concentration. PCT clearance at T15' therefore seems to be linked to the CVVH techniques but not to the inflammatory status. The low variability of these results has also been confirmed at T60' and T6h. There was no significant change in plasma clearance over time: 34.7 ± 4.1 ml/min at T60', and 31.5 ± 7 ml/min at T6h (not significant). The sieving coefficient of PCT was low, 0.07 at T15' and 0.09 at T60', but significantly increased to 0.19 at T6h (P < 0.05). The ultrafiltrate clearance was 1.85 ± 1.7 ml/min at T15', with a significant increase to 2.3 ± 1.8 ml/min at T60' and 5 ± 2.3 ml/min at T6h (P < 0.01). There was no difference for plasma and ultrafiltrate clearance when we compared the AN69 group and the polyamide group during the follow-up period. The calculated adsorbed mass was 38% of the total inlet mass at T15', 31% at T60' and 25% at T6h, with a significant decrease of this ratio during the follow-up period. The results are presented in Table 2.
Discussion
In the present study, we demonstrate that PCT is partially removed from the plasma to the ultrafiltrate, with elimination of a significant mass of this 13,000 Da protein. Plasma clearance was calculated from 37.8 to 30 ml/min without a significant difference from T15' to T6h during CVVH. For all patients, the CVVH procedure was constant. Most of the mass of PCT is eliminated by convective flow, with no doubt that clearance in convective treatment essentially depends on the ultrafiltration and substitution rate. In similar conditions of CVVH (AN69 membrane, 0.9 m2, substitution rate of 2 l/hour), Brunet and coworkers demonstrated that β2-microglobulin (MW 12,000 Da near from PCT) had a clearance value in the same range, confirming the fact that convection is more efficient than diffusion in removing middle molecular weight solutes [13]. Using a substitution rate from 1.5 to 2.5 l/hour, so-called 'conventional' CVVH, one could easily adapt the clearance (ml/min) to the effusion flow (ml/hour) [13].
Our results were in accordance with most of the previous studies on PCT clearance in the same range of ultrafiltration rate. In the study by Meisner and colleagues (26 septic patients, 1.25 m2 polysulfone filter, 1.2 l/hour ultrafiltration rate, 10 ml/min blood flow), the plasmatic clearance was 10 ml/min after 12 hours of CVVH and 17 ml/min after 24 hours of CVVH, with an ultrafiltrate clearance from 2 to 5 ml/min in the same interval [14]. Dahaba and colleagues also found similar results for plasmatic and ultrafiltrate clearance in similar conditions of CVVH [15]. In the present study we used the postdilution technique, which reduces clearances of most solutes by about 15% at an ultrafiltrate flow of 2000 ml/hour [13].
Adsorption to the membrane also contributes to elimination of PCT. This mechanism is major during the first hours of CVVH. The total adsorbed mass/total inlet mass ratios are 38% at T15', 31% at T60' and 25% at T6h (P < 0.01). This observation probably reflects the progressive coating and saturation of the filter in the early phase of CVVH. The sieving coefficients were low (0.07 and 0.09) at T15' and T60', with a significant increase at T6h to 0.19 (P < 0.05). These results are in the expected range described for other solutes of similar MW: M100 [Hospal SA], creatinin (MW 113 Da) = 1, β2-microglobulin (MW 13,000 DA) = 0.65, myoglobin (MW 17,000 Da) = 0.35, IL-1β (MW17,000) = 0.18, tumor necrosis factor alpha, trimeric (MW 54,000 Da) = 0.06, albumin (MW69,000 Da)<0.01; Polyflux 14S [Gambro SA], creatinin = 1, β2-microglobulin = 0.63, myoglobin = 0.3, albumin < 0.01. The sieving coefficient describes the passage of a solute through the membrane, with a maximal value of 1 when the filtration is complete.
Our results thus indicate that about 20% of PCT is removed throughout the membrane. The membrane structure strongly affects the results in convective therapy [16]. Moreover, recent data clearly show that a synthetic membrane appears to confer a significant survival advantage over a cellulose-based membrane [17]. In our study, we used AN69 or polyamide membranes, two synthetic and biocompatible high-flux permeability membranes. The geometry and properties of theses two membrane with a symmetric (AN69) or an asymmetric (polyamide) structure and neutral or negative electric charges easily explain its adsorptive capacity [18]. The electrostatic interaction is also a function of the pH and the flow through the hemofilter. For instance, adsorption of a globally positive molecule such as cytochrome C (MW 12,300 Da) on the AN69 membrane is maximal at pH 7.4 and linearly increases with the wall shear rate and the electrical differential potential [19]. The cut-off of 35–40 kDa allows the filtration of PCT but, according to the European Renal Association guidelines, AN69 is classified as of very high adsorptive capacity while polyamide is defined only as of intermediate adsorptive capacity [20]. In our study, we found no difference in patients treated with the AN69 membrane or with the polyamide membrane.
As shown in Fig. 1, the ultrafiltrate clearance significantly increased while the plasma clearance remained stable and the adsorbed mass/total inlet mass ratio significantly decreased. This relation between the ultrafiltration rate and the plasma clearance has been previously described in animal models of cytokine mass transfer, and has been described with the concept of additional clearance. In this hypothesis, the total clearance increased with ultrafiltration flow but also with a progressive coating and adsorption on the membrane, which is itself optimized with the increase of the substitution volume during the course of CVVH [10]. An increase in the middle MW range solutes clearance has also been demonstrated recently with a reduction of the inner diameter of hollow fibers in the polysulfone hemofilter, so-called 'internal filtration' [21].
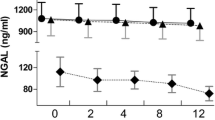
Procalcitonin (PCT) plasma concentration and clearance kinetics during continuous venovenous hemofiltration (CVVH). (a) PCT ultrafiltrate clearances (± standard deviation, ml/min) at 15 min (T15'), 60 min (T60') and 6 hours (T6h) after setup of CVVH (*P < 0.01). (b) PCT plasma clearances (± standard deviation, ml/min) at T15', T60' and T6h (** not significant). (c) PCT plasma concentration (± standard deviation, ng/ml) at the beginning of CVVH (T0) and every 24 hours during 4 days (J1–J4) (*** not significant). Cluf, ultrafiltrate clearance; Clp, plasma clearance.
Figure 1 demonstrates that median plasma levels of PCT were not significantly altered during CVVH in all patients. PCT levels also remained essentially unchanged in blood that was related to the serious illness of the patients. The kinetics of PCT during CVVH, however, is difficult to interpret. The production of PCT essentially depends on the evolution of the sepsis and its response to infectious injury while it does not seem to be induced by an extracorporeal circuit, as has been shown in studies in patients with cardiopulmonary bypass [14]. On the contrary, the observed decrease of PCT plasma concentration is probably more the consequence of an adequate antibiotherapy than of the elimination by CVVH.
Dahada and colleagues differ from us in this opinion and tried to demonstrate a significant decrease of PCT, IL-6 and tumor necrosis factor alpha in septic patients during the first 12 hours of CVVH (AN69 membrane, 0.9 m2, 2 l/hour, 100 ml/min blood flow) [15]. In their study, they proposed to change the hemofilters every 12 hours and explained their efficiency with the very high adsorptive capacity of AN69. From this point of view, a coupled plasma filtration adsorption with a resin or carbon column is another interesting solution [22]. If there is no doubt that cytokines could be removed by CVVH then reports demonstrating a significant fall in the serum levels of inflammatory mediators are scarce, especially during CVVH with a 'conventional' substitution rate (< 2.5 l/hour) [10, 23–27]. The PCT clearance measurement and its impact on plasma concentration should be evaluated in studies with high-volume hemofiltration.
Conclusions
We conclude that PCT is removed from the plasma of patients with septic shock during CCVH. Most of the mass is eliminated by convective flow, but adsorption also contributes to elimination during the first hours of CVVH. The effect of PCT removal with a conventional CVVH substitution fluid rate (< 2.5 l/hour) on the PCT plasma concentration seems to be limited, and PCT remains a useful diagnostic marker in these septic patients. The impact of high-volume hemofiltration on the PCT clearance, the mass transfer and the plasma concentration should be evaluated in further studies.
Key messages
-
PCT is partially removed from the plasma of patients with septic shock during CVVH
-
Sieving coefficients are low, from 0.07 at T15' to 0.19 at T6h after the beginning of CVVH
-
With a conventional substitution fluid rate (≤ 2.5 l/hour), the effect on plasma concentration is limited
-
PCT remains a useful marker in the management of septic patients treated with CVVH
Abbreviations
- Ci:
-
Ci = inlet filter plasma concentration
- Co:
-
Co = outlet filter plasma concentration
- Cuf:
-
Cuf = ultrafiltrate concentration
- CVVH:
-
CVVH = continuous venovenous hemofiltration
- IL:
-
IL = interleukin
- MW:
-
MW = molecular weight
- PCT:
-
PCT = procalcitonin
- T0:
-
T0 = beginning of CVVH
- T15:
-
T15' = after 15 min of CVVH
- T60:
-
T60' = after 60 min of CVVH
- T6h:
-
T6h = after 6 hours of CVVH.
References
Utgarte H, Silva E, Mercan D, De Mendonca A, Vincent JL: Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med 1999, 27: 498-504. 10.1097/00003246-199903000-00024
Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C: High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993, 341: 515-518. 10.1016/0140-6736(93)90277-N
Meisner M, Tschaikowsky K, Palmaers T, Schmidt J: Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit Care 1999, 3: 45-50. 10.1186/cc306
Brunkhorst FM, Heinz U, Forycki ZF: Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med 1998, 24: 888-892. 10.1007/s001340050683
Hoffmann G, Czechowski M, Schoesser M, Schobersberger W: Procalcitonin amplifies inductible nitric oxyde synthase gene expression and nitric oxide production in vascular smooth muscle cells. Crit Care Med 2002, 30: 2091-2095. 10.1097/00003246-200209000-00023
Level C, Chauveau P, Delmas Y, Lasseur C, Pelle G, Peuchant E, Montaudon D, Combe C: Procalcitonin: a new marker of inflammation in haemodialysis patients? Nephrol Dial Transplant 2001, 16: 980-986. 10.1093/ndt/16.5.980
Meisner M, Schmidt J, Hüntter H, Tschaikowsky K: The natural elimination rate of procalcitonin in patients with normal and impaired renal function. Intensive Care Med 2000, 26: S212-S216. 10.1007/s001340051146
Liano F, Junco E, Pascual J, Madero R, Verde E: The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. Kidney Int 1998, 53: 16-24.
Yhijs A, Thijs L: Pathogenis of renal failure in sepsis. Kidney Int 1998, 53: 34-37.
Honoré PM, Jamez J, Wauthier M, Pirenne B, Pelgrim J, Dugernier T: Removal of mediators by hemofiltration in septic shock: where do we stand? Réanim Urgences 2000, 9: 289-297.
Nylen ES, Whang KT, Snider RH, Steinwald P, White J, Becker K: Mortality is increased by procalcitonin and decreased by an antiserum reactive to procalcitonin in experimental sepsis. Crit Care Med 1998, 26: 1001-1006. 10.1097/00003246-199806000-00015
Bone RC, Balk R, Cerra F, Dellinger R, Fein A, Knaus W, Schein R, Sibbald WJ, for the ACCP/SCCM Conference Consensus: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 1992, 101: 1644-1655.
Brunet S, Leblanc M, Geadah D, Parent D, Couteau S, Cardinal J: Diffusive and convective solute clearances during continuous replacement therapy at various dialysate and ultrafiltration flow rates. Am J Kidney Dis 1999, 34: 486-492.
Meisner M, Hüttmann E, Lohs T, Kasakov L, Reinhart K: Plasma concentrations and clearance of procalcitonin during continuous veno-venous hemofiltration in septic patients. Shock 2001, 15: 171-175.
Dahaba A, Elawady G, Rehak P, List W: Procalcitonin and proinflammatory cytokine clearance during continuous venove-nous haemofiltration in septic patients. Anaesth Intensive Care 2002, 30: 269-274.
Matson J, Lee P: Evolving concepts of therapy for sepsis and septic shock and the use of hyperpermeable membranes. Curr Opin Crit Care 2000, 6: 431-436. 10.1097/00075198-200012000-00011
Subramanian S, Venkataraman R, Kellum JA: Influence of dialysis membrane on outcomes in acute renal failure: a meta-analysis. Kidney Int 2002, 62: 1819-1823. 10.1046/j.1523-1755.2002.00608.x
Subramanian S, Kellum JA: Convection or diffusion in continuous renal replacement therapy for sepsis. Curr Opin Crit Care 2000, 6: 426-430. 10.1097/00075198-200012000-00010
Valette P, Thomas M, Déjardin P: Adsorption of low molecular weight proteins to hemodialysis membranes: experimental results and simulations. Biomaterials 1999, 20: 1621-1634. 10.1016/S0142-9612(99)00070-8
European Best Practice Guidelines Expert Group on Haemodialysis: Section III. Biocompatibility. Nephrol Dial Transplant 2002, 17: 32-44.
Ronco C, Brendolan A, Lupi A, Metry G, Levin N: Effects of a reduced inner diameter of hollow fibers in haemodialyzers. Kidney Int 2000, 58: 809-817. 10.1046/j.1523-1755.2000.00230.x
Tetta C, Bellomo R, Brendolan A, Piccini P, Digito A, Dan M, Irone M, Lonneman G, Moscato D, Buades J, Lagreca G, Ronco C: Use of adsorptive mechanisms in continuous renal replacement therapies in the critically ill. Kidney Int 1999, 56: S15-S19.
De Vriese ANS, Colardyn F, Philippé J, Vanholder R, Desutter J, Lameire N: Cytokine removal during continuous hemofiltration in septic patients. J Am Soc Nephrol 1999, 10: 846-853.
Vincent JL, Tielemans C: Continuous hemofiltration in severe sepsis: is it beneficial? J Crit Care 1995, 10: 27-32. 10.1016/0883-9441(95)90028-4
Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccini P, Lagreca P: Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 2000, 356: 26-30. 10.1016/S0140-6736(00)02430-2
Brause M, Neuman A, Schumacher T, Grabense B, Heering P: Effect of filtration volume of continuous veno-venous hemofiltration in the treatment of patients with acute renal failure in intensive care units. Crit Care Med 2003, 31: 841-846. 10.1097/01.CCM.0000054866.45509.D0
Honore PM, Jamez J, Wauthier M, Lee PA, Dugernier T, Pirenne B, Hannique G, Matson JF: Prospective evaluation of short-term, high-volume isovolemic hemofiltration on the hemodynamic course and outcome in patients with intractable circulatory failure resulting from septic shock. Crit Care Med 2000, 28: 3581-3587.
Acknowledgments
The authors thank Miss Béatrice Martin, Miss Alice Steel and Mr David Brittmann for their help reviewing the manuscript, and the nursing staff of the intensive care unit for their assistance during this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Level, C., Chauveau, P., Guisset, O. et al. Mass transfer, clearance and plasma concentration of procalcitonin during continuous venovenous hemofiltration in patients with septic shock and acute oliguric renal failure. Crit Care 7, R160 (2003). https://doi.org/10.1186/cc2372
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc2372