Abstract
Introduction
Severe traumatic brain injury (TBI) in childhood is associated with a high mortality and morbidity. Decompressive craniectomy has regained therapeutic interest during past years; however, treatment guidelines consider it a last resort treatment strategy for use only after failure of conservative therapy.
Patients
We report on the clinical course of six children treated with decompressive craniectomy after TBI at a pediatric intensive care unit. The standard protocol of intensive care treatment included continuous intracranial pressure (ICP) monitoring, sedation and muscle relaxation, normothermia, mild hyperventilation and catecholamines to maintain an adequate cerebral perfusion pressure. Decompressive craniectomy including dura opening was initiated in cases of a sustained increase in ICP > 20 mmHg for > 30 min despite maximally intensified conservative therapy (optimized sedation and ventilation, barbiturates or mannitol).
Results
In all cases, the ICP normalized immediately after craniectomy. At discharge, three children were without disability, two children had a mild arm-focused hemiparesis (one with a verbal impairment), and one child had a spastic hemiparesis and verbal impairment. This spastic hemiparesis improved within 6 months follow-up (no motor deficit, increased muscle tone), and all others remained unchanged.
Conclusion
These observational pilot data indicate feasibility and efficacy of decompressive craniectomy in malignant ICP rise secondary to TBI. Further controlled trials are necessary to evaluate the indication and standardization of early decompressive craniectomy as a 'second tier' standard therapy in pediatric severe head injury.
Similar content being viewed by others
Introduction
Severe traumatic brain injury (TBI) (Glasgow Coma Scale < 8) occurs in 60% of polytraumatized children after car accidents or child abuse, and it is associated with a high mortality and morbidity [1, 2]. The primary therapeutic aim is to maintain an adequate cerebral blood flow (estimated from cerebral perfusion pressure) and brain oxygenation. Intensive care management of severe head injury in cases of refractory intracranial pressure (ICP) is not based on controlled, randomized studies. Studies in adults report more side effects than positive benefits [3].
Decompressive craniectomy has regained some therapeutic interest during the past decade. However, treatment guidelines for traumatic brain injury from German, European (European Brain Injury Consortium [4]) North-American (Brain Trauma Foundation [5]) and international (pediatric neurosurgery) [6] medical societies consider decompressive craniectomy only as last resort treatment strategy after failure of conservative therapy. In the pediatric population, a mere handful of case reports, cohort studies and pilot studies discuss the indication for decompressive craniectomy [7, 8]. We report on the clinical course of six pediatric patients enrolled in a pilot study secondary to decompressive craniectomy after TBI.
Patients
All patients were immediately treated by the medical emergency team at the accident site and transferred to the Pediatric Intensive Care Unit (see Table 1 for details on diagnosis, treatment and follow-up). Early parameters of treatment at admission were transcutaneous oxygen saturation > 92% and an estimated cerebral perfusion pressure of at least 50 mmHg.
Standard protocol of treatment
After emergent clinical evaluation with stabilization of ventilation and hemodynamics, a computed tomography (CT) scan was initiated. Significant traumatic masses were treated surgically on an emergency basis. In all other cases, an external ventriculostomy was performed and/or an ICP monitor was inserted. Insertion of an external ventriculostomy was performed in cases with accessible ventricles on admission for cerebrospinal fluid drainage as required by ICP monitoring. The ICP was monitored continuously by an intraparenchymal probe (MicroSensor; Codman, Johnson & Johnson, Raynham, MA, USA) in all cases. The treatment protocol generally applied was sedation and continuous muscle relaxation, 15–30° elevation of the upper part of the body, normothermia (36–37°C), and mild hyperventilation (pCO2 = 30–35 mmHg). To maintain a sufficient cerebral perfusion pressure (50–60 mmHg; see [9]), all patients received catecholamines (epinephrine and norepinephrine) as needed.
Intensified treatment protocol
Standard therapy was intensified in cases of an ICP increase > 20 mmHg for at least 30 min. Body position, body temperature, blood pressure, fluid management and ventilation as well as analgosedation were evaluated and optimized in order to lower the ICP. In each case of an unexpected and sustained elevation of ICP, a current CT scan was evaluated to rule out new space occupying intracranial lesions [10]. Continuing and sustained deviation of the ICP > 20 mmHg for longer than 30 min was treated by single doses of barbiturate (2–5 mg/kg) and by infusion of mannitol (0.5 g/kg in 15 min). No treatment response within 30 min or even a further increase of ICP lead to immediate surgical treatment (decompressive craniectomy).
Surgical procedure
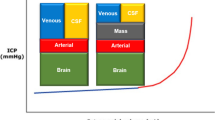
A unilateral or bilateral fronto-temporo-parietal craniectomy was performed depending on the extent and location of the brain swelling. The removed bone flap was stored by kryopreservation until secondary cranioplasty. The dura was opened and enlarged by an autologous galeal flap or by a Goretex patch. In patient 1, dura enlargement was restricted to one side despite bilateral decompression. In patient 6 (cerebellar contusion), a suboccipital craniectomy and duraplasty was performed because of a cerebellar swelling and altered somatosensory evoked potentials (SEP), in addition to a severe head trauma after blunt injury to the craniocervical junction.
Results
Immediate postoperative course
In five out of six patients, the ICP normalized (< 12 mmHg) immediately after craniectomy and no secondary elevation in ICP was noticed. The continuous sedation and muscle relaxation could be tapered and stopped on day 5 or day 6 after surgical decompression.
Special clinical courses
Patient 2 showed a secondary brain swelling with an increase of ICP level intractable to intensified medical treatment on day 4 after unilateral decompression. A craniectomy of the contralateral side was therefore performed, with subsequent normalization of the ICP. Figure 1 presents the CT scan before and Figure 2 after bilateral craniectomy of patient 2.
Complications
There was neither infection nor disturbance of wound healing, nor mortality.
One patient (patient 3) developed a late aseptic necrosis of the replaced bone flap. In this case, a post-traumatic hydrocephalus led to subgaleal cerebrospinal fluid collection with surgical revision and transient insertion of a ventriculo-peritoneal shunt. This might have caused insufficient fixation of the bone flap and a lack of revascularization with subsequent partial necrosis. The shunt was removed 3 months after trauma and the bony defect was covered by an autologous calvarial split graft.
Neurological outcome
The neurological outcomes at discharge and at 6 months follow-up are presented in Table 2. Furthermore, SEP of the median nerve before and after decompressive craniectomy are described in Table 2.
Patient 1 suffered from a severe transitional syndrome after discontinuation of sedation. The neurological status was normal after recovery, in spite of pathological SEP of the median nerve. At discharge from the intensive care unit, patient 2 showed a hemiparesis, predominantly of the left arm, which resolved to normal strength in the following weeks.
None of the patients with severe head injury suffered from post-traumatic seizures or received anticonvulsive medication. Based on findings for SEP of the median nerve, a favorable and stable long-term outcome could be predicted for all of our patients suffering from TBI, confirming previously published data [11]. The SEP 1 week after trauma correlated with the neurological outcome 6 months after trauma, except for patient 1. Mild disturbances of SEP were seen in patient 1, but revealed no deterioration during follow-up.
Discussion
After exclusion or surgical removal of traumatic hematomas and other space occupying lesions, prevention of secondary brain injury is the mainstay of intensive care treatment in pediatric severe head injury. Diffuse brain swelling and multiple cerebral contusions are the most common cause of morbidity and death after severe head injury in pediatric patients [12].
Standardized treatment protocols have been suggested for the management of severe head injury in children [13], including drainage of cerebrospinal fluid, mild hyperventilation (pCO2 lower threshold of 30 mmHg) and mannitol bolus (unless serum osmolality exceeds 320 mosmol/l) as generally accepted baseline therapies for the pediatric population [6]. In cases of sustained elevated ICP (> 20 mmHg) and reduced critically cerebral perfusion pressure (< 50 mmHg), despite optimal medical therapy including controlled hyperventilation, further management using 'second tier' therapy is a matter of controversy [6] and has to follow the different stages of postinjury cerebral insults.
Brain swelling and intracranial hypertension in the early post-traumatic period has been proposed to induced by cerebral hyperemia (i.e. increased cerebral blood flow [CBF]), especially in children [14, 15]. However, the impact of hyperemia on outcome has been rated controversially. Beneficial [16, 17] as well as detrimental effects have been discussed [18].
'Second tier' intensified conservative treatment will have to rely on specific prognostic monitoring parameters. Therefore, CBF-dependent therapy has been studied [19]. But, as cerebral blood flow is age dependent in the unaffected child (normal range from 40 to > 100 ml/100 g/min [20], absolute cerebral hyperemia may only be defined within narrow age ranges [21]. CBF thresholds cannot be taken from adult studies for the initiation of therapeutic interventions in the pediatric population.
Monitoring of cerebral metabolic parameters has been reported for treatment in adult patients. In children, an early decrease in the cerebral metabolic rate of oxygen and the arterio-venous difference for oxygen has been reported to occur 1–3 days after trauma [14]. Recently, Cruz and colleagues [15] predicted clinical outcome based on monitoring of the ICP and the cerebral extraction rate for oxygen (CEO2) in children. In their observational study of 45 children, an increased ICP and a decreased CEO2 indicated cerebral hyperemia during the first 5 days after head injury. An unfavorable outcome occurred in children with higher ICP and lower CEO2 (< 17%). Monitoring of the CEO2 (or oxygen saturation at the jugular vein bulb for hemoglobin > 12 g/l) might therefore be used to direct ventilation and medical therapy in children in the future. However, two out of 45 patients died prior to intended decompressive surgery while being monitored for CEO2, which points towards the need for shortened monitoring intervals and early surgical decompression.
Prolonged barbiturate therapy inherits a high risk of unwanted therapeutic effects, and revealed small benefits in the outcome in children [22]. In a proven state of refractory absolute hyperemia, selective reduction of the CBF by cerebral vasomodulation (dihydroergotamine, metoprolol and clonidin [22], or a monotherapy dihydroergotamine respectively [23]) might be considered, but these treatment options are still not for routine application and require very intensive multimodal monitoring.
Brain edema associated with cerebral ischemia requires optimized cerebral perfusion and fluid management. Experimental medical treatment is proposed to lower the ICP and to reestablish sufficient CBF after failure of mannitol and vasopressors to support sufficient CBF. Hypertonic saline (7.2%) as a bolus or an infusion decreased the ICP in adults and children, and may therefore be indicated preferably in hypovolemia [24–26].
As a surgical 'second tier' option, controlled lumbar drainage of cerebrospinal fluid has been proposed. This regimen necessitates an external ventriculostomy and discernible basal cisterns on CT with careful control of both external drainage systems. In a study cohort of 16 pediatric head injury patients, Levy and colleagues [27] reported good control of refractory intracranial hypertension without drainage-related mortality.
Surgical decompression using craniectomy is largely seen as a last resort therapeutic option. This may be due to disappointment from previous anecdotic results based on late intervention. Encouraging results have been reported from studies in adolescent and adult patients indicating an early time point of decompression as extremely important to achieve a favorable outcome [3, 8, 28].
In addition to the 'optimal' time point for decompression, the extent of brain decompression seems to be important [3]. Restoration of cerebral perfusion by surgical enlargement of the intracranial space is the primary goal of decompression [3]. This may necessitate a large craniotomy with duraplasty. Prospective controlled, randomized studies on the effect of surgical decompression in TBI in childhood are missing. A pilot study by Taylor and colleagues [8] demonstrated an improved neurological outcome of patients who were treated with an early decompressive craniectomy in a cohort of 27 children compared with historical controls. In contrast to our patients, only a small temporal craniectomy without opening the dura was performed. The risk of transtentorial herniation can be lowered in this way, but restoration of the cerebral perfusion can hardly be achieved. However, a benefit from temporal craniectomy without duraplasty has been shown by Taylor and colleagues, which underlines the potential of a larger decompression. Studies in adults demonstrated a greater decrease of the ICP after duraplasty than in cases with craniectomy only [3, 29].
Neither in these studies nor in our cohort was a higher rate of complications such as infections or hygroma noted due to duraplasty. Immediate normalization of the ICP after supratentorial surgical decompression was achieved in all patients from our study cohort. A good neurological outcome was achieved in all our patients suffering from TBI treated with decompressive craniectomy and duraplasty. Due to the early timepoint of decompression after failure of first-line treatment options, unwanted effects of prolonged medical therapy (e.g. barbiturate coma) or brain herniation with secondary brain stem compromise could be prevented, and all children survived.
There currently seems to be no specific treatment regimen in children compared with adults in severe head injury [21], and there is no preference for a special 'second tier' treatment strategy in pediatric head injury [6]. The presented pilot trial adds an additional argument for surgical decompression at an early stage in case of treatment-refractory intracranial hypertension, and calls for a controlled trial that includes this treatment option in pediatric severe head injury patients.
Key messages
-
In case of sustained increase in ICP (>20 mmHg) under intensified conservative therapy conditions and early decompressive craniectomy including duraplasty has to be considered
Conclusion
This pilot trial and the favorable results from the study by Taylor and colleagues [8] demonstrate the necessity of a multicenter, controlled, randomized study to evaluate the indication and standardization of early decompressive craniectomy as a 'second tier' standard therapy in children with severe head injury.
Abbreviations
- CBF:
-
CBF = cerebral blood flow
- CEO:
-
CEO2 = cerebral extraction rate for oxygen
- CT:
-
CT = computed tomography
- ICP:
-
ICP = intracranial pressure
- SEP:
-
SEP = somatosensory evoked potentials
- TBI:
-
TBI = traumatic brain injury.
References
Berger MS, Pitts LH, Lovely M, Edwards MS, Bartkowski HM: Outcome from severe head injury in children and adolescents. J Neurosurg 1985, 62: 194-199.
Marshall LF, Gautille T, Klauber MR, Eisenberg HM, Jane JA, Luerssen TG, Marmarou A, Foulkes MA: The outcome of severe closed head injury. J Neurosurg 1991, 75: 528-536.
Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ: Surgical decompression for traumatic brain swelling: indication and results. J Neurosurg 1999, 90: 187-196.
Maas AI, Dearden M, Teasdale GM, Braakman R, Cohadon F, Jannotti F, Karimi A, Lapierre F, Murray G, Ohmann J, Persson L, Servadei F, Stochetti N, Unterberg A: EBIC-guidelines for management of severe head injury in adults. European Brain Injury consortium. Acta Neurochir 1997, 139: 286-294.
Brain Trauma Foundation, American Association of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care: Guidelines for the management of severe head injury. J Neurotrauma 1996, 13: 641-734.
Rekate HL: Head injuries: management of primary injuries and prevention of secondary damage. Childs Nerv Syst 2000, 17: 632-634. 10.1007/s003810100487
Dam Hieu P, Sizun J, Person H, Besson G: The place of decompressive surgery in the treatment of uncontrollable post-traumatic intracranial hypertension in children. Childs Nerv Syst 1996, 12: 270-275.
Taylor A, Butt W, Rosenfeld J, Shann F, Ditchfield M, Lewis E, Klug G, Wallace D, Henning R, Tibballs J: A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst 2001, 17: 154-162. 10.1007/s003810000410
Downard C, Hulka F, Mullins RJ, Piatt J, Chesnut R, Quint P, Mann N: Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. J Trauma 2000, 49: 654-658.
Bruce DA: Imaging after head trauma: why, when and which. Childs Nerv Syst 2000, 16: 755-759. 10.1007/s003810000336
Carter BG, Taylor A, Butt W: Severe brain injury in children: long-term outcome and its prediction using somatosensory evoked potentials (SEPs). Intensive Care Med 1999, 25: 722-728. 10.1007/s001340050936
Marshall LF, Toole BM, Bowers SA: The national traumatic coma data bank. Part 2: patients who talk and deteriorate: implications for treatment. J Neurosurg 1983, 59: 285-288.
Chiaretti A, Piastra M, Pulitanò S, Pietrini D, De Rosa G, Barbaro R, Di Rocco C: Prognostic factors and outcome of children with severe head injury: an 8-year experience. Childs Nerv Syst 2002, 18: 129-136. 10.1007/s00381-002-0558-3
Sharples PM, Stuart AG, Mathews DSF, Synsley-Green A, Eyre JA: Cerebral blood flow and metabolism in severely head-injured children: Part I – relationship to age, Glascow coma score, outcome, intracranial pressure and time after surgery. J Neurol Neurosurg Psychiatry 1995, 58: 145-152.
Cruz J, Nakayama P, Imamura JH, Rosenfeld KGW, De Souza HS, Giorgetti GVF: Cerebral extraction of oxygen and intracranial hypertension in severe, acute, pediatric brain trauma: preliminary novel management strategies. Neurosurgery 2002, 50: 774-780.
Lang DA, Teasdale GM, Macpherson P, Lawrence A: Diffuse brain swelling after head injury: more often malignant in adults than in children? J Neurosurg 1994, 80: 675-680.
Sakas DE, Bullock MR, Patterson J, Hadley D, Wyper DJ, Teasdale GM: Focal cerebral hyperemia after focal head injury in humans: a benign phenomenon? J Neurosurg 1995, 83: 277-284.
Feickert HJ, Drommer S, Heyer R: Severe head injury in children: impact of risk factors on outcome. J Trauma 1999, 47: 33-38.
Siotos PJ, Orozco JA, Carter LP, Weinand ME, Hamilton AJ, Williams FC: Continuous regional cerebral cortical blood flow monitoring in head-injured patients. Neurosurgery 1995, 36: 943-950.
Susuki K: The changes of regional cerebral blood flow with advancing age in normal children. Nagoya Med J 1990, 34: 159-170.
Zwienenberg M, Muizelaar JP: Severe pediatric head injury: the role of hyperemia revisited. J Neurotrauma 1999, 16: 937-943.
Ecker C, Asgeirsson B, Grände PO, Schalen W, Nordström CH: Improved outcome after severe injury with a new therapy based on principles for brain volume regulation and preserved microcirculation. Crit Care Med 1998, 26: 1881-1886.
Orliaguet GA, Meyer PG, Renier D, Blanot S, Carli PA: Successful treatment of uncontrollable posttraumatic intracranial hypertension with dihydroergotamine in a child. Anesth Analg 1997, 85: 1218-1220.
Simma B, Burger R, Falk M, Sacher P, Fanconi S: A prospective, randomized, and controlled study of fluid management in children with severe head injury. Crit Care Med 1998, 26: 1265-1270. 10.1097/00003246-199807000-00032
Horn P, Munch E, Vajkoczy P, Herrmann P, Qintel M, Schilling L, Schmiedek P, Schurer L: Hypertonic saline solution for control of elevated intracranial pressure in patients with exhausted response to mannitol and barbiturates. Neurol Res 1999, 21: 758-764.
Munar F, Ferrer AM, de Nadal M, Poca MA, Pedraza S, Sahuquillo J, Garnacho A: Cerebral hemodynamic effects of 7.2% hypertonic saline in patients with head injury and raised intracranial pressure. J Neurotrauma 2000, 17: 41-51.
Levy DI, Rekate HL, Cherny B, Manwaring K, Moss D, Baldwin HZ: Controlled lumbar drainage in pediatric head injury. J Neurosurg 1995, 83: 453-460.
Polin RS, Shaffrey ME, Bogaev CA, Tisdale N, Germanson T, Bocchi Jane JA: Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery 1997, 41: 84-92.
Yoo DS, Kim DS, Cho KS, Huh PW, Park CK, Kang JK: Ventrikular pressure monitoring during bilateral decompression with dural expansion. J Neurosurg 1999, 91: 953-959.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ruf, B., Heckmann, M., Schroth, I. et al. Early decompressive craniectomy and duraplasty for refractory intracranial hypertension in children: results of a pilot study. Crit Care 7, R133 (2003). https://doi.org/10.1186/cc2361
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc2361