Abstract
Introduction
The ideal measures to prevent postoperative delirium remain unestablished. We conducted this systematic review and meta-analysis to clarify the significance of potential interventions.
Methods
The PRISMA statement guidelines were followed. Two researchers searched MEDLINE, EMBASE, CINAHL and the Cochrane Library for articles published in English before August 2012. Additional sources included reference lists from reviews and related articles from 'Google Scholar'. Randomized clinical trials (RCTs) on interventions seeking to prevent postoperative delirium in adult patients were included. Data extraction and methodological quality assessment were performed using predefined data fields and scoring system. Meta-analysis was accomplished for studies that used similar strategies. The primary outcome measure was the incidence of postoperative delirium. We further tested whether interventions effective in preventing postoperative delirium shortened the length of hospital stay.
Results
We identified 38 RCTs with interventions ranging from perioperative managements to pharmacological, psychological or multicomponent interventions. Meta-analysis showed dexmedetomidine sedation was associated with less delirium compared to sedation produced by other drugs (two RCTs with 415 patients, pooled risk ratio (RR) = 0.39; 95% confidence interval (CI) = 0.16 to 0.95). Both typical (three RCTs with 965 patients, RR = 0.71; 95% CI = 0.54 to 0.93) and atypical antipsychotics (three RCTs with 627 patients, RR = 0.36; 95% CI = 0.26 to 0.50) decreased delirium occurrence when compared to placebos. Multicomponent interventions (two RCTs with 325 patients, RR = 0.71; 95% CI = 0.58 to 0.86) were effective in preventing delirium. No difference in the incidences of delirium was found between: neuraxial and general anesthesia (four RCTs with 511 patients, RR = 0.99; 95% CI = 0.65 to 1.50); epidural and intravenous analgesia (three RCTs with 167 patients, RR = 0.93; 95% CI = 0.61 to 1.43) or acetylcholinesterase inhibitors and placebo (four RCTs with 242 patients, RR = 0.95; 95% CI = 0.63 to 1.44). Effective prevention of postoperative delirium did not shorten the length of hospital stay (10 RCTs with 1,636 patients, pooled SMD (standard mean difference) = -0.06; 95% CI = -0.16 to 0.04).
Conclusions
The included studies showed great inconsistencies in definition, incidence, severity and duration of postoperative delirium. Meta-analysis supported dexmedetomidine sedation, multicomponent interventions and antipsychotics were useful in preventing postoperative delirium.
Similar content being viewed by others
Introduction
An estimated 36.8% of surgical patients suffer from postoperative delirium [1]. The incidence is much higher in patients 70 years of age and older [2]. Delirium is associated with increased morbidity and mortality [3], prolonged hospital stay and persistent functional and cognitive decline [4]. Postoperative delirium is also a major burden to medical services with costs in US dollars ranging from $38 to $152 billion per year [5].
Prevention may be the most effective strategy for minimizing the occurrence of postoperative delirium and its adverse outcomes but it is untested or unproven. In hospitalized patients, 30 to 40% cases of delirium are thought to be preventable [6, 7]. Multimodal strategies have been used in an effort to counter delirium resulting from diverse causes such as neurotransmitter imbalance, neuroinflammation, pain, infection, metabolic abnormalities and sleep disorders [8, 9]. Widely applicable therapeutic countermeasures for delirium have not yet been discovered. It is not presently clear whether a single intervention for patients with different risk factors is a realistic goal, or whether there is an optimal treatment for specific groups of patients.
The purposes of this study were 1) to critically review available randomized clinical trials (RCTs) that assessed the effects of multiple kinds of interventions to prevent postoperative delirium in adult patients, 2) to determine the efficacy of interventions, and 3) to explore whether interventions successful in preventing postoperative delirium also shortened the length of hospital stay.
Materials and methods
This systematic review and meta-analysis was conducted following the guidelines of the PRISMA statement (Additional file 1) [10, 11].
Search strategy
We conducted a literature search of MEDLINE, EMBASE, CINAHL and the Cochrane Library databases for articles published in English before August, 2012. Search key words were delirium (including delirium, confusion, acute confusional state or acute confusional syndrome) and postoperative (including postoperative, operation, surgery, anaesthesia or anesthesia). We only searched articles reporting results from adult patients. Case reports were excluded from our primary search. The search strategy we used for MEDLINE was as follows: 1) delirium; 2) deliri*; 3) confusion; 4) acute confusional state; 5) acute confusional syndrome; 6) postoperative; 7) operation*; 8) surgery; 9) surgical; 10) anaesthesia; 11) anesthesia; 12) 1 OR 2 OR 3 OR 4 OR 5; 13) 6 OR 7 OR 8 OR 9 OR 10 OR 11; 14) 12 OR 13; 15) 'English' (Language); 16) 14 AND 15; 17) 'case reports' (Publication Type); 18) 16 NOT 17; 19) 'Adult' (Mesh); 20) 18 AND 19. Additional studies were identified by reviewing the reference lists of reviews and meta-analyses and searching the related articles of identified studies using 'Google Scholar'.
Study selection
The initial search returned 2,813 articles. After title and abstract review, 198 potential articles with full texts were further independently reviewed by two coauthors (HZ and YL) to determine the eligibility according to the predefined selection and exclusion criteria. Disagreements between reviewers were resolved by including another coauthor (XS). Completed studies that met all the following criteria were considered eligible for inclusion in the systematic review and meta-analysis: 1) RCTs assessing interventions to prevent postoperative delirium; 2) delirium identified by validated methods including the Diagnostic and Statistical Manual of Mental Disorders, 1987 (DSM-III), DSM-III-R (1994), DSM-IV (1999), the 10th revision of the International Statistical Classification of Diseases and Related Health Problems, 1992 (ICD-10), and clinical diagnostic tools based on these such as the Confusion Assessment Method (CAM), Delirium Rating Scale (DRS) and NEECHAM Confusion Scale [12]; 3) incidence, severity and duration of delirium analyzed independently of other neurologic events such as emergence delirium and dementia. Research articles were excluded if they recruited 1) patients with delirium before surgery; 2) patients with alcohol withdrawal syndrome; 3) groups that also included nonsurgical patients (for example patients in the intensive care unit or ward without surgery); 4) homogeneous populations of patients with certain central nervous system diseases or mental disorders (for example stroke, dementia, schizophrenia and depression).
Data extraction
Data extraction was completed by two coauthors (ML and ZZ) using a predesigned piloted data extraction form. Disagreements were resolved by the third coauthor (XS) consultation. The following study characteristics were collected: primary author, publication year, country of origin, PubMed identifier (if possible), types of surgery, participant characteristics (gender, age, number, existing illness, inclusion and exclusion criteria), intervention (type, dosage, duration and frequency), criteria for delirium, incidence, severity and duration of delirium, P value, duration and frequency of follow-up and the length of hospital stay. Dichotomous data were converted to incidences for data synthesis and continuous data were recorded using mean and standard deviation (SD).
Quality scoring of included trials
The validity and quality of included trials was evaluated independently by two coauthors (FX and LW) using a scoring system (Table 1) that combined the modified Jadad scale [13] and the delirium-specific score we developed for the current study. The quality review system included eight items with a maximal score of 12. Studies with a score ≤ 5 were arbitrarily defined as low-quality studies with high risk of within-study bias. We designed this delirium-specific scoring system because postoperative delirium is defined subjectively with validated methods such as DSM-IV and ICD-10, has certain risk factors (for example age, sex, comorbidities and medications) and mostly occurs 24 to 72 hours after surgery [1, 12, 14–16]. Disagreements were resolved by including a third author (XS) for discussion. Studies were not excluded or weighted based on quality scores in the meta-analysis.
Data analysis
The analyses focused on the incidence of postoperative delirium as the primary outcome measure. We further tested the hypothesis that interventions reducing postoperative delirium would shorten the length of hospital stay. Only studies reporting significant differences in the incidences of postoperative delirium between two interventions (P < 0.05, two-tailed) were included. Placebo and control procedures were also considered as interventions when a study aimed to compare the effects between interventions and placebos. Interventions were divided into two groups (interventions with less delirium and interventions with more delirium) and the length of hospital stay was synthesized for comparison.
Meta-analysis was performed when two or more than two studies using similar interventions were identified. Statistical analysis was performed using STATA 11 (StataCorp, College Station, TX, USA). A test for heterogeneity was performed using a standard chi-square (χ2) and I-square (I2) statistic. Significant heterogeneity was considered present at χ2 P < 0.10 or I2 > 50%. Where no heterogeneity was found, a fixed-effects parametric approach (weighted with inverse variance) was taken. Otherwise a random-effects model was used. For the incidence of postoperative delirium, both pooled relative risk (RR) and incidence with 95% confidence intervals (CI) were calculated. Sample size calculations of different interventions (n1 = n2, α = 0.05 and β = 0.1, two-tailed) were performed based on reported or pooled incidences. For the length of hospital stay, SMD (standard mean difference) was used due to that there was a big intertrial difference. We intended to conduct a subgroup analysis, where possible, to explore 1) the effects of different interventions, or 2) the effects of single intervention in patients with different surgeries. Publication bias was assessed by visually inspecting funnel plot and Begg's test. Meta-regression was performed to help investigate the origin of heterogeneity. For all the analyses, a P value of less than 0.05 (two-tailed) was considered statistically significant.
Results
Study selection
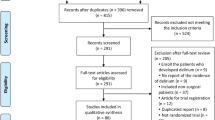
The process of literature identification, screening and selection is summarized by Figure 1. Our primary search yielded 2,813 articles. After screening, 198 studies potentially met the inclusion criteria. After examining the full texts, 160 articles were excluded: 25 studies were not clinical trials; three studies had no control group; 40 studies did not include postoperative delirium as a study variable; three studies tested the diagnosis methods of delirium; 47 studies did not screen postoperative delirium using validated tools; seven studies recruited both surgical and nonsurgical patients; three studies did not provide the delirium data; 11 studies included patients with delirium prior to surgery; 12 studies included homogeneous patients with brain diseases, mental disorders or alcohol withdrawal syndrome; seven studies described ongoing trials; one study was retracted, and one study was identified as a duplicated publication. We ultimately included 38 RCTs [17–54] in our systematic review and meta-analysis.
Study characteristics
The characteristics of 38 included studies were listed in Table 2 and Table 3. These single-centered studies [17–54] included from 11 [38] to 457 [54] patients. The earliest study was published in 1987 [17]. The average ages of the participants were all above 60 except two studies by Leung [33] and Maldonado [41]. Two studies included patients only at high risk for delirium [29, 43]. One study included patients only with subsyndromal delirium [52]. The surgery types included orthopedic (n = 18) [17, 19, 20, 23, 25, 27, 29, 30, 33, 35, 37, 39, 42, 43, 45, 47, 48, 53], cardiovascular (n = 9) [18, 21, 36, 40, 41, 44, 46, 51, 52], abdominal (n = 7) [22, 24, 26, 28, 31, 32, 50], noncardiovascular (n = 2) [34, 54], and thoracic (n = 2) surgeries [38, 49]. Interventions could be divided into two categories. Category 1, perioperative procedures and drugs (n = 18, Table 2) including controlled hypotension [23], anesthesia [17, 20, 27, 28, 31, 34, 40, 47, 51], analgesia [19, 24, 32, 43, 53] and sedation [41, 44, 46]. Category 2, pharmacological, psychological or multicomponent interventions (n = 20, Table 3) including acetylcholinesterase inhibitors [30, 37, 39, 48], antipsychotics [22, 29, 36, 45, 52, 54], anticonvulsants [33, 50], sleep restoration by drug [26] or bright light [38, 49], psychological intervention [18], music [42], multicomponent interventions [25, 35] and histamine H2 receptor blockers [21]. Thirty-six studies [17–41, 43–49, 51–54] reported incidences of postoperative delirium. The reported incidences ranged from 0 [18, 28, 33] to 75.3% [35]. Inpatient time was reported in 22 studies [18, 20, 21, 24–26, 29, 30, 32, 34–37, 39–41, 44, 47, 49, 51, 52, 54]. The duration of postoperative delirium was reported in 10 studies [25, 29, 30, 35, 41, 43–45, 47, 52] and the severity of delirium was reported in 11 studies [25, 28, 29, 38, 42, 43, 45, 46, 48, 50, 53].
Quality scores of included studies
The scores of included studies were shown in Table 4. The scores ranged from 3 [18] to 12 [36, 52, 54]. The average score was 8.3 with a standard deviation of 2.2. A score lower than 6 was found in four studies [17, 18, 21, 22]. Three studies got the full score 12 [36, 52, 54].
Quantitative review and meta-analysis
Category 1. Perioperative procedures and drugs (Table 2)
1.1 Controlled hypotension
Williams-Russo et al. [23] tested the effects of induced hypotension by epidural anesthesia on delirium in patients accepting hip replacement surgery. Intraoperative mean arterial blood pressure (MAP) was maintained in the range of 45 to 55 (n = 117) or 55 to 70 mmHg (n = 118). They found no difference in the incidences of postoperative delirium (8.5% vs. 4.2%; MAP 45 to 55 vs. MAP 55 to 70, P = 0.167). Power calculations suggested that 675 patients per group would be needed to observe a significant difference in delirium occurrence based on the reported incidences but this study included a total of 235 patients.
1.2 Neuraxial anesthesia versus general anesthesia
We identified four studies with 511 patients [17, 20, 27, 31] that compared the effects of different anesthesia methods on postoperative delirium. Meta-analysis using a fixed-effects model (χ2(3) = 4, P = 0.261, I2 = 25%) revealed no difference between neuraxial and general anesthesia (pooled RR = 0.99, 95% CI = 0.65 to 1.50, P = 0.962, Figure 2A). The pooled incidences based on a random-effects model were 17.1% (95% CI = 7.8% to 37.8%) for neuraxial anesthesia and 17.1% (95% CI = 9.3% to 31.4%) for general anesthesia.
1.3 Sedation depth during spinal anesthesia
Sieber et al. [47] tested whether patients receiving deep sedation during spinal anesthesia would suffer from more postoperative delirium. Bispectral index (BIS) was kept at approximately 50 in the deep sedation group (n = 57) and at 80 or higher in the light sedation group (n = 57) during surgery. The study showed an increased incidence of postoperative delirium (40.4% vs. 19.3%; deep sedation vs. light sedation, P = 0.014) and a significant longer duration of delirium (1.4 ± 4.0 vs. 0.5 ± 1.5 days; deep sedation vs. light sedation, P = 0.01) in the deep-sedated patients.
1.4 General anesthetics
Patients receiving propofol for general anesthesia showed higher Delirium Rating Scale (DRS) scores compared to patients receiving sevoflurane (6 ± 3 vs. 2 ± 1, P = 0.002) in the study by Nishikawa et al. [28]. There was no difference in the incidences of postoperative delirium (16% vs. 0, propofol vs. sevoflurane, P = 0.110). Power calculations suggested that 58 patients per group would be needed to achieve a significant difference in the incidences of delirium but the current study only included 25 patients per group.
Royse et al. [51] tested the influence of either propofol or desflurane on the incidence of postoperative delirium in patients undergoing coronary artery bypass surgery. Seven of 89 patients receiving propofol and 12 of 92 patients receiving desflurane developed delirium. No difference was found in incidence between the two groups (7.9% vs. 13.2%, propofol vs. desflurane, P = 0.245). Power calculations suggested that 732 patients per group would be needed to observe a significant difference in delirium occurrence based on the reported incidences but a total of 171 patients were enrolled in the study.
Less postoperative delirium was found in patients receiving additional ketamine (0.5 mg/kg intravenously, single bolus) for anesthesia induction compared to standard methods (3.45% vs. 31.03%; ketamine vs. standard, P = 0.012). Hudetz et al. [40] recruited 29 patients undergoing cardiac surgery per group for this study.
Leung et al. [34] found no additional effect of N2O on the development of postoperative delirium compared to standard anesthesia in older patients undergoing noncardiac surgery. Forty-four of 105 patients (41.9%) exposed to additional N2O and 46 of 105 patients (43.8%) receiving standard anesthesia developed delirium. Power calculations suggested that 14,524 patients per group would be needed to get a difference in delirium occurrence based on the reported incidences.
1.5 Epidural analgesia versus intravenous analgesia
Three RCTs with 167 patients [19, 24, 32] tested whether epidural analgesia was superior to intravenous analgesia in preventing postoperative delirium in older patients undergoing major orthopedic and abdominal surgeries. Meta-analysis using a fixed-effects model (χ2(2) = 0.14, P = 0.932, I2 = 0) found no difference between epidural and intravenous analgesia (pooled RR = 0.93, 95% CI = 0.61 to 1.43, P = 0.751, Figure 2B). The pooled incidences utilizing a fixed-effects model were 33.4% (95% CI = 23.8% to 47.0%) for epidural analgesia and 36.7% (95% CI = 26.6% to 50.4%) for intravenous analgesia. Power calculations suggested that 4,391 patients per group would be needed to observe a significant difference in delirium occurrence based on the pooled incidences but a total of 167 patients were recruited in the identified three trials.
1.6 Additional fascia iliaca compartment block
Mouzopoulos et al. [43] investigated the effects of additional fascia iliaca compartment block (FICB, 0.25% bupivacaine 0.3 mL/kg) on postoperative delirium in hip surgery patients who were at intermediate or high risk for delirium. Patients included had to have at least one of the four predictive risk factors (severity of illness, cognitive impairment, index of dehydration and visual impairment) as described by Inouye et al. [55, 56]. There were 102 patients receiving additional FICB plus standard analgesia and 105 patients receiving standard analgesia only. The FICB prophylaxis group showed decreased incidence (10.8% vs. 23.8%; additional FICB vs. standard, P < 0.001), reduced severity (DRS scale, 14.34 ± 3.6 vs. 18.61 ± 3.4; additional FICB vs. standard, P < 0.001) and shortened duration of delirium (5.22 ± 4.28 vs. 10.97 ± 7.16 days, additional FICB vs. standard, P < 0.001). The study was accompanied with insufficient allocation concealment, blinding and no intention-to-treat (ITT) analysis.
1.7 Long-acting morphine
Musclow et al. [53] reported increased severity of postoperative delirium using the NEECHAM scale in patients receiving long-acting morphine (28.70 ± 1.82 vs. 29.14 ± 0.61; morphine vs. placebo, P = 0.02) which was administered at an oral dose of 30 mg, twice daily for three days. There was no difference in the incidences of delirium (10.3% vs. 3.4%; morphine vs. placebo, P = 0.082). Power calculations suggested that 524 patients would be needed to observe a difference in delirium occurrence based on the reported incidences but this study enrolled 190 patients.
1.8 Postoperative sedation using alpha-2 adrenoreceptor agonists
Three RCTs with 445 patients [41, 44, 46] tested whether alpha-2 adrenoreceptor agonists (dexmedetomidine and clonidine) were superior to other sedatives in preventing postoperative delirium in patients undergoing cardiovascular surgery. Meta-analysis using a random-effects model (χ2(2) = 5.71, P = 0.057, I2 = 65) found no difference between alpha-2 adrenoreceptor agonists and other sedatives (pooled RR = 0.55, 95% CI = 0.23 to 1.28, P = 0.163, Figure 2C). The pooled incidences utilizing a fixed-effects model were 15.2% (95% CI = 5.3% to 43.6%) for alpha-2 adrenoreceptor agonists and 25.1% (95% CI = 10.1% to 62.1%) for other sedatives. Power calculations suggested that 686 patients would be needed to get a difference in delirium occurrence based on the pooled incidences but a total of 445 patients were included. Subgroup analysis found that dexmedetomidine was more effective than other sedatives in preventing postoperative delirium (pooled RR = 0.39, 95% CI = 0.16 to 0.95, P = 0.039). Besides the effects on the incidences of delirium, Maldonado et al. [41] found that dexmedetomidine (loading dose: 0.4 μg/kg, maintenance drip of 0.2 to 0.7 μg/kg/hour) had no effect on the duration of delirium in patients with delirium (2.0 ± 0 vs. 3.0 ± 3.1 vs. 5.4 ± 6.6 days; dexmedetomidine vs. propofol vs. midazolam, P = 0.82). Shehabi et al. [44] found dexmedetomidine (0.1 to 0.7 μg/kg/hour) was superior to propofol in shortening the duration of delirium (2 ± 4 vs. 5 ± 8 days; dexmedetomidine vs. propofol, P = 0.032). Rubino et al. [46] found supplemental clonidine (loading dose: 0.5 μg/kg, maintenance drip of 1 to 2 μg/kg/hour) was able to reduce the severity of delirium (DDS, 0.6 ± 0.7 vs. 1.8 ± 0.8, additional clonidine vs. standard, P < 0.001).
Category 2. Pharmacological, psychological or multicomponent interventions (Table 3)
2.1 Acetylcholinesterase inhibitors
Four RCTs with 242 patients tested whether elevating brain acetylcholine levels by acetylcholinesterase inhibitors (AchEI) would be helpful for preventing postoperative delirium in patients accepting major orthopedic surgeries [30, 37, 39, 48]. Three studies used oral donepezil (5 mg/day, 4 to 30 days) [30, 37, 48] and one study used oral rivastigmine (4.5 mg/day, 7 days) [39]. Meta-analysis using fixed-effects model (χ2(3) = 2.83, P = 0.419, I2 = 0) found no difference between the two groups on the incidences of postoperative delirium (pooled RR = 0.95, 95% CI = 0.63 to 1.44, P = 0.825, Figure 3A). The pooled incidences utilizing fixed-effects model were 28.5% (95% CI = 20.6% to 39.5%) for patients taking acetylcholinesterase inhibitors and 36.1% (95% CI = 26.7% to 48.7%) for patients taking placebos. Power calculations suggested that 794 patients per group would be needed to observe a significant difference in delirium occurrence based on the pooled incidences and 121 patients per group in the existing four studies were included. Besides no effects on the incidences of delirium, Liptzin et al. [30] found that donepezil failed to shorten the duration of delirium (1 ± 0 vs. 1.3 ± 1.2 days, donepezil vs. placebo, P = 0.12). Marcantonio et al. [48] reported that donepezil did not reduce the severity of delirium (Memorial Delirium Assessment Scale (MDAS) changes,1.3 ± 2.5 vs. 1.6 ± 5.2, donepezil vs. placebo, P = 0.91) but only 16 patients were included in the study.
2.2 Antipsychotics
We identified six trials with 1,592 patients which tested the role of antipsychotics on preventing postoperative delirium. Three trials used the typical antipsychotic haloperidol [22, 29, 54]. The doses varied from 1.5 mg/day to 5 mg/day with a duration of one to five days. The other three trials used atypical antipsychotics risperidone and olanzapine [36, 45, 52]. Risperidone was given sublingually once after surgery at a dose of 1 mg in the study by Prakanrattana et al. [36]. Hakim et al. [52] recruited patients with subsyndromal delirium only and risperidone was continually given orally at a dose of 1 mg/day until 24 hours after subsidence of subsyndromal delirium or a score of more than 3 on the Intensive Care Delirium Screening Checklist (ICDSC) was obtained. Oral olanzapine was given at a dose of 5 mg just before and after surgery in the study by Larsen et al. [45]. Meta-analysis using a random-effects model (χ2(5) = 13.82, P = 0.017, I2 = 63.8) found a significant difference between antipsychotics and placebo (pooled RR = 0.50, 95% CI = 0.34 to 0.73, P = 0.000, Figure 3B). Meta-regression showed that the heterogeneity came from the kind of antipsychotics used (typical or atypical; REML estimate of between-study variance = 0, proportion of between-study variance explained = 100%). Subgroup analysis suggested that both typical and atypical antipsychotics were able to prevent postoperative delirium (RR = 0.71, 95% CI = 0.54 to 0.93 for typical antipsychotics and RR = 0.36, 95% CI = 0.26 to 0.50 for atypical antipsychotics). Indirect comparison using ITC tools [57] found a superior role of atypical antipsychotics in preventing delirium compared to typical antipsychotics (estimated RR = 1.95, 95% CI = 1.28 to 2.96, P = 0.072). The pooled incidences utilizing a fixed-effects model were 14.5% (95% CI = 12.1% to 17.3%) for patients receiving antipsychotics and 28.4% (95% CI = 21.0% to 38.5%) for patients taking placebo. Besides the effects on the incidences of delirium, Kalisvaart et al. [29] found that haloperidol reduced the severity (DRS, 14.40 ± 3.5 vs. 18.41 ± 4.4, haloperidol vs. placebo, P < 0.001) and shortened the duration of delirium (5.41 ± 4.91 vs. 11.85 ± 7.56 days, haloperidol vs. placebo, P < 0.001) in patients suffering from delirium. Larsen et al. [45] reported that olanzapine increased the severity (DRS), 16.44 ± 3.7 vs. 14.5 ± 2.7, haloperidol vs. placebo, P = 0.02) and duration of delirium (2.2 ± 1.3 vs. 1.6 ± 0.7 days, haloperidol vs. placebo, P = 0.02). Hakim et al. [52] found that risperidone had no effect on the duration of delirium in patients with postoperative delirium (3 ± 1.5 vs. 3 ± 0.8 days, risperidone vs. placebo, P = 0.669).
2.3 Anticonvulsants
Leung et al. [33] tested whether oral gabapentin (900 mg/day, for four days) was helpful in preventing postoperative delirium in older patients undergoing spine surgery. Delirium was identified in none of the nine patients receiving gabapentin and in five of the twelve patients receiving placebo (P = 0.045). Pesonen et al. [50] randomly assigned oral pregabalin (150 mg/day, for six days, n = 35) or placebo (n = 35) to patients accepting cardiac surgery. Their study found that pregabalin was able to reduce the severity of delirium (CAM-ICU, 24 ± 8 vs. 21 ± 19, P = 0.04).
2.4 Sleep restoration using diazepam, flunitrazepam and pethidine
Aizawa et al. [26] tested whether restoring sleep-wake cycle with medications after surgery was useful to prevent postoperative delirium. The researchers randomly divided 40 patients accepting major abdominal surgeries into two groups. The experimental group (n = 20) received standard treatment plus diazepam/flunitrazepam/pethidine (DFP) for three days to improve sleep disorders and the control group underwent standard treatment (n = 20). Less delirium was developed in the DFP group (5% vs. 35%, DFP vs. standard, P = 0.023).
2.5 Sleep restoration using bright light
We identified two studies [38, 49] with 33 patients that tested the hypothesis that improving the sleep-wake cycle using bright light (two hours per day in the morning, 2500 to 5000 lx) would be useful to prevent delirium. Meta-analysis using a fixed-effects model (χ2(1) = 0.15, P = 0.703, I2 = 0) found no difference between bright light and control (pooled RR = 0.30, 95% CI = 0.07 to 1.26, P = 0.099). The pooled incidence utilizing a fixed-effects model were 13.0% (95% CI = 2.1% to 78.8%) for bright light and 41.3% (95% CI = 20.9% to 81.5%) for control. Power calculations suggested that 50 patients per group would be needed to get a significant difference in delirium occurrence based on the pooled incidences but only a total of 33 patients were included in the two trials. Besides the effects on the incidences of delirium, Taguchi et al. [38] found bright light therapy reduced the severity of delirium (NEECHAM, 6.7 ± 0.7 vs. 21.1 ± 7, bright light vs. standard, P = 0.014).
2.6 Psychological intervention
Schindler et al. [18] detected the role of active daily psychological intervention on postoperative delirium in patients undergoing cardiac surgery. No difference was found on the incidences of delirium between the two groups (0 vs. 12.5%; psychological intervention vs. standard, P = 0.227). Power calculations suggested that a total of 154 patients were needed to observe a difference in delirium occurrence based on the reported incidences but the study included only 33 patients.
2.7 Music
McCaffrey et al. [42] recruited 22 patients (11 patients per group) and evaluated the effects of music on delirium prevention. The patients in the music group received standard hospital care plus listening to soothing lullaby music at least four times a day for one hour. Delirium severity was detected using NEECHAM confusion scale on each of the first three postoperative days. They found that listening to music decreased the severity of delirium (NEECHAM, 24 ± 0.97 vs. 22.5 ± 1.22, music vs. standard, P = 0.000).
2.8 Multicomponent interventions
Multicomponent interventions that combined both pharmacological and non-pharmacological strategies were performed to prevent postoperative delirium in two RCTs with 325 patients [25, 35]. Meta-analysis using a fixed-effects model (χ2(1) = 0.55, P = 0.608, I2 = 0) found that multicomponent interventions decreased the incidence of postoperative delirium (pooled RR = 0.71, 95% CI = 0.58 to 0.86, P = 0.000). The pooled incidences were 43.3% for multicomponent interventions and 62.4% for standard treatment. In addition, Marcantonio et al. [25] found multicomponent interventions reduced the number of patients with severe delirium (7/62 (11.3%) vs. 18/64 (28.1%); multicomponent interventions vs. standard, P = 0.02) and had no effect on the duration of delirium in patients suffering from delirium (2.9 ± 2 vs. 3.1 ± 2.3 days; multicomponent interventions vs. standard, P = 0.73). Lundstrom et al. [35] reported that multicomponent interventions shortened the duration of delirium (5.0 ± 7.1 vs. 10.2 ± 13.3 days; multicomponent interventions vs. standard, P = 0.009).
2.9 Histamine H2 receptor blockers
Kim et al. [21] found no difference in the incidences of postoperative delirium between cimetidine and ranitidine in postoperative cardiac surgical patients. The incidences were close (24.5% vs. 25.9%, cimetidine vs. ranitidine, P = 0.872). More than 20,000 patients for each group would be needed to observe a significant difference in the incidences of delirium and a total of 111 patients were included in the study.
Interventions effective in preventing postoperative delirium did not shorten the length of hospital stay
We identified 10 studies with 1,636 patients reporting both different incidences of postoperative delirium between the two interventions and inpatient time [25, 26, 35, 36, 40, 41, 44, 47, 52, 54]. Meta-analysis using a fixed-effects model (χ2(9) = 12.1, P = 0.208, I2 = 25.6%) found no significant difference in the length of hospital stay between interventions with lower or higher incidences of postoperative delirium (pooled SMD = -0.06, 95% CI = -0.16 to 0.04, P = 0.159, Figure 4A). The pooled incidences based on the fixed-effects model were 16.1% for interventions with less delirium and 35.4% for interventions with more delirium. No significant publication bias was found by Begg's test (z = 0.54, P > |z| = 0.592) and by visual inspection of the funnel plot (Figure 4B).
Interventions successful in preventing postoperative delirium failed to shorten the length of hospital stay. (A) Summary standard mean differences (SMDs) for the length of hospital stay between interventions with less delirium and interventions with more delirium. (B) Begg's funnel plot with effect measures (SMD) as a function of its standard error (SE) for the length of hospital stay.
Discussion
Summary of evidence
The main findings to emerge from this systematic review and meta-analysis include: 1) There was a huge heterogeneity of interventions among trials. Most of the interventions suffered from only a handful of studies and small sample sizes. Some studies suffered from methodological defects and were at high risk of bias [17, 18, 21, 22]. These shortcomings disallowed sufficient interpretation of the effectiveness of interventions. 2) The meta-analysis showed that dexmedetomidine sedation, multicomponent interventions and antipsychotics were useful in preventing postoperative delirium. 3) Based on the result and quality of individual study, it appeared that light sedation during spinal anesthesia, additional ketamine during anesthesia induction, additional fascia iliaca compartment block, anticonvulsants and sleep restoration using diazepam/flunitrazepam/pethidine were useful in preventing postoperative delirium. 4) The meta-analysis found that interventions useful for preventing postoperative delirium did not shorten the patients' time spent in the hospital. Table 5 provides a summary of the efficacy of interventions for diminishing delirium.
Consistent with our results, Lin et al. [58, 59] found that dexmedetomidine sedation was inversely related with the incidence of delirium in patients with cardiac surgery. Contrarily, Tan et al. [60] concluded that the use of dexmedetomidine in critically ill adult patients had no effect on delirium in their meta-analysis. Besides the different doses and durations of dexmedetomidine use, patients with different illnesses might develop delirium due to different reasons [61], which might help explain the discrepancy.
Lonergan et al. [62] found that both typical antipsychotics (haloperidol) and atypical antipsychotics (olanzapine, risperidone and quetiapine) were effective in treating delirium in their meta-analysis. These results were consistent with the current meta-analysis which tested the role of antipsychotics on delirium prevention. Studies by Devlin et al. [63, 64] and Skrobik et al. [65] also found a positive role of quetiapine and olanzapine in treating delirium in critically ill patients. Campbell et al. [66] found no superiority for second-generation antipsychotics over haloperidol in managing delirium. Devlin et al. [67] had critically reviewed six studies [54, 65, 68–71] which used haloperidol to prevent or treat delirium in noncritically or critically ill patients. Only the study by Wang et al. [54] showed that low-dose haloperidol reduced the incidence of delirium compared to placebo. The inconsistent results of haloperidol might be due to the following reasons: 1) There was a great heterogeneity of the patient populations among the six studies [54, 65, 68–71]. These studies included patients with severe illnesses (AIDS and cancer) and patients accepting different surgeries or critically ill patients. 2) The comparator of haloperidol was different among studies. Girard el al. [71] used both atypical antipsychotics and placebo as the control. Wang et al. [54] used placebo as the control. Chlorpromazine and lorazepam were used as the control for haloperidol in the study by Breitbart et al. [68]. Another three studies [65, 69, 70] used atypical antipsychotics (olanzapine or risperidone or ziprasidone) as the control. 3) The dosage and duration of haloperidol differed greatly among studies.
In support of the National Institute for Health and Clinical Excellence guidelines recommending an individual multicomponent intervention package aiming to prevent delirium [72, 73], two studies included in our meta-analysis supported multicomponent interventions as a useful way to prevent postoperative delirium [25, 35]. A recent study also showed a 30% reduction of delirium by multimodal geriatric consultation versus usual care in older adults with recent hip fracture [74]. Additional similar studies are being performed, which might add evidence to the finding [75–77].
Our meta-analysis data found no difference in the length of patient hospital stay between interventions with higher incidences of delirium (pooled incidence, 35.4%) and interventions with lower incidences (pooled incidence,16.1%). This finding was contrary to results from previous observational studies which showed that patients with postoperative delirium stayed longer in the hospital [4, 78–80]. Resolving these differences is important as prolonged hospital stay is a heavy burden on the health care system [5] and should also be included as an important clinical outcome during delirium prevention [67]. However, as only 21 of the 38 included trials reported the inpatient time, there was a potential publication bias. Furthermore, our meta-analysis included heterogeneous studies with huge differences in both the incidences of postoperative delirium and the time of hospital stay. Further clinical trials with homogenous patients receiving similar interventions might help to clarify this issue.
Limitations
This systematic review and meta-analysis had several limitations. 1) We included different types of surgeries for a single intervention and this possibly affected the heterogeneity. 2) Multiple methods and different frequencies of postoperative delirium screening across the studies were another source of heterogeneity [1]. 3) For the same intervention, the dose and duration varied greatly among studies and this might account for different effects [70]. 4) Application of the scoring system developed for this study revealed methodological defects in some studies. These defects added a degree of uncertainty to the present results. For example, given that delirium is a multifactorial disorder, similar baseline data were essential when comparing the effects of two interventions. However, 18 [17–19, 21, 25, 27, 28, 30, 32, 35, 37, 38, 42, 45, 46, 48, 49, 53] of the 38 studies did not adjust the risk factors before grouping. 5) Publication bias might account for some of the effects reported here. Most of the included studies were small-sampled single-centered studies with less methodological rigor than large-sampled studies. This factor might contribute to an overestimation of effect sizes in small trials. 6) We excluded homogeneous populations of patients with dementia in our study [81, 82]. In addition, only two studies [25, 35] stated that they included a small subpopulation of patients with dementia. Considering the high morbidity of dementia in the adults and the overlap of dementia with, and contribution to, delirium [3, 83–85], we have excluded a large group of patients who were susceptible to postoperative delirium. This exclusion should be seen as a source of potential selection bias and could limit the interpretation of our findings.
Future directions
Our review raised several questions that need to be addressed in future studies: 1) There were three types of postoperative delirium: hyperactive (25%), hypoactive (50%) and mixed (25%) delirium which had different causes and consequences [1, 16, 86]. However, none of the existing studies tried to distinguish them or tested the specific effects of interventions. Future studies should include screening tools such as the Richmond Agitation-Sedation Scale (RASS) to classify the subtypes of delirium [87] and test their reactions to various interventions. 2) The severity and duration of delirium needed more attention. Moreover, the severity and duration of delirium should be averaged for all patients but not only for patients with delirium. 3) High-risk and low-risk patients might show different sensitivity to precipitating factors and interventions. Thus, there is a need for future studies that stratify high-risk patients and low-risk patients in delirium assessment. We identified only two studies stratifying the risk of delirium [29, 43]. One study only included patients with subsyndromal delirium [52]. Further studies using valid risk-stratifying tools for delirium [12] can make a contribution to this important clinical problem.
Conclusions
Heterogeneity and small sample sizes precluded conclusions regarding the interventions that are likely to prevent postoperative delirium. The limited data suggested that the efficacious way to prevent postoperative delirium included dexmedetomidine sedation, multicomponent interventions and antipsychotics comprising haloperidol, olanzapine and risperidone. Anesthesia types and analgesia methods had no bearing on delirium. Acetylcholinesterase inhibitors were ineffective in preventing delirium. Interventions effective in preventing postoperative delirium did not shorten the length of hospital stay. Considered together, these findings suggested an urgent need for high-quality large-scale RCTs.
Key messages
-
Multiple strategies including perioperative management procedures, pharmacological and nonpharmacological interventions have been used in an effort to prevent postoperative delirium.
-
There is a consensus in the data that dexmedetomidine sedation, multicomponent interventions and antipsychotics are useful in preventing postoperative delirium.
-
Anesthesia types and analgesia methods have no bearing on postoperative delirium.
-
Acetylcholinesterase inhibitors are ineffective in preventing postoperative delirium.
-
Reduced postoperative delirium is not related with shortened hospital stay.
Abbreviations
- AchEI:
-
acetylcholinesterase inhibitors
- BIS:
-
bispectral index
- CAM:
-
Confusion Assessment Method
- CI:
-
confidence interval
- DDS:
-
Delirium Detection Score
- DFP:
-
diazepam/flunitrazepam/pethidine
- DRS:
-
Delirium Rating Scale
- DSI:
-
Delirium Symptom Interview
- DSM:
-
Diagnostic and Statistical Manual of Mental Disorders
- FICB:
-
fascia iliaca compartment block
- ICD:
-
International Statistical Classification of Diseases and Related Health Problems
- ICDSC:
-
Intensive Care Delirium Screening Checklist
- ICU:
-
intensive care unit
- ITT:
-
intention-to-treat
- MAP:
-
mean arterial blood pressure
- MDAS:
-
Memorial Delirium Assessment Scale
- N/A:
-
not available
- NS:
-
not significant
- OBS:
-
organic brain syndrome
- POD:
-
postoperative day
- pre-:
-
preoperative day
- RASS:
-
Richmond Agitation-Sedation Scale: RCT: randomized controlled trial
- RR:
-
risk ratio
- SD:
-
standard definition
- SE:
-
standard error
- SMD:
-
standard mean difference.
References
McDaniel M, Brudney C: Postoperative delirium: etiology and management. Current opinion in critical care 2012, 18: 372-376. 10.1097/MCC.0b013e3283557211
Marcantonio ER, Goldman L, Mangione CM, Ludwig LE, Muraca B, Haslauer CM, Donaldson MC, Whittemore AD, Sugarbaker DJ, Poss R, Hass S, Cook EF, Orav EJ, Lee TH: A clinical prediction rule for delirium after elective noncardiac surgery. JAMA 1994, 271: 134-139. 10.1001/jama.1994.03510260066030
Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA: Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010, 304: 443-451. 10.1001/jama.2010.1013
Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN: Cognitive trajectories after postoperative delirium. The New England journal of medicine 2012, 367: 30-39. 10.1056/NEJMoa1112923
Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK: One-year health care costs associated with delirium in the elderly population. Archives of internal medicine 2008, 168: 27-32. 10.1001/archinternmed.2007.4
Fong TG, Tulebaev SR, Inouye SK: Delirium in elderly adults: diagnosis, prevention and treatment. Nature reviews Neurology 2009, 5: 210-220. 10.1038/nrneurol.2009.24
Siddiqi N, House AO, Holmes JD: Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age and ageing 2006, 35: 350-364. 10.1093/ageing/afl005
Chaput AJ, Bryson GL: Postoperative delirium: risk factors and management: continuing professional development. Canadian journal of anaesthesia 2012, 59: 304-320. 10.1007/s12630-011-9658-4
Skrobik Y: Delirium prevention and treatment. Anesthesiology clinics 2011, 29: 721-727. 10.1016/j.anclin.2011.09.010
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D: The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009, 339: b2700. 10.1136/bmj.b2700
Moher D, Liberati A, Tetzlaff J, Altman DG: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009, 339: b2535. 10.1136/bmj.b2535
Steiner LA: Postoperative delirium. part 2: detection, prevention and treatment. European journal of anaesthesiology 2011, 28: 723-732. 10.1097/EJA.0b013e328349b7db
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ: Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled clinical trials 1996, 17: 1-12. 10.1016/0197-2456(95)00134-4
Mangnall LT, Gallagher R, Stein-Parbury J: Postoperative delirium after colorectal surgery in older patients. American journal of critical care 2011, 20: 45-55. 10.4037/ajcc2010902
Whitlock EL, Vannucci A, Avidan MS: Postoperative delirium. Minerva anestesiologica 2011, 77: 448-456.
Allen SR, Frankel HL: Postoperative complications: delirium. The Surgical clinics of North America 2012, 92: 409-431. x 10.1016/j.suc.2012.01.012
Berggren D, Gustafson Y, Eriksson B, Bucht G, Hansson LI, Reiz S, Winblad B: Postoperative confusion after anesthesia in elderly patients with femoral neck fractures. Anesthesia and analgesia 1987, 66: 497-504.
Schindler BA, Shook J, Schwartz GM: Beneficial effects of psychiatric intervention on recovery after coronary artery bypass graft surgery. General hospital psychiatry 1989, 11: 358-364. 10.1016/0163-8343(89)90124-2
Williams-Russo P, Urquhart BL, Sharrock NE, Charlson ME: Post-operative delirium: predictors and prognosis in elderly orthopedic patients. Journal of the American Geriatrics Society 1992, 40: 759-767.
Williams-Russo P, Sharrock NE, Mattis S, Szatrowski TP, Charlson ME: Cognitive effects after epidural vs general anesthesia in older adults. A randomized trial. JAMA 1995, 274: 44-50. 10.1001/jama.1995.03530010058035
Kim KY, McCartney JR, Kaye W, Boland RJ, Niaura R: The effect of cimetidine and ranitidine on cognitive function in postoperative cardiac surgical patients. International journal of psychiatry in medicine 1996, 26: 295-307. 10.2190/CBUA-RL4V-5UN8-MWJ3
Kaneko T: Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta medica 1999, 42: 179-184.
Williams-Russo P, Sharrock NE, Mattis S, Liguori GA, Mancuso C, Peterson MG, Hollenberg J, Ranawat C, Salvati E, Sculco T: Randomized trial of hypotensive epidural anesthesia in older adults. Anesthesiology 1999, 91: 926-935. 10.1097/00000542-199910000-00011
Mann C, Pouzeratte Y, Boccara G, Peccoux C, Vergne C, Brunat G, Domergue J, Millat B, Colson P: Comparison of intravenous or epidural patient-controlled analgesia in the elderly after major abdominal surgery. Anesthesiology 2000, 92: 433-441. 10.1097/00000542-200002000-00025
Marcantonio ER, Flacker JM, Wright RJ, Resnick NM: Reducing delirium after hip fracture: a randomized trial. Journal of the American Geriatrics Society 2001, 49: 516-522. 10.1046/j.1532-5415.2001.49108.x
Aizawa K, Kanai T, Saikawa Y, Takabayashi T, Kawano Y, Miyazawa N, Yamamoto T: A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery. Surgery today 2002, 32: 310-314. 10.1007/s005950200044
Kudoh A, Takase H, Takazawa T: A comparison of anesthetic quality in propofol-spinal anesthesia and propofol-fentanyl anesthesia for total knee arthroplasty in elderly patients. Journal of clinical anesthesia 2004, 16: 405-410. 10.1016/j.jclinane.2003.10.003
Nishikawa K, Nakayama M, Omote K, Namiki A: Recovery characteristics and post-operative delirium after long-duration laparoscope-assisted surgery in elderly patients: propofol-based vs. sevoflurane-based anesthesia. Acta anaesthesiologica Scandinavica 2004, 48: 162-168. 10.1111/j.0001-5172.2004.00264.x
Kalisvaart KJ, de Jonghe JF, Bogaards MJ, Vreeswijk R, Egberts TC, Burger BJ, Eikelenboom P, van Gool WA: Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. Journal of the American Geriatrics Society 2005, 53: 1658-1666. 10.1111/j.1532-5415.2005.53503.x
Liptzin B, Laki A, Garb JL, Fingeroth R, Krushell R: Donepezil in the prevention and treatment of post-surgical delirium. The American journal of geriatric psychiatry 2005, 13: 1100-1106.
Papaioannou A, Fraidakis O, Michaloudis D, Balalis C, Askitopoulou H: The impact of the type of anaesthesia on cognitive status and delirium during the first postoperative days in elderly patients. European journal of anaesthesiology 2005, 22: 492-499. 10.1017/S0265021505000840
Beaussier M, Weickmans H, Parc Y, Delpierre E, Camus Y, Funck-Brentano C, Schiffer E, Delva E, Lienhart A: Postoperative analgesia and recovery course after major colorectal surgery in elderly patients: a randomized comparison between intrathecal morphine and intravenous PCA morphine. Regional anesthesia and pain medicine 2006, 31: 531-538.
Leung JM, Sands LP, Rico M, Petersen KL, Rowbotham MC, Dahl JB, Ames C, Chou D, Weinstein P: Pilot clinical trial of gabapentin to decrease postoperative delirium in older patients. Neurology 2006, 67: 1251-1253. 10.1212/01.wnl.0000233831.87781.a9
Leung JM, Sands LP, Vaurio LE, Wang Y: Nitrous oxide does not change the incidence of postoperative delirium or cognitive decline in elderly surgical patients. British journal of anaesthesia 2006, 96: 754-760. 10.1093/bja/ael106
Lundstrom M, Olofsson B, Stenvall M, Karlsson S, Nyberg L, Englund U, Borssen B, Svensson O, Gustafson Y: Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging clinical and experimental research 2007, 19: 178-186.
Prakanrattana U, Prapaitrakool S: Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesthesia and intensive care 2007, 35: 714-719.
Sampson EL, Raven PR, Ndhlovu PN, Vallance A, Garlick N, Watts J, Blanchard MR, Bruce A, Blizard R, Ritchie CW: A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. International journal of geriatric psychiatry 2007, 22: 343-349. 10.1002/gps.1679
Taguchi T, Yano M, Kido Y: Influence of bright light therapy on postoperative patients: a pilot study. Intensive & critical care nursing 2007, 23: 289-297. 10.1016/j.iccn.2007.04.004
Gamberini M, Bolliger D, Lurati Buse GA, Burkhart CS, Grapow M, Gagneux A, Filipovic M, Seeberger MD, Pargger H, Siegemund M, Carrel T, Seiler WO, Berres M, Strebel SP, Monsch AU, Steiner LA: Rivastigmine for the prevention of postoperative delirium in elderly patients undergoing elective cardiac surgery--a randomized controlled trial. Critical care medicine 2009, 37: 1762-1768. 10.1097/CCM.0b013e31819da780
Hudetz JA, Patterson KM, Iqbal Z, Gandhi SD, Byrne AJ, Hudetz AG, Warltier DC, Pagel PS: Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. Journal of cardiothoracic and vascular anesthesia 2009, 23: 651-657. 10.1053/j.jvca.2008.12.021
Maldonado JR, Wysong A, van der Starre PJ, Block T, Miller C, Reitz BA: Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics 2009, 50: 206-217. 10.1176/appi.psy.50.3.206
McCaffrey R: The effect of music on acute confusion in older adults after hip or knee surgery. Applied nursing research 2009, 22: 107-112. 10.1016/j.apnr.2007.06.004
Mouzopoulos G, Vasiliadis G, Lasanianos N, Nikolaras G, Morakis E, Kaminaris M: Fascia iliaca block prophylaxis for hip fracture patients at risk for delirium: a randomized placebo-controlled study. Journal of orthopaedics and traumatology 2009, 10: 127-133. 10.1007/s10195-009-0062-6
Shehabi Y, Grant P, Wolfenden H, Hammond N, Bass F, Campbell M, Chen J: Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study). Anesthesiology 2009, 111: 1075-1084. 10.1097/ALN.0b013e3181b6a783
Larsen KA, Kelly SE, Stern TA, Bode RH Jr, Price LL, Hunter DJ, Gulczynski D, Bierbaum BE, Sweeney GA, Hoikala KA, Cotter JJ, Potter AW: Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics 2010, 51: 409-418.
Rubino AS, Onorati F, Caroleo S, Galato E, Nucera S, Amantea B, Santini F, Renzulli A: Impact of clonidine administration on delirium and related respiratory weaning after surgical correction of acute type-A aortic dissection: results of a pilot study. Interactive cardiovascular and thoracic surgery 2010, 10: 58-62. 10.1510/icvts.2009.217562
Sieber FE, Zakriya KJ, Gottschalk A, Blute MR, Lee HB, Rosenberg PB, Mears SC: Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clinic proceedings Mayo Clinic 2010, 85: 18-26. 10.4065/mcp.2009.0469
Marcantonio ER, Palihnich K, Appleton P, Davis RB: Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. Journal of the American Geriatrics Society 2011, Suppl 2: S282-288.
Ono H, Taguchi T, Kido Y, Fujino Y, Doki Y: The usefulness of bright light therapy for patients after oesophagectomy. Intensive & critical care nursing 2011, 27: 158-166. 10.1016/j.iccn.2011.03.003
Pesonen A, Suojaranta-Ylinen R, Hammaren E, Kontinen VK, Raivio P, Tarkkila P, Rosenberg PH: Pregabalin has an opioid-sparing effect in elderly patients after cardiac surgery: a randomized placebo-controlled trial. British journal of anaesthesia 2011, 106: 873-881. 10.1093/bja/aer083
Royse CF, Andrews DT, Newman SN, Stygall J, Williams Z, Pang J, Royse AG: The influence of propofol or desflurane on postoperative cognitive dysfunction in patients undergoing coronary artery bypass surgery. Anaesthesia 2011, 66: 455-464. 10.1111/j.1365-2044.2011.06704.x
Hakim SM, Othman AI, Naoum DO: Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology 2012, 116: 987-997. 10.1097/ALN.0b013e31825153cc
Musclow SL, Bowers T, Vo H, Glube M, Nguyen T: Long-acting morphine following hip or knee replacement: a randomized, double-blind and placebo-controlled trial. Pain research & management 2012, 17: 83-88.
Wang W, Li HL, Wang DX, Zhu X, Li SL, Yao GQ, Chen KS, Gu XE, Zhu SN: Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial*. Critical care medicine 2012, 40: 731-739. 10.1097/CCM.0b013e3182376e4f
Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME: A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Annals of internal medicine 1993, 119: 474-481.
Inouye SK, Charpentier PA: Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 1996, 275: 852-857. 10.1001/jama.1996.03530350034031
Wells GA, Sultan SA, Chen L, Khan M, D C: Indirect treatment comparison [computer program]. Version 2.0. Ottawa: Canadian Agency for Drugs and Technologies in Health;http://www.cadthca/en/resources/about-this-guide/download-software2009 Available from: []
Lin YY, He B, Chen J, Wang ZN: Can dexmedetomidine be a safe and efficacious sedative agent in post-cardiac surgery patients? a meta-analysis. Critical care 2012, 16: R169. 10.1186/cc11646
Lin Y, Chen J, Wang Z: Meta-analysis of factors which influence delirium following cardiac surgery. Journal of cardiac surgery 2012, 27: 481-492. 10.1111/j.1540-8191.2012.01472.x
Tan JA, Ho KM: Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive care medicine 2010, 36: 926-939. 10.1007/s00134-010-1877-6
Devlin JW, Skrobik Y: Antipsychotics for the prevention and treatment of delirium in the intensive care unit: what is their role? Harvard Review of Psychiatry 2011, 19: 59-67. 10.3109/10673229.2011.565247
Lonergan E, Britton AM, Luxenberg J, Wyller T: Antipsychotics for delirium. Cochrane database of systematic reviews 2007, (2):CD005594.
Devlin JW, Roberts RJ, Fong JJ, Skrobik Y, Riker RR, Hill NS, Robbins T, Garpestad E: Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Critical care medicine 2010, 38: 419-427. 10.1097/CCM.0b013e3181b9e302
Devlin J, Skrobik Y, Riker R, Hinderleider E, Roberts R, Fong J, Ruthazer R, Hill N, Garpestad E: Impact of quetiapine on resolution of individual delirium symptoms in critically ill patients with delirium: a post-hoc analysis of a double-blind, randomized, placebo-controlled study. Critical care 2011, 15: R215. 10.1186/cc10450
Skrobik YK, Bergeron N, Dumont M, Gottfried SB: Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive care medicine 2004, 30: 444-449. 10.1007/s00134-003-2117-0
Campbell N, Boustani MA, Ayub A, Fox GC, Munger SL, Ott C, Guzman O, Farber M, Ademuyiwa A, Singh R: Pharmacological management of delirium in hospitalized adults-a systematic evidence review. Journal of general internal medicine 2009, 24: 848-853. 10.1007/s11606-009-0996-7
Devlin JW, Al-Qadhee NS, Skrobik Y: Pharmacologic prevention and treatment of delirium in critically ill and non-critically ill hospitalised patients: a review of data from prospective, randomised studies. Best practice & research Clinical anaesthesiology 2012, 26: 289-309. 10.1016/j.bpa.2012.07.005
Breitbart W, Marotta R, Platt MM, Weisman H, Derevenco M, Grau C, Corbera K, Raymond S, Lund S, Jacobson P: A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. The American journal of psychiatry 1996, 153: 231-237.
Han CS, Kim YK: A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics 2004, 45: 297-301. 10.1016/S0033-3182(04)70170-X
Grover S, Kumar V, Chakrabarti S: Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. Journal of psychosomatic research 2011, 71: 277-281. 10.1016/j.jpsychores.2011.01.019
Girard TD, Pandharipande PP, Carson SS, Schmidt GA, Wright PE, Canonico AE, Pun BT, Thompson JL, Shintani AK, Meltzer HY, Bernard GR, Dittus RS, Ely EW, for the MIND Trial Investigators: Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Critical care medicine 2010, 38: 428-437. 10.1097/CCM.0b013e3181c58715
O'Mahony R, Murthy L, Akunne A, Young J, Guideline Development G: Synopsis of the National Institute for Health and Clinical Excellence guideline for prevention of delirium. Annals of internal medicine 2011, 154: 746-751.
Popp J, Arlt S: Prevention and treatment options for postoperative delirium in the elderly. Current opinion in psychiatry 2012, 25: 515-521. 10.1097/YCO.0b013e328357f51c
Deschodt M, Braes T, Flamaing J, Detroyer E, Broos P, Haentjens P, Boonen S, Milisen K: Preventing delirium in older adults with recent hip fracture through multidisciplinary geriatric consultation. Journal of the American Geriatrics Society 2012, 60: 733-739. 10.1111/j.1532-5415.2012.03899.x
Mouchoux C, Rippert P, Duclos A, Fassier T, Bonnefoy M, Comte B, Heitz D, Colin C, Krolak-Salmon P: Impact of a multifaceted program to prevent postoperative delirium in the elderly: the CONFUCIUS stepped wedge protocol. BMC geriatrics 2011, 11: 25. 10.1186/1471-2318-11-25
Saltvedt I, Prestmo A, Einarsen E, Johnsen LG, Helbostad JL, Sletvold O: Development and delivery of patient treatment in the Trondheim Hip Fracture Trial. A new geriatric in-hospital pathway for elderly patients with hip fracture. BMC research notes 2012, 5: 355. 10.1186/1756-0500-5-355
Wyller TB, Watne LO, Torbergsen A, Engedal K, Frihagen F, Juliebo V, Saltvedt I, Skovlund E, Raeder J, Conroy S: The effect of a pre- and post-operative orthogeriatric service on cognitive function in patients with hip fracture. The protocol of the Oslo Orthogeriatrics Trial. BMC geriatrics 2012, 12: 36. 10.1186/1471-2318-12-36
Edelstein DM, Aharonoff GB, Karp A, Capla EL, Zuckerman JD, Koval KJ: Effect of postoperative delirium on outcome after hip fracture. Clinical orthopaedics and related research 2004, 422: 195-200.
Gonzalez M, Martinez G, Calderon J, Villarroel L, Yuri F, Rojas C, Jeria A, Valdivia G, Marin PP, Carrasco M: Impact of delirium on short-term mortality in elderly inpatients: a prospective cohort study. Psychosomatics 2009, 50: 234-238. 10.1176/appi.psy.50.3.234
Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M: Postoperative delirium in the elderly: risk factors and outcomes. Annals of surgery 2009, 249: 173-178. 10.1097/SLA.0b013e31818e4776
Boustani MA, Campbell NL, Khan BA, Abernathy G, Zawahiri M, Campbell T, Tricker J, Hui SL, Buckley JD, Perkins AJ, Farber MO, Callahan CM: Enhancing care for hospitalized older adults with cognitive impairment: a randomized controlled trial. Journal of general internal medicine 2012, 27: 561-567. 10.1007/s11606-012-1994-8
Stenvall M, Berggren M, Lundstrom M, Gustafson Y, Olofsson B: A multidisciplinary intervention program improved the outcome after hip fracture for people with dementia--subgroup analyses of a randomized controlled trial. Archives of gerontology and geriatrics 2012, 54: e284-289. 10.1016/j.archger.2011.08.013
Holtta E, Laakkonen ML, Laurila JV, Strandberg TE, Tilvis R, Kautiainen H, Pitkala KH: The overlap of delirium with neuropsychiatric symptoms among patients with dementia. The American journal of geriatric psychiatry 2011, 19: 1034-1041. 10.1097/JGP.0b013e31820dcbb6
Landreville P, Voyer P, Carmichael PH: Relationship between delirium and behavioral symptoms of dementia. International psychogeriatrics/IPA 2013, 25: 635-643. 10.1017/S1041610212002232
Silverstein JH, Deiner SG: Perioperative delirium and its relationship to dementia. Progress in neuro-psychopharmacology & biological psychiatry 2012, 43C: 108-115.
Steiner LA: Postoperative delirium. Part 1: pathophysiology and risk factors. European journal of anaesthesiology 2011, 28: 628-636. 10.1097/EJA.0b013e328349b7f5
Robinson TN, Raeburn CD, Tran ZV, Brenner LA, Moss M: Motor subtypes of postoperative delirium in older adults. Archives of surgery (Chicago, Ill: 1960) 2011, 146: 295-300. 10.1001/archsurg.2011.14
Acknowledgements
We thank Professor Ralph Lydic (Deparment of Anesthesiology, University of Michigan, Ann Arbor) for suggestions and help regarding the writing in English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Dr. Xue-Yin Shi receives grants from the National Natural Science Foundation of China (No. 81070880), the 12th Five-Year Key Project of PLA (No. BWS12J027) and Key Basic Research Projects of the Science and Technology Commission of Shanghai Municipality, China (No. 12JC1410902). The three grants financed this article. Hao Zhang, Yan Lu, Meng Liu, Zui Zou, Long Wang and Feng-Ying Xu declared no competing interests.
Authors' contributions
HZ, YL and XS conceived and designed the study. HZ and YL carried out the literature search. ML, ZZ and XS carried out the data extraction. FX, LW and XS carried out the quality assessment. HZ and YL analyzed and interpreted the data. HZ, YL and XS prepared and revised the manuscript. All authors have read and approved the manuscript.
Hao Zhang, Yan Lu contributed equally to this work.
Electronic supplementary material
13054_2012_1691_MOESM1_ESM.DOC
Additional file 1: PRISMA Checklist. This file contains a table of the PRISMA 2009 Checklist in which we checked and noted what had been done according to the guidelines of the PRISMA statement for the current systematic review and meta-analysis. (DOC 66 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Zhang, H., Lu, Y., Liu, M. et al. Strategies for prevention of postoperative delirium: a systematic review and meta-analysis of randomized trials. Crit Care 17, R47 (2013). https://doi.org/10.1186/cc12566
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc12566