Abstract
Introduction
Hyperglycemia, hypoglycemia, and increased glycemic variability have each beenindependently associated with increased risk of mortality in critically illpatients. The role of diabetic status on modulating the relation of these threedomains of glycemic control with mortality remains uncertain. The purpose of thisinvestigation was to determine how diabetic status affects the relation ofhyperglycemia, hypoglycemia, and increased glycemic variability with the risk ofmortality in critically ill patients.
Methods
This is a retrospective analysis of prospectively collected data involving 44,964patients admitted to 23 intensive care units (ICUs) from nine countries, betweenFebruary 2001 and May 2012. We analyzed mean blood glucose concentration (BG),coefficient of variation (CV), and minimal BG and created multivariable models toanalyze their independent association with mortality. Patients were stratifiedaccording to the diagnosis of diabetes.
Results
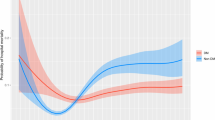
Among patients without diabetes, mean BG bands between 80 and 140 mg/dl wereindependently associated with decreased risk of mortality, and mean BG bands> 140 mg/dl, with increased risk of mortality. Among patients withdiabetes, mean BG from 80 to 110 mg/dl was associated with increased risk ofmortality and mean BG from 110 to 180 mg/dl with decreased risk of mortality. Aneffect of center was noted on the relation between mean BG and mortality.Hypoglycemia, defined as minimum BG <70 mg/dl, was independently associatedwith increased risk of mortality among patients with and without diabetes andincreased glycemic variability, defined as CV > 20%, was independentlyassociated with increased risk of mortality only among patients without diabetes.Derangements of more than one domain of glycemic control had a cumulativeassociation with mortality, especially for patients without diabetes.
Conclusions
Although hyperglycemia, hypoglycemia, and increased glycemic variability is eachindependently associated with mortality in critically ill patients, diabeticstatus modulates these relations in clinically important ways. Our findingssuggest that patients with diabetes may benefit from higher glucose target rangesthan will those without diabetes. Additionally, hypoglycemia is independentlyassociated with increased risk of mortality regardless of the patient's diabeticstatus, and increased glycemic variability is independently associated withincreased risk of mortality among patients without diabetes.
See related commentary by Krinsley,http://ccforum.com/content/17/2/131
See related commentary by Finfer and Billot,http://ccforum.com/content/17/2/134
Similar content being viewed by others
Introduction
Stress-induced hyperglycemia during intensive care unit (ICU) admission has a strong andconsistent relation with mortality [1–3]. Nevertheless, hyperglycemia in these populations of patients was not alwaystreated with insulin infusion until the publication of a landmark single-center study in2001 [4]. This trial demonstrated reductions in mortality when continuous intravenousinsulin was used to achieve blood glucose (BG) from 80 to 110 mg/dl, compared withconventional therapy. Although these findings were corroborated in a large single-centercohort study [5], they were not confirmed by subsequent randomized trials [6–10].
One possible explanation for the divergent results among such trials may relate to theincidence of severe hypoglycemia sustained by patients in the interventional arms ofrandomized trials [6–11]. Data from observational [12–17] and interventional studies [4, 6, 11] demonstrated a strong and independent relation between hypoglycemia andmortality, even at milder thresholds, such as BG <70 mg/dl. Glycemic variability, notconsidered in the design or implementation of these trials, has also been independentlyassociated with mortality in observational [18–24] and prospective [25] investigations. These findings have led to the emergence of the concept thatthree domains of glycemic control in the critically ill (hyperglycemia, hypoglycemia,and glycemic variability [26, 27]) must be addressed to optimize glycemic control.
These factors, however, may not apply to all patients and, in particular, to those withthe diagnosis of diabetes, presumably related to adaptive mechanisms developed in thesetting of chronic hyperglycemia [28]. Observational cohort studies demonstrated that the relation betweenhyperglycemia and mortality is much stronger among patients without diabetes than inthose with diabetes [3, 29–31], and other observational data suggested that diabetes is not independentlyassociated with increased risk of mortality and may actually have a modest protectiveeffect [32–36].
The purpose of this study was to assess how diabetic status modulates the relation ofthe three domains of glycemic control to mortality in a large and diverse group ofcritically ill patients. We hypothesized that an association would exist betweenmortality and each of the three domains of glycemic control, but that a premorbiddiagnosis of diabetes would attenuate the strength of these associations compared withthose observed in patients without diabetes.
Materials and methods
Patient cohorts and clinical settings
Table 1 provides an overview of the nine different patientcohorts (Amsterdam (AM), Austin (AU), BayCare (BC), Birmingham (BI), Geelong (GE),Okayama (OK), Stamford (ST), Tufts (TU), and Vienna (VI)), the organizationalstructure of the ICUs, and the glycemic-control practices of the differentcenters.
Outcomes
The primary end point for this analysis was all-cause hospital mortality, defined asdeath before hospital discharge.
Definitions and statistical analysis
Patients were classified as having preexisting diabetes by documentation in theirmedical records. Disease severity was assessed by using APACHE II scores [37]. Descriptive statistics were calculated for all variables of interest.Continuous variables were summarized by using means and standard deviations, whereascategoric variables were summarized by using counts and percentages.
The primary outcome, mortality, was assessed in relation to the glycemic-controlmetric and control variables by using a logistic regression model adjusting forcorrelation among observations taken at the same center (that is, a generalizedestimating equation (GEE) model. Three models were run, one for each glycemicmeasure: hyperglycemia, hypoglycemia, and glycemic variability. The models included avariable denoting diabetic status, the glycemic measure, and the key interaction termof diabetic status and glycemic measure. Each model controlled for mean BG, age,APACHE II score, mechanical ventilation, ICU length of stay (LOS), as well asadjusting for center effects. The models on hyperglycemia and glycemic variabilityalso controlled for hypoglycemia (minimum BG <70 mg/dl). Each model was stratifiedby diagnostic category: medical or surgical. Patients admitted with trauma diagnoseswere included in the surgical cohort.
Before analysis, the set of variables was assessed for the presence ofmulticollinearity. A tolerance statistic less than or equal to 0.4 was considered toindicate the presence of multicollinearity, and in such cases, only one member of acorrelated set would be retained for the multivariable model.
The estimates of each model were presented by using odds ratios and their associated95% confidence intervals. A Bonferroni correction was applied to account for multipletesting. As the greatest number of pairwise comparisons presented for aglycemic-control variable was 10, the standard P value of 0.05 was adjustedto 0.005 to denote statistical significance for all analyses.
Analyses were run by using SAS Version 9.2 (SAS Institute, Cary, NC, USA) and MedCalcV12.4.0.0 (Ostend, Belgium).
The institutional review boards of the different centers approved the investigation.The requirement for informed consent was waived because of the retrospective natureof the study and because the data were deidentified.
Results
In Table 2a and b, we present the considerable heterogeneity inbaseline clinical characteristics of the nondiabetic and diabetic cohorts in the ninedifferent centers. The percentage of patients with diabetes in the different centersranged from 14.0% (AM) to 38.6% (BC).
Glycemic control
Patients with diabetes had higher mean BG, higher CV, and higher rates ofhypoglycemia than did patients without diabetes. The nine centers demonstratedconsiderable variation in the frequency of BG monitoring as well as in the intensityof glycemic control, as reflected by mean BG.
Three domains of glycemic control: unadjusted mortality data, nine centers
Mean BG
Figure 1A and 1B displays theunadjusted relation between mean BG and mortality for the nine centers. Additionalfile 1, Table S1 in the online supplement delineates thenumber of patients in each "band" of mean BG, as well as their mean (95%confidence interval (CI)) mortality. Among patients without diabetes, mortalitywas lowest when mean BG was 80 to 110 and 110 and 140 mg/dl and increased athigher levels. The mortality rate of the 200 patients with mean BG <80 mg/dl(0.62% of the total of 32,084 patients without diabetes) was 47.0%. Among patientswith diabetes, the shape of the relation between mean BG and mortality wascharacterized as a shallow trough, with modestly higher mortality in the aggregatewith mean BG 80 to 110 and > 180 mg/dl than with mean BG in the 110- to180-mg/dl range. The mortality rate of the 71 patients with mean BG <80 mg/dl(0.55% of the total of 12,880 patients with diabetes) was 42.3%.
Hypoglycemia
Figure 2A and 2B illustrates theunadjusted relation between hypoglycemia and mortality. Hypoglycemia wasassociated with increased mortality in patients with diabetes as well as inpatients without diabetes, although the relation was stronger among patientswithout diabetes.
Glycemic variability
Figure 3A and 3B displays theunadjusted relation between CV and mortality. Among patients without diabetes, therelation between increasing CV and increasing mortality was steep, with more thana threefold higher mortality among the entire cohort with CV > 40%compared with those with CV <20%. This relation was similar, albeit attenuated,among patients with diabetes.
Cumulative derangements in the three domains of glycemic control and theirassociation with mortality
Figure 4A and 4B illustrates thecumulative impact of derangements in the three domains of glycemic control. Amongpatients without diabetes who had mean BG between 80 and 110, 110 and 140, and 140and 180 mg/dl, increasing CV and the occurrence of hypoglycemia were associated withincreased mortality, and their effect was cumulative. Among patients without diabeteswith mean BG > 180 mg/dl, no incremental impact was found of additionalderangements of glycemic control. Among patients with diabetes, hypoglycemia wasconsistently associated with increased mortality, but mean BG and CV did not have aconsistent, cumulative impact on mortality.
Cumulative derangements of three domains. The relation of cumulativederangements of the three domains of glycemic control to mortality innondiabetes (A) and diabetes (B) patients. Patients arestratified first by mean BG during ICU stay, then by increasing coefficient ofvariation (CV), and then by the presence or absence of hypoglycemia, defined asminimum BG <70 mg/dl during ICU stay. "Bands" with fewer than 20 patientsare not reported.
Multivariable analysis
Figure 5A through F displays the resultsof multivariable analysis, assessing the independent association of bands within eachdomain with mortality.
Forest plots of bands of the independent association of mean BG,hypoglycemia, and coefficient of variation to mortality, for diabetes andnondiabetes patients. This figure illustrates the independentassociation of mean BG, hypoglycemia, and coefficient of variation tomortality, for diabetes and nondiabetes patients, including stratificationbased on medical versus surgical status. Pair-wise comparisons of odds ratio(95% CI) for each domain of glycemic control are presented.
Mean BG
An effect of center was seen on the relation between mean BG and mortality. Amongpatients without diabetes, mean BG of 110 to 140 mg/dl was independentlyassociated with reduced risk of mortality compared with mean BG of 140 to 180 and> 180 mg/dl, and similar risk compared with mean BG of 80 to 110mg/dl.
The medical and surgical patients demonstrated different patterns. Among medicalpatients, bands of mean BG of 80- to 140-mg/dl range were independently associatedwith the lowest risk of mortality, with increased risk of mortality at higherbands. In contrast, among surgical patients, a mean BG of 80 to 110 mg/dl wasindependently associated with increased risk of mortality compared with bands ofmean BG of 110 to 180 mg/dl.
The relation of mean BG to mortality was somewhat different among patients withdiabetes. Among the entire cohort of patients with diabetes, as well as for bothmedical and surgical subpopulations, mean BG of 80 to 110 mg/dl was independentlyassociated with increased risk of mortality compared with the bands of mean BG of110 to 180 mg/dl, those with mean BG of 110 to 140, 140 to 180, and< 180 mg/dl had a reduced risk of mortality.
Hypoglycemia
Severe (minimum BG <40 mg/dl) and mild to moderate (BG of 40 to 69 mg/dl)hypoglycemia were independently associated with increased risk of mortality, forthe entire cohort, as well as for the medical and surgical subpopulations.
Glycemic variability
Among patients without diabetes, low glycemic variability (CV <20%) wasindependently associated with decreased risk of mortality compared with bands ofCV of 20% to 40% and > 40% for the entire cohort; this relation was morerobust in medical patients than in surgical patients. However, among patients withdiabetes, multivariable analysis demonstrated that increased CV was notindependently associated with increased risk of mortality.
Diabetes
Diabetes was independently associated with decreased risk of mortality for theentire cohort (OR (95% CI)) 0.93 (0.87 to 0.97); P = 0.0030. Figure 6 displays the results of multivariable analysis assessing theindependent association of diabetes with mortality, stratified by individual bandsof the three domains of glycemic control. Among patients with mean BG of 80 to 110mg/dl, diabetes was independently associated with increased risk of mortality forthe entire cohort and the medical subgroup of <80 to > 110 mg/dl.However, for all other bands of mean BG, diabetes was associated with decreasedrisk of mortality for the entire cohort and the medical subgroup. Diabetes was notindependently associated with mortality in the surgical subgroup. Similarly, amongthe entire cohort with hypoglycemia and in the medical subgroup with hypoglycemia,diabetes was independently associated with decreased mortality; diabetes was notindependently associated with mortality among hypoglycemic surgical patients.
Forest plots describing the independent association of diabetes withmortality, for each of the three domains of glycemic control. Thisfigure illustrate the independent association of diabetic status withmortality associated with each of the three domains of glycemic control. Forexample, Figure 6a demonstrates that, among patients with mean BG 80 to 110mg/dl, diabetes was independently associated with increased risk ofmortality, but among patients with mean BG of 110 to 140 mg/dl, diabetes wasindependently associated with decreased risk of mortality.
Finally, diabetes was independently associated with decreased mortality among theentire cohort and both subgroups in patients with increased glycemic variability,defined as CV > 20%.
Discussion
Salient findings
This multicenter investigation demonstrates clinically important differences betweencritically ill patients with diabetes and patients without diabetes in regard to therelation between the three domains of glycemic control and mortality. Among patientswithout diabetes, the lowest mortality occurred in patients with mean BG of 80 to 140mg/dl. In contrast, among patients with diabetes, mean BG of 80 to 110 mg/dl wasindependently associated with increased risk of mortality compared with patients witha mean BG of 110 to 140, 140 to 180, and even > 180 mg/dl. Hypoglycemia wasindependently associated with increased risk of mortality among patients withdiabetes as well as among those without diabetes. Increased glycemic variability (CV>20%), however, was independently associated with increased risk of mortality amongpatients without diabetes but not among patients with diabetes. Derangements in morethan one domain of glycemic control were associated with cumulative increase inmortality among nondiabetes patients but not among patients with diabetes. Finally,for the entire cohort of 44,964 patients, diabetes was independently associated withdecreased risk of mortality.
Relation to prior literature
Hyperglycemia is associated with increased mortality in the critically ill [2, 3, 14, 29–31]. Increments of mean BG levels above 80 mg/dl are clearly associated withincreasing mortality among patients without diabetes. In contrast, a blunted relationexists between increasing mean BG levels above 80 mg/dl and mortality among patientswith diabetes [3, 29–31]. It is likely that changes in glycemic-control practice over time havealtered the observed relation between mean BG and mortality. The currentinvestigation reflects contemporary practice; all patients were admitted to ICUspracticing at least "moderate" glycemic control; the range of mean BG values of thepatients without diabetes in the different centers (119 to 137 mg/dl) contrastssharply with the mean morning BG of the patients in the control arm of the firstLeuven trial of IIT (153 mg/dl) [4].
Hypoglycemia was the second of the three domains to be associated with increased riskof mortality in critically ill patients. Although most of the literature hasdescribed an independent association of severe hypoglycemia (minimum BG <40 mg/dl)with mortality [12–15, 22], recent observational studies [16, 17] and prospective trial data [11] have identified mild hypoglycemia (minimum BG <70 mg/dl) as beingindependently associated with increased risk of mortality. Our findings confirm theseobservations for patients with and without diabetes.
Glycemic variability was the third of the three domains to be independentlyassociated with mortality in the critically ill [18–25]. One observational study suggested that glycemic variability wasindependently associated with mortality only among critically ill patients withoutdiabetes [24]; our study confirms these findings.
Finally, the independent impact of diabetic status, without reference to glycemiccontrol, on the mortality of critically ill patients has been the subject of recentobservational studies that concluded that patients with diabetes did not experiencehigher mortality, and diabetes may, in fact, be protective [30–36]. We demonstrated here that diabetes is independently associated withdecreased risk of mortality.
Strengths and weaknesses
The clearest strength of this study is its size. The 44,964 patients include patientsadmitted with a large array of medical, surgical, and trauma diagnoses, treated witha variety of glycemic-control protocols, substantially enhancing the generalizabilityof the investigation. Moreover, this is a modern cohort of patients treated in an eracharacterized by attention to glycemic control. Each of the nine centers maintained arobust database characterized by prospective data collection, creating an additionalimportant strength of this investigation: the breadth of demographic, clinicaloutcome, and glycemic data available for analysis. The absence of information aboutinsulin therapy is an important limitation. It is likely that important differencesexist between insulin-treated and insulin-naive patients regarding the relation ofthe three domains of glycemic control to mortality.
Another potential limitation is that the identification of diabetic status was madeon clinical grounds, based on all information available at the time of ICU admission.It is likely that some patients designated as without diabetes may actually have haddiabetes; HgbA1c levels were not obtained routinely, and, of course,glucose-tolerance testing could not be performed. Furthermore, we are unable todetermine whether the diabetes patients were categorized as type I or type II.Although most were likely type II, important differences may exist between the twogroups in their response to derangements in the domains of glycemic control.Additionally, we cannot provide details of nutritional therapy and cannot thereforeanalyze the interactions among glycemic control, nutritional therapy, and insulintreatment of hyperglycemia. Moreover, many of the glycemia data from several of thecenters included in this study were derived from capillary blood measured onpoint-of-care devices, a method associated with increased analytic inaccuracy [38–41]. Nevertheless, any degree of measurement imprecision would only serve todampen the observed relations between glycemia and diabetic status.
Finally, we acknowledge that the observational nature of this investigation mandatesthat its conclusions must be considered to be hypothesis generating, rather thanproof of causality. Nevertheless, it would be unethical to randomize patients toinduced hyperglycemia, hypoglycemia, or increased glycemic variability.
Biological plausibility
Considerable evidence suggests that diabetes may alter the relation between glycemiaand mortality in critically ill patients [28]. Diabetes patients may develop a tolerance to hyperglycemia, and amoderate degree of hyperglycemia that might exert toxicity in a patient withoutdiabetes may be well tolerated in a patient with diabetes. This may explain thestrong relation seen between increasing mean BG levels and mortality in patientswithout diabetes, detailed in several large observational studies, but not amongthose with diabetes [3, 29–31, 36, 42]. In a recent study [43], diabetes patients with poor preadmission glycemic control, reflected byhigh HgbA1c levels, had higher mortality when mean BG was tightly controlled duringICU stay compared with patients with high premorbid HgbA1c levels who had a highermean BG during ICU stay. These intriguing data parallel the results of largeinterventional studies in outpatient populations with type II diabetes [44, 45]. An extensive body of literature has explored the physiological basis ofthe deleterious impact of hypoglycemia [46–51] demonstrated in interventional [4, 6, 11, 25] and observational [12–17] studies; none of these has focused explicitly on the different impact thathypoglycemia may exert on patients with diabetes compared with those withoutdiabetes. Similarly, although various physiological mechanisms underlying the harmfuleffect of increased glycemic variability detailed in interventional [4, 6, 25] and observational [18–24] studies have been proposed [52–56], the reasons that glycemic variability has no or a muted independentassociation with risk of mortality in patients with diabetes compared with thestriking relation seen in patients without diabetes requires furtherclarification.
Clinical implications
The central findings of the current investigation have important implications for thecare of critically ill patients. Hyperglycemia does not have the same associationwith mortality among critically ill patients without diabetes compared with thosewith diabetes. The euglycemic range was independently associated with the lowest riskof mortality among patients without diabetes but with higher mortality among patientswith diabetes. Additionally, important differences were noted when comparing medicaland surgical populations. These findings call into question the "one size fits all"strategy for glycemic control of critically ill patients. It may be most appropriateto establish lower glycemic target ranges for medical patients without diabetes thanfor patients with diabetes or for surgical patients without diabetes.
In addition, our observations call into question the appropriateness of recentlypublished glycemic-control guidelines that recommend a glycemic target range of 140to 180 mg/dl [57] or 140 to 200 mg/dl [58] for all critically ill patients. Furthermore, premorbid glycemic controlin diabetes may have an important impact on the consequences of glycemic control inthe ICU [43]. The optimal glycemic-control protocol may result not only fromstratifying patients by diabetic status, but also by additionally stratifyingpatients with diabetes based on the degree of preadmission glycemic control. Incontrast, the deleterious association of hypoglycemia with mortality, even atthreshold levels of <70 mg/dl, was observed in patients with diabetes and in thosewithout diabetes. Because hypoglycemia can never be the subject of a randomizedtrial, the data from this investigation, when combined with the findings fromprevious interventional [4, 6, 10, 11, 25] and observational [12–17] studies, provide the strongest evidence basis for the goal of avoidinghypoglycemia in all critically ill patients.
Finally, increased glycemic variability, defined as CV > 20%, was identifiedin the current study as having a strong independent association with increased riskof mortality in patients without diabetes. These data provide strong impetus for thecreation of insulin-dosing strategies and the development of new technologies [59] for accurate continuous or near-continuous BG monitoring, with the goal ofreducing glycemic variability in critically ill patients. Further investigationshould stratify patient outcomes by specific admitting diagnosis; importantdifferences may be found within the broad medical and surgical categories that thecurrent investigation was underpowered to assess.
The design of future trials of IIT should include consideration of all three domainsof glycemic control as well as recognition of the differences in their associationwith mortality based on premorbid diabetes status.
Conclusions
This large international cohort study evaluated the relation of diabetic status to theassociation of hyperglycemia, hypoglycemia, and increased glycemic variability in aheterogeneous population of critically ill patients. We found that diabetic statusmodulates the relation between the three domains of glycemic control and mortality inclinically important ways. Our findings suggest that patients with diabetes may benefitfrom higher glucose target ranges than those without diabetes. Additionally,hypoglycemia is independently associated with increased risk of mortality, regardless ofthe patient's diabetic status, and increased glycemic variability is independentlyassociated with increased risk of mortality among patients without diabetes. Thesefindings may inform the implementation of glycemic-control protocols in the intensivecare unit, as well as for the design of future interventional trials of intensivemonitoring and treatment of dysglycemia in the critically ill.
Key messages
-
Diabetic status modulates the relation between the three domains ofglycemic control (hyperglycemia, hypoglycemia, and glycemic variability) and mortalityin critically ill patients in clinically important ways.
-
The range of mean BG from 80 to 140 mg/dl is associated with thelowest severity adjusted mortality among nondiabetes patients. In contrast, among thosewith diabetes, a mean BG of 80 to 110 mg/dl is associated with higher mortality riskthan is the range of 110 to 180 mg/dl.
-
A single episode of hypoglycemia (BG <70 mg/dl) is independentlyassociated with increased risk of mortality among those without as well as those withdiabetes.
-
Increased glycemic variability, defined as CV > 20%, isindependently associated with increased risk of mortality among those without, but notamong those with diabetes.
Abbreviations
- ABG:
-
arterial blood gas
- APACHE:
-
acute physiology and chronic health evaluation
- BG:
-
blood glucose
- CV:
-
coefficient of variation
- DM:
-
diabetes mellitus
- ICU:
-
intensive careunit
- IIT:
-
intensive insulin therapy
- LOS:
-
length of stay
- OR:
-
odds ratio.
- Participating centers in this investigation:
-
AM: Amsterdam
- AU:
-
Austin
- BC:
-
BayCare
- BI:
-
Birmingham
- GE:
-
Geelong
- OK:
-
Okayama
- ST:
-
Stamford
- TU:
-
Tufts
- VI:
-
Vienna.
References
Dungan KM, Braithwaite SS, Preiser JC: Stress hyperglycaemia. Lancet 2009, 373: 1798-1807. 10.1016/S0140-6736(09)60553-5
Krinsley JS: Association between hyperglycemia and increased hospital mortality in aheterogeneous population of critically ill patients. Mayo Clin Proc 2003, 78: 1471-1478. 10.4065/78.12.1471
Falciglia M, Freyberg RW, Almenoff PL, D'Alessio DA, Render ML: Hyperglycemia-related mortality in critically ill patients varies with admissiondiagnosis. Crit Care Med 2009, 37: 3001-3009. 10.1097/CCM.0b013e3181b083f7
Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R: Intensive insulin therapy in the critically ill patients. N Engl J Med 2001, 345: 1359-1367. 10.1056/NEJMoa011300
Krinsley JS: Effect of an intensive glucose management protocol on the mortality of criticallyill adult patients. Mayo Clin Proc 2004, 79: 992-1000. 10.4065/79.8.992
Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R: Intensive insulin therapy in the medical ICU. N Engl J Med 2006, 354: 449-461. 10.1056/NEJMoa052521
Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hargog C, Natanson C, Loeffler M, Reihart K for the German Competence Network Sepsis: Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008, 358: 125-139. 10.1056/NEJMoa070716
Preiser JC, Devos P, Ruiz-Santana S, Mélot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, Chioléro R: A prospective randomised multi-centre controlled trial on tight glucose control byintensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 2009, 35: 1738-1748. 10.1007/s00134-009-1585-2
Arabi YM, Dabbagh OC, Tamim HM, Al-Shimemeri AA, Memish ZA, Haddad SH, Syed SJ, Giridhar HR, Rishu AH, Al-Daker MO, Salim K, Riette B, Maram S: Intensive versus conventional insulin therapy: a randomized controlled trial inmedical and surgical critically ill patients. Crit Care Med 2008, 36: 3190-3197. 10.1097/CCM.0b013e31818f21aa
The NICE-SUGAR Study Investigators: Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009, 360: 1283-1297.
The NICE-SUGAR Study Investigators: Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012, 367: 1108-1118.
Vriesendorp TM, DeVries JH, van Santen S, Moeniralam HS, de Jonge E, Roos YB, Schultz MJ, Rosendaal FR, Hoekstra JB: Evaluation of short-term consequences of hypoglycemia in an intensive careunit. Crit Care Med 2006, 34: 2714-2718. 10.1097/01.CCM.0000241155.36689.91
Krinsley JS, Grover A: Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med 2007, 35: 2262-2267. 10.1097/01.CCM.0000282073.98414.4B
Bagshaw SM, Egi M, George C, Bellomo R: Early blood glucose control and mortality in critically ill patients inAustralia. Crit Care Med 2009, 37: 463-470. 10.1097/CCM.0b013e318194b097
Hermanides J, Bosman RJ, Vriesendorp TM, Dotsch R, Rosendaal FR, Zandstra DF, Hoekstra JB, DeVries JH: Hypoglycemia is associated with intensive care unit mortality. Crit Care Med 2010, 38: 1430-1434. 10.1097/CCM.0b013e3181de562c
Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, Hegarty C, Bailey M: Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc 2010, 85: 217-224. 10.4065/mcp.2009.0394
Krinsley JS, Schultz MJ, Spronk PE, Harmsen RE, van Braam Houckgeest F, van der Sluijs JP, Melot C, Preiser JC: Mild hypoglycemia is independently associated with increased mortality in thecritically ill. Crit Care 2011, 15: R173. 10.1186/cc10322
Egi M, Bellomo R, Stachowski E, French CJ, Hart G: Variability of blood glucose concentration and short-term mortality in criticallyill patients. Anesthesiology 2006, 105: 244-252. 10.1097/00000542-200608000-00006
Krinsley JS: Glycemic variability: a strong independent predictor of mortality in criticallyill patients. Crit Care Med 2008, 36: 3008-3013. 10.1097/CCM.0b013e31818b38d2
Ali NA, O'Brien JM, Dungan K, Phillips G, Marsh CB, Lemeshow S, Connors AF, Preiser JC: Glucose variability and mortality in patients with sepsis. Crit Care Med 2008, 36: 2316-2321. 10.1097/CCM.0b013e3181810378
Dossett LA, Cao H, Mowery NT, Dortch MJ, Morris JM, May AK: Blood glucose variability is associated with mortality in the surgical intensivecare unit. Am Surg 2008, 74: 679-685.
Bagshaw SM, Bellomo R, Jacka MJ, Egi M, Hart GK, George C: The impact of early hypoglycemia and blood glucose variability on outcome incritical illness. Crit Care 2009, 13: R91. 10.1186/cc7921
Hermanides J, Vriesendorp TM, Bosman RJ, Zandstra DF, Hoekstra JB, Devries JH: Glucose variability is associated with intensive care unit mortality. Crit Care Med 2010, 38: 838-842. 10.1097/CCM.0b013e3181cc4be9
Krinsley JS: Glycemic variability and mortality in critically ill patients: the impact ofdiabetes. J Diabetes Sci Technol 2009, 3: 1292-1301.
Meyfroidt G, Keenan DM, Wang X, Wouters PJ, Veldhuis JD, Van den Berghe G: Dynamic characteristics of blood glucose time series during the course of criticalillness: effects of intensive insulin therapy and relative association withmortality. Crit Care Med 2010, 38: 1021-1029. 10.1097/CCM.0b013e3181cf710e
Mackenzie IM, Whitehouse T, Nightingale PG: The metrics of glycaemic control in critical care. Intensive Care Med 2011, 37: 435-443. 10.1007/s00134-010-2103-2
Krinsley JS: Understanding glycemic control in the critically ill: 3 domains are better thanone. Intensive Care Med 2011, 37: 382-384. 10.1007/s00134-010-2110-3
Krinsley JS, Meyfroidt G, van den Berghe G, Egi M, Bellomo R: The impact of premorbid diabetic status on the relationship between the 3 domainsof glycemic control and mortality in critically ill patients. Curr Opin Clin Nutr Metab Care 2012, 15: 151-160. 10.1097/MCO.0b013e32834f0009
Rady MY, Johnson DJ, Patel BM, Larson JS, Helmers RA: Influence of individual characteristics on outcome of glycemic control inintensive care unit patients with or without diabetes mellitus. Mayo Clin Proc 2005, 80: 1558-1567. 10.4065/80.12.1558
Krinsley JS: Glycemic control, diabetic status, and mortality in a heterogeneous population ofcritically ill patients before and during the era of intensive glycemicmanagement: six and one-half years experience at a university-affiliated communityhospital. Semin Thorac Cardiovasc Surg 2006, 18: 317-325. 10.1053/j.semtcvs.2006.12.003
Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Hegarty C, Bailey M: Blood glucose concentration and outcome of critical illness: the impact ofdiabetes. Crit Care Med 2008, 36: 2249-2255. 10.1097/CCM.0b013e318181039a
Graham BB, Keniston A, Gajic O, Trillo Alvarez CA, Medvedev S, Douglas IS: Diabetes mellitus does not adversely affect outcomes from a critical illness. Crit Care Med 2010, 38: 16-24. 10.1097/CCM.0b013e3181b9eaa5
Vincent JL, Preiser JC, Sprung CL, Moreno R, Sakr Y: Insulin-treated diabetes is not associated with increased mortality in criticallyill patients. Crit Care 2010, 14: R12. 10.1186/cc8866
Stegenga ME, Vincent JL, Vail GM, Xie J, Haney DJ, Williams MD, Bernard GR, van der Poll T: Diabetes does not alter mortality or hemostatic and inflammatory responses inpatients with severe sepsis. Crit Care Med 38: 539-545.
Esper AM, Moss M, Martin GS: The effect of diabetes mellitus on organ dysfunction with sepsis: anepidemiological study. Crit Care 2009, 13: R18. 10.1186/cc7717
Krinsley JS, Fiser M: The diabetes paradox: diabetes is not independently associated with increasedmortality in critically ill patients. Hosp Pract 2012, in press.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE: APACHE II: a severity of disease classification system. Crit Care Med 1985, 13: 818-829. 10.1097/00003246-198510000-00009
Finkielman JD, Oyen LJ, Afessa B: Agreement between bedside blood and plasma glucose measurement in the ICUsetting. Chest 2005, 127: 1749-1751. 10.1378/chest.127.5.1749
Desachy A, Vuagnat AC, Ghazali AD, Baudin OT, Longuet OH, Calvat SN, Gissot V: Accuracy of bedside glucometry in critically ill patients: influence of clinicalcharacteristics and perfusion index. Mayo Clin Proc 2008, 83: 400-405. 10.4065/83.4.400
Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, Fergusson D, McIntyre LA, Hebert PC: Reliability of point-of-care testing for glucose measurement in critically illadults. Crit Care Med 2005, 33: 2778-2785. 10.1097/01.CCM.0000189939.10881.60
Corstjens AM, Ligtenberg JJ, van der Horst IC, Spanjersberg R, Lind JS, Tulleken JE, Meertens JH, Zijlstra JG: Accuracy and feasibility of point-of-care and continuous blood glucose analysis incritically ill ICU patients. Crit Care 2006, 10: R135. 10.1186/cc5048
Krinsley JS: Moving closer to untangling a sweet web: hyperglycemia, diabetic status, andmortality in the critically ill. Crit Care Med 2010, 38: 295-296. 10.1097/CCM.0b013e3181bfe9e5
Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, Hegarty C, Bailey M: The interaction of chronic and acute glycemia with mortality in critically illpatients with diabetes. Crit Care Med 2011, 39: 105-111. 10.1097/CCM.0b013e3181feb5ea
Action to Control Cardiovascular Risk in Diabetes Study Group: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008, 358: 2545-2559.
ADVANCE Collaborative Group: Intensive blood glucose control and vascular outcomes in patients with type 2diabetes. N Engl J Med 2008, 358: 2560-2572.
Dotson S, Freeman R, Failing HJ, Adler GK: Hypoglycemia increases serum interleukin-6 levels in healthy men and women. Diabetes Care 2008, 31: 1222-1223. 10.2337/dc07-2243
Schlenk F, Graetz D, Nagel A, Schmidt M, Sarrafzadeh AS: Insulin-related decrease in cerebral glucose despite normoglycemia in aneurysmalsubarachnoid hemorrhage. Crit Care 2008, 12: R9. 10.1186/cc6776
Keller-Wood ME, Shinsako J, Dallman MF: Inhibition of the adrenocorticotropin and corticosteroid responses to hypoglycemiaafter prior stress. Endocrinology 1983, 113: 491-496. 10.1210/endo-113-2-491
Herlein JA, Morgan DA, Phillips BG, Haynes WG, Sivitz WI: Antecedent hypoglycemia, catecholamine depletion, and subsequent sympatheticneural responses. Endocrinology 2006, 147: 2781-2788. 10.1210/en.2005-1247
Dieguez G, Fernandez N, Garcia JL, Garcia-Villalon AL, Monge L, Gomez B: Role of nitric oxide in the effects of hypoglycemia on the cerebral circulation inawake goats. Eur J Pharmacol 1997, 330: 185-193. 10.1016/S0014-2999(97)00181-7
Dagogo-Jack SE, Craft S, Cryer PE: Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus:recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, anddefense against subsequent hypoglycemia. J Clin Invest 1993, 91: 819-828. 10.1172/JCI116302
Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A: Intermittent high glucose enhances apoptosis related to oxidative stress in humanumbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidaseactivation. Diabetes 2003, 52: 2795-2804. 10.2337/diabetes.52.11.2795
Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C: Activation of oxidative stress by acute glucose fluctuations compared withsustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295: 1681-1687. 10.1001/jama.295.14.1681
Watada H, Azuma K, Kawamori R: Glucose fluctuation on the progression of diabetic macroangiopathy: new findingsfrom monocyte adhesion to endothelial cells. Diabetes Res Clin Pract 2007,77(Suppl 1):S58-S61.
Azuma K, Kawamori R, Toyofuku Y, Kitahara Y, Sato F, Shimizu T, Miura K, Mine T, Tanaka Y, Mitsumata M, Watada H: Repetitive fluctuations in blood glucose enhance monocyte adhesion to theendothelium of rat thoracic aorta. Arterioscler Thromb Vasc Biol 2006, 26: 2275-2280. 10.1161/01.ATV.0000239488.05069.03
Risso A, Mercuri F, Quagliaro L, Damante G, Ceriello A: Intermittent high glucose enhances apoptosis in human umbilical vein endothelialcells in culture. Am J Physiol Endocrinol Metab 2001, 281: E924-E930.
American Diabetes Association: Standards of medical care in diabetes. Diabetes Care 2010,33(Suppl 1):S11-S61.
Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P: Use of intensive insulin therapy for the management of glycemic control inhospitalized patients: a clinical practice guideline from the American College ofPhysicians. Ann Intern Med 2011, 154: 260-267. 10.7326/0003-4819-154-4-201102150-00007
Joseph JI, Hipszer B, Mraovic B, Chervoneva I, Joseph M, Grunwald Z: Clinical need for continuous glucose monitoring in the hospital. J Diabetes Sci Technol 2009, 3: 1309-1318.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Dr. Krinsley reported receiving consultant fees from Medtronic Inc., Edwards LifeSciences, Roche Diagnostics, OptiScan Biomedical, and Alere and research support fromOptiScan Biomedical. He also received royalty payments for sales of ICU Tracker. Dr.Amin reported receiving speaker fees from BioMerueux. Ms. Maurer works as a consultantfor Alere, the distributor of ICU Tracker. Dr. Schultz reported receiving consultantfees from Medtronic Inc., GlySure Ltd., and Roche Diagnostics, and research support fromMedtronic Inc. and OptiScan Biomedical. Dr. van Hooijdonk reported consultant fees fromMedtronic Inc. and GlySure Ltd., and research support from Medtronic Inc. and OptiScanBiomedical. Dr. Annane reported serving on advisory board meetings for Edwards LifeSciences but did not receive compensation. Dr. Nasraway reported receiving consultantfees from GlySure Ltd., OptiScan Biomedical, and Edwards Life Sciences, and consultingfees and stock options from Echo Therapeutics. Dr. Holzinger reported receivingconsultant fees from Medtronic Inc. and speaker fees from NovoNordisk. Dr. Preiserreported receiving consultant fees from Medtronic Inc., Edwards Life Sciences, andOptiScan Biomedical.
Dr. Egi, Dr. Kiss, Dr. Amin, Dr. Schuetz, Dr. Kiyoshi, Dr. Mackenzie, Dr. Stow, Ms.Holewinski, Dr. Vincent, and Dr. Bellomo reported no relevant interests.
Authors' contributions
JK conceived of the study concept and design, wrote the draft of the manuscript, hadfull access to all of the data in the study, and takes responsibility for the integrityof the data and the accuracy of the data analysis. JK, ME, DA, PS, PM MS, RvH, KM, IM,PS, SN, SH, UH, and RM participated in data acquisition. JK, ME, MS, JP, and RBperformed analysis and interpretation of the data. ME, DA, PS, MS, IA, DA, SN, RvH, JP,UH, JP, JV, and RB performed critical revision of the manuscript for importantintellectual content. AK and JK completed the statistical analysis. All of the authorsread and approved the final draft of the manuscript.
Electronic supplementary material
13054_2012_1682_MOESM1_ESM.DOC
Additional file 1: Table S1. Mortality (percentage, 95% CI) and number of patients forindividual cohorts, nondiabetes, and diabetes patients, for each of thethree domains of glycemic control. This file contains data detailing thenumber of patients from each of the nine centers, their mortalitypercentage, and the 95% CI of this percentage, stratified by diabeticstatus, for each of the "bands" of the three domains of glycemic controldescribed in the manuscript. (DOC 124 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Krinsley, J.S., Egi, M., Kiss, A. et al. Diabetic status and the relation of the three domains of glycemic control tomortality in critically ill patients: an international multicenter cohort study. Crit Care 17, R37 (2013). https://doi.org/10.1186/cc12547
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc12547