Abstract
Introduction
Assessment of treatments for acute respiratory distress syndrome (ARDS) has focused on short-term outcomes (for example, mortality); little information exists regarding long-term effects of ARDS treatment. Survivors of ARDS episodes may have long-term obstructive/restrictive pulmonary abnormalities and pulmonary gas exchange impairment. A 2004 prospective randomized placebo-controlled trial assessed the efficacy and safety of inhaled nitric oxide (iNO) in patients with non-septic ARDS; the primary endpoint was days alive and off assisted breathing. This analysis examined potential effects of iNO or placebo on pulmonary function six months post-treatment in ARDS survivors from that original study.
Methods
ARDS survivors (N = 92) from a large-scale randomized, placebo-controlled study evaluating mortality after either 5 ppm iNO or placebo for up to 28 days were assessed six months post-treatment. Pulmonary function testing across seven parameters was conducted.
Results
At 6 months post-treatment, results indicated significantly better absolute values for iNO versus placebo for mean ± SD total lung capacity (TLC, 5.54 ± 1.42 vs. 4.81 ± 1.00; P = 0.026). There were also significantly better values for mean ± SD percent predicted values for a) forced expiratory volume in 1 second (FEV1, 80.23 ± 21.21 vs. 69.51 ± 28.97; P = 0.042), b) forced vital capacity (FVC, 83.78 ± 19.37 vs. 69.84 ± 27.40; P = 0.019), c) FEV1/FVC (96.14 ± 13.79 vs. 87.92 ± 19.77; P = 0.033), and d) TLC (93.33 ± 18.21 vs. 76.10 ± 21.84; P < 0.001). Nonsignificant differences were found in absolute FEV1, FEV1/FVC, FVC, forced expiratory flow from 25% to 75% of FVC, functional residual capacity, and CO diffusion.
Conclusions
ARDS patients surviving after treatment with low-dose iNO had significantly better values for select pulmonary function tests at six months post-treatment than placebo-treated patients. Further trials are warranted to determine the effects of iNO on chronic lung function in ARDS survivors, a factor in long-term morbidity and quality of life in this population.
Trial Registration
A Double-blind, Randomized, Placebo-controlled, Dose-response Study of Inhaled Nitric Oxide in the Treatment of Acute Respiratory Distress Syndrome. NCT number: ISRCTN53268296
Similar content being viewed by others
Introduction
Inhaled nitric oxide (iNO) is a vasodilator indicated for treatment of term and near-term neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension. In these patients, iNO has been shown to improve oxygenation and reduce the need for extracorporeal membrane oxygenation therapy [1]. NO binds to and activates cytosolic guanylate cyclase, thereby increasing intracellular levels of cyclic guanosine 3',5'-monophosphate (cGMP). This, in turn, relaxes vascular smooth muscle, leading to vasodilatation. iNO selectively dilates the pulmonary vasculature, with minimal systemic vasculature effect as a result of efficient hemoglobin scavenging. In acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), increases in partial pressure of arterial oxygen (PaO2) are believed to occur secondary to pulmonary vessel dilation in better-ventilated lung regions. As a result, pulmonary blood flow is redistributed away from lung regions with low ventilation/perfusion ratios toward regions with normal ratios [1–3].
The incidence of ARDS has been estimated to be approximately 75 cases per 100,000 population, although this figure is impacted by ambiguity in the causes and manifestations of ARDS [4, 5]. Mortality rates in ARDS are substantial, with estimates ranging from 34% to 68% [4], highlighting the need for effective treatment.
Many pharmacologic treatments have been investigated in ARDS patients, including alprostadil [6], acetylcysteine [7], corticosteroids [8], surfactant [9], dazoxiben [10] and acyclovir [11]. All studies to date have focused on mortality as the primary endpoint. A meta-analysis of trials completed through 2004 indicated limited mortality benefit with any of the above-mentioned treatments [12].
Patients surviving an episode of ARDS may have long-term obstructive and restrictive pulmonary abnormalities as well as pulmonary gas exchange impairment [13–15]. These long-term effects may contribute to decreased quality of life (QoL), repeatedly demonstrated by ARDS survivors [13, 16, 17]. The importance of long-term effects following an ARDS episode has recently emerged, with clinicians noting that assessing short-term survival of ARDS is only part of its clinical impact. Therefore, treatments provided in the ICU that improve long-term ARDS outcomes (without improving immediate survival) and clinical studies examining treatment effects on later outcomes may be relevant [18].
A large-scale, randomized, blinded, placebo-controlled study carried out in the ICUs of 46 US hospitals evaluated the efficacy of low-dose (5 ppm) iNO in 385 patients with moderately severe ALI. The primary endpoint was number of days alive and off assisted breathing. Results of an intent-to-treat analysis revealed that iNO had no significant benefit versus control (nitrogen gas) as it related to mortality (23% versus 20%, respectively), days alive and off assisted breathing (mean, 10.7 versus 10.6 days), or days alive and meeting oxygenation criteria for extubation (mean, 16.7 versus 17.0 days). Treatment, however, resulted in a significant increase (P < 0.05) in PaO2 during the initial 24 hours of treatment, with improvement resolved by 48 hours [19].
Safety results for the initial 28-day study period have been reported [19] and are summarized briefly here. A total of 630 adverse events (AEs) were reported for patients treated with iNO versus 666 events for those receiving placebo. Respiratory system AEs occurred in 51% versus 61% of patients receiving iNO and placebo, respectively, primarily due to higher frequencies of pneumonia, pneumothorax, and apnea in the placebo group. Frequency of other AEs was similar in both groups [19].
The present analysis was developed a priori as part of the original study protocol and assessed long-term pulmonary function differences between iNO and placebo at six months post-treatment. The original rationale for long-term follow-up of pulmonary function in survivors was to assess both the safety of iNO use in ARDS and to examine any potential efficacy on the incidence of chronic lung disease in survivors. This study is the first prospective long-term analysis of pulmonary function in ARDS survivors participating in a randomized interventional clinical trial comparing iNO and placebo.
Materials and methods
The protocol, amendments to the protocol, and local Informed Consent Forms were reviewed and approved by each of the participating hospitals' Institutional Review Board prior to initiation of patient accrual.
Inclusion and exclusion criteria and treatment details for this analysis are described elsewhere [19]; they are summarized briefly here.
Patients
Patients had moderately severe ALI, defined by a modification of American-European Consensus Conference criteria (PaO2/fraction of inspired oxygen [FiO2] ratio of ≤ 250 mm Hg), due to causes other than severe sepsis. Patients with evidence of non-pulmonary system failure at the time of randomization and sepsis-induced ARDS were excluded. Patients were also excluded if they had sustained hypotension requiring vasopressor support, hemodynamic profiles supporting severe sepsis, severe head injury, severe burns or evidence of other significant organ system dysfunction at baseline [19].
Treatment
Patients were randomly assigned to receive either inhaled placebo (nitrogen) or 5 ppm of iNO (INO Therapeutics Inc., Port Allen, LA, USA). Patients, healthcare professionals, and investigators were blinded to the assigned treatment. iNO was administered via INOvent® delivery system (Datex-Ohmeda, Madison, WI, USA) that blended the treatment drug (nitrogen or NO at 100-ppm balance nitrogen) 1:20 with ventilator gases to achieve a target ppm value in the inspiratory limb of the ventilator [19].
All patients using the iNO delivery system received mechanical ventilatory support. Treatment continued with active or placebo gas until one of the following criteria was met: 1) end of trial (28 days); 2) death; or 3) adequate oxygenation (arterial oxygen saturation by pulse oximetry [SpO2] ≥ 92% or PaO2 of ≥ 63 mm Hg) without treatment at ventilator settings of FiO2 ≤ 0.4 and positive end-expiratory pressure (PEEP) of ≤ 5 cm H2O. Decreases in treatment gas continued in 20% decrements (titrated down by 1 ppm for iNO) every 30 minutes until either the drug concentration reached 0% or oxygenation criteria were not satisfied. If oxygenation criteria were not met, drug concentration was titrated up until they were again achieved. Increments of upward titration were determined by the clinician, based on degree of arterial desaturation [19].
Respiratory parameters measured during hospitalization
Baseline oxygenation measures included PaO2, arterial partial pressure of CO2 (PaCO2), SpO2, FiO2, PEEP, PaO2/FiO2 ratio, ventricular rate, tidal volume, and mean airway pressure. Respiratory parameters (FiO2, PEEP, and PaO2/FiO2 ratio) were recorded on case report forms every 12 hours during mechanical ventilation.
Long-term pulmonary function measures
Pulmonary function testing (PFT) at six months post-treatment was required in both iNO- and placebo-treated patients as part of the original study design. PFTs included FEV1, FEV1 % predicted, FVC, FVC % predicted, the FEV1/FVC ratio, FEV1/FVC ratio % predicted, forced expiratory flow (FEF) from 25% to 75% of FVC (FEF25-75%), FEF25-75% % predicted, functional residual capacity (FRC), FRC % predicted, total lung capacity (TLC), TLC % predicted, CO diffusion, and CO diffusion % predicted.
Statistical methods
All between-group differences in PFT results were evaluated using the Wilcoxon rank sum test. Between-group differences in baseline clinical and demographic characteristics were assessed with either Fisher's exact test or chi-square test for categorical variables and with Wilcoxon rank sum test for continuous variables. Baseline oxygenation and respiratory/oxygenation parameters in the two groups were compared using Wilcoxon rank sum tests. The areas under the curve (AUCs) of FiO2 and PEEP were calculated using the trapezoidal rule to demonstrate the total exposure of both groups during days of mechanical ventilation to supplemental oxygen and PEEP from Baseline through day 28 or day of discharge, inclusive. The null hypothesis that the respective AUCs were normally distributed was rejected employing the Shapiro-Wilk test. A Wilcoxon rank sum test was utilized to assess the differences in each median AUC between treatment groups. A P value < 0.05 was considered significant.
Results
Demographics and baseline characteristics
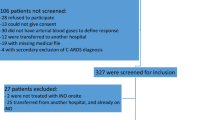
Final disposition of all subjects in the original study and six-month follow-up is shown in Figure 1. A total of 302 patients were survivors (alive, on or off assisted breathing) after the initial 28-day treatment period (148 in the iNO group, 154 placebo). Of these, 92 (30%) were capable of and participated in the six-month follow-up pulmonary function evaluations, 51 (55%) in the iNO group and 41 (45%) in the placebo group. The remaining surviving subjects (n = 210) either died prior to follow-up (n = 20), were lost to follow-up (n = 47), or did not have available PFT data (n = 143). Baseline patient characteristics are summarized in Table 1. The two treatment groups were well matched for all demographic variables. There were no significant differences between groups with respect to ARDS etiology, severity of illness, frequency of co-morbid chronic respiratory conditions or use of inhaled corticosteroids. More subjects had a history of tobacco use in the iNO group (26 versus 17, P = 0.41). There were more placebo patients who had evidence of morbid obesity (actual body weight is ≥ 35% over ideal body weight) (19 versus 13, P = 0.028). Regardless of the presence or absence of morbid obesity, analyses showed that the treatment effect remained the same.
Baseline oxygenation parameters
Baseline oxygenation parameters, including PaO2, PaCO2, SpO2, FiO2, PEEP, and PaO2/FiO2 ratio are summarized in Table 2. The patients included in this analysis were severely ill with mean baseline PaO2/FiO2 ratios of 140.5 ± 43.4 (iNO) and 136.1 ± 40.4 (placebo). Except for a clinically insignificant difference in SpO2, there were no significant between-group differences with respect to baseline oxygenation parameters.
Baseline respiratory parameters
Baseline respiratory parameters, including ventilator rate, tidal volume, and mean airway pressure are also summarized in Table 2. There were no significant differences between groups for any of these measures.
Respiratory parameters during mechanical ventilation
There were no significant differences between groups for aggregate daily per-patient changes from baseline parameters in supplemental oxygen, PEEP, or PaO2/FiO2 ratio for patients receiving mechanical ventilation (Figures 2, 3 and 4).
However, when calculating the duration of exposure (AUC) over the length of mechanical ventilation for total FiO2 (6.3 ± 4.5 versus 7.6 ± 4.7 (%days) for iNO and placebo groups, respectively; P = 0.151) and total PEEP (96.3 ± 75.9 versus 113.4 ± 81.1 (mmHgdays), P = 0.261), although not statistically significant, the iNO group did have less cumulative exposure to both variables (Table 3).
Pulmonary function tests at six months
Results for PFTs at six months post-treatment with placebo or iNO are summarized in Table 4 and presented as comparison percent between treatment groups in Figure 5. Study results indicated significantly better values for patients treated with iNO versus placebo for FEV1 % predicted (P = 0.042), FVC % predicted (P = 0.019), FEV1/FVC % predicted (P = 0.033), TLC (P = 0.026), and TLC % predicted (P < 0.001). No significant differences were observed in FEV1, FEV1/FVC, FVC, FEF25-75%, FRC, or CO diffusion values, nor for percent predicted values for FEF25-75%, FRC, and CO diffusion.
Baseline characteristics of survivors and patients with no follow-up at six months
Table 5 shows that the two treatment groups were well matched for all demographic variables except for a slight but statistically significant difference in age and the incidence of other types of preexisting lung disease with the patients without six month follow-up data being slightly older and having a greater incidence of preexisting lung disease of 'other' etiologies.
Discussion
Clinical trials evaluating numerous interventions have repeatedly failed to demonstrate significant benefit in decreasing mortality in ARDS patients [12, 20]. Endpoints such as long-term morbidity, or a shift of focus to short- and long-term respiratory changes in survivors of ARDS, may be important when evaluating established and emerging ARDS treatments. In a study evaluating 50 long-term ARDS survivors, assessed a median of 5.5 years after ICU discharge, 54% had impairment (defined as < 80% predicted value) in at least one pulmonary function measure, including decreases in FEV1/FVC ratio consistent with airflow obstruction in 16 (32%), residual volume in 14 (28%), TLC in 10 (20%), and diffusing capacity in 8 (16%) patients. Seven patients (14%) had multiple pulmonary function abnormalities. Overall, ARDS survivors described a 25% reduction in physical and physical role function compared with age- and sex-matched controls (P < 0.001). In addition, those with more than one pulmonary function abnormality had significantly decreased health-related QoL parameters (that is, general/mental health, physical/social function, and vitality) compared with those having one or no abnormalities [21].
While part of a larger study examining the short-term (28-day) effects of iNO on mortality and need for assisted breathing, this six-month follow-up assessment is the first prospective analysis evaluating iNO effects on long-term pulmonary function in ARDS survivors. The original clinical trial [19], as well as a meta-analysis of 12 randomized controlled trials in ALI or ARDS patients [20], indicated no significant benefit of iNO in decreasing mortality and only transient effects on physiological endpoints, such as PaO2/FiO2 ratio. The results of this six-month follow-up indicate an association between iNO and better PFT results at six months post-treatment. The patients receiving iNO had less restrictive defect as reflected by higher FEV% predicted, FVC % predicted and TLC. This association of iNO treatment with reduced restrictive defect was not accompanied by a significantly higher CO diffusion and raises the question of potential systemic effect of iNO on muscle function. The effects of iNO on systemic tissue beds is a rapidly expanding, but only recently recognized, area of research discovery.
The clinical significance of longer-term lung function and QoL in ARDS survivors has been examined. Although not linked in all studies, results from one long-term follow-up of ARDS survivors indicated that both FEV1 and FVC at 12 months post-episode were correlated with the physical function domain of two validated QoL questionnaires [14].
Additionally, the cumulated aggregate per-subject values for FiO2, PEEP, and PaO2/FiO2 exposure days, while not reaching statistical significance, were less in the iNO-treated patients compared with those in the group treated with placebo. Taken together, the differences in six-month PFTs, as well as the aggregate oxygenation exposure data, suggest a potential long-term physiologic effect of iNO on lung function in ARDS survivors.
There was an increased incidence of morbid obesity in placebo patients in the study. As patients with morbid obesity have an increased risk for pulmonary complications, this could be hypothesized to partially explain some of the results. Regardless of the presence or absence of morbid obesity, analyses showed that the treatment effect remained the same (Additional files 1, 2, 3 and 4). Thus, the effects seen at six months are not explained by the imbalance in number of patients with morbid obesity between placebo and treatment groups.
iNO exerts its physiologic effects via cGMP-mediated relaxation of the vascular smooth muscle and selective dilation of the pulmonary vasculature. In addition, several other potential mechanisms may underlie better performance of iNO on pulmonary function. ARDS is associated with pronounced elevations in multiple inflammatory markers [22, 23] and several studies have suggested that these may be attenuated by iNO. Studies with experimental animals [24] and ARDS patients [25] have shown that iNO significantly decreases pulmonary concentrations of IL-8 and neutrophils, as well as significantly inhibiting the formation of platelet-leukocyte aggregates (an effect correlated with an NO-dependent inhibition of platelet P-selectin expression). This may potentially lead to improvement of microcirculation in vascular beds, including muscle [26]. In another study with ARDS patients, iNO was found to significantly decrease H2O2 production and β2-integrin CD11b/CD18 expression by polymorphonuclear leukocytes. In addition, iNO decreased IL-6 and IL-8 concentrations in bronchoalveolar lavage (BAL) fluid [27]. The well-known mechanism of action for iNO (for example, cGMP-mediated vasodilatation) likely explains the decreased duration of FiO2 and PEEP exposure in the original 28-day trial; the aforementioned insight into other mechanisms of iNO in ARDS patients offers additional focus for research regarding long-term effects in ARDS survivors.
In the original study, iNO did not improve short-term mortality in patients with ARDS, despite transient physiologic benefit [19]. With ARDS patients in general, 26% to 44% of deaths typically occur within 72 hours of ARDS onset; these deaths are more often attributable to events such as sepsis with multiple organ failure (30% to 50%) than to respiratory failure (13% to 19%) [28]. In addition, changes in oxygenation sustained for only 24 hours with iNO have been shown to be insufficient to alter mortality in patients with ARDS/ALI [20]. Given these data, short-term outcomes with treatments such as iNO may not prove clinically impactful; however, the longer-term impact of iNO treatment in ARDS survivors, based on the data herein and the potential mechanisms of iNO in ARDS patients, warrant additional investigation.
Limitations
This analysis had limitations, primarily: 1) the large percentage of subjects lost to follow-up who did not have PFTs performed at six months; and 2) the inability to obtain premorbid PFTs. Even though the former constituted a protocol violation, the reasons for this occurring are not available, potentially influencing the results via significant bias or confounding. Additionally, while the lack of premorbid PFTs would have provided valuable insight, from a practical study standpoint, obtaining these values was not possible. The fact that the baseline characteristics between groups were very similar, especially with respect to severity of illness, co-morbid chronic respiratory conditions and use of inhaled corticosteroids, suggests that these potential influences may have been minimized.
There was a small but statistically significant difference in baseline SpO2 that favored the iNO group. Tidal volume was higher, and ventilator rate and mean airway pressure were lower in patients receiving iNO; however, there was no consistent pattern of these small, non-significant differences that would support an influence on pulmonary function at the six-month follow-up. Finally, the inclusion criteria of the original study did not exclude preexisting lung disease and treatment assignment was not stratified on that basis [19].
Conclusions
While current clinical research regarding ARDS treatment has focused on mortality and short-term effects of treatment, it is important to consider chronic lung effects in ARDS survivors, which could be a cause of long-term morbidity and reduction of QoL in this population. Results from this six-month analysis show that ARDS survivors who received iNO had significantly better PFT parameters versus those who received placebo, as indicated by decreased restrictive defect.
These results support consideration of further clinical trials to determine the longer-term effects of iNO on the incidence and severity of chronic lung disease in ARDS patients. Additional outcomes that should be explored include measures of health-related QoL, healthcare utilization, and overall patient management cost.
Key messages
-
1.
Multiple clinical trials have failed to show a survival benefit of inhaled nitric oxide (INO) in ARDS.
-
2.
Inhaled nitric oxide has known actions that might be associated with long term improvement in pulmonary function
-
3.
Long term functional outcome differences might be present for acute interventions in ARDS that target 28 day mortality even if primary outcome endpoint is not met.
-
4.
In a large randomized clinical trial that failed to demonstrate survival benefits when ARDS was treated with INO, PFTs performed at 180 days in a survivor sample demonstrated less restrictive defect in survivors given INO.
-
5.
Additional studies are needed to prove or disprove this secondary endpoint analysis.
Abbreviations
- AE:
-
adverse event
- ALI:
-
acute lung injury
- ARDS:
-
acute respiratory distress syndrome
- AUC:
-
area under the curve
- cGMP:
-
cyclic guanosine 3',5'-monophosphate
- FEF:
-
forced expiratory flow
- FEF25-75%:
-
forced expiratory flow from 25% to 75% of forced vital capacity
- FEV1:
-
forced expiratory volume in 1 second
- FiO2:
-
fraction of inspired oxygen
- FRC:
-
functional residual capacity
- FVC:
-
forced vital capacity
- IL:
-
interleukin
- iNO:
-
inhaled nitric oxide
- PaCO2:
-
arterial partial pressure of CO2
- PaO2:
-
partial pressure of arterial oxygen
- PEEP:
-
positive end-expiratory pressure
- PFT:
-
pulmonary function testing
- QoL:
-
quality of life
- SpO2:
-
arterial oxygen saturation by pulse oximetry
- TLC:
-
total lung capacity.
References
INOmax [package insert] Clinton, NJ: INO Therapeutics; 2009.
Klinger JR: Inhaled nitric oxide in ARDS. Crit Care Clin 2002, 18: 45-68. vi 10.1016/S0749-0704(03)00064-2
Blanch L, Joseph D, Fernandez R, Mas A, Martinez M, Vallés J, Diaz E, Baigorri F, Artigas A: Hemodynamic and gas exchange responses to inhalation of nitric oxide in patients with the acute respiratory distress syndrome and in hypoxemic patients with chronic obstructive pulmonary disease. Intensive Care Med 1997, 23: 51-57. 10.1007/s001340050290
Ware LB, Matthay MA: The acute respiratory distress syndrome. N Engl J Med 2000, 342: 1334-1349. A published erratum appears in N Engl J Med 2000, 343:520 10.1056/NEJM200005043421806
Valta P, Uusaro A, Nunes S, Ruokonen E, Takala J: Acute respiratory distress syndrome: frequency, clinical course, and costs of care. Crit Care Med 1999, 27: 2367-2374. 10.1097/00003246-199911000-00008
Bone RC, Slotman G, Maunder R, Silverman H, Hyers TM, Kerstein MD, Ursprung JJ: Randomized double-blind, multicenter study of prostaglandin E1 in patients with the adult respiratory distress syndrome Prostaglandin E1 Study Group. Chest 1989, 96: 114-119. 10.1378/chest.96.1.114
Suter PM, Domenighetti G, Schaller MD, Laverriere MC, Ritz R, Perret C: N-acetylcysteine enhances recovery from acute lung injury in man. A randomized, double-blind, placebo-controlled clinical study. Chest 1994, 105: 190-194. 10.1378/chest.105.1.190
Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M, National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network: Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006, 354: 1671-1684.
Spragg RG, Lewis JF, Walmrath HD, Johannigman J, Bellingan G, Laterre PF, Witte MC, Richards GA, Rippin G, Rathgeb F, Häfner D, Taut FJ, Seeger W: Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med 2004, 351: 884-892. 10.1056/NEJMoa033181
Reines HD, Halushka PV, Olanoff LS, Hunt PS: Dazoxiben in human sepsis and adult respiratory distress syndrome. Clin Pharmacol Ther 1985, 37: 391-395. 10.1038/clpt.1985.60
Tuxen DV, Wilson JW, Cade JF: Prevention of lower respiratory herpes simplex virus infection with acyclovir in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 1987, 136: 402-405. 10.1164/ajrccm/136.2.402
Adhikari N, Burns KE, Meade MO: Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med 2004, 3: 307-328. 10.2165/00151829-200403050-00005
Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS, Canadian Critical Care Trials Group: One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003, 348: 683-693. 10.1056/NEJMoa022450
Heyland DK, Groll D, Caeser M: Survivors of acute respiratory distress syndrome: relationship between pulmonary dysfunction and long-term health-related quality of life. Crit Care Med 2005, 33: 1549-1556. 10.1097/01.CCM.0000168609.98847.50
Neff TA, Stocker R, Frey HR, Stein S, Russi EW: Long-term assessment of lung function in survivors of severe ARDS. Chest 2003, 123: 845-853. 10.1378/chest.123.3.845
Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP: Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA 1999, 281: 354-360. 10.1001/jama.281.4.354
Angus DC, Clermont G, Linde-Zwirble WT, Musthafa AA, Dremsizov TT, Lidicker J, Lave JR, NO-06 Investigators: Healthcare costs and long-term outcomes after acute respiratory distress syndrome: a phase III trial of inhaled nitric oxide. Crit Care Med 2006, 34: 2883-2890.
Schein RM, Quartin AA: Acute respiratory distress syndrome and long-term outcomes: what should we follow? Crit Care Med 2005, 33: 1656-1658. 10.1097/01.CCM.0000170176.22020.76
Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K Jr, Kelly KM, smith TC, Small RJ, Inhaled Nitric Oxide in ARDS Study Group: Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA 2004, 291: 1603-1609. 10.1001/jama.291.13.1603
Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO: Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ 2007, 334: 779. 10.1136/bmj.39139.716794.55
Schelling G, Stoll C, Vogelmeier C, Hummel T, Behr J, Kapfhammer HP, Rothenhäusler HG, Haller M, Durst K, Krauseneck T, Briegel J: Pulmonary function and health-related quality of life in a sample of long-term survivors of the acute respiratory distress syndrome. Intensive Care Med 2000, 26: 1304-1311. 10.1007/s001340051342
Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR: Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1996, 154: 602-611.
Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F, Park DR, Pugin J, Skerrett SJ, Hudson LD, Martin TR: Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001, 164: 1896-1903.
El Kebir D, Hubert B, Taha R, Troncy E, Wang T, Gauvin D, Gangal M, Blaise G: Effects of inhaled nitric oxide on inflammation and apoptosis after cardiopulmonary bypass. Chest 2005, 128: 2910-2917. 10.1378/chest.128.4.2910
Gries A, Herr A, Kirsch S, Günther C, Weber S, Szabo G, Holzmann A, Böttiger BW, Martin E: Inhaled nitric oxide inhibits platelet-leukocyte interactions in patients with acute respiratory distress syndrome. Crit Care Med 2003, 31: 1697-1704. 10.1097/01.CCM.0000063446.19696.D3
Kawabata A, Kuroda R, Nishikawa H, Asai T, Kataoka K, Taneda M: Enhancement of vascular permeability by specific activation of protease-activated receptor-1 in rat hindpaw: a protective role of endogenous and exogenous nitric oxide. Br J Pharmacol 1999, 126: 1856-1862. 10.1038/sj.bjp.0702513
Chollet-Martin S, Gatecel C, Kermarrec N, Gougerot-Pocidalo MA, Payen DM: Alveolar neutrophil functions and cytokine levels in patients with the adult respiratory distress syndrome during nitric oxide inhalation. Am J Respir Crit Care Med 1996, 153: 985-990.
Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP: Causes and timing of death in patients with ARDS. Chest 2005, 128: 525-532. 10.1378/chest.128.2.525
Acknowledgements
This study was sponsored by INO Therapeutics LLC (formerly Ohmeda PPD, Inc.), a subsidiary of Ikaria, Inc. and statistical analysis of the data was conducted by Ikaria, Inc.
Dr. Dellinger, Dr. Goldstein, and Mr. Young had full access to the data and will take responsibility for the integrity of the data analysis. Editorial support for this article was provided by Albert M. Balkiewicz of Peloton Advantage, LLC and was funded by INO Therapeutics LLC, a subsidiary of Ikaria, Inc.
All of the authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Cooper University Hospital has been, in the past, reimbursed by Ikaria for consulting time by R. Phillip Dellinger. Robert W. Taylor, Janice L. Zimmerman, and Gerard J. Criner have no financial disclosure. Stephen Trzeciak receives material support for an ongoing clinical trial of inhaled nitric oxide from Ikaria, Inc. Mr. Young, Dr Usansky (former) and Dr. Goldstein are employees of Ikaria, Inc. Mr. Young and Dr Goldstein have stock in that company. This study was sponsored by INO Therapeutics LLC (formerly Ohmeda PPD, Inc.). Editorial support for this article was provided by Albert M. Balkiewicz, MSc, of Peloton Advantage, LLC and was funded by INO Therapeutics LLC, a subsidiary of Ikaria, Inc. Statistical analysis was provided by Ikaria, Inc.
Authors' contributions
GC, PD, RT, JZ and JY participated in the study concept and design. GC and RT participated in the acquisition of data. JY performed the statistical analysis. GC, RP, BG, RT, ST, HU and JY participated in the analysis and interpretation of the data. PD, BG and JY participated in drafting the manuscript. All authors participated in the critical revision of the manuscript for important intellectual content and read and approved the final version.
Electronic supplementary material
13054_2011_474_MOESM1_ESM.RTF
Additional file 1:Pulmonary function test results at six months in subjects with morbid obesity. Demonstrates full pulmonary function studies in enrolled patients who were morbidly obese at time of enrollment. (RTF 87 KB)
13054_2011_474_MOESM2_ESM.RTF
Additional file 2:Pulmonary function test results at six months in subjects without morbid obesity. Demonstrates full pulmonary function studies in enrolled patients who were not morbidly obese at time of enrollment. (RTF 87 KB)
13054_2011_474_MOESM3_ESM.RTF
Additional file 3:Obesity effect on pulmonary function test results at six months in subjects treated with placebo. This data demonstrate the effect of obesity on pulmonary function tests performed six months from the enrollment in the study who were treated with placebo. (RTF 87 KB)
13054_2011_474_MOESM4_ESM.RTF
Additional file 4:Obesity effect on pulmonary function test results at six months in subjects treated with INO. This data demonstrate the effect of obesity on pulmonary function tests performed 6 months from the enrollment in the study who were treated with inhaled nitric oxide. (RTF 87 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Dellinger, R.P., Trzeciak, S.W., Criner, G.J. et al. Association between inhaled nitric oxide treatment and long-term pulmonary function in survivors of acute respiratory distress syndrome. Crit Care 16, R36 (2012). https://doi.org/10.1186/cc11215
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc11215