Abstract
Introduction
Previous meta-analyses of magnesium sulphate infusion in the treatment of aneurysmal subarachnoid hemorrhage (SAH) have become outdated due to recently published clinical trials. Our aim was thus to perform an up-to-date systemic review and meta-analysis of published data on the use of magnesium sulphate infusion in aneurysmal SAH patients.
Methods
A systemic review and meta-analysis of the literature was carried out on published randomized controlled clinical trials that investigated the efficacy of magnesium sulphate infusion in aneurysmal SAH patients. The results were analyzed with regard to delayed cerebral ischemia (DCI), delayed cerebral infarction, and favorable neurological outcomes at three and six months. The risks of bias were assessed using the Jadad criteria, with a Jadad score >3 indicating a lower such risk. Meta-analyses are presented in terms of relative risk (RR) with 95% confidence intervals (CIs).
Results
Six eligible studies with 875 patients were reviewed. The pooled RR for DCI was 0.87 (95% CI, 0.36 to 2.09; P = 0.75). That for delayed cerebral infarction was 0.58 (95% CI, 0.35 to 0.97; P = 0.04), although this result did not persist if only randomized clinical trials with a lower risk of bias were included (RR 0.61, 95% CI, 0.31 to 1.22; P = 0.17). The pooled RR for a favorable outcome at three months was 1.14 (95% CI, 0.99 to 1.31; P = 0.07), and that for a favorable outcome at six months was 1.08 (95% CI, 0.94 to 1.24; P = 0.29).
Conclusions
The present findings do not lend support to a beneficial effect of magnesium sulphate infusion on delayed cerebral infarction. The reduction in DCI and improvement in the clinical outcomes of aneurysmal SAH patients following magnesium sulphate infusion observed in previous pilot studies are not confirmed, although a beneficial effect cannot be ruled out because of sample size limitation.
Similar content being viewed by others
Introduction
Although spontaneous subarachnoid hemorrhage (SAH) accounts for only 3 to 5% of all strokes and 4.4% of deaths from stroke [1, 2], the relative youth of the individuals affected means that it is actually responsible for approximately 25% of all years of life lost as a result of stroke [3]. Such complications as early brain injury and delayed ischemic neurological deficits remain a major cause of morbidity and mortality in this group of patients.
Pilot clinical trials using magnesium sulphate in patients with acute aneurysmal SAH have reported a trend toward a reduction in clinical deterioration due to delayed cerebral ischemia (DCI) and an improvement in clinical outcomes [4–11], although two recently completed clinical trials failed to demonstrate any improvement in neurological outcomes [12, 13]. Interestingly, however, a German trial found a significant decrease in delayed cerebral infarction but no improvement in neurological outcomes, in contrast to other retrospective analyses [13–15].
Previous meta-analyses of the use of magnesium sulphate infusion to treat aneurysmal SAH have become outdated due to recently published clinical trials [16–18]. Hence, we conducted an up-to-date literature review and meta-analysis of published data on patients with DCI, delayed cerebral infarction, or neurological outcomes.
Materials and methods
Type of studies
We included only randomized controlled clinical trials comparing magnesium sulphate infusion to placebo infusion for patients with acute aneurysmal SAH.
Types of outcome measures
The primary outcome was dichotomized neurological outcome using Glasgow Outcome Scale (GOS) [19] and modified Rankin Scale (mRS) [20] at three and six months. We also assessed surrogate outcome with DCI and delayed cerebral infarction according to a recent consensus paper [15].
Definition of outcome measures
In our systemic review and meta-analysis, DCI was defined as the occurrence of: clinical deterioration, which was manifested clinically as a new focal neurological deficit (motor or speech deficit) that developed after SAH or a decrease in the Glasgow Coma Scale of two or more points for more than six hours; and/or delayed cerebral infarction, which was defined as a new cerebral infarction within three weeks that was not related to post-treatment (coiling or clipping) complications, the ventricular catheter track, a rebleed, or hydrocephalus [15].
Another outcome, delayed cerebral infarction, was similarly defined as a new cerebral infarction within three weeks that was not related to post-treatment (coiling or clipping) complications, the ventricular catheter track, a rebleed, or hydrocephalus.
Neurological outcomes were defined by the GOS [19] and mRS [20]. A favorable outcome was defined as a GOS score of 4 to 5 or a mRS score of 0 to 2.
Search strategy
Cochrane Central Register of Controlled Trials (Clinical Trials), EMBASE, PubMed, and Ovid MEDLINE searches (using the keywords magnesium AND subarachnoid hemorrhage) of studies employing randomized controlled trials and published between 1 January, 1980 and 15 June, 2010 were carried out. The references listed in these publications were also searched for relevant studies.
Risk of bias assessment
The Jadad criteria were used to assess the risk of bias [21]. The criteria included randomization, blinding, and an explanation of withdrawal or loss to follow up. Clinical trials with Jadad scores of 3 or above were considered to be of high quality. No funnel plot was employed to test for asymmetry, because fewer than 10 clinical trials were ultimately included.
Statistical analysis
Statistical analyses were generated using SPSS for Windows Version 15.0 (SPSS Inc., Chicago, Illinois, USA) and Review Manager 5 (Cochrane Collaboration, Oxford, UK). Statistical significance was taken as a P value less than 0.05 or a 95% confidence interval (CI) of relative risk (RR) not including 1. Data are represented using numbers (percentages) for the categorical variables and mean +/- standard deviations for the numerical variables. The I2 value for heterogeneity describes the proportion of total variation in a study estimated to be due to heterogeneity. The random-effects model was employed to pool studies when statistical heterogeneity occurred (the P value was less than 0.1) or when the I2 value was larger than 0.3; otherwise, the fixed-effects model was used. Results are presented using the RRs and 95% CIs.
Results
Search results
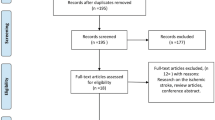
A PubMed literature search (using the keywords magnesium AND subarachnoid hemorrhage) of studies employing randomized controlled trials and published between 1 January, 1980 and 15 June, 2010 yielded 20 publications. Additional searches of the Cochrane Central Register of Controlled Trials (Clinical Trials), EMBASE, and Ovid MEDLINE (using the keywords magnesium AND subarachnoid hemorrhage and considering only human studies) yielded 51, 72, and 91 publications, respectively. The references listed in these publications were also searched for relevant studies. Examination of the abstracts and/or manuscripts revealed seven completed randomized controlled clinical trials on the use of magnesium in patients with aneurysmal SAH [6, 8–13] and one methodology-only abstract [22]. None of these studies detected a statistically significant improvement in primary clinical outcome measures.
Study descriptions
Veyna and colleagues [6] reported the results of a 40-patient prospective single-blinded clinical trial of high-dose magnesium sulphate infusion therapy (a bolus of 6 g followed by 2 g per hour intravenous infusion, with a target magnesium level of 4 to 5.5 mg/dl) following spontaneous SAH. They enrolled patients with Hunt and Hess grades II to IV at admission and presenting within 72 hours after spontaneous SAH. In the magnesium treatment group, they maintained the magnesium sulphate infusion for 10 days. They presented no data on DCI or delayed cerebral infarction. A favorable outcome (good recovery or moderate disability, as defined by GOS 4 to 5) was achieved in 13 of 20 (65%) patients receiving magnesium sulphate infusion and 10 of 20 (50%) patients receiving the placebo treatment. No data were available for excellent outcome (good recovery, as defined by GOS 5 or mRS 0 to 1).
Van den Bergh and colleagues [8] reported the magnesium group results for the Magnesium and Acetylsalicylic Acid in Subarachnoid Hemorrhage (MASH) trial, a randomized, double-blinded, placebo-controlled multicenter trial with a factorial design. The salicylic acid-related data were not complete at this stage. A total of 283 patients were randomized within four days of aneurysmal SAH. Magnesium treatment consisted of a continuous intravenous dose of 64 mmol/day, begun within four days of SAH and continued until 14 days after occlusion of the aneurysm. CT hypointensities (with clinical features of a decreased consciousness level or new focal neurological deficit) occurred in 22 of 139 (16%) magnesium-treated patients and 35 of 144 (24%) placebo-treated patients, but no data were made available for DCI and delayed cerebral infarction according to the recent consensus paper [15]. Unfavorable outcomes occurred in 38 of 139 (27%) magnesium-treated patients and 51 of 144 (35%) placebo-treated patients. There were no separate outcome data on excellent outcomes.
In our single-center pilot study [9], 60 patients were randomly allocated to receive either magnesium sulphate infusion (80 mmol/day) or saline infusion for 14 days. We did not record DCI or cerebral infarction as a separate outcome measure during this pilot study. A favorable outcome was achieved in 20 of 30 (67%) patients receiving magnesium sulphate infusion and 16 of 30 (53%) patients receiving the placebo treatment, and an excellent outcome in 14 of 30 (47%) of the former and 10 of 30 (33%) of the latter.
Muroi and colleagues [11] reported the results of a prospective, randomized, patient-blinded, placebo-controlled pilot study of 58 patients with aneurysmal SAH predominantly treated by microsurgical clipping (97%). The patients allocated to the treatment group received a bolus of 16 mmol magnesium sulphate administered over 15 minutes, followed by a continuous intravenous infusion of 64 mmol per day for 14 days. To maintain the serum magnesium level at twice the baseline, with a maximum of 2.0 mmol/L until the 14th day after SAH, subsequent dosage adjustments were made every 12 hours. Delayed cerebral infarction occurred in 3 of 31 (10%) patients in the treatment group and 6 of 27 (22%) in the placebo group. A favorable neurological outcome was achieved in 20 of 31 (64%) patients in the treatment group and 13 of 27 (48%) in the placebo group, and an excellent outcome was achieved in 18 of 31 (58%) in the former and 12 of 27 (44%) in the latter.
The Asian-Australasian Intravenous Magnesium Sulphate for Aneurysmal Subarachnoid Hemorrhage (IMASH) trial was a randomized, double-blinded, placebo-controlled, multicenter phase III trial [12], under the protocol of which patients who were diagnosed with acute aneurysmal SAH (within 48 hours of ictus) were randomly assigned to receive either intravenous magnesium sulphate infusion or normal saline infusion (placebo). For patients receiving the active treatment, 20 mmol of magnesium sulphate was administered over 30 minutes, followed by a continuous infusion of 80 mmol of magnesium sulphate per day for up to 14 days after the hemorrhage. The plasma magnesium concentration was measured regularly. The infusion was adjusted to raise the plasma magnesium concentration to approximately twice the baseline value and less than 2.5 mmol/L. The patients in the control group received an equivalent volume of normal saline. The proportions of patients with a favorable outcome were similar: 108 of 169 (64%) in the magnesium sulphate group and 100 of 158 (63%) in the saline group. So too were those with an excellent outcome: 77 of 169 (46%) in the magnesium sulphate group and 71 of 158 (45%) in the saline group. The proportions of patients with DCI and delayed cerebral infarction were also similar.
Westermaier and colleagues [13] recently reported the results of another single-center, randomized controlled clinical trial. Patients were randomly allocated to receive either magnesium sulphate infusion (with a target level of 2.0 to 2.5 mmol/L) or saline infusion for 14 days. This study reported negative clinical outcomes, but showed a reduction in DCI and delayed cerebral infarction with magnesium sulphate infusion. A favorable outcome was reported for 34 of 54 (63%) patients in the treatment group and 27 of 53 (51%) in the placebo group, and an excellent outcome for 27 of 54 (50%) in the former and 18 of 53 (34%) in the latter. DCI occurred in 20 of 53 (37%) patients in the magnesium group and 35 of 53 (66%) in the control group. Delayed cerebral infarction occurred in 12 of 54 (22%) patients in the magnesium group and 27 of 53 (51%) in the control group.
Our pilot study [9], IMASH [12], the magnesium component of the MASH study [8], and the studies published by Veyna and colleagues [6], Muroi and colleagues [11], and Westermaier and colleagues [13] were analyzed. The study by Schmid-Elsasser and colleagues [10] was excluded because of the unconventional omission of nimodipine in the magnesium group. That by Pravedello and colleagues [23] was excluded from the analysis because no measures of DCI, cerebral infarction, or clinical outcomes were reported. The six eligible studies were reviewed according to the terminologies defined in the Methods section (Table 1) The target of the magnesium arm of the six studies was to produce a similar degree of hypermagnesemia, namely, double the baseline value. All of the studies recruited patients with SAH during the acute phase, within 48 to 96 hours of the aneurysmal SAH. The magnesium infusion was maintained for 10 to 14 days, and the neurological outcome was measured using the GOS or mRS at three months [6, 8, 11] or six months [9, 12, 13], but not both.
Assessment of risks of bias in included studies
The six eligible studies were assessed for risks of bias using the Jadad criteria (Table 2). Four of the six trials were found to have lower bias risks (Jadad score above 3) [8, 9, 12, 13]. The authors of this systemic review acknowledged potential risk of bias because we were authors of two of the included studies in this systemic review [9, 12].
Meta-analysis of eligible randomized clinical trials with relevant data available according to the consensus paper definitions
Two studies with 434 patients were available for analysis of DCI. The pooled RR for DCI was 0.87 (95% CI = 0.36 to 2.09; P = 0.75; Figure 1). Three studies with 492 patients were available for analysis of delayed cerebral infarction. The pooled RR for delayed cerebral infarction was 0.58 (95% CI = 0.35 to 0.97; P = 0.04; Figure 2) [15].
Three studies with 381 patients and 3 studies with 494 patients were available for analyses of favorable outcome at three months and six months, respectively. The pooled RR for a favorable outcome at three months was 1.14 (95% CI = 0.99 to 1.31; P = 0.07; Figure 3), and that for a favorable outcome at six months was 1.08 (95% CI = 0.94 to 1.24; P = 0.29; Figure 4).
When only the four high-quality randomized clinical trials (those with a Jadad score above 3) were considered, two studies with 434 patients were available for analysis of DCI. The beneficial effect on delayed cerebral infarction did not persist (RR = 0.61, 95% CI = 0.31 to 1.22; P = 0.17; Figure 5), although the other outcomes remained statistically similar.
Discussion
The results of our up-to-date meta-analysis suggest that the present findings do not lend support to a beneficial effect of magnesium sulphate infusion in reducing DCI and leading to better clinical outcome. Some would argue that these negative findings could be due to a lack of tight neuro-intensive monitoring and treatment, thereby resulting in variation in management outcomes and diluting the neuro-protective effects of magnesium sulphate infusion [24]. However, whether tight parameter control, such as glycemic control, would result in better neurological outcomes remains debatable [24].
The recent meta-analysis by Ma and colleagues suggested that intravenous magnesium therapy reduced the risk of DCI and poor outcome after aneurysmal SAH [18]. However, they have not taken into account the widely varied outcome definitions and time points among studies and simply pooled for assessments. Moreover, two more studies had been published since the meta-analysis by Ma and colleagues. The Asian-Australasian IMASH study was the first international multi-center randomized controlled clinical trial in testing the efficacy of intravenous magnesium sulphate infusion in aneurysmal SAH with negative results in all clinical and surrogate outcomes [12]. Westermaier and colleagues found a significant 29% decrease in delayed cerebral infarction and a nonsignificant 9% decrease in unfavorable outcomes with magnesium sulphate infusion [13]. However, in the landmark British aneurysm nimodipine trial and recently published IMASH report, the differences in the proportions of delayed cerebral infarction paralleled those in the proportions of favorable outcomes [12, 25]. Whether these decreases represent a change in the pattern of detected delayed cerebral infarction with the evolution of endovascular treatment and neuro-intensive care remains to be investigated.
There are several limitations to the meta-analysis presented in this paper. The regimens of magnesium sulphate infusion varied across the different studies, and caution should thus be exercised in extrapolating the results. Other problems include the wide variation in the definitions of outcome measures and the time points of assessment, making a smaller number of studies available for meta-analyses of different outcome measures. Furthermore, there may be a publication bias toward the reporting of positive trends and spuriously inflated effects in smaller studies. Statistical heterogeneities were noted in the meta-analyses for DCI and delayed cerebral infarction. The risk of bias was assessed with Jadad criteria [22]; the other option would have been to employ domain-based evaluation [26]. However, any proposed tool would have difficulties to validate, and realistic assessment is eventually open to subjectivity. The recent consensus is that cerebral infarction on plain computed tomography (CT) scan is the most objective measure of DCI, based on the British Aneurysm Nimodipine Trial and other retrospective analyzes [15, 5], in which the data were obtained primarily from microsurgically treated intracranial aneurysms. However, identifying delayed cerebral infarction from among all causes of CT hypointensities can be difficult with plain CT alone [27].
Although our post-hoc analysis of the IMASH data did not suggest a higher achieved plasma magnesium concentration to be associated with a better clinical outcome [28], the current evidence suggests that the development of a clinical trial targeting reductions in symptomatic vasospasm and cerebral infarction, and an improvement in clinical outcomes, would require a large sample size (as many as 2,445 patients) to demonstrate the efficacy of such treatment in improving neurological outcomes, even with a treatment effect size of 50% [29]. Finally, the relatively small number of patients in the present meta-analysis could mask a possible smaller beneficial effect of magnesium sulphate infusion. Another European multi-center trial (Magnesium in Aneurysmal Subarachnoid Hemorrhage: MASH II) employing a slightly lower dosage regimen is ongoing and should contribute further data for future meta-analysis of the use of magnesium sulphate infusion in patients with aneurysmal SAH [30].
Conclusions
The reduction in DCI and improvement in clinical outcomes for aneurysmal SAH patients with magnesium sulphate infusion observed in previous pilot studies has not been confirmed although a beneficial effect cannot be ruled out because of sample size limitation.
Key messages
-
Pilot studies have suggested the possible beneficial effects of magnesium sulphate infusion in treating patients with aneurysmal SAH, but previous meta-analyses have become outdated by recently published clinical trials.
-
An up-to-date systemic review and meta-analysis showed that magnesium sulphate infusion does not reduce DCI or improve neurological outcomes although a beneficial effect cannot be ruled out because of sample size limitation.
Abbreviations
- CI:
-
confidence interval
- CT:
-
computed tomography
- DCI:
-
delayed cerebral ischemia
- GOS:
-
Glasgow Outcome Scale
- IMASH:
-
Intravenous Magnesium Sulphate for Aneurysmal Subarachnoid Hemorrhage trial
- MASH:
-
Magnesium and Acetylsalicylic Acid in Subarachnoid Hemorrhage trial
- MASH:
-
Magnesium and Acetylsalicylic Acid in Subarachnoid Hemorrhage
- mRS:
-
modified Rankin Scale
- RR:
-
relative risk
- SAH:
-
subarachnoid hemorrhage.
References
Sudlow CL, Warlow CP: Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. Stroke 1997, 28: 491-499.
Wong GK, Ng RY, Poon WS: Aneurysmal subarachnoid haemorrhage. Surgical Practice 2008, 12: 51-55. 10.1111/j.1744-1633.2008.00397.x
Johnston SC, Selvin S, Gress DR: The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology 1998, 50: 1413-1418.
Boet R, Mee E: Magnesium sulphate in the management of patients with Fisher grade 3 subarachnoid hemorrhage: a pilot study. Neurosurgery 2000, 47: 602-607. 10.1097/00006123-200009000-00014
Chia RY, Hughes RS, Morgan MK: Magnesium: a useful adjunct in the prevention of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Clin Neurosci 2002, 9: 279-281. 10.1054/jocn.2001.1039
Veyna RS, Seyfried D, Burke DG, Zimmerman C, Mlynarek M, Nichols V, Marrocco A, Thomas AJ, Mitsias PD: Magnesium sulphate therapy after aneurysmal subarachnoid hemorrhage. J Neurosurg 2002, 96: 510-514. 10.3171/jns.2002.96.3.0510
van den Bergh WM, Albrecht KW, Berkelbach van der Sprenkel JW, Rinkel GJ: Magnesium therapy after aneurysmal subarachnoid haemorrhage: a dose-finding study for long term treatment. Acta Neurochir (Wien) 2003, 145: 195-199. 10.1007/s00701-002-1064-9
van den Bergh WM, on behalf of the MASH Study Group: Magnesium sulphate in aneurysmal subarachnoid hemorrhage. Stroke 2005, 36: 1011-1015. 10.1161/01.STR.0000160801.96998.57
Wong GK, Chan MT, Boet R, Poon WS, Gin T: Intravenous magnesium sulphate after aneurysmal subarachnoid hemorrhage: a prospective randomized pilot study. J Neurosurg Anesthesiol 2006, 18: 142-148. 10.1097/00008506-200604000-00009
Schmid-Elsaesser R, Kunz M, Zausinger S, Prueckner S, Briegel J, Steiger HJ: Intravenous magnesium versus nimodipine in the treatment of patients with aneurysmal subarachnoid hemorrhage: a randomized study. Neurosurgery 2006, 58: 1054-1065. 10.1227/01.NEU.0000215868.40441.D9
Muroi C, Terzic A, Fortunati M, Yonekawa Y, Keller E: Magnesium sulphate in the management of patients with aneurysmal subarachnoid hemorrhage: a randomized, placebo-controlled, dose-adapted trial. Surg Neurol 2008, 69: 33-39. 10.1016/j.surneu.2007.07.015
Wong GK, Poon WS, Boet R, Chan MT, Gin T, Ng SC, Zee B, IMASH investigators: Intravenous magnesium sulphate after aneurysmal subarachnoid hemorrhage: a multi-center phase III study. Stroke 2010, 41: 921-926. 10.1161/STROKEAHA.109.571125
Westermaier T, Stetter C, Vince GH, Pham M, Tejon JP, Eriskat J, Kunze E, Matthies C, Ernestus RI, Solymosi L, Roosen K: Prophylactic intravenous magnesium sulfate for treatment of aneurysmal subarachnoid hemorrhage: a randomized placebo-controlled, clinical study. Crit Care Med 2010, 38: 1284-1290. 10.1097/CCM.0b013e3181f17878
Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL: Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke 2007, 38: 2315-2321. 10.1161/STROKEAHA.107.484360
Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, Terbrugge KG, Macdonald RL, Diringer MN, Broderick JP, Drier JP, Roos YB: Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observation studies: proposal of a multidisciplinary research group. Stroke 2010, 41: 2391-2395. 10.1161/STROKEAHA.110.589275
Dorhout Mees SM, Rinkel GJ, Feign VL, Algra A, van den Bergh WM, Vermeulen M, van Gijn J: Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2007, (3):CD000277.
Zhao XD, Zhou YT, Zhang X, Zhuang Z, Shi JX: A meta-analysis of treating subarachnoid hemorrhage with magnesium sulfate. J Clin Neurosci 2009, 16: 1394-1397. 10.1016/j.jocn.2009.05.001
Ma L, Lu WG, Zhang JM, Chen G, Fan J, Sheng HS: Magnesium sulphate in the management of patients with aneurysmal subarachnoid haemorrhage: a meta-analysis of prospective controlled trials. Brain Injury 2010, 24: 730-735. 10.3109/02699051003610516
Wilson JTL, Pettigrew LEL, Teasdale GM: Structured interviews for the Glasgow Outcome Scale and Extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma 1998, 15: 573-585. 10.1089/neu.1998.15.573
Wilson JTL, Hareendran A, Grant M, Baird T, Schulz UG, Muir KW, Bone I: Improving the assessment of outcomes in stroke: use of a structural interview to assign grades on the modified Rankin Scale. Stroke 2002, 33: 2243-2246. 10.1161/01.STR.0000027437.22450.BD
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavanghan DJ, McQuay HJ: Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996, 17: 1-12. 10.1016/0197-2456(95)00134-4
Macedo SK, Nuss RM, Pereira S, Siqueira CM, Siquerira SB, Lima DP: Effect of magnesium on prophylaxis of vasospasm, morbidity, and mortality in subarachnoid hemorrhage patients. Critical Care 2009, 13: P54. 10.1186/cc7856
Prevedello DM, Cordeiro JG, de Morais AL, Saucedo NS Jr, Chen IB, Araujo JC: Magnesium sulfate: role as possible attenuating factor in vasospasm morbidity. Surg Neurol 2006, 65 Suppl 1: S1:14-S1:20. 10.1016/j.surneu.2005.11.035
Keller E, Muroi C: Magnesium sulfate for subarachnoid hemorrhage: a piece of the mosaic. Stroke 2010, 41: e576. author reply e577 10.1161/STROKEAHA.110.589903
Pickard JD, Murray GD, Illingworth R, Shaw MD, Teasdale GM, Foy PM, Humphrey PR, Lang DA, Nelson R, Richards P, Sinar J, Bailey S, Skene A: Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ 1989, 298: 636-642. 10.1136/bmj.298.6674.636
Higgins JP, Altman DG, (editors): Assessing risk of bias in included studies. In Cochrane Handbook for Systemic Reviews of Interventions. Edited by: Higgins JP, Green S. Chichester (UK): John Wiley & Sons; 2008.
Hopyan J, Ciarallo A, Dowlatshahi D, Howard P, John V, Yeung R, Zhang L, Kim J, MacFarlane G, Lee TY, Aviv RI: Certainty of stroke diagnosis: incremental benefit with CT perfusion over noncontrast CT and CT angiography. Radiology 2010, 255: 142-153. 10.1148/radiol.09091021
Wong GK, Poon WS, Boet R, Chan MT, Gin T, Ng SC, Zee B, IMASH investigators: Plasma magnesium concentrations and clinical outcomes in aneurysmal subarachnoid hemorrhage patients: post-hoc analysis of Intravenous Magnesium Sulphate for Aneurysmal Subarachnoid Hemorrhage (IMASH) Trial. Stroke 2010, 41: 1841-1844. 10.1161/STROKEAHA.110.585232
Kreiter KT, Mayer SA, Howard G, Knappertz V, Ilodigwe D, Sloan MA, Macdonald RL: Sample size estimates for clinical trials of vasospasm in subarachnoid hemorrhage. Stroke 2009, 40: 2362-2367. 10.1161/STROKEAHA.109.547331
van den Bergh WM: Magnesium in subarachnoid haemorrhage: proven beneficial? Magnes Res 2009, 22: 121-126.
Acknowledgements
We would especially like to thank the patients and their relatives who agreed to participate in the clinical trials discussed herein, as well as all of the medical and nursing staff in the participating centers who supported these trials. The IMASH trial was partly supported by the Research Grants Council of Hong Kong [CUHK Ref. No. CUHK4183/02M].
IMASH was approved by the CUHK-NTEC Clinical Research Ethics Committee [CRE-2002.078-T], and informed written consent was obtained from all IMASH patients participating in the study and for the publication of the manuscripts, tables and/or figures. Copies of their written consent forms are available for review from the editor-in-chief of Critical Care.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All of the authors contributed to the design of the study. GKW, SCN, and BZ were responsible for the statistical analysis. GKW drafted the manuscript. All of the authors critically revised the manuscript and agreed on the submitted version.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Wong, G.K., Boet, R., Poon, W.S. et al. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage: an updated systemic review and meta-analysis. Crit Care 15, R52 (2011). https://doi.org/10.1186/cc10017
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc10017