Abstract
Background
The androgen-regulated proteins prostate-specific antigen (PSA) and prostate-specific acid phosphatase (PSAP) are present in high concentrations in normal prostate and prostatic cancer and are considered to be tissue-specific to prostate. These markers are commonly used to diagnose metastatic prostate carcinoma at various sites including the male breast. However, expression of these two proteins in tumors arising in tissues regulated by androgens such as male breast carcinoma has not been thoroughly evaluated.
Methods
In this study we analyzed the expression of PSA, PSAP and androgen receptor (AR) by immunohistochemistry in 26 cases of male breast carcinomas and correlated these with the expression of other prognostic markers.
Results
AR, PSA and PSAP expression was observed in 81%, 23% and 0% of carcinomas, respectively. Combined expression of AR and PSA was observed in only four tumors.
Conclusion
Although the biological significance of PSA expression in male breast carcinomas is not clear, caution should be exercised when it is used as a diagnostic marker of metastatic prostate carcinoma.
Similar content being viewed by others
Introduction
Male breast carcinoma (MBC) is rare and represents less than 1% of all mammary carcinomas [1]. Although less frequent, it tends to be more aggressive than its female counterpart [2, 3]. One of the reasons for this is the relatively small size of the male breast, which may permit earlier and/or easier access for the tumor cells to the chest wall. Because of the rarity of the disease, randomized clinical trials have not been performed and the treatment of MBC has not been standardized [4]. MBCs are treated, like female breast carcinomas (FBCs), with radical mastectomy followed by adjuvant chemotherapy and radiation. Tamoxifen has also been used in estrogen receptor (ER)-positive cases.
It is presumed that MBC is biologically similar to FBC. However, there are differences between the two: a higher frequency of expression of ER (81%) and progesterone receptor (PR) (74%) is in MBC (for review see [5]). In contrast to women, men do not have a higher incidence of ER-positive tumors with advancing age. The relationship of ER with overall survival is uncertain. In a multi-institutional study, Donegan and colleagues [6] found that ER was associated with better survival; however, in a series of 229 patients from the Princess Margaret Hospital no significance was found in multivariate analysis [7]. Wang-Rodriguez and colleagues found better survival in ER-positive patients but did not find a benefit from treatment with Tamoxifen [8]. Proliferative activity is higher in ER+/PR+MBCs than in ER-/PR- tumors [9]. More recently, Pich and colleagues [4] found no correlation between the expression of ER, PR or androgen receptor (AR) with overall survival. They concluded that MBCs are biologically different from FBCs and questioned the rationale for the use of anti-hormonal (Tamoxifen) therapy in MBCs.
The male sex hormones (5α-androstan-3α,17β-diol dipropionate; 2α-methyl dihydro testosterone; and testosterone propionate) have been shown to cause regression of 7,12-dimethylbenz[a]anthracene-induced breast cancers in rats [10] and to suppress the growth of breast cancer cell lines. Additionally, an increased risk of breast cancer is seen in patients with hypoandrogenism and gynecomastia. They have therefore been considered protective agents in the etiopathogenesis of male breast cancer [5, 6, 11]. Androgens in the prostate stimulate the secretion of androgen-dependent proteins such as prostate-specific antigen (PSA) and prostate-specific acid phosphatase (PSAP). Similarly, the production of PSA is seen in some breast cancer cell lines after treatment with androgens [12, 13], suggesting an intact androgen pathway. In this retrospective study we attempt to analyze the functional status of the AR pathway in MBCs by assessing the expression of the androgen-regulated proteins PSA and PSAP.
Materials and methods
Twenty-six cases of MBC were retrieved from the archives of the Department of Pathology at Northwestern Memorial Hospital and Lakeside VA Hospital, Chicago, IL, between the years 1977 and 1999. Clinical charts were reviewed to obtain follow-up information. Archival paraffin slides were reviewed to confirm histologic grade in accordance with the modified Scarff–Bloom–Richardson system [14]. Data about the expression status in these tumors of ER/PR, Her-2/Neu, epidermal growth factor receptor and Ki-67 were also available for comparison.
One block from each case was selected to analyze the expression of PSA, PSAP and AR on serial sections by immunohistochemical methods. Paraffin-embedded sections (4 μm thick) after peroxide blocking (3% hydrogen peroxide in methanol) and antigen retrieval (a microwave oven pressure cooker with Citra [BioGenex®]) were incubated with serum block to reduce non-specific staining before exposure to the primary antibody. Polyclonal antibodies against PSA and PSAP (both from Dako Corp®; 1:1800), and monoclonal antibody against AR (Novocastra®, 1:10) were used. Biotin-labeled secondary antibody and ABC complex (Vector®) followed by exposure to diaminobenzidine tetrahydrochloride (Sigma Corp®) were used to detect the antigen. The slides were counter-stained with hematoxylin and mounted in Permount. Phosphate-buffered saline and rabbit or mouse isotypic antibody were used to confirm specificity of the staining. Normal prostatic tissue (from other patients) was used for positive control for AR, PSA and PSAP.
The criteria used to evaluate expression were as follows. Expression of PSA and PSAP was classified as focal or diffuse depending on the fraction of the tumor cells stained. The criteria for AR positivity were based on the intensity and percentage of tumor cells showing expression. The intensity was graded as negative, weak, moderate or strong. Tumors that had more than 10% of cells exhibiting moderate or strong intensity of expression were considered positive.
We reviewed the databases of the Northwestern Memorial Hospital as well as Lakeside VA Hospital to obtain the follow-up information of MBC. Follow-up information was available for 17 cases. The overall follow-up ranged from 12 to 168 months; the median follow-up was 48 months.
The expression of the antigens was correlated with clinical and histological features including tumor grade, mitotic activity, tumor size, histological grade, steroid receptors, axillary lymph node status, and clinical outcome. Significance of the association was assessed by the χ2 test for independence with the use of the Prophet® Statistical Program (BBN Systems and Technology). P values less than 0.05 were considered significant.
Results
Histologically, 25 of the tumors were infiltrating ductal carcinomas; the remaining tumor was an infiltrating lobular carcinoma. Three cases were grade I, 11 cases were grade II and 12 cases were grade III (Table 1). AR-positive cases were histologically classified as grade I (3 of 3 cases), grade II (7 of 11 cases) and grade III (9 of 12 cases). ER was expressed in 22 of the 26 cases (84.6%), whereas PR expression was seen in 13 cases (50%). All the cases that expressed PR were ER-positive. Expression of Her-2 was seen in 18 cases, epidermal growth factor receptor in 12 cases and p53 in one case. Ki-67 expression in more than 30% of the cells was seen in 21 cases. Of the 19 cases with axillary dissection, 10 showed lymph node metastases.
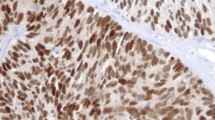
AR expression, like that of ER and PR, was seen in the nuclei of breast epithelial cells (Fig. 1) and in scattered stromal cells. The expression in tumor cells was seen in 19 of 26 cases (73%).
In contrast, PSA expression was cytoplasmic (Fig. 2). Normal breast tissue, both ducts and stromal elements (including adipocytes and vascular endothelium), did not show any expression of this protein. Expression was observed in six (23%) cases of tumors; five of these (19%) showed focal expression, and one (4%) demonstrated diffuse staining. These PSA-positive cases were histologically grade II and III tumors (three cases each). PSAP expression was not detected in any of the cases studied.
Correlation between the expression of PSA and AR with other parameters is shown in Tables 2 and 3 respectively. PSA expression was not correlated with AR expression (P = 0.61), ER (P = 0.16), or PR (P = 0.35). Similarly, expression of AR did not correlate with any of these parameters. Neither of these markers (PSA and AR) was significantly associated with positive lymph node status. There was no significant association of PSA or AR with overall follow-up, and neither was able to predict the clinical behavior in these patients.
Discussion
Because MBC is rare, it has often been suggested that metastatic carcinoma from other sites should be considered before diagnosis of a primary tumor [15]. Prostatic carcinoma is one of the many primary sites from which metastases can arise. It has been suggested that immunohistochemistry should be performed to rule out this possibility [15]. In the present study we demonstrated the expression of PSA in 6 of 26 cases of MBC. We were unable to observe any expression of PSAP in these cases. Similar findings were reported by Gatalica and colleagues [16] in their cases of MBC and gynecomastia. Cho and Epstein [17] reported coexpression of PSAP and PSA in 23 of 25 cases of metastatic prostatic carcinoma involving supra-diaphragmatic lymph nodes. Taken together, these findings suggest that PSAP might be a better marker for excluding metastases from a prostatic primary at this site. The reasons for the differential expression of PSA and PSAP are not known; however, similar divergence of expression has previously been recorded.
PSA and PSAP are androgen-regulated proteins and are thought to be relatively specific to the prostate. Although serum PSA in males is predominantly derived from the prostate, there are additional sources of this protein: low levels are found even in females [18–20]. PSA has been shown to be present in several sites including salivary glands and the breast. Whether PSA is expressed in normal healthy breast is controversial [21], although Yu and colleagues [22], using quantitative methods and polymerase chain reaction, were able to demonstrate PSA in normal breast tissues in 33% of cases. PSA has been demonstrated in milk from lactating breast [18–20], in nipple aspiration fluid [23], and in apocrine foci within fibrocystic disease of the breast [24] in addition to breast cancer (for review see [25]). In male breast, Gatalica and colleagues [16] have reported focal strong PSA expression in normal and hyperplastic ductal epithelium in 5 of 18 cases of gynecomastia. In our study we did not observe any immunoreactivity for PSA in peritumoral breast tissues.
PSA from breast tumors has the same molecular characteristics as seminal or prostatic PSA. In the prostatic secretions PSA is important in the dissolution of the gel structure of freshly ejaculated semen [26], through the specific proteolysis of both high molecular mass semenogelin and fibronectin. PSA, like some other proteases, can also digest other substrates. It can, for example, digest insulin-like growth factor-binding proteins, leading to the release of these growth factors [27]. Similarly, digestion of the basement membrane and extracellular matrix proteins might facilitate cell migration and invasion [27]. However, PSA has also been shown to inhibit the endothelial cell response to angiogenic stimulation by fibroblast growth factor-2 and vascular endothelial growth factor [28]. It has been suggested that PSA might be an endogenous anti-angiogenic compound. This might contribute to the association between PSA and prognosis seen in some studies [29, 30].
PSA expression in FBC has been associated with younger age [31, 32] in some studies but with postmenopausal status in others [33]. Alanen and colleagues [33] and Howarth and colleagues [34] found a correlation of PSA expression in FBCs with differentiated tumor types and low tumor grade. Similarly, Yu and colleagues [30] reported that the presence of PSA was significantly associated with smaller tumors, tumors with low S-phase fraction, diploid tumors, younger patient age, and tumors with lower cellularity. In their study, PSA expression was associated with good prognosis in FBC even after multivariate analysis. All the MBC cases in our study that expressed PSA were histologically of grade II or III. Our study, although limited by size and follow-up information, could not identify a prognostic role for PSA.
PSA expression in FBCs is strongly correlated with expression of ER and AR. Most PSA-producing FBCs are positive for steroid hormone receptor (ER/AR), but not all tumors that are positive for steroid hormone receptors produce PSA [35]. Hall and colleagues [36] have reported an even stronger association of AR and PSA expression with AR expression seen in 98% of FBCs expressing PSA. In the present study, of the six cases that were positive for PSA, AR expression was seen in only four. These observations are consistent with the hypothesis [4] that MBC is biologically different from FBC. The presence of PSA expression in breast carcinomas (both FBCs and MBCs) in the absence of AR expression would suggest the presence of an AR-independent pathway for PSA expression. Yu and colleagues [37] have shown the expression of PSA in normal breast tissue from a woman taking Brevicon, a progesterone-containing contraceptive. Of the two cases in our series that were PSA+/AR+, only one was PR+. However, both expressed ER. This is consistent with the reported expression of PSA in breast cancer cell lines after stimulation by norethindrone or ethinylestradiol [37].
The expression of AR in FBC, although not associated with smaller tumor size or negative lymph node status [38, 39], is significantly associated with longer disease-free and/or overall survival [40–43]. This has been suggested to be due in part to its association with ER status and therefore its better response to hormone therapy [40, 41, 44]. In MBC, the relationship between AR and ER is more variable, from positive association [4, 45] to no association (this study), and even a significant inverse correlation [46]. Additionally, ER status in MBCs, in contrast to FBCs, does not seem to have a significant effect on prognosis [4, 47]. Although this might be due in part to a higher frequency of expression, it has been suggested that ERs in MBCs do not have the same function as in FBCs [48].
Conclusion
In the present study the expression of PSAP was not observed in any of the 26 cases examined; this suggests that this marker might have a greater utility in distinguishing primary breast carcinomas from metastatic prostatic tumors. Further studies to confirm these findings are necessary. Male breast cancers can express PSA. The expression of PSA did not correlate with AR expression, suggesting the existence of alternative pathways for the control of PSA expression. The expression of neither of the two proteins was correlated with other clinical and pathologic tumor parameters. These observations are unlike those seen in FBCs and support the contention that MBC is biologically different from FBC [4].
Abbreviations
- AR:
-
androgen receptor
- ER:
-
estrogen receptor
- FBC:
-
female breast carcinoma
- MBC:
-
male breast carcinoma
- PR:
-
progesterone receptor
- PSA:
-
prostate-specific antigen
- PSAP:
-
prostate-specific acid phosphatase.
References
Hecht J, Winchester D: Male breast cancer. Am J Clin Pathol. 1994, 102: 25-30.
Ribeiro G: Male breast cancer: review of 301 cases from Christie Hospital and Holt Radium Institute, Manchester. Br J Cancer. 1985, 65: 115-119.
Salvadori B, Saccozzi R, Manzari A, Andreola S, Conti RA, Cusumano F, Grassi M: Prognosis of breast cancer in males. An analysis of 170 cases. Eur J Cancer. 1994, 30: 930-935.
Pich A, Margaria E, Chiusa L, Candelaresi G, Dal Canton O: Androgen receptor expression in male breast carcinoma: lack of clinicopathological association. Br J Cancer. 1999, 79: 959-964. 10.1038/sj.bjc.6690153.
Giordano SH, Buzdar AU, Hortobagyi GN: Breast cancer in men. Ann Intern Med. 2002, 137: 678-687.
Donegan WL, Redlich PN: Breast cancer in men. Surg Clin N Am. 1996, 76: 343-363.
Goss PE, Reid C, Pintilie M, Lim R, Miller N: Male breast carcinoma: a review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955–1996. Cancer. 1999, 85: 629-639. 10.1002/(SICI)1097-0142(19990201)85:3<629::AID-CNCR13>3.0.CO;2-V.
Wang-Rodriguez J, Cross K, Gallagher S, Djahanban M, Armstrong JM, Wiedner N, Shapiro DH: Male breast carcinoma: correlation of ER, PR, Ki-67, Her2-Neu, and p53 with treatment and survival, a study of 65 cases. Mod Pathol. 2002, 15: 853-861.
Munoz de Toro MM, Maffini MV, Kass L, Luque EH: Proliferative activity and steroid hormone receptor status in male breast carcinoma. J Steroid Biochem Mol Biol. 1998, 67: 333-339. 10.1016/S0960-0760(98)00124-1.
Teller MN, Stock CC, Stohr G, Merker PC, Kaufman RJ, Escher GC, Bowie M: Biologic characteristics and chemotherapy of 7,12-dimethylbenz(a)anthracene-induced tumors in rats. Cancer Res. 1966, 26: 245-252.
Lobaccaro JM, Lumbroso S, Belon C, Galtier-Dereure F, Bringer J, Lesimple T, Namer M, Cutuli BF, Pujol H, Sultan C: Androgen receptor gene mutation in male breast cancer. Hum Mol Genet. 1993, 2: 1799-1802.
Zarghami N, Grass L, Diamandis EP: Steroid hormone regulation of prostate-specific antigen gene expression in breast cancer. Br J Cancer. 1997, 75: 579-588.
Yu H, Diamandis EP, Zarghami N, Grass L: Induction of prostate specific antigen production by steroids and tamoxifen in breast cancer cell lines. Breast Cancer Res Treat. 1994, 32: 291-300.
ADASP: Recommendations for the reporting of breast carcinoma. Association of Directors of Anatomic and Surgical Pathology. Mod Pathol. 1996, 9: 77-81.
Green LK, Klima M: The use of immunohistochemistry in metastatic prostatic adenocarcinoma to the breast. Hum Pathol. 1991, 22: 242-246.
Gatalica Z, Norris BA, Kovatich AJ: Immunohistochemical localization of prostate-specific antigen in ductal epithelium of male breast. Potential diagnostic pitfall in patients with gynecomastia. Appl Immunohistochem Mol Morphol. 2000, 8: 158-161. 10.1097/00022744-200006000-00011.
Cho KR, Epstein JI: Metastatic prostatic carcinoma to supradiaphragmatic lymph nodes. A clinicopathologic and immunohistochemical study. Am J Surg Pathol. 1987, 11: 457-463.
Alanen KA, Kuopio T, Koskinen PJ, Nevalainen TJ: Immunohistochemical labelling for prostate specific antigen in non-prostatic tissues. Pathol Res Pract. 1996, 192: 233-237.
Yu H, Diamandis EP: Prostate-specific antigen in milk of lactating women. Clin Chem. 1995, 41: 54-58.
Yu H, Berkel H: Prostate-specific antigen (PSA) in women. J La State Med Soc. 1999, 151: 209-213.
Zaviacic M, Ablin RJ, Ruzickova M, Stvrtina S, Danihel L, Zaviacic T, Pohlodek K, Holoman K: The normal female and the male breast epithelium does not express prostate-specific antigen. Preliminary immunohistochemical observations of autopsy breast tissues. Gen Physiol Biophys. 1999, 18 (Suppl 1): 41-44.
Yu H, Diamandis EP, Levesque M, Giai M, Roagna R, Ponzone R, Sismondi P, Monne M, Croce CM: Prostate specific antigen in breast cancer, benign breast disease and normal breast tissue. Breast Cancer Res Treat. 1996, 40: 171-178.
Black MH, Diamandis EP: The diagnostic and prognostic utility of prostate-specific antigen for diseases of the breast. Breast Cancer Res Treat. 2000, 59: 1-14. 10.1023/A:1006380306781.
Papotti M, Paties C, Peveri V, Moscuzza L, Bussolati G: Immunocytochemical detection of prostate-specific antigen (PSA) in skin adnexal and breast tissues and tumors. Basic Appl Histochem. 1989, 33: 25-29.
Lopez-Otin C, Diamandis EP: Breast and prostate cancer: an analysis of common epidemiological, genetic, and biochemical features. Endocr Rev. 1998, 19: 365-396. 10.1210/er.19.4.365.
Lilja H, Oldbring J, Rannevik G, Laurell C: Seminal vesicle-secreted proteins and their reactions during gelation and liquefaction of human semen. J Clin Invest. 1987, 80: 281-285.
Peehl D: Prostate specific antigen role and function. Cancer. 1995, Suppl 75: 2021-2026.
Fortier AH, Nelson BJ, Grella DK, Holaday JW: Antiangiogenic activity of prostate-specific antigen. J Natl Cancer Inst. 1999, 91: 1635-1640. 10.1093/jnci/91.19.1635.
Diamandis EP, Helle SI, Yu H, Melegos DN, Lundgren S, Lonning PE: Prognostic value of plasma prostate specific antigen after megestrol acetate treatment in patients with metastatic breast carcinoma. Cancer. 1999, 85: 891-898. 10.1002/(SICI)1097-0142(19990215)85:4<891::AID-CNCR17>3.0.CO;2-K.
Yu H, Levesque MA, Clark GM, Diamandis EP: Prognostic value of prostate-specific antigen for women with breast cancer: a large United States cohort study. Clin Cancer Res. 1998, 4: 1489-1497.
Diamandis EP, Yu H, Sutherland DJ: Detection of prostate-specific antigen immunoreactivity in breast tumors. Breast Cancer Res Treat. 1994, 32: 301-310.
Giai M, Yu H, Roagna R, Ponzone R, Katsaros D, Levesque MA, Diamandis EP: Prostate-specific antigen in serum of women with breast cancer. Br J Cancer. 1995, 72: 728-731.
Alanen KA, Kuopio T, Collan YU, Kronqvist P, Juntti L, Nevalainen TJ: Immunohistochemical labelling for prostate-specific antigen in breast carcinomas. Breast Cancer Res Treat. 1999, 56: 169-176. 10.1023/A:1006210627219.
Howarth DJ, Aronson IB, Diamandis EP: Immunohistochemical localization of prostate-specific antigen in benign and malignant breast tissues. Br J Cancer. 1997, 75: 1646-1651.
Yu H, Giai M, Diamandis EP, Katsaros D, Sutherland DJ, Levesque MA, Roagna R, Ponzone R, Sismondi P: Prostate-specific antigen is a new favorable prognostic indicator for women with breast cancer. Cancer Res. 1995, 55: 2104-2110.
Hall RE, Clements JA, Birrell SN, Tilley WD: Prostate-specific antigen and gross cystic disease fluid protein-15 are coexpressed in androgen receptor-positive breast tumours. Br J Cancer. 1998, 78: 360-365.
Yu H, Diamandis EP, Monne M, Croce CM: Oral contraceptive-induced expression of prostate-specific antigen in the female breast. J Biol Chem. 1995, 270: 6615-6618. 10.1074/jbc.270.12.6615.
Allegra JC, Lippman ME, Thompson EB, Simon R, Barlock A, Green L, Huff KK, Do HM, Aitken SC, Warren R: Distribution, frequency, and quantitative analysis of estrogen, progesterone, androgen, and glucocorticoid receptors in human breast cancer. Cancer Res. 1979, 39: 1447-1454.
Miller WR, Telford J, Dixon JM, Hawkins RA: Androgen receptor activity in human breast cancer and its relationship with oestrogen and progestogen receptor activity. Eur J Cancer Clin Oncol. 1985, 21: 539-542.
Birrell SN, Roder DM, Horsfall DJ, Bentel JM, Tilley WD: Medroxyprogesterone acetate therapy in advanced breast cancer: the predictive value of androgen receptor expression. J Clin Oncol. 1995, 13: 1572-1577.
Bryan RM, Mercer RJ, Bennett RC, Rennie GC, Lie TH, Morgan FJ: Androgen receptors in breast cancer. Cancer. 1984, 54: 2436-2440.
Kuenen-Boumeester V, Van der Kwast TH, Claassen CC, Look MP, Liem GS, Klijn JG, Henzen-Logmans SC: The clinical significance of androgen receptors in breast cancer and their relation to histological and cell biological parameters. Eur J Cancer. 1996, 32A: 1560-1565. 10.1016/0959-8049(96)00112-8.
Langer M, Kubista E, Schemper M, Spona J: Androgen receptors, serum androgen levels and survival of breast cancer patients. Arch Gynecol Obstet. 1990, 247: 203-209.
Nomura Y, Yamagata J, Takenaka K, Tashiro H: Steroid hormone receptors and clinical usefulness in human breast cancer. Cancer. 1980, 46 (Suppl 12): 2880-2883.
Pacheco MM, Oshima CF, Lopes MP, Widman A, Franco EL, Brentani MM: Steroid hormone receptors in male breast diseases. Anticancer Res. 1986, 6: 1013-1017.
Sasano H, Kimura M, Shizawa S, Kimura N, Nagura H: Aromatase and steroid receptors in gynecomastia and male breast carcinoma: an immunohistochemical study. J Clin Endocrinol Metab. 1996, 81: 3063-3067. 10.1210/jc.81.8.3063.
Pich A, Margaria E, Chiusa L: Proliferative activity is a significant prognostic factor in male breast carcinoma. Am J Pathol. 1994, 145: 481-489.
Weber-Chappuis K, Bieri-Burger S, Hurlimann J: Comparison of prognostic markers detected by immunohistochemistry in male and female breast carcinomas. Eur J Cancer. 1996, 32: 1686-1692. 10.1016/0959-8049(96)00154-2.
Acknowledgements
The study is partly supported by a gift from the Avon Products Foundation Breast Cancer Research and Care Program. SB was also supported by the Pathology Core of the SPORE grant 1P 50 89018-01.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Rights and permissions
About this article
Cite this article
Kidwai, N., Gong, Y., Sun, X. et al. Expression of androgen receptor and prostate-specific antigen in male breast carcinoma. Breast Cancer Res 6, R18 (2003). https://doi.org/10.1186/bcr733
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/bcr733