Abstract
Cross-communication between different signalling systems is critical for the integration of multiple and changing environmental influences on individual cells. The epidermal growth factor receptor (EGFR) has been identified as a key element in the complex signalling network that is utilized by various classes of cell-surface receptors. This nonclassical mode of signalling system cross-talk, in distinction to receptor activation induced by cognate ligands, has been termed 'signal transactivation'. With the EGFR as the convergence point and distribution focus, this scenario may involve signals emitted by other members of the tyrosine kinase family, cytokine receptors, ion channels, G-protein-coupled receptors and integrins.
Similar content being viewed by others
Introduction
In complex organisms individual cells communicate through a variety of molecular messengers and actions, such as growth factors, hormones, cytokines, neuropeptides or cell-cell contact, which are recognized by diverse signal-generating cell-surface receptors and thereby regulate cellular growth, differentiation and survival. One important membrane-spanning protein that integrates the information flux from multiple sources is the EGFR, which was the first mammalian signalling protein to be fully characterized [1]. The EGFR belongs to a family of four closely related receptor tyrosine kinases (RTKs): EGFR/ErbB1, HER2/ErbB2/neu, HER3/ErbB3 and HER4/ErbB4. These RTKs are able to form homodimers or heterodimers, and regulate a large diversity of biological processes [2,3]. Deregulation of this tightly controlled signalling network by receptor overexpression, autocrine ligand stimulation or activating mutations has been frequently implicated in several types of human cancers, especially of the breast, ovary, lung and prostate [4,5].
Generally, ligands for the EGFR such as EGF, transforming growth factor-α or heparin-binding EGF-like growth factor (HB-EGF) are synthesized as transmembrane precursors and are proteolytically cleaved by metalloproteinases to yield the mature growth factor, which subsequently activates receptors on the same or on adjacent cells [6]. Following ligand binding, the EGFR dimerizes and becomes tyrosine phosphorylated on distinct residues by the intrinsic kinase activity of the receptor [7]. These residues represent docking sites for first stage signal transducers with enzymatic activity, such as phospholipase Cγ, and adapter proteins, such as Shc, Grb2 and Nck, which couple receptor activation to downstream pathways, calcium metabolism, and protein kinase C (PKC) signalling, transcription and phospholipid turnover. Apart from these established functions, the EGFR has been found to be a critical down-stream element of signalling systems, including those employed by G-protein-coupled receptors (GPCRs), cytokine receptors, integrins and membrane-depolarizing or stress-inducing agents [8,9,10,11].
The present review addresses aspects of cross-communication between cell-surface receptors and the EGFR, and the implications of these cellular signalling network connections for signal generation and diversification.
G-protein-coupled receptor-induced epidermal growth factor receptor transactivation
Historical background
The classical functions of GPCRs are the generation of second messengers such as cAMP, diacylglycerol and inositol trisphosphate, and the modulation of ion channel function [12]. In addition, GPCR agonists such as lysophosphatidic acid (LPA), thrombin, endothelin-1, carbachol or bombesin regulate cellular growth, differentiation and gene transcription through the Ras-mitogen-activated protein kinase (MAPK) signalling pathway [13]. Because activating mutations in GPCRs or G-proteins have been associated with cellular transformation [14,15], human endocrine tumours [16] and thyroid adenomas [17], understanding the mechanisms of G-protein-mediated growth control is essential for targeted disease intervention strategies. As an early point of convergence with RTK-induced mitogenic signalling events, GPCRs stimulate the formation and membrane recruitment of protein complexes that involve the adapter proteins Shc, Grb2 and the guanine nucleotide exchange factor Sos, which results in the activation of the small GTPase Ras [18,19]. Because these processes are sensitive to the nonspecific tyrosine kinase inhibitor genistein, the upstream activation of kinases was proposed to be essential for G-protein-mediated MAPK activation [20]. More recent studies have identified the EGFR tyrosine kinase [21], as well as other candidate mediators, including the cytoplasmic tyrosine kinase Pyk2 [22] and members of the Src family [22,23]. It is generally accepted that, depending on the expression pattern, cell type and the activated receptor, these kinases appear to contribute with varying extent to the activation of the Ras-MAPK pathway by GPCRs. Less well understood are the molecular mechanisms of how these tyrosine kinases can be activated by heterotrimeric G proteins after GPCR stimulation.
General characteristics of the transactivated epidermal growth factor receptor
Since Daub et al [21] identified the EGFR to be essential for endothelin-1, thrombin and LPA-induced MAPK activation and c-fos gene transcription in 1996, various studies [24,25,26] have demonstrated the critical role of this inter-receptor cross-talk for mitogenic G-protein signalling (Fig. 1). Common to all these reports is the observation that GPCR-induced tyrosine phosphorylation of the EGFR is very rapid, transient and comparable to stimulation with low amounts of EGF [21,24*,25,26]. Furthermore, the same signalling capacity of the transactivated and EGF-activated EGFR [24*], carbachol-induced EGFR dimerization [27] and the critical dependence on the intrinsic tyrosine kinase activity of the EGFR [21,24*,25,26,27,28,29] points to an analogous mechanism of EGFR activation by G-proteins or exogenous EGF. However, the speed of transactivation and the absence of detectable amounts of EGF-like ligands after G-protein activation [25,27] led to the conclusion that GPCR-induced EGFR activation is exclusively mediated by intracellular elements [8,9,10,11].
EGFR transactivation by GPCRs and cytokine receptors. Various signalling stimuli induce EGFR tyrosine phosphorylation to activate different downstream signalling pathways. Apart from the activation of the classical Ras-MAP kinase pathway, GPCR-EGFR cross-talk is critical for G-protein-mediated stress fibre formation, ion channel modulation or PI-3K-stimulated phospholipid turnover. In contrast to cytokine-induced EGFR tyrosine phosphorylation by janus kinase (Jak)2, GPCR-induced EGFR transactivation is dependent on the intrinsic kinase activity of the receptor, and several cytoplasmic mediators have been controversially discussed in the literature.
Involvement of Src kinases
Potential candidates as mediators of GPCR-induced EGFR transactivation are members of the Src family of cytoplasmic tyrosine kinases [30]. In COS-7 cells, Src was reported to induce EGFR tyrosine phosphorylation after stimulation of the G i -coupled LPA and α2A-adrenergic receptors or overexpression of Gβγ subunits, independently of the intrinsic kinase activity of EGFR [31]. In the same cell line, however, the Src kinase inhibitor PP1 only slightly reduced LPA-induced EGFR transactivation [24*], and recent data [32] suggest Src as a point of convergence downstream of RTK transactivation and G-protein-mediated focal adhesion complex assembly. A number of reports have demonstrated a critical function of Src in GPCR-induced Shc and Gab1 tyrosine phosphorylation [24*], MAPK activation by GPCR agonists [24*,31,33] and agonist-dependent α2-adrenergic receptor endocytosis [34] as an early step in G-protein-mediated signal generation. Furthermore, the association of Src with Shc, Grb2 and the EGFR after angiotensin II stimulation [25], together with previously described Src-mediated phosphorylation of tyrosine residues Y845 and Y1101 of the EGFR [35,36], suggest an additional means of receptor activation utilized by GPCRs that is distinct from EGFR autophosphorylation. Although the functional connection between Src and the EGFR is still unclear, EGFR tyrosine phosphorylation by Src was shown to be crucial for EGF-induced and LPA-induced mitogenic responses [37]. This confirms previous studies [38] that implicated EGFR-Src complex formation in malignant transformation of breast cancer cell lines.
Calcium-dependent epidermal growth factor transactivation
Elevation of the intracellular calcium level and the activation of the Ser/Thr PKC were both shown to be required for Gq-coupled receptors to induce EGFR transactivation. Although EGFR transactivation in response to carbachol or uridine triphosphate stimulation of PC12 cells is sensitive to PKC inhibition [27,39], bradykinin-stimulated EGFR tyrosine phosphorylation in COS-7 cells was shown to be PKC-independent, but bradykinin treatment increased PKC activity in parallel [40]. Nevertheless, in this case both pathways critically contribute to Gq-mediated MAPK activation.
Recently, calcium influx has been shown to be sufficient to transactivate the EGFR and is necessary for various GPCRs to increase the tyrosine phosphorylation of the EGFR [25,28,41]. In PC12 cells [28,39], rat vascular smooth muscle cells [25] or rat cardiac fibroblasts [42], overexpression of the dominant-negative EGFR mutant HER-CD533 or inhibition with the EGFR selective tyrphostin AG1478 strongly interfered with GPCR-induced MAPK activation or mitogenic responses. Although in PC12 cells the presence of extracellular calcium is necessary for bradykinin-induced EGFR tyrosine phosphorylation, complexation of intracellular calcium upon treatment with the chelating agent BAPTA-AM was sufficient to inhibit angiotensin II-induced EGFR activation in cardiac fibroblasts.
Because elevation in intracellular calcium or GPCR stimulation can activate the PYK2 tyrosine kinase [39,43,44*], its possible role as a link between GPCRs and the EGFR was considered [39]. Nevertheless, in PC12 cells tetracycline-inducible expression of a kinase-inactive PYK2 mutant did not effect EGFR transactivation by bradykinin or membrane-depolarization [44*]. Interestingly, pharmacological inhibition of calcium/calmodulin-dependent protein kinases completely abrogates EGFR tyrosine phosphorylation in response to membrane depolarization or angiotensin II stimulation. This finding led to the identification of these Ser/Thr kinases as possible mediators of EGFR transactivation, at least in some cell types [42,44*].
Downstream of the transactivated epidermal growth factor receptor
The signalling capacity of the transactivated EGFR is not restricted to coupling GPCRs to the Ras-MAPK pathway, but rather functions as a critical intermediate for Gq-, Gi-and G12/13-coupled receptors to induce a multitude of biological responses. Several isoforms of phosphatidyl inositol-3 kinase (PI-3K) have been shown to be involved in Gβγ-dependent, LPA- or noradrenaline-induced MAPK activation, but are likely to act downstream of or separate from transactivated EGFR [24*,45,46,47]. Moreover, inhibition of EGFR function blocks LPA-induced PI-3K activity in COS-7 cells [24*]. The complex formation of the docking protein Gab1 with the EGFR and p85, the catalytic subunit of PI-3K, was essential for LPA-stimulated PI-3K lipid product generation [48*]. Furthermore, the p70 S6 kinase, a known effector of PI-3K that is critical for cell cycle progression, is activated by endothelin-1 via EGFR-dependent pathways [49].
Another cellular event regulated by various GPCRs that depends on the EGFR function is the rearrangement of the actin cytoskeleton and subsequent stress fibre formation via Gα13 but not Gα12 subunits [50,51*]. Finally, the EGFR-dependent modulation of a potassium channel or regulation of chloride secretion after carbachol stimulation [26,27] represent additional responses within the complex signalling network utilized by GPCRs, with EGFR as the major signal integrator.
Triple membrane-passing signal mechanism of epidermal growth factor receptor transactivation
Generally, GPCR-induced EGFR transactivation has been, so far, considered to be a ligand-independent process, with several cytoplasmic players controversially discussed as mediators of this inter-receptor cross-talk.
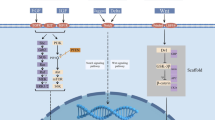
Experiments with different stable Rat-1 cell lines [52**] have revealed the critical involvement of the EGFR ligand-binding domain for EGFR transactivation, as well as the possibility of intercellular transactivation between cocultured cells in cell-density dependent manner. This again raised the question whether ligands for the EGFR can contribute to GPCR-induced transactivation. Moreover, LPA, carbachol and bombesin induce shedding of the pro-HB-EGF growth factor precursor in COS-7 cells. Inhibition of this cleavage by the metalloproteinase inhibitor batimastat completely abolished GPCR-induced EGFR transactivation, as well as further downstream signalling steps such as Shc and Gab1 tyrosine phosphorylation in COS-7 and HEK293 cells [52**]. Because proteolytic processing affects various transmembrane proteins, this critical role of metalloproteinases extends the repertoire of known G-protein effectors and might open a new dimension of GPCR signalling. Furthermore, the ability of HB-EGF to bind to cell-surface heparan sulphate proteoglycans prevents the immediate release of the ligand after precursor cleavage, which might explain why mature growth factors are not released into the culture medium after GPCR stimulation. Additionally, transactivation and the critical involvement of metalloproteinases for this cross-communication were shown in prostate cancer cells, which are known to regulate their growth via autocrine pathways requiring either the bombesin or the EGFR [52**]. This suggests a link between GPCR and EGFR autocrine signals, which further emphasizes that EGFR transactivation may contribute to pathophysiological disease states. Although this triple membrane-passing signal model provides new insights into mechanistic details of transactivation (Fig. 2), it raises a variety of new questions. The nature of the metalloproteinase(s) involved and its regulation by heterotrimeric G-proteins will be the key issue in the future.
Triple membrane-passing signal mechanism of EGFR transactivation. GPCR-induced EGFR transactivation involves as key elements pro-HB-EGF, an EGF-like growth factor precursor, and a metalloproteinase activity that is induced upon G-protein activation. Therefore, this new mechanistic concept for ligand-dependent GPCR-EGFR cross-talk entails three membrane-passing signals and couples G-protein signalling to activation of the Ras-MAPK pathway.
Cross-talk between cytokine and epidermal growth factor receptor family members
In addition to GPCRs, growth hormone and prolactin, members of the cytokine superfamily, have been reported to increase the EGFR tyrosine phosphorylation and EGFR-Grb2 association in mouse liver in vivo [53**]. In this case the EGFR was directly phosphorylated by the Janus tyrosine kinase Jak2, which couples cytokine stimulation to MAPK activation and c-fos gene transcription via the transactivated EGFR. In contrast to GPCR-induced effects, growth hormone only induces phosphorylation of Tyr1068, the major Grb2 binding site of the EGFR, independently of the intrinsic kinase activity of the receptor. Furthermore, interleukin-6 was shown [54*] to stimulate the autophosphorylation of HER2 in prostate cancer cells, leading to increased HER2 and HER3 tyrosine phosphorylation, MAPK activation and cell proliferation. Clustering of the interleukin-6 receptor β-subunit, gp 130 and HER2 was proposed to initiate these tyrosine phosphorylation events.
Cross-talk between integrins and epidermal growth factor
Adhesion of cells to the extracellular matrix is mediated through the integrin family of cell-surface receptors and leads to the activation of multiple biological responses, including calcium influx, increased protein tyrosine phosphorylation and activation of MAPK cascade [55]. Integrins, which are devoid of any intrinsic enzymatic activity, have been demonstrated [56**] to transactivate the EGFR in order to generate further cellular responses, most notably adhesion-dependent cell survival. In accordance with the first studies of the GPCR-EGFR cross-talk, those investigators suggested, on the basis of medium-transfer experiments, a ligand-independent mechanism. It would be interesting to investigate the requirement of metalloproteinases in this process.
Cross-talk between platelet-derived growth factor and epidermal growth factor receptor
Inter-receptor communication was not only demonstrated between heterologous receptor classes, but also among different members of the RTK family. The EGFR and the platelet-derived growth factor (PDGF)-β receptor are known to interact physically [57], and EGF stimulation has been shown to increase the tyrosine phosphorylation of PDGF-β receptor and subsequent recruitment of PI-3K to the PDGF receptor [58]. In contrast to tyrosine phosphorylation events, signal regulation by serine or threonine phosphorylation has been poorly characterized, but is generally thought to influence negatively the signalling capacity of RTKs such as the EGFR and its relative HER2/neu [59]. Interestingly, Bagowski et al [60] provided evidence that PDGF stimulates threonine phosphorylation of T654 and T669 of the EGFR, thus negatively regulating EGF-induced c-jun amino-terminal kinase activation, and c-jun transcription and cellular transformation. In this context the PKC-related Ser/Thr kinase protein kinase D has been identified as a potential mediator, but the detailed molecular mechanism of this intracellular RTK-RTK cross-talk still remains elusive.
Conclusion
Signal transduction was often considered to be organized in discrete signalling cassettes, linking receptor activation to cell division and gene transcription in a linear manner. Recent studies have broadened this view to encompass a complex network, which allows cross-communication between separate signalling units with the EGFR as a key element for signal integration and diversification from a multitude of stimuli. We are just beginning to understand the molecular mechanisms of RTK transactivation. On the basis of identification of critical players, such as Tyr or Ser/Thr kinases, metalloproteinases and growth factor precursors, however, one must expect a general role of this cross-talk in developmental and normal physiological processes, as well in pathophysiological disorders such as cancer.
References
Ullrich A, Coussens L, Hayflick JS, et al: Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984, 309: 418-425.
Alroy I, Yarden Y: The ErbB signaling network in embryogenesis and oncogenesis:signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997, 410: 83-86. 10.1016/S0014-5793(97)00412-2.
Riesell DJ, Stern DF: Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998, 20: 41-48. 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V.
Van Agthoven T, Timmermans M, Dorssers LC, Henzen-Logmans SC: Expression of estrogen, progesterone and epidermal growth factor receptors in primary and metastatic breast cancer. Int J Cancer. 1995, 63: 790-793.
Earp HS, Dawson TL, Li X, Yu H: Heterodimerization and functional interaction between EGF receptor family members: a new signaling paradigm with implications for breast cancer research. Breast Cancer Res Treat. 1995, 35: 115-132.
Massagué J, Pandiella A: Membrane-anchored growth factors. Annu Rev Biochem. 1993, 62: 515-541. 10.1146/annurev.bi.62.070193.002503.
Schlessinger J, Ullrich A: Growth factor signaling by receptor tyrosine kinases. Neuron. 1992, 9: 383-391.
Hackel PO, Zwick E, Prenzel N, Ullrich A: Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr Opin Cell Biol. 1999, 11: 184-189. 10.1016/S0955-0674(99)80024-6.
Luttrell LM, Daaka Y, Lefkowitz RJ: Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999, 11: 177-183. 10.1016/S0955-0674(99)80023-4.
Zwick E, Hackel PO, Prenzel N, Ullrich A: The EGF receptor as central transducer of heterologous signalling systems. Trends Pharmacol Sci. 1999, 20: 408-412. 10.1016/S0165-6147(99)01373-5.
Carpenter G: Employment of the epidermal growth factor receptor in growth factor-independent signaling pathways. J Cell Biol. 1999, 146: 697-702. 10.1083/jcb.146.4.697.
Hamm HE: The many faces of G-protein signaling. J Biol Chem. 1998, 273: 669-672. 10.1074/jbc.273.2.669.
Dhanasekaran N, Tsim S-T, Dermott JM, Onesime D: Regulation of cell proliferation by G-proteins. Oncogene. 1998, 17: 1383-1394. 10.1038/sj.onc.1202242.
Xu N, Voyno-Yasenetskaya T, Gutkind S: Potent transforming activity of the G13 α subunit defines a novel family of oncogenes. Biochem Biophys Res Commun. 1994, 201: 603-609. 10.1006/bbrc.1994.1744.
Allen LF, Lefkowitz RJ, Caron MG, Cotecchia S: G-protein-coupled receptor genes as protooncogenes: constitutively activating mutation of the α1B- adrenergic receptor enhances mitogenesis and tumorigenicity. Proc Natl Acad Sci USA. 1991, 88: 11354-11358.
Lyons JL, Landis CA, Harsh G, et al: Two G protein oncogenes in human endocrine tumors. Science. 1990, 249: 655-659.
Parma J, Duprez L, Van Sande J, et al: Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature. 1993, 365: 649-651. 10.1038/365649a0.
Howe LR, Marshall CJ: Lysophosphatidic acid stimulates mitogenactivated protein kinase activation via a G-protein-coupled pathway requiring p21ras and p74raf-1. J Biol Chem. 1993, 268: 20717-20720.
Crespo P, Xu N, Simonds WF, Gutkind S: Ras-dependent activation of MAP kinase pathway mediated by G-protein βγsubunits. Nature. 1994, 369: 418-420. 10.1038/369418a0.
Van Corven EJ, Hordijk PL, Medema RH, Bos JL, Moolenaar WH: Pertussis toxin-sensitive activation of p21ras by G-protein-coupled receptor agonists in fibroblasts. Proc Natl Acad Sci USA. 1993, 90: 1257-1261.
Daub H, Weiss FU, Wallasch C, Ullrich A: Role of transactivation of the EGF receptor in signaling by G-protein-coupled receptors. Nature. 1996, 379: 557-560. 10.1038/379557a0.
Dikic J, Tokina G, Lev S, Courtneidge SA, Schlessinger J: A role for Pyk2 and Src in linking G-protein-coupled receptors win MAPkinase activation. Nature. 1996, 383: 547-550. 10.1038/383547a0.
Luttrell LM, Hawes BE, van Biesen T, et al: Role of c-Src tyrosine kinase in G protein-coupled receptor-and Gβγsubunit mediated activation mitogen activated protein kinases. J Biol Chem. 1996, 271: 19443-19450. 10.1074/jbc.271.21.12133.
Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A: Signal characteristics of G-protein-transactivated EGF receptor. EMBO J. 1997, 16: 101-113. 10.1093/emboj/16.23.7032.
Eguchi S, Numaguchi K, Iwasaki H, et al: Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem. 1998, 273: 8890-8896. 10.1074/jbc.273.15.8890.
Keely SJ, Uribe JM, Barrett KE: Carbachol stimulates transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T84 cells. J Biol Chem. 1998, 273: 27111-27117. 10.1074/jbc.273.42.27111.
Tsai W, Morielli AD, Peralta EG: The m1 muscarinic acetylcholine receptor transactivates the EGF receptor to modulate log channel activity. EMBO J. 1997, 16: 4597-4605. 10.1093/emboj/16.15.4597.
Zwick E, Daub H, Aoki N, et al: Critical role of calcium-dependent epidermal growth factor receptor transactivation in PC12 cell membrane depolarization and bradykinin signaling. J Biol Chem. 1997, 272: 24767-24770. 10.1074/jbc.272.40.24767.
Cunnick JM, Dorsey JF, Standley J, et al: Role of tyrosine kinase activity of epidermal growth factor receptor in the lysophosphatidic acid-stimulated mitogen-activated protein kinase pathway. Biol Chem. 1998, 273: 14468-14475. 10.1074/jbc.273.23.14468.
Schwartzberg PL: The many faces of Src: multiple functions of a prototypical tyrosine kinase. Oncogene. 1998, 17: 1463-1468. 10.1038/sj.onc.1202176.
Luttrell LM, Della Rocca GJ, vanBiesen T, Luttrell DK, Lefkowitz RH: Gβγsubunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1997, 272: 4637-4644. 10.1074/jbc.272.50.31648.
Della Rocca GJ, Maudsley S, Daaka Y, Lefkowitz RJ, Luttrell LM: Pleiotropic coupling of G protein-coupled receptors to the mitogen-activated protein kinase cascade. J Biol Chem. 1999, 274: 13978-13984. 10.1074/jbc.274.20.13978.
Vaingankar SM, Martins-Green M: Thrombin activation of the 9E3/CEF4 chemokine involves tyrosine kinases including c-src and the epidermal growth factor receptor. J Biol Chem. 1998, 273: 5226-5234. 10.1074/jbc.273.9.5226.
Luttrell LM, Ferguson SSG, Daaka Y, et al: β-Arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science. 1999, 283: 655-661. 10.1126/science.283.5402.655.
Stover DR, Becker M, Liebetanz J, Lydon NB: Src phosphorylation of the epidermal growth factor receptor at novel sites mediates receptor interaction with src and p85α. J Biol Chem. 1995, 270: 15591-15597. 10.1074/jbc.270.26.15591.
Biscardi JS, Maa M, Tice DA, et al: C-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999, 274: 8335-8344. 10.1074/jbc.274.12.8335.
Tice DA, Biscardi JS, Nickles AL, Parson SJ: Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA. 1999, 96: 1415-1420. 10.1073/pnas.96.4.1415.
Luttrell DK, Lee A, Lansing TJ, et al: Involvement of pp60c-src with two major signaling pathways in human breast cancer. Proc Natl Acad Sci USA. 1994, 91: 83-87.
Soltoff SP: Related adhesion focal tyrosine kinase and the epidermal growth factor receptor mediate the stimulation of mitogen-activated protein kinase by the G-protein-coupled P2Y2 receptor. J Biol Chem. 1998, 273: 23110-23117. 10.1074/jbc.273.36.23110.
Adomeit A, Graness A, Gross S, et al: Bradykinin B2 receptor-mediated mitogen-activated protein kinase activation in COS-7 cells requires dual signaling via both protein kinase C pathway and epidermal growth factor receptor transactivation. Mol Cell Biol. 1999, 19: 5289-5297.
Rosen LB, Greenberg ME: Stimulation of growth factor receptor signal transduction by activation of voltage-sensitive calcium channels. Proc Natl Acad Sci USA. 1996, 93: 1113-1118. 10.1073/pnas.93.3.1113.
Murasawa S, Mori Y, Nozawa Y, et al: Angiotensin II type 1 receptor induced extracellular signal-regulated protein kinase activation is mediated by Ca2+/calmodulin dependent transactivation of epidermal growth factor receptor. Circ Res. 1998, 82: 1338-1348.
Lev S, Moreno H, Martinez R, et al: Protein tyrosine kinase PYK2 involved in Ca(2+) induced regulation of ion channel and MAP kinase functions. Nature. 1995, 376: 737-745. 10.1038/376737a0.
Zwick E, Wallasch C, Daub H, Ullrich A: Distinct calcium-dependent pathways of epidermal growth factor receptor transactivation and PYK2 tyrosine phosphorylation in PC12 cells. J Biol Chem. 1999, 274: 20989-20996. 10.1074/jbc.274.30.20989.
Lopez-llasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R: Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI3-kinase γ. Science. 1997, 275: 394-397. 10.1126/science.275.5298.394.
Hu Z-W, Shi X-Y, Lin RZ, Hoffman BB: α1 adrenergic receptors activate phosphatidylinositol 3-kinase in human vascular smooth muscle cells. J Biol Chem. 1996, 271: 8977-8982. 10.1074/jbc.271.15.8977.
Hawes BE, Luttrell LM, van Biesen T, Lefkowitz RJ: Phosphatidylinositol 3-kinase is an early intermediate in the Gβγ-mediated mitogen-activated protein kinase signaling pathway. J Biol Chem. 1996, 271: 12133-12136. 10.1074/jbc.271.21.12133.
Laffargue M, Raynal P, Yart A, et al: An epidermal growth factor receptor/Gab1 signaling pathway is required for activation of phopshoinositide 3-kinase by lysophosphatidic acid. J Biol Chem. 1999, 274: 32835-32841. 10.1074/jbc.274.46.32835.
Iwasaki H, Eguchi S, Ueno H, Marumo F, Hirata Y: Endothelin-mediated vascular growth requires p42/p44 mitogen-activated protein kinase and p70 S6 kinase cascades via transactivation of epidermal growth factor receptor. Endocrinology. 1999, 140: 4659-4668. 10.1210/en.140.10.4659.
Gohla A, Harhammer R, Schultz G: The G-protein G13 but not G12 mediates signalling from lysophosphatidic acid receptor via epidermal growth factor receptor to rho. J Biol Chem. 1998, 273: 4653-4659. 10.1074/jbc.273.8.4653.
Gohla A, Offermanns S, Wilkie TM, Schultz G: Differential involvement of Gα12 and Gα13 in receptor-mediated stress fiber formation. J Biol Chem. 1999, 274: 17901-17907. 10.1074/jbc.274.25.17901.
Prenzel N, Zwick E, Daub H, et al: EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999, 402: 884-888. 10.1038/47260.
Yamauchi T, Ueki K, Tobe K, et al: Tyrosine phosphorylation of the EGF receptor by the kinase Jak2 is induced by growth hormone. Nature. 1997, 390: 91-96. 10.1038/36369.
Qiu Y, Ravi L, Kung HJ: Requirement of ErbB2 for signaling by interleukin-6 in prostate carcinoma cells. Nature. 1998, 393: 83-85. 10.1038/30012.
Giancotti FG, Ruoslahti E: Integrin signaling. Science. 1999, 285: 1028-1032. 10.1126/science.285.5430.1028.
Moro L, Venturino M, Bozzo C, et al: Integrins Induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 1998, 17: 6622-6632. 10.1093/emboj/17.22.6622.
Liu P, Anderson RGW: Spatial organization of EGF receptor transmodulation by PDGF. Biochem Biophys Res Commun. 1999, 261: 695-700. 10.1006/bbrc.1999.1082.
Habib AA, Högnason T, Stefansson K, Ratan RR: The epidermal growth factor receptor associates with and recruits phosphatidylinositol 3-kinase to the platelet-derived growth factor βreceptor. J Biol Chem. 1998, 273: 6885-6891. 10.1074/jbc.273.12.6885.
Seedorf K, Shearman M, Ullrich A: Rapid and long term effects of protein kinase C on receptor tyrosine kinase phosphorylation and degradation. J Biol Chem. 1995, 270: 18953-18960. 10.1074/jbc.270.32.18953.
Bagowski CP, Stein-Gerlach M, Choidas A, Ullrich A: Cell-type specific phosphorylation of threonines T654 and T669 by PKD defines the signal capacity of the EGF receptor. EMBO J. 1999, 18: 5567-5576. 10.1093/emboj/18.20.5567.
Author information
Authors and Affiliations
Corresponding author
Additional information
Articles of particular interest have been highlighted as:
• of special interest
•• of outstanding interest
Rights and permissions
About this article
Cite this article
Prenzel, N., Zwick, E., Leserer, M. et al. Tyrosine kinase signalling in breast cancer: Epidermal growth factor receptor - convergence point for signal integration and diversification. Breast Cancer Res 2, 184 (2000). https://doi.org/10.1186/bcr52
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/bcr52