Abstract
Introduction
Very few studies have investigated whether the time elapsed between surgical resection and tissue fixation or the difference between core-cut and excision biopsies impact on immunohistochemically measured biomarkers, including phosphorylated proteins in primary breast cancer. The aim of this study was to characterise the differences in immunoreactivity of common biomarkers that may occur (1) as a result of tissue handling at surgery and (2) between core-cuts and resected tumours.
Methods
Core-cuts taken from surgical breast cancer specimens immediately after resection (sample A) and after routine X-ray of the excised tumour (sample B) were formalin-fixed and paraffin-embedded and compared with the routinely fixed resection specimen (sample C). The variation in immunohistochemical expression of Ki67, oestrogen receptor (ER), progesterone receptor (PgR), human epidermal growth factor 2 (HER2), p-Akt and p-Erk1/2 were investigated.
Results
Twenty-one tissue sets with adequate tumour were available. Median time between collection of core-cuts A and B was 30 minutes (range, 20 to 80 minutes). None of the markers showed significant differences between samples A and B. Similarly, Ki67, ER, PgR and HER2 did not differ significantly between core-cuts and main resection specimen, although there was a trend for lower resection values for ER (P = 0.06). However, p-Akt and p-Erk1/2 were markedly lower in resections than core-cuts (median, 27 versus 101 and 69 versus 193, respectively; both P < 0.0001 [two-sided]). This difference was significantly greater in mastectomy than in lumpectomy specimens for p-Erk1/2 (P = 0.01).
Conclusions
The delay in fixation in core-cuts taken after postoperative X-ray of resection specimens has no significant impact on expression of Ki67, ER, PgR, HER2, p-Akt or p-Erk1/2. However, extreme loss of phospho-staining can occur during routine fixation of resection specimens. These differences are likely attributable to suboptimal fixation and may have major repercussions for clinical research involving these markers.
Similar content being viewed by others
Introduction
Stratification of therapy is a prime goal of current research. Tissue biomarkers are expected to provide indices enabling selection of therapy. Assurance of the validity of biomarker measurement is critical for their accurate application and interpretation, particularly in the context of presurgical studies, which are being increasingly used to speed drug development [1].
A variety of tissue sample types (e.g., core-cuts, punch biopsies, excisions) are used in biomarker studies, and comparative measurements of a marker between tissue types may occur within a single trial (e.g., core-cut at diagnosis/pretreatment versus excision/posttreatment). There are, however, few data available on the impact of sample type, even for frequently measured biomarkers such as Ki67, which has been used as a primary end point of some trials [2]. It is essential that any differences that arise from the expression of such markers in trials should be solely attributable to the effect of treatment with the drug and not due to potential artefacts such as those caused by delays to tissue fixation in different sample types.
Protein kinases are targets for approximately one third of drugs in development for oncology. Their phosphorylated products provide pharmacodynamic end points during clinical development and are likely, at least in some cases, to be determinants/indices of treatment efficacy and thus to become biomarkers in routine practice. Some previous studies have indicated that some phosphoproteins are labile during fixation [3, 4]. We undertook a systematic assessment of immunoreactive expression of several established or developmental biomarkers for breast cancer, including two centrally important phosphorylated proteins: p-Akt and p-Erk1/2. To address these issues, we evaluated two situations that arise in the increasingly popular "window of opportunity" studies in primary breast cancer that exploit the approximately 2 weeks between diagnosis and surgery: (1) the delay of starting fixation between tumour excision and its return to theatre after X-ray to assess calcification and margin clearance, and (2) differences between core-cuts fixed immediately on tumour resection and histopathological sections from routinely fixed primary breast cancers.
Materials and methods
Sample collection
Twenty-eight patients were studied at resection of primary breast cancer; 29 specimens were available (one patient with two tumours). Basic demographics were median age, 54 years; median tumour size, 29 mm; lumpectomy versus mastectomy, 16 versus 13, respectively; node negative versus positive, 16 and 12, respectively (one was not available). Two 14-gauge core-cuts were taken immediately after tumour resection (sample A); one was placed in neutral-buffered formalin and one into RNAlater (Applied Biosystems/Ambion, Austin, TX, USA). The tumour was sent for X-ray at ambient temperature; on return to theatre after a recorded time, two more core-cuts were taken and handled similarly (sample B). The resected specimen (sample C) was placed in formalin and subjected to the histopathology department's routine fixation for breast tumours: lumpectomy specimens were left unsliced until the next morning, and mastectomy specimens were sliced at intervals of about 10 mm to allow penetration of formalin. Radioactive specimens were left unsliced for 48 hours to allow for isotope decay. Ethical approval was provided by the Royal Marsden Hospital (RMH). All patients gave written, informed consent.
Immunohistochemical determination of Ki67, ER, PgR, HER2, p-Akt and p-Erk1/2
Four-micron sections of formalin-fixed, paraffin-embedded (FFPE) tissues were deparaffinised in xylene for 5 minutes and gradually rehydrated in decreasing grades (100%, 90%, 80% and 70%) of industrial methylated spirits (IMS) and water washes. Table 1 provides further details of the immunohistochemical methodology used for all markers. In all cases, endogenous peroxidase blocking, incubation with primary and secondary antibodies and signal amplification and detection steps of the immunohistochemical procedure were conducted on the Autostainer Immunostaining System (Dako, Carpinteria, CA, USA). Slides were then washed in running tap water for 5 minutes, counterstained in Mayer's haematoxylin for 1 minute and washed again for 5 minutes. Sections were dehydrated in gradual IMS washes (70%, 80%, 90% and 100%), incubated twice in xylene for 5 minutes and mounted on the CTM6 Glass Coverslipper (Thermo Fisher Scientific/Microm, Walldorf, Germany). All sections within the same set (i.e., samples A, B and C) were stained in the same batch to minimise any batch-to-batch variations that might occur which could affect staining intensity and lead to erroneous results.
Assessment of p-Akt, p-Erk1/2, ER and PgR expression across 10 high-power fields was done by H score, in which the percentage of invasive cells staining in each intensity category (0, zero; 1, faint; 2, moderate; or 3, strong) was derived, multiplied by its intensity and summed (range, 0-300). Ki67 expression was assessed by percentage of positive invasive cells staining in any category 1-3 (percentage positive score) across 10 high-power fields. HER2 assessment was done by IHC categories 0, 1+, 2+ and 3+ as per HercepTest™ (DakoCytomation, Glostrup, Denmark) and the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines [5]. The assessment of all markers was blinded with regard to sample time point and sample set.
Statistical analysis
Comparison of IHC scores between samples A, B and C was conducted using the Wilcoxon signed-rank test. Spearman regression analyses were conducted between the difference in expression between samples A and B and the time elapsed between their collection. Data from samples placed in RNAlater will be published separately. P values were two-sided.
Results
Twenty complete sets of samples with adequate invasive tissue and one set missing sample B were available. Median time from collection of samples A and B was 30 minutes (range, 20-80 minutes). No significant differences were found between samples A and B for Ki67, ER, PgR, p-Akt, p-Erk1/2 or HER2 (Table 2). There were no significant correlations between time elapsed and the differences between samples A and B (all P > 0.20). Comparisons with sample C were therefore made with mean expression values for samples A and B (mean A,B).
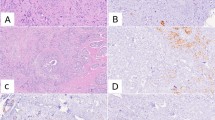
There were no significant differences between these scores for Ki67, ER and PgR, although a trend to a lower ER value in the resection was seen (Figure 1a, P = 0.19; Figure 1b, P = 0.06; and Figure 1c, P = 0.89, respectively). For HER2, 12 of 15 cases categorised as 1+ on at least one of sample A or B were scored 0 in the resection, but no differences in HER2 positive or negative status occurred (Figure 1d). However, p-Akt and p-Erk1/2 were highly significantly lower in sample C than in mean A,B (Figures 2a and 2b, respectively; P < 0.0001). Near-complete absence of staining (H score <5) occurred in 6 of 21 samples C for p-Akt and 8 of 21 for p-Erk1/2, despite mean A,B values for some of these being among the highest. Nonetheless, there was a significant correlation between values for mean A,B and C samples for both phosphoproteins (R = 0.68, P = 0.0008; R = 0.52, P = 0.015, respectively).
The mean difference in staining between mean A,B and C samples was greater for both phosphoproteins in mastectomy than lumpectomy samples (Figures 3a and 3b), but only significantly so for p-Erk1/2 (P = 0.01).
Expression of p-Akt and p-Erk1/2 in lumpectomy versus mastectomy specimens. Percentage difference between resections (sample C) and core-cuts (mean of samples A and B) expression values for (a) p-Akt and (b) p-Erk1/2. Percentage difference defined as (sample C - mean of samples A and B) × 100/mean of samples A and B.
Discussion
The data described reveal that the immunohistochemical detection of certain biomarkers is highly comparable between core-cut biopsies and routinely fixed resections. The modest difference in ER and the lack of difference in Ki67 and PgR is consistent with the absence of a difference between core-cuts and resection samples in the placebo arm of some of our earlier reports on short-term presurgical studies [6, 7]. While there was no effect on the p-Akt or p-Erk1/2 staining over the 20- to 80-minute delay in fixation of core-cuts, the difference between core-cuts and excision samples was extreme and could lead to major erroneous interpretation. For example, if these results were obtained in a "window-of-opportunity" trial that included only a treatment arm, they could be taken as strong evidence of the pharmacological effectiveness of that treatment.
The differences between core-cut and excision samples could be influenced by sampling differences of heterogeneously expressed markers but are most likely due to differences in time to fixation: the correlation between core-cut and excision values indicates a systematic difference. At room temperature and without fixation accelerators, formalin, by far the most widely used histopathological fixative, penetrates tissue at approximately 1 mm/hour [8]. Thus fixation will be initiated rapidly throughout core-cuts (small volume) after immersion whilst penetration of the larger volume main specimen will be slower, resulting in greater loss of biomarker expression. Manoeuvres are therefore necessary to allow rapid initiation of fixation throughout excision biopsies. This is further supported by the reduction in phospho-staining between cores and the main resection being more noticeable in mastectomy than in lumpectomy specimens, especially for p-Erk1/2.
We recently reported that the routine fixation procedure at RMH provides excellent correlations for ER and HER2 status (i.e., positive or negative) between diagnostic core-cuts and resection specimens and those results are supported here [9]. Approximately 15% discordance was noted for PgR in that study on the basis of positive-negative categorisation. Using the cutoff of H score 20, no such discordance was seen in the current study, but the numbers of subjects are too low to exclude that level of discordance. The trend to a quantitative difference reported here for ER and the significant difference we reported previously between excisions and cores are unlikely to lead to erroneous exclusion of responsive patients from endocrine therapy [6]. However, recent data indicate that quantitative levels of ER may be usefully incorporated into algorithms for predicting patient outcome [10]; such assessments will benefit from optimal fixation procedures.
The major differences seen in the two phosphoproteins have major implications for clinical research and future patient management. Past research reports that assessed expression of these markers in samples without special attention to fixation, including studies from our own group [11], may have reached erroneous conclusions [12–19]. One recent publication on p-Akt in relation to the clinical benefit of paclitaxel in the adjuvant treatment of breast cancer recognised the potential for loss of immunoreactivity because of variations in specimen fixation; an optimised antigen retrieval technique was stated to have been used, but its effectiveness was not reported [20]. False-negative results (when inconsistent fixation has occurred) or false-positive results (when systematic bias has resulted from consistently different fixation as alluded to above for window-of-opportunity studies) may both occur. There are also implications for future clinical management: if any such labile markers emerge, as is anticipated, from current translational research efforts, stored routinely fixed tissues are unlikely to be valid for their measurement. Although guidelines for optimal fixation have been published [5], it is clear that these are not uniformly adhered to. This practice needs to be markedly improved to prepare for clinical application of future agents.
Conclusions
Immunohistochemical staining for ER, PgR, HER2 and Ki67 is similar between core-cuts and excision biopsies but is markedly reduced for p-Akt and p-Erk1/2 in routinely fixed excisions. This effect could lead to both false-negative and false-positive findings according to study design and should be considered both in study design and when interpreting published data.
Abbreviations
- DAB:
-
3,3"-diaminobenzidine
- ER:
-
oestrogen receptor
- FFPE:
-
formalin-fixed paraffin-embedded, HER2: human epidermal growth factor receptor 2
- HRP:
-
horseradish peroxidase
- IHC:
-
immunohistochemistry
- IMS:
-
industrial methylated spirits
- PGR:
-
progesterone receptor
- ASCO:
-
American Society of Clinical Oncology
- CAP:
-
College of American Pathologists.
References
Dowsett M: Preoperative models to evaluate endocrine strategies for breast cancer. Clin Cancer Res. 2003, 9: 502s-
Smith IE, Walsh G, Skene A, Llombart A, Mayordomo JI, Detre S, Salter J, Clark E, Magill P, Dowsett M: A phase II placebo-controlled trial of neoadjuvant anastrozole alone or with gefitinib in early breast cancer. J Clin Oncol. 2007, 25: 3816-3822. 10.1200/JCO.2006.09.6578.
Baker AF, Dragovich T, Ihle NT, William R, Fenoglio-Presiser C, Powis G: Stability of phosphoprotein as a biological marker of tumour signalling. Clin Cancer Res. 2005, 11: 4338-4340. 10.1158/1078-0432.CCR-05-0422.
De Cecco L, Musella V, Veneroni S, Cappelletti V, Bongarzone I, Callari M, Valeri B, Pierotti MA, Daidone MG: Impact of biospecimens handling on biomarker research in breast cancer. BMC Cancer. 2009, 9: 409-10.1186/1471-2407-9-409.
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LS, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, Vijver M, Wheeler TM, Hayes DF: American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. J Clin Oncol. 2007, 25: 118-145. 10.1200/JCO.2006.09.2775.
Dowsett M, Bundred NJ, Decensi A, Sainsbury RC, Lu Y, Hills MJ, Cohen FJ, Veronesi P, O'Brien MER, Scott T, Muchmore DB: Effect of raloxifene on breast cancer cell Ki67 and apoptosis: a double-blind, placebo-controlled, randomized clinical trial in postmenopausal patients. Cancer Epidemiol Biomarkers Prev. 2001, 10: 961-966.
Dowsett M, Dixon JM, Horgan K, Salter J, Hills M, Harvey E: Antiproliferative effects of idoxifene in a placebo-controlled trial in primary human breast cancer. Clin Cancer Res. 2000, 6: 2260-2267.
Goldstein NS, Ferkowicz M, Odish E, Mani A, Hastah F: Minimum formalin fixation time for consistent estrogen receptor immunohistochemical staining of invasive breast carcinoma. Am J Clin Pathol. 2003, 120: 86-92. 10.1309/QPHDRB00QXGMUQ9N.
Arnedos M, Nerurkar A, Osin P, A'Hern R, Smith IE, Dowsett M: Discordance between core needle biopsy (CBN) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC). Ann Oncol. 2009, 20: 1948-1952. 10.1093/annonc/mdp234.
Cuzick J, Dowsett M, Wale C, Salter J, Quinn E, Zabaglo L, Howell A, Buzdar A, Forbes J: Prognostic value of a combined ER, PgR, Ki67, HER2 immunohistochemical (IHC4) score and comparison with the GHI recurrence score: results from TransATAC. Cancer Res. 2009, 69 (Suppl): 503s-
Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J, Allred DC, Schiff R, Osborne CK, Dowsett M: Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005, 23: 2469-2476. 10.1200/JCO.2005.01.172.
Svensson S, Jirstrom K, Ryden L, Roos G, Emdin S, Ostrowski MC, Landberg G: ERK phosphorylation is linked to VEGFR2 expression and Ets-2 phosphorylation in breast cancer and is associated with tamoxifen treatment resistance and small tumours with good prognosis. Oncogene. 2005, 24: 4370-4379. 10.1038/sj.onc.1208626.
Frogne T, Laenkholm AV, Lyng MB, Henriksen KL, Lykkesfeldt AE: Determination of HER2 phosphorylation at tyrosine 1221/1222 improves prediction of poor survival for breast cancer patients with hormone receptor-positive tumors. Breast Cancer Res. 2009, 11: R11-10.1186/bcr2230.
Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, Bartlett J: AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol. 2005, 207: 139-146. 10.1002/path.1829.
Modi S, DiGiovanna MP, Lu Z, Moskowitz C, Panageas KS, Van Poznak C, Hudis CA, Norton L, Tan L, Stern DF, Carter D, Seidman AD: Phosphorylated/activated HER2 as a marker of clinical resistance to single agent taxane chemotherapy for metastatic breast cancer. Cancer Invest. 2005, 23: 483-487. 10.1080/07357900500201301.
Haas-Kogan DA, Prados MD, Lamborn KR, Tihan T, Berger MS, Stokoe D: Biomarkers to predict response to epidermal growth factor receptor inhibitors. Cell Cycle. 2005, 4: 1369-1372.
Al-Bazz YO, Underwood JC, Brown BL, Dobson PR: Prognostic significance of Akt, phospho-Akt and BAD expression in primary breast cancer. Eur J Cancer. 2009, 45: 694-704. 10.1016/j.ejca.2008.11.044.
Cappuzzo F, Magrini E, Ceresoli GL, Bartolini S, Rossi E, Ludovini V, Gregorc V, Ligorio C, Cancellieri A, Damiani S, Spreafico A, Paties CT, Lombardo L, Calandri C, Bellezza G, Tonato M, Crinò L: Akt phosphorylation and gefitinib efficacy in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2004, 96: 1133-1141. 10.1093/jnci/djh217.
Haas-Kogan DA, Prados MD, Tihan T, Eberhard DA, Jelluma N, Arvold ND, Baumber R, Lamborn KR, Kapadia A, Malec M, Berger MS, Stokoe D: Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005, 97: 880-887. 10.1093/jnci/dji161.
Yang SX, Costantino JP, Kim C, Mamounas EP, Nguyen D, Jeong JH, Wolmark N, Kidwell K, Paik S, Swain SM: Akt phosphorylation at Ser473 predicts benefit of paclitaxel chemotherapy in node-positive breast cancer. J Clin Oncol. 2010, 28: 2974-2981. 10.1200/JCO.2009.26.1602.
Acknowledgements
This work was supported by Cancer Research UK (grant number C406/A8962), Breakthrough Breast Cancer Research, and the National Institute for Health Research Royal Marsden Hospital Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
IP coordinated sample collection, conducted immunohistochemical determination and scoring of markers Ki67, p-Akt and p-Erk1/2, acquired data on clinical-pathological parameters, conducted statistical analysis and interpretation of data for all markers and assisted in drafting the manuscript; FM selected eligible patients, collected samples in theatre and assisted in drafting the manuscript; MH sectioned tissue blocks and conducted immunohistochemical determination and scoring of markers ER, PgR and HER2; SD assisted in the immunohistochemical determination of phospho-markers; RA supervised the statistical analysis and assisted in drafting the manuscript; AN and PO supervised the histopathological processing of samples and contributed towards the histopathological discussions; JS provided training and supervision of the immunohistochemical tests; IS contributed to the design of the study and its interpretation; and MD conceived and designed the study, interpreted data and drafted the manuscript. All authors approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Pinhel, I.F., MacNeill, F.A., Hills, M.J. et al. Extreme loss of immunoreactive p-Akt and p-Erk1/2 during routine fixation of primary breast cancer. Breast Cancer Res 12, R76 (2010). https://doi.org/10.1186/bcr2719
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/bcr2719