Abstract
Breast cancer is the most common cancer among women worldwide. Estimates suggest up to 35% of cases may be preventable through diet and lifestyle modification. Growing research on the role of fats in human health suggests that early exposure in life to specific fatty acids, when tissues are particularly sensitive to their environment, can have long-term health impacts. The present review examines the role of dietary fat in mammary gland development and breast cancer throughout the lifecycle. Overall, n-3 polyunsaturated fatty acids have promising cancer-preventive effects when introduced early in life, and warrant further research to elucidate the mechanisms of action.
Similar content being viewed by others
Introduction
Cancer is one of the leading causes of mortality worldwide, having significant impact on individuals and healthcare systems. Breast cancer (BC) is one of the top five cancers contributing to overall cancer mortality worldwide, and the incidence is predicted to continue to rise [1–3].
Although the causes of BC are widely unknown, anthropometric, diet and other traits observed at specific stages of life have been associated with BC risk. Birth weights of 4 kg or more, height, level of obesity, early puberty onset and later age at first pregnancy or nulli-parity have been positively correlated with BC risk [4–6]. These observations support the notion that timing and critical periods of development modify BC risk, which is also mirrored in experimental studies [7–9]. Epidemiological studies have provided early clues and highlight the impact that our environment, which includes dietary factors, has on BC risk.
Asian women, who typically have a low rate of BC and a high level of fish oil consumption compared with western women, experience an increase in BC incidence as well as obesity after emigration to the West. In Asian women who have immigrated to the United States, BC rates increase to a level equal to westerners within a generation, and obesity increases twofold between first and second US -born generations [10–12]. This observation suggests that the environment, which includes changes to dietary habits, may contribute to an increase in BC risk and risk factors. Since this change was evident in the generation born post emigration, diet exposure as early as in utero should be considered a factor that modifies BC risk. Findings such as these provide evidence suggesting that early life exposure to environmental factors such as diet may modify disease risk later in life.
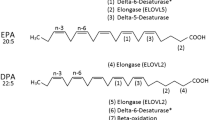
Dietary factors, including specific classes of fatty acids, may be important modifiers of BC risk. Dietary fat is composed of a complex matrix of fatty acids, with unique chemical and biophysical properties that underlie their impact on health and disease. Saturated fatty acids (SFA) (see Figure 1a), monounsaturated fatty acids (MUFA) (see Figure 1b) and trans fatty acids (TFA) (see Figure 1c) have all been found to be potentially associated with an increase in cancer risk in rodent models [13] and in humans [14, 15]. Specific polyunsaturated fatty acids (PUFA), however, may have anticancer effects. There are two major classes of PUFA: omega-6 polyunsaturated fatty acids (n-6 PUFA) and omega-3 polyunsaturated fatty acids (n-3 PUFA). Linoleic acid (LA, 18:2n6) (see Figure 1d) and arachidonic acid (AA, 20:4n6) are the two most common n-6 PUFA in typical western diets [16, 17] and have been suggested to have cancer-promoting effects. In contrast, n-3 PUFA may have anticancer effects. α-Linolenic acid (ALA, 18:3n3) (see Figure 1e) is the predominant form of n-3 PUFA in western diets [18]. The metabolism of ALA, involving sequential desaturation and elongation steps, gives rise to two important long-chain n-3 PUFA: eicosapentaenoic acid (EPA, 20:5n3) (see Figure 1f) and docosahexaenoic acid (DHA, 22:6n3) (see Figure 1g). In addition, EPA and DHA can be obtained directly from seafood and fish oils [19]. For a more in-depth look at fatty acid metabolism, see the article by Shanklin and Cahoon [20].
Representation of molecular connectivity of different dietary fatty acid classes. (a) Saturated fatty acids. (b) Monounsaturated fatty acids, specifically oleic acid. (c) Trans fatty acids. (d) Polyunsaturated fatty acids, specifically linoleic acid. (e) α-Linolenic acid. (f) Eicosapentaenoic acid. (g) Docosahexaenoic acid.
A growing body of research focusing on the role of diet in early life has emerged; in particular, on the consumption of specific fatty acids and cancer risk. The purpose of the present review is therefore to evaluate the linkages between dietary fatty acids and cancer risk at different life stages of the lifecycle and the contribution of specific families of fatty acids and their individual members. Attention will also be given to how specific fatty acids modify the development of the mammary gland (MG), which may underlie how fatty acids modify the BC risk.
Role of fatty acids in mammary gland development and impact on breast cancer risk
Development of the MG is unique and continues throughout life. The ductal branching system within the MG originates at the nipple with a primary duct that extends and branches posteriorly throughout the fat pad in childhood at a slow, gradual pace until the onset of puberty, at which point the process rapidly accelerates [21]. Although the rate of branching is reduced post puberty, it continues throughout the reproductive years of the female. At the ends of each ductal branch are zones of high cell proliferation called terminal ductal lobular units, which differentiate into lobuloalveolar units during pregnancy and later stages of development [21].
The development of the MG is comparable between humans and rodents, thus rodents are often used in MG-related research [21]. The equivalent structures for human terminal ductal lobular units and lobuloalveolar units in rodents are terminal end buds (TEB) and alveolar buds, respectively [10]. The highly proliferative structures have been identified as the initiation sites in tumorigenesis [21].
In humans, cessation of branching begins around 35 years of age or at pregnancy, when differentiation occurs and the gland prepares for lactation. Each stage may represent a sensitive developmental window during which exposure to environmental factors modifies BC risk.
Our understanding of mammary carcinogenesis continues to advance through basic research using rodent models [13, 21–24]. Recent evidence suggests that timing of exposure to dietary fats is an important factor in modulating cancer risk at multiple stages, including in utero, prepuberty/puberty, as well as during pregnancy and lactation, which are probably periods most sensitive to dietary exposure [7–9]. The following sections detail how dietary fatty acids may affect MG development at specific stages throughout the lifecycle.
In uterostage
In utero development may be influenced via maternal interaction and exposure to the surrounding environment. A direct link between the developing fetus and the environment is the transfer of placental nutrients from the maternal diet. Maternal nutrition is therefore of great importance in the development of the fetus, which includes the MG.
When pregnant rats were fed high-fat diets using oils high in n-6 PUFA, serum estradiol levels increased in the mothers and resulted in the female offspring having significantly higher incidence of mammary tumors (Table 1) [25, 26]. The increase in tumor incidence was attributed to changes in the MG of the female offspring. The effects of the high n-6 PUFA diet were similar to animals injected with estradiol during pregnancy, which suggests that n-6 PUFA induces adipose cells to produce and release estrogen in the body, leading to the promotion of tumor growth [25, 26]. Additionally, female offspring exposed to maternal high -fat diets had earlier menarche than controls, which is a marker of increased BC risk, and is associated with significantly higher numbers of TEB (Table 1) [25, 26]. Moreover, the MG of female rodents exposed to estradiol or a high n-6 PUFA diet developed TEB that persisted longer, and with reduced differentiation to alveolar buds (Table 1) [25].
In a human study following pregnant mothers' diets and circulating fatty acids and hormone levels, PUFA had a significant positive association with levels of estrogenic compounds (Table 2) [27]. When long-chain n-3 PUFA levels were examined, however, a significant inverse relationship with estrogenic levels was found (Table 2) [27].
Combined, these factors linked to n-6 PUFA consumption potentially increase the risk of carcinogenesis by prolonging the presence of greater numbers of highly proliferative cells that are sites for tumorigenesis. When high-fat n-6 PUFA diets were co-supplemented with n-3 PUFA via fish oil during pregnancy in rats, a decrease in BC risk in the offspring was observed (Table 1) [28], suggesting that maternal intake of PUFA requires an optimal balance between n-6 PUFA and n-3 PUFA to mitigate the BC risk.
The opposing effects of these families of PUFA relate to their competition for similar enzymes involved in their metabolism, including desaturases, elongases and enzymes involved in eicosanoid synthesis such as cyclo-oxygenases and lypoxygenases. The metabolism of n-6 PUFA produces proinflammatory eicosanoids such as prostaglandin E2, but the presence of n-3 PUFA inhibits their synthesis [29]. The ratio of n -6/n-3 PUFA is thus of considerable importance. There is some controversy as to whether or not this fatty acid ratio is truly important [30], but historical trends suggest that a lower n-6/n-3 ratio is potentially associated with a decreased incidence of cancer [31, 32].
Birth to pubescence
Physical status at birth as well as early nutritional intake are potentially telling of future cancer risk. Studies have shown that high birth weights of 4 kg or more are positively associated with BC risk, nearly doubling the risk when compared with normal weights of 2.5 to 2.9 kg (Table 1) [5, 33, 34]. n-6 PUFA consumption has been shown to be positively associated with birth weight in humans [27].
Breastfeeding has been shown to reduce an infant's risk of developing BC before menopause by up to 35% (Table 1) [33]. The long-chain PUFA DHA, present in breast milk, is known to be important for supporting neuronal and visual development, constituting, on average, 2.88% of an infant's total energy (Table 1) [35]. As will be discussed, the presence or supply of DHA at this early stage of life may also impact on the long-term development of the MG and consequently modify future cancer risk. The supply of DHA is further modified by maternal intake of TFA, which may interfere with DHA synthesis, thus reducing the DHA concentration in breast milk (Table 1) [36]. As DHA plays an important role in fetal development, interactions such as these stress the importance of being aware of interactive effects between different fatty acids - particularly in the maternal diet, as negative effects on fetal development can result.
The literature on estrogenic effects during prepubescence is more complex than that for other developmental stages. Estrogen in rodent fetal development translates to an increase in BC risk [25, 26], but studies in both humans [37] and rodents [8, 38] indicate that estrogen may be protective in the prepubescent period. Rat studies have shown that continuous postnatal exposure to estradiol during the first 30 days after birth decreases the susceptibility of the MG to carcinogen-induced tumorigenesis (Table 1) [39, 40]. Similar effects have been observed on the MG when estradiol exposure is replaced with high n-6 PUFA diets (Table 1) [8]. Additionally, fat intake and a high body mass index surrounding puberty, which is correlated with increased production of estrogens, have been linked to MG development [8]. It has been reported that a higher body mass index during childhood and adolescence reduces the risk of both premenopausal and postmenopausal BC (Table 1) [8, 37, 41, 42]. Some studies, however, suggest no association exists between high body mass index during childhood or adolescence and BC risk [43]. Adding to the complexity of effects at this stage, higher childhood body mass index is linked to early menarche [44], which is associated with increased BC risk (Table 1) [4, 45].
The type of fat consumed is also another modifying factor in this complex relationship. A rodent study that provided high n-6 PUFA diets to rats during various stages of life found that all rats exposed to the high n-6 PUFA diets in utero or postnatally prior to pubescence experienced a significant increase in mammary tumor incidence, when compared with rats that were only exposed to high n-6 PUFA post puberty (Table 1) [9]. When considering long-chain n-3 PUFA, there is an inverse association with estrogenic levels (Table 2) [27], which may be beneficial to the MG during early developmental stages. Consuming a low-fat n-3 PUFA diet during prepubescence resulted in a protective effect against mammary carcinogenesis in rats (Table 1) [46]. In contrast, in the same study, a high-fat n-3 PUFA diet led to lower TEB numbers relative to n-6 PUFA controls, but immunohistochemistry revealed that cells comprising the TEB had higher levels of cell proliferation and increased incidence of mammary tumorigenesis (Table 2) [46]. Such observations may be attributable to the overall high fat content of the diet, as high-fat diets have been linked to mammary tumors that have increased protein kinase C activity [47–49].
Pregnancy and late-stage mammary gland development
Pregnancy and lactation is a time of considerable remodeling of the MG in the mother. During pregnancy, the breast tissue undergoes rapid proliferation followed by differentiation in preparation for lactation [50]. The timing of first pregnancy has been observed to affect BC risk, which may be due to the accumulation of mutations. First pregnancy later in life results in more time having elapsed for mutations to develop, which become potential tumor sites in the developing MG [45]. In fact, if the first full-term pregnancy occurs before the age of 18, BC risk is decreased to 40% that of nulliparous women (Table 1) [4, 50]. In contrast, having a first birth after 35 years of age increases risk by 20% over nulliparous women [50]. A maternal diet high in fat increases BC risk for the mother by raising estrogenic levels (Table 1) [26]. In addition, high pregnancy weight gain, where women gained more than the recommended 11 to 15 kg throughout pregnancy, increased risk by 62% (Table 1) [45].
Effect of fatty acids on breast cancer
Depending on the type, fatty acids may differentially promote BC or, alternatively, may play a role in inhibiting BC. Cell culture studies have found that SFA, MUFA and n-6 PUFA promote BC cell growth [13], whereas ALA, EPA and DHA inhibit BC cell growth [51–53] (Table 2).
An exception within the MUFA class is oleic acid (18:1n9) (see Figure 1b), which has been shown to exert anti-tumorigenic effects by suppressing overexpression of human epidermal growth factor receptor-2 (HER2) (Table 2) [54]. Additionally, oleic acid intake levels are high in the Mediterranean diet, which boasts low cancer levels [55].
The n-6 PUFA, including LA and AA, are observed to promote a tumor-enhancing environment in the MG (Table 2) [16, 46]. AA is a long-chain n-6 PUFA, linked to cell proliferation within the MG, and is associated with enhancing early stages of angiogenesis (Table 2) [16]. AA is the direct precursor of eicosanoids, which mediate proinflammatory processes contributing to tumorigenesis in women [29] and in female rodents [56] (Table 2). Studies have also shown that n-6 PUFA are positively associated with the HER2 oncogene, present in 40% of BC cases (Table 2) [52, 57].
In contrast, n-3 PUFA have been shown to reduce the risk of mammary carcinogenesis (Table 2) [58, 59]. Cell culture work found that ALA strongly suppressed over-expression of HER2-positive mammary carcinomas (Table 2) [52]. The use of flaxseed treatments (57% ALA) on MCF-7 cells in ovariectomized mice produced a significant reduction in tumor growth when compared with a diet containing no flaxseed (Table 2) [60]. In conjunction with these effects, it was found that flaxseed consumption actually enhanced the inhibitory effects of tamoxifen, a drug used in BC treatment [60]. A study on mice treated with MDA-MB-231 BC cells comparing diets of corn oil (1% ALA) with diets of canola oil (10% ALA) found the tumor growth rate to be significantly reduced in canola-fed mice (Table 2) [61].
The n-3 PUFA are thought to be linked to increased susceptibility to peroxidative damage, leading to cell death (Table 2) [62]. In one study, when compared with an n-6 PUFA diet, the n-3 PUFA diet decreased the tumor growth rate by 66% in mice carrying human BC xenografts (Table 2) [63]. These benefits are seen particularly with the long-chain n-3 PUFA EPA and DHA, which occur in high concentrations in fish oils [29, 59, 64]. Despite these supportive studies, a few limited studies on rodents [13] and humans [65, 66] have shown that fish oil and n-3 PUFA are not always consistently shown to inhibit BC.
Little is known in terms of the mechanistic pathways by which n-3 PUFA inhibit cancer growth, but several mechanisms have been proposed. EPA and DHA have both been found to inhibit the production of AA-derived eicosanoids in tumors [64]. Lipid peroxidation, which induces apoptosis, is another potential mechanism by which n-3 PUFA suppress cell proliferation in tumors (Table 2) [62, 67–70]. Numerous experimental studies have looked at apoptotic effects induced by n-3 PUFA mediated by peroxisome proliferator-activated receptor gamma (PPARγ) [57, 62, 67, 68, 71–74]. n-3 PUFA bind and activate PPARγ, leading to activation of the proteoglycan syndecan-1 in human BC cells, which promotes apoptosis leading to cell growth inhibition [73, 74]. n-3 PUFA containing low-density lipoproteins deliver both EPA and DHA to cells and cause a significant twofold increase in syndecan-1 synthesis (Table 2) [74]. These mechanistic studies help to explain potential mechanisms by which n-3 PUFA inhibits cancer cell growth, which supports a role for n-3 PUFA as an effective means of treating BC. Further investigation is necessary, however, as a study conducted recently showed that both the amount and type of fatty acid have an impact on cellular activity, with low-fat n-3 PUFA diets decreasing cell proliferation via PPARγ expression and high-fat n-3 PUFA diets increasing cell proliferation via cyclin D1 expression [75].
Fish oil is a mixture of EPA and DHA, and specific individual effects of each fatty acid remain to be fully clarified. In support of EPA as the principal effector, a study examining cancer progression in mice with induced mammary tumorigenesis found that 4% EPA in the diet had greater efficacy than 4% DHA in inhibiting lung metastases (Table 2) [64]. EPA was also found to suppress an inhibitory G protein-coupled free fatty acid receptor-mediated signal transduction pathway involved in cell proliferation in MCF-7 cell xenografts on mice (Table 2) [76]. Moreover, EPA was observed to slow the tumor growth rate and to reduce metastasis when an EPA diet was compared with a LA diet in mice inoculated with KPL-1 human BC cells (Table 2) [53]. Evidence of DHA being the primary effector in inhibiting tumorigenesis was observed in patients with localized BC. In this study, adipose tissue with high concentrations of DHA showed the greatest extent of tumor regression (Table 2) [77]. DHA itself has also been linked to downregulation of the HER2 oncogene (Table 2) [52] and to the reduction of cancer cell viability (Table 2) [51]. Additionally, DHA has been shown to synergistically enhance the cytotoxic activity of taxanes, in some cases by up to 13-fold (Table 2) [51]. Recent research has shown that DHA is the precursor to potent lipid mediators, identified as resolvins, that regulate inflammation [78, 79]. Further attention is warranted in order to distinguish and understand the effects of individual n-3 PUFA.
Human studies and n-3 PUFA
While a considerable amount of cell culture and animal-based research suggests that n-3 PUFA may modify BC risk and provide protection against mammary tumorigenesis [8, 46, 59, 80, 81], human cohort, case-control studies have failed to provide similar strong support, often finding null relationships between EPA and DHA consumption and BC risk [19, 82–84]. Nevertheless, a limited number of studies support a beneficial relationship between n-3 PUFA and BC risk, many of which are conducted in Asian populations that typically consume higher levels of n-3 PUFA (Table 2) [80, 85–89]. One could argue that if such a beneficial effect truly existed then it should be quite obvious. The wide disparity in findings may reflect methodological limitations, specifically the use of dietary measures reliant on self- reports that are often biased [8, 13, 26, 49, 90–92]. Moreover, North American populations may not be the most appropriate study population for epidemiological research to investigate the link between n-3 PUFA and BC, as the dynamic range between low consumers and high consumers is not very great and is therefore too low to elicit any health benefits, as compared with studies conducted in Asian countries that show a strong link [12, 86]. A recent study conducted on Korean women found BC risk in both premenopausal women and postmenopausal women to be reduced with EPA and DHA consumption (Table 2) [80]. Furthermore, longitudinal studies have only focused on dietary effects in adulthood; potential effects during pregnancy/fetal developmental period and puberty have therefore yet to be rigorously examined.
Another factor that may contribute to the disparity between North American and Asian studies is the source of dietary n-3 PUFA. While Asian countries have higher intake of marine foods containing EPA and DHA, the North American diet contains mainly ALA. Conversion of ALA to EPA and DHA is limited in humans, yielding only ~5.0% and ~0.5%, respectively [19]. Unfortunately, despite numerous dietary options, daily intake of ALA in North American women is low, in some instances averaging less than one-half of their recommended intake of 1.1 g/day [19, 93]. Additional research is needed to determine the specific biological effects of ALA, EPA and DHA in order to ascertain their relative effectiveness in modifying BC risk.
The effects of other fatty acids on BC, including SFA, MUFA and TFA, in human studies are also limited. Studies conducted on BC cell lines and rodents have shown SFA to promote carcinogenesis by inhibiting cellular responses to DNA damage (Table 2) [13, 94], and SFA consumption in humans is observed to be associated with increased BC risk (Table 2) [95]. What is not clear, however, is whether these are truly direct effects of SFA or a marker of a poor diet and obesity, which is associated with cancer risk. A rat study comparing trans and cis fatty acids showed no significant difference between groups (Table 2) [96]. In contrast, French data from a large-scale European health and nutrition prospective cohort study (Etude Epidémiologique de femmes de la Mutuelle Générale de l'Education Nationale (E3N) 1989-2002) found that BC risk was positively associated with TFA, but was not associated with cis-MUFA (Table 2) [14]. Despite negative connotations with TFA to human health, conjugated LAs are an exception. Conjugated LAs, naturally occurring TFA found in ruminant fat products such as dairy and beef products, have been shown to exert anti-carcinogenic effects in BC cell lines and animal models (Table 2) [97, 98]. Further studies conducted on E3N data found ALA to be inversely associated with BC risk, but only when the source was from vegetable oils or fruits and vegetables (Table 2) [99]. Conversely, an increase in BC incidence was noted in relation to ALA when it was obtained through processed foods (Table 2) [99].
Directions for future research
The present review has outlined the widely varying effects dietary fatty acids have on MG development and BC. There remains much inconsistency in the literature regarding the effects of specific classes of fatty acids and their role in BC. The lack of support may not truly be due to a lack of effect, but rather to a lack of our understanding of temporal effects and of specific dietary fatty acids during critical periods of development. Most promising is the ability of n-3 PUFA to alter MG development in the early stages of life, thus protecting against mammary tumorigenesis later in life. The role of dietary fat in BC is indeed a complex relationship. Given that there is mechanistic evidence and emerging data supporting a role for nutrition during pregnancy and early stages of life that modify BC risk, further investigations are warranted. A better appreciation for diet during early life and critical periods of development may help enhance our ability to conduct more rigorous human studies to ascertain the effect of dietary components in the development of BC.
This review identifies evidence suggesting that n-3 PUFA may have a beneficial role for both the prevention and treatment of BC. Future research in this field should focus on the effect of early n-3 PUFA exposure on long-term BC risk, targeting early critical windows in development as discussed throughout the present review. Furthermore, a closer look at the role of individual fatty acids in MG development and BC risk, specifically ALA-enriched, EPA-enriched and DHA-enriched diets, is justified to elucidate differential effects. Such studies are warranted since prevention of BC, as opposed to treatment modalities, will not only have the most impact for individuals but is also economically sustainable.
Overall, the present review highlights additional complexities underlying the relationship between diet and BC and new directions for future research to improve our understanding of the role of dietary fatty acids in BC prevention and treatment.
Abbreviations
- AA:
-
arachidonic acid
- ALA:
-
α-linolenic acid
- BC:
-
breast cancer
- DHA:
-
docosahexaenoic acid
- E3N:
-
Etude Epidémiologique de femmes de la Mutuelle Générale de l'Education Nationale
- EPA:
-
eicosapentaenoic acid
- HER2:
-
human epidermal growth factor receptor-2
- LA:
-
linoleic acid
- MG:
-
mammary gland
- MUFA:
-
monounsaturated fatty acids
- n-3 PUFA:
-
omega-3 polyunsaturated fatty acids
- n-6 PUFA:
-
omega-6 polyunsaturated fatty acids
- PPARγ:
-
peroxisome proliferator-activated receptor gamma
- PUFA:
-
polyunsaturated fatty acids
- SFA:
-
saturated fatty acids
- TEB:
-
terminal end buds
- TFA:
-
trans fatty acids.
References
Breast Cancer Society of Canada. [http://www.bcsc.ca/p/46/1/129/t/statistics]
World Health Organization. [http://www.who.int/mediacentre/factsheets/fs297/en/index.html]
Canadian Cancer Society. [http://www.cancer.ca/Canada-wide/About%20cancer/Cancer%20statistics/Stats%20at%20a%20glance/Breast%20cancer.aspx?sc_lang=en]
Kelsey JL, Gammon MD, John EM: Reproductive factors and breast cancer. Epidemiol Rev. 1993, 15: 36-47.
Michels KB, Trichopoulos D, Robins JM, Rosner BA, Manson JE, Hunter DJ, Colditz GA, Hankinson SE, Speizer FE, Willett WC: Birthweight as a risk factor for breast cancer. Lancet. 1996, 348: 1542-1546.
van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, Marshall JR, Miller AB, Rohan T, Smith-Warner SA, Speizer FE, Willett WC, Wolk A, Hunter DJ: Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000, 152: 514-527.
De AS, Hilakivi-Clarke L: Timing of dietary estrogenic exposures and breast cancer risk. Ann N Y Acad Sci. 2006, 1089: 14-35.
Hilakivi-Clarke L, Cho E, deAssis S, Olivo S, Ealley E, Bouker KB, Welch JN, Khan G, Clarke R, Cabanes A: Maternal and prepubertal diet, mammary development and breast cancer risk. J Nutr. 2001, 131: 154S-157S.
Lo CY, Hsieh PH, Chen HF, Su HM: A maternal high-fat diet during pregnancy in rats results in a greater risk of carcinogen-induced mammary tumors in the female offspring than exposure to a high-fat diet in postnatal life. Int J Cancer. 2009, 125: 767-773.
Popkin BM, Udry JR: Adolescent obesity increases significantly in second and third generation U.S. immigrants: the National Longitudinal Study of Adolescent Health. J Nutr. 1998, 128: 701-706.
Singh GK, Hiatt RA: Trends and disparities in socioeconomic and behavioural characteristics, life expectancy, and cause-specific mortality of native-born and foreign-born populations in the United States, 1979- 2003. Int J Epidemiol. 2006, 35: 903-919.
Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, Wu-Williams AH, Kolonel LN, Horn-Ross PL, Rosenthal JF, Hyer MB: Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993, 85: 1819-1827.
Fay MP, Freedman LS, Clifford CK, Midthune DN: Effect of different types and amounts of fat on the development of mammary tumors in rodents: a review. Cancer Res. 1997, 57: 3979-3988.
Chajes V, Thiebaut AC, Rotival M, Gauthier E, Maillard V, Boutron-Ruault MC, Joulin V, Lenoir GM, Clavel-Chapelon F: Association between serum transmonounsaturated fatty acids and breast cancer risk in the E3N-EPIC study. Am J Epidemiol. 2008, 167: 1312-1320.
Sasaki S, Horacsek M, Kesteloot H: An ecological study of the relationship between dietary fat intake and breast cancer mortality. Prev Med. 1993, 22: 187-202.
Fiorio PA, Grange C, Antoniotti S, Tomatis C, Merlino A, Bussolati B, Munaron L: Arachidonic acid-induced Ca2+ entry is involved in early steps of tumor angiogenesis. Mol Cancer Res. 2008, 6: 535-545.
Horrocks LA, Yeo YK: Health benefits of docosahexaenoic acid (DHA). Pharmacol Res. 1999, 40: 211-225.
Burdge G: Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care. 2004, 7: 137-144.
Harris WS, Mozaffarian D, Lefevre M, Toner CD, Colombo J, Cunnane SC, Holden JM, Klurfeld DM, Morris MC, Whelan J: Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr. 2009, 139: 804S-819S.
Shanklin J, Cahoon EB: Desaturation and related modifications of fatty acids. Annu Rev Plant Physiol Plant Mol Biol. 1998, 49: 611-641.
Russo J, Russo IH: Molecular Basis of Breast Cancer: Prevention and Treatment. 2004, Heidelberg: Springer-Verlag
Ball SM: The development of the terminal end bud in the prepubertal-pubertal mouse mammary gland. Anat Rec. 1998, 250: 459-464.
Cardiff RD, Wellings SR: The comparative pathology of human and mouse mammary glands. J Mammary Gland Biol Neoplasia. 1999, 4: 105-122.
Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, van Zwieten MJ: Comparative study of human and rat mammary tumorigenesis. Lab Invest. 1990, 62: 244-278.
Hilakivi-Clarke L, Clarke R, Onojafe I, Raygada M, Cho E, Lippman M: A maternal diet high in n-6 polyunsaturated fats alters mammary gland development, puberty onset, and breast cancer risk among female rat offspring. Proc Natl Acad Sci USA. 1997, 94: 9372-9377.
Hilakivi-Clarke L, Clarke R, Lippman M: The influence of maternal diet on breast cancer risk among female offspring. Nutrition. 1999, 15: 392-401.
Nagata C, Iwasa S, Shiraki M, Sahashi Y, Shimizu H: Association of maternal fat and alcohol intake with maternal and umbilical hormone levels and birth weight. Cancer Sci. 2007, 98: 869-873.
Su HM, Hsieh PH, Chen HF: A maternal high n-6 fat diet with fish oil supplementation during pregnancy and lactation in rats decreases breast cancer risk in the female offspring. J Nutr Biochem. 2010, : 1033-1037.
Bourre JM: Dietary omega-3 fatty acids for women. Biomed Pharmacother. 2007, 61: 105-112.
Goyens PL, Spilker ME, Zock PL, Katan MB, Mensink RP: Conversion of alphalinolenic acid in humans is influenced by the absolute amounts of alphalinolenic acid and linoleic acid in the diet and not by their ratio. Am J Clin Nutr. 2006, 84: 44-53.
Simopoulos AP: Evolutionary aspects of diet and essential fatty acids. World Rev Nutr Diet. 2001, 88: 18-27.
Simopoulos AP: The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008, 233: 674-688.
Potischman N, Troisi R: In-utero and early life exposures in relation to risk of breast cancer. Cancer Causes Control. 1999, 10: 561-573.
Sanderson M, Williams MA, Malone KE, Stanford JL, Emanuel I, White E, Daling JR: Perinatal factors and risk of breast cancer. Epidemiology. 1996, 7: 34-37.
Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM: Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007, 85: 1457-1464.
Innis SM: Trans fatty intakes during pregnancy, infancy and early childhood. Atheroscler Suppl. 2006, 7: 17-20.
Li J, Humphreys K, Eriksson L, Czene K, Liu J, Hall P: Effects of childhood body size on breast cancer tumour characteristics. Breast Cancer Res. 2010, 12: R23-
Peng JH, Zhu JD, Mi MT, Li FJ, Cai L, Dong JZ, Zhang HX, Zhao Y, Xue RL: Prepubertal genistein exposure affects erbB2/Akt signal and reduces rat mammary tumorigenesis. Eur J Cancer Prev. 2010, 19: 110-119.
Cabanes A, Wang M, Olivo S, deAssis S, Gustafsson JA, Khan G, Hilakivi-Clarke L: Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis. 2004, 25: 741-748.
Nagasawa H, Yanai R, Shodono M, Nakamura T, Tanabe Y: Effect of neonatally administered estrogen or prolactin on normal and neoplastic mammary growth and serum estradiol-17 beta level in rats. Cancer Res. 1974, 34: 2643-2646.
Berkey CS, Frazier AL, Gardner JD, Colditz GA: Adolescence and breast carcinoma risk. Cancer. 1999, 85: 2400-2409.
Baer HJ, Colditz GA, Rosner B, Michels KB, Rich-Edwards JW, Hunter DJ, Willett WC: Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast Cancer Res. 2005, 7: R314-R325.
Okasha M, McCarron P, Gunnell D, Smith GD: Exposures in childhood, adolescence and early adulthood and breast cancer risk: a systematic review of the literature. Breast Cancer Res Treat. 2003, 78: 223-276.
Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS: The relation of menarcheal age to obesity in childhood and adulthood: the Bogalusa heart study. BMC Pediatr. 2003, 3: 3-
Kinnunen TI, Luoto R, Gissler M, Hemminki E, Hilakivi-Clarke L: Pregnancy weight gain and breast cancer risk. BMC Womens Health. 2004, 4: 7-
Olivo SE, Hilakivi-Clarke L: Opposing effects of prepubertal low- and high-fat n-3 polyunsaturated fatty acid diets on rat mammary tumorigenesis. Carcinogenesis. 2005, 26: 1563-1572.
Goodstine SL, Zheng T, Holford TR, Ward BA, Carter D, Owens PH, Mayne ST: Dietary (n-3)/(n-6) fatty acid ratio: possible relationship to premenopausal but not postmenopausal breast cancer risk in U.S. women. J Nutr. 2003, 133: 1409-1414.
Hilakivi-Clarke L, Stoica A, Raygada M, Martin MB: Consumption of a high-fat diet alters estrogen receptor content, protein kinase C activity, and mammary gland morphology in virgin and pregnant mice and female offspring. Cancer Res. 1998, 58: 654-660.
Hilakivi-Clarke L, Clarke R: Timing of dietary fat exposure and mammary tumorigenesis: role of estrogen receptor and protein kinase C activity. Mol Cell Biochem. 1998, 188: 5-12.
MacMahon B, Cole P, Lin TM, Lowe CR, Mirra AP, Ravnihar B, Salber EJ, Valaoras VG, Yuasa S: Age at first birth and breast cancer risk. Bull World Health Org. 1970, 43: 209-221.
Menendez JA, Lupu R, Colomer R: Exogenous supplementation with omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA; 22:6n-3) synergistically enhances taxane cytotoxicity and downregulates Her-2/neu (c-erbB-2) oncogene expression in human breast cancer cells. Eur J Cancer Prev. 2005, 14: 263-270.
Menendez JA, Vazquez-Martin A, Ropero S, Colomer R, Lupu R: HER2 (erbB-2)-targeted effects of the omega-3 polyunsaturated fatty acid, alpha-linolenic acid (ALA; 18:3n-3), in breast cancer cells: the 'fat features' of the 'Mediterranean diet' as an 'anti-HER2 cocktail'. Clin Transl Oncol. 2006, 8: 812-820.
Senzaki H, Iwamoto S, Ogura E, Kiyozuka Y, Arita S, Kurebayashi J, Takada H, Hioki K, Tsubura A: Dietary effects of fatty acids on growth and metastasis of KPL-1 human breast cancer cells in vivo and in vitro. Anticancer Res. 1998, 18: 1621-1627.
Colomer R, Menendez JA: Mediterranean diet, olive oil and cancer. Clin Transl Oncol. 2006, 8: 15-21.
Simopoulos AP: The Mediterranean diets: what is so special about the diet of Greece? The scientific evidence. J Nutr. 2001, 131: 3065S-3073S.
Rose DP, Connolly JM, Rayburn J, Coleman M: Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J Natl Cancer Inst. 1995, 87: 587-592.
Yee LD, Young DC, Rosol TJ, Vanbuskirk AM, Clinton SK: Dietary (n-3) polyunsaturated fatty acids inhibit HER-2/neu-induced breast cancer in mice independently of the PPARγ ligand rosiglitazone. J Nutr. 2005, 135: 983-988.
Caygill CP, Charlett A, Hill MJ: Fat, fish, fish oil and cancer. Br J Cancer. 1996, 74: 159-164.
Stoll BA: N-3 fatty acids and lipid peroxidation in breast cancer inhibition. Br J Nutr. 2002, 87: 193-198.
Chen J, Power KA, Mann J, Cheng A, Thompson LU: Flaxseed alone or in combination with tamoxifen inhibits MCF-7 breast tumor growth in ovariectomized athymic mice with high circulating levels of estrogen. Exp Biol Med (Maywood). 2007, 232: 1071-1080.
Hardman WE: Dietary canola oil suppressed growth of implanted MDA-MB 231 human breast tumors in nude mice. Nutr Cancer. 2007, 57: 177-183.
Gonzalez MJ: Fish oil, lipid peroxidation and mammary tumor growth. J Am Coll Nutr. 1995, 14: 325-335.
Hardman WE, Sun L, Short N, Cameron IL: Dietary omega-3 fatty acids and ionizing irradiation on human breast cancer xenograft growth and angiogenesis. Cancer Cell Int. 2005, 5: 12-
Rose DP, Connolly JM, Coleman M: Effect of omega-3 fatty acids on the progression of metastases after the surgical excision of human breast cancer cell solid tumors growing in nude mice. Clin Cancer Res. 1996, 2: 1751-1756.
Kaizer L, Boyd NF, Kriukov V, Tritchler D: Fish consumption and breast cancer risk: an ecological study. Nutr Cancer. 1989, 12: 61-68.
Terry PD, Rohan TE, Wolk A: Intakes of fish and marine fatty acids and the risks of cancers of the breast and prostate and of other hormone-related cancers: a review of the epidemiologic evidence. Am J Clin Nutr. 2003, 77: 532-543.
Begin ME, Ells G, Horrobin DF: Polyunsaturated fatty acid-induced cytotoxicity against tumor cells and its relationship to lipid peroxidation. J Natl Cancer Inst. 1988, 80: 188-194.
Gonzalez MJ, Schemmel RA, Dugan L, Gray JI, Welsch CW: Dietary fish oil inhibits human breast carcinoma growth: a function of increased lipid peroxidation. Lipids. 1993, 28: 827-832.
Hardman WE, Munoz J, Cameron IL: Role of lipid peroxidation and antioxidant enzymes in omega 3 fatty acids induced suppression of breast cancer xenograft growth in mice. Cancer Cell Int. 2002, 2: 10-
Welsch CW: Review of the effects of dietary fat on experimental mammary gland tumorigenesis: role of lipid peroxidation. Free Radic Biol Med. 1995, 18: 757-773.
Edwards IJ, O'Flaherty JT: Omega-3 fatty acids and PPARγ in cancer. PPAR Res. 2008, 2008: 358052-
Hilakivi-Clarke L, Olivo SE, Shajahan A, Khan G, Zhu Y, Zwart A, Cho E, Clarke R: Mechanisms mediating the effects of prepubertal (n-3) polyunsaturated fatty acid diet on breast cancer risk in rats. J Nutr. 2005, 135: 2946S-2952S.
Jump DB, Clarke SD, Thelen A, Liimatta M, Ren B, Badin MV: Dietary fat, genes, and human health. Adv Exp Med Biol. 1997, 422: 167-176.
Sun H, Berquin IM, Edwards IJ: Omega-3 polyunsaturated fatty acids regulate syndecan-1 expression in human breast cancer cells. Cancer Res. 2005, 65: 4442-4447.
Olivo-Marston SE, Zhu Y, Lee RY, Cabanes A, Khan G, Zwart A, Wang Y, Clarke R, Hilakivi-Clarke L: Gene signaling pathways mediating the opposite effects of prepubertal low-fat and high-fat n-3 polyunsaturated fatty acid diets on mammary cancer risk. Cancer Prev Res (Phila). 2008, 1: 532-545.
Sauer LA, Dauchy RT, Blask DE, Krause JA, Davidson LK, Dauchy EM: Eicosapentaenoic acid suppresses cell proliferation in MCF-7 human breast cancer xenografts in nude rats via a pertussis toxin-sensitive signal transduction pathway. J Nutr. 2005, 135: 2124-2129.
Bougnoux P, Germain E, Chajes V, Hubert B, Lhuillery C, Le FO, Body G, Calais G: Cytotoxic drugs efficacy correlates with adipose tissue docosahexaenoic acid level in locally advanced breast carcinoma. Br J Cancer. 1999, 79: 1765-1769.
Calder PC: Polyunsaturated fatty acids and inflammatory processes: new twists in an old tale. Biochimie. 2009, 91: 791-795.
Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN: Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003, 278: 14677-14687.
Kim J, Lim SY, Shin A, Sung MK, Ro J, Kang HS, Lee KS, Kim SW, Lee ES: Fatty fish and fish omega-3 fatty acid intakes decrease the breast cancer risk: a case-control study. BMC Cancer. 2009, 9: 216-
La GM, Giammanco S, Di MD, Tabacchi G, Tripoli E, Giammanco M: Omega 3 fatty acids: biological activity and effects on human health. Panminerva Med. 2005, 47: 245-257.
MacLean CH, Newberry SJ, Mojica WA, Khanna P, Issa AM, Suttorp MJ, Lim YW, Traina SB, Hilton L, Garland R, Morton SC: Effects of omega-3 fatty acids on cancer risk: a systematic review. JAMA. 2006, 295: 403-415.
Schulz M, Hoffmann K, Weikert C, Nothlings U, Schulze MB, Boeing H: Identification of a dietary pattern characterized by high-fat food choices associated with increased risk of breast cancer: the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Br J Nutr. 2008, 100: 942-946.
Witt PM, Christensen JH, Schmidt EB, Dethlefsen C, Tjonneland A, Overvad K, Ewertz M: Marine n-3 polyunsaturated fatty acids in adipose tissue and breast cancer risk: a case-cohort study from Denmark. Cancer Causes Control. 2009, 20: 1715-1721.
Bagga D, Anders KH, Wang HJ, Glaspy JA: Long-chain n-3-to-n-6 polyunsaturated fatty acid ratios in breast adipose tissue from women with and without breast cancer. Nutr Cancer. 2002, 42: 180-185.
Gago-Dominguez M, Yuan JM, Sun CL, Lee HP, Yu MC: Opposing effects of dietary n-3 and n-6 fatty acids on mammary carcinogenesis: the Singapore Chinese Health Study. Br J Cancer. 2003, 89: 1686-1692.
Kuriki K, Hirose K, Wakai K, Matsuo K, Ito H, Suzuki T, Hiraki A, Saito T, Iwata H, Tatematsu M, Tajima K: Breast cancer risk and erythrocyte compositions of n-3 highly unsaturated fatty acids in Japanese. Int J Cancer. 2007, 121: 377-385.
Maillard V, Bougnoux P, Ferrari P, Jourdan ML, Pinault M, Lavillonniere F, Body G, Le FO, Chajes V: N-3 and N-6 fatty acids in breast adipose tissue and relative risk of breast cancer in a case-control study in Tours, France. Int J Cancer. 2002, 98: 78-83.
Shannon J, King IB, Moshofsky R, Lampe JW, Gao DL, Ray RM, Thomas DB: Erythrocyte fatty acids and breast cancer risk: a case-control study in Shanghai, China. Am J Clin Nutr. 2007, 85: 1090-1097.
Prentice RL: Measurement error and results from analytic epidemiology: dietary fat and breast cancer. J Natl Cancer Inst. 1996, 88: 1738-1747.
Wynder EL, Cohen LA, Muscat JE, Winters B, Dwyer JT, Blackburn G: Breast cancer: weighing the evidence for a promoting role of dietary fat. J Natl Cancer Inst. 1997, 89: 766-775.
Wynder EL, Cohen LA, Winters BL: The challenges of assessing fat intake in cancer research investigations. J Am Diet Assoc. 1997, 97: S5-S8.
Astorg P, Arnault N, Czernichow S, Noisette N, Galan P, Hercberg S: Dietary intakes and food sources of n-6 and n-3 PUFA in French adult men and women. Lipids. 2004, 39: 527-535.
Zeng L, Wu GZ, Goh KJ, Lee YM, Ng CC, You AB, Wang J, Jia D, Hao A, Yu Q, Li B: Saturated fatty acids modulate cell response to DNA damage: implication for their role in tumorigenesis. PLoS One. 2008, 3: e2329-
Kallianpur AR, Lee SA, Gao YT, Lu W, Zheng Y, Ruan ZX, Dai Q, Gu K, Shu XO, Zheng W: Dietary animal-derived iron and fat intake and breast cancer risk in the Shanghai Breast Cancer Study. Breast Cancer Res Treat. 2008, 107: 123-132.
Selenskas SL, Ip MM, Ip C: Similarity between trans fat and saturated fat in the modification of rat mammary carcinogenesis. Cancer Res. 1984, 44: 1321-1326.
Amaru DL, Field CJ: Conjugated linoleic acid decreases mcf-7 human breast cancer cell growth and insulin-like growth factor-1 receptor levels. Lipids. 2009, 44: 449-458.
Parodi PW: Cows' milk fat components as potential anticarcinogenic agents. J Nutr. 1997, 127: 1055-1060.
Thiebaut AC, Chajes V, Gerber M, Boutron-Ruault MC, Joulin V, Lenoir G, Berrino F, Riboli E, Benichou J, Clavel-Chapelon F: Dietary intakes of omega-6 and omega-3 polyunsaturated fatty acids and the risk of breast cancer. Int J Cancer. 2009, 124: 924-931.
Acknowledgements
Funding from a Canadian Breast Cancer Research Alliance/Canadian Institutes of Health Research operating grant (MOP-89971) and from the Canadian Foundation for Innovation with Matching from the Ontario Research Fund was provided to DWLM. MM is supported by a Canadian Institutes of Health Research Graduate Scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
MacLennan, M., Ma, D.W. Role of dietary fatty acids in mammary gland development and breast cancer. Breast Cancer Res 12, 211 (2010). https://doi.org/10.1186/bcr2646
Published:
DOI: https://doi.org/10.1186/bcr2646