Abstract
Introduction
The phosphoinositide-3 kinase (PI3K)/Akt pathway, operating downstream of epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor (HER)2, is implicated in cell migration and survival. EGFR and HER2 are expressed in circulating tumor cells, but the activation status of downstream signaling molecules has not yet been reported.
Methods
To investigate expression levels of EGFR, HER2, PI3K, and Akt in circulating tumor cells, we used peripheral blood mononuclear cells from 32 cytokeratin-19 mRNA-positive patients with early (n = 16) and metastatic (n = 16) breast cancer.
Peripheral blood mononuclear cell cytospins were double stained with cytokeratin antibody along with one of the following: EGFR, phospho-EGFR, HER2, phospho-PI3K, or phospho-Akt antibodies.
Results
EGFR and HER2 were expressed in circulating tumor cells of 38% and 50% patients with early and 44% and 63% patients with metastatic disease, respectively. Interestingly, phospho-PI3K and phospho-Akt expression levels were similar at 88% (14 out of 16) and 81% (13 out of 16), respectively, in circulating tumor cells of patients with early and metastatic disease. Phospho-EGFR was observed in circulating tumor cells of two (33%) early and six (86%) metastatic EGFR-positive patients. Immunomagnetic separation of peripheral blood mononuclear cells, using EpCAM antibody, and subsequent double-staining experiments of circulating tumor cells showed that EGFR was co-expressed with HER2, phospho-Akt and phospho-PI3K kinases, indicating activation of the corresponding survival signaling pathway.
Conclusions
Our findings demonstrate that circulating tumor cells express receptors and activated signaling kinases of the EGFR/HER2/PI3K/Akt pathway, which could be used as targets for their effective elimination.
Similar content being viewed by others
Introduction
Circulating tumor cells (CTCs) have been identified in the blood of patients bearing a wide range of malignancies [1, 2], but not in healthy individuals or in patients with nonmalignant diseases [1]. CTCs have also been identified in significant proportions of patients with both early and metastatic breast cancer, and their presence carries significant prognostic information [3, 4]. Indeed, the detection of CTCs before adjuvant chemotherapy as well as the persistence of CTCs after the completion of systemic adjuvant treatment is associated with an unfavorable clinical outcome [4–6]. Similarly, in patients with metastatic disease, elevated CTC numbers before or soon after the initiation of chemotherapy is an indicator of poor prognosis [7, 8].
The malignant nature of CTCs is supported by the presence of chromosomal alterations [9–12]. However, it appears that only a small proportion of CTCs are capable of forming overt tumor deposits [13]. The molecular characteristics of these cells may play an important role in their survival and could therefore be used to guide effective treatment strategies.
Epidermal growth factor receptor (EGFR; human epidermal growth factor receptor [HER]1) is a member of the ErbB family of receptors that also includes HER2, HER3, and HER4. EGFR ligand binding induces the formation of homodimers and heterodimers [14, 15] and triggers the activation of downstream signaling pathways, such as the phosphoinositide-3 kinase (PI3K)/Akt pathway (among others), which control cell proliferation, survival, and migration [16]. HER2 is the preferred partner for heterodimerization with the other members of ErbB family of receptors [17], and its over-expression has been reported to amplify EGFR signaling [18]. EGFR and ligands such as transforming growth factor-α and amphiregulin are over-expressed in a large subset of primary breast carcinomas [19, 20]. Co-expression of these factors in breast cancer confers poor prognosis and resistance to hormonal therapy [21]. Moreover, inappropriate activation [22] or over-expression [23] of EGFR was associated with poor patient outcome.
Recent studies have reported expression of growth factor receptors on CTCs of patients with breast and prostate cancer [15, 24–26]. However, little is known about the presence of activated receptors and downstream signaling kinases that regulate pro-survival pathways in the CTCs of breast cancer patients. The objective of this study was to investigate whether EGFR and phosphorylated EGFR are expressed on CTCs isolated from the blood of patients with breast cancer. The expression of HER2 and the activation status of PI3K and Akt kinases operating downstream of EGFR were also evaluated in adjuvant as well as in metastatic settings.
Materials and methods
Patient samples and cytospin preparation
A total of 38 patients with detectable cytokeratin (CK)-19 mRNA positive cells [27, 28] in peripheral blood were screened using immunofluorescence, and 32 (84.2%) of them with early (n = 16) and metastatic (n = 16) breast cancer who were found to harbor occult tumor cells were enrolled in the study. In addition 20 female normal blood donors were included in the study as negative control individuals. Specifically, peripheral blood (10 ml in EDTA) was obtained before the initiation of adjuvant treatment (usually 3 to 4 weeks after primary surgery) or first-line chemotherapy for metastatic disease. All blood samples were obtained at the middle of vein puncture after the first 5 ml of blood was discarded. These precautions was undertaken in order to avoid contamination of the blood sample with epithelial cells from the skin during sample collection. All patients gave their informed consent to participate in the study, which has been approved by the Ethics and Scientific Committees of our institution.
Peripheral blood mononuclear cells (PBMCs) were isolated with Ficoll-Hypaque density gradient (d = 1,077 g/mol) centrifugation at 1,800 rpm for 30 minutes. The PBMCs were washed three times with phosphate-buffered saline and centrifuged at 1,500 rpm for 10 minutes. Aliquots of 250,000 cells were centrifuged at 2,000 rpm for 2 minutes on glass slides. Cytospins were dried up and stored at -80°C. Four to five slides from each patient were used for staining experiments.
Cell cultures
For control experiments we used two different breast cancer cell lines, namely MCF7 and SKBR3, which express EGFR, HER2, and the other examined signaling molecules. The SKBR3 breast cancer cell line, which expresses high levels of HER2 and EGFR, was used as a positive control for these molecules, and the MCF7 cell line as a positive control for phosphorylated Akt (pAkt) and phosphorylated PI3K (pPI3K) [29–32]. The MCF7 mammary adenocarcinoma cells (obtained from the American Type Culture Collection, Manassas, VA, USA) were cultured in (vol:vol) 1:1 Dulbecco's modified Eagle medium/Ham's F12 medium (GIBCO-BRL NY, USA), supplemented with 10% fetal bovine serum (GIBCO-BRL NY, USA), 2 mmol/l L-glutamine (GIBCO-BRL NY, USA.), 30 mmol/l NaHCO3, 16 ng/ml insulin, and 50 mg/ml penicilline/streptomycin (GIBCO-BRL NY, USA). SKBR3 cells were cultured in McCoy's (GIBCO-BRL Co.), enriched with 10% fetal bovine serum and 2 mmol/l L-glutamine supplemented with 50 mg/ml penicilline/streptomycin.
Cells were maintained in a humidified atmosphere of 5% CO2/95% air. Subcultivation for all cell lines was performed with 0.25% trypsin and 5 mmol/l EDTA (GIBCO-BRL Co.). All of the experiments were performed during the logarithmic growth phase. At 15 to 20 hours before the experiments, cells were transferred in serum-starved medium containing only L-glutamine, NaHCO3, and penicillin/streptomycin.
Confocal laser scanning microscopy
The presence of CK-positive cells in PBMCs of cytospin preparations was investigated using two different antibodies, according to the origin of the examined second antibody: the mouse A45-B/B3 (detecting CK8, CK18, and CK19; Micromet, Munich, Germany) for the rabbit anti-EGFR, anti-pEGFR and anti-pAKT, and for the goat anti-pPI3K; and the rabbit anti-pancytokeratin (Santa Cruz, Santa Cruz, CA, USA) for the mouse anti-HER2. The pancytokeratin and HER2 antibodies were validated and their specificity was confirmed in normal donors and in CK-negative patients, as previously reported [26]. Cytospins were also double stained with anti-CD45 (common leukocyte antigen; Santa Cruz, Santa Cruz, CA, USA) and A45-B/B3 antibodies in order to exclude possible ectopic expression of cytokeratins on hematopoietic cells. The cytomorphological criteria proposed by Meng and coworkers [33] (high nuclear/cytoplasmic ratio, larger cells than white blood cells, and so on) were used to characterize a CK-positive cell as a CTC.
Cytospins from the same patients were also stained for EGFR (Santa Cruz, Santa Cruz, CA, USA), pEGFR (Invitrogen, Carlsbad, CA, USA), HER2 (Oncogene, Dermstadt, Germany), pPI3K (Santa Cruz), and pAkt (Cell Signaling, Boston, MA, USA) expressions in double-staining experiments and assessed using confocal laser scanning microscopy [26]. Specific staining can be easily distinguished by double immunofluorescence because of the differential intracellular distribution of the examined molecules compared with nonspecific staining, as reported to Fehm and coworkers [34]. PBMC cytospins were fixed with cold aceton:methanol 9:1 for 20 minutes and stained for cytokeratin with a pancytokeratin antibody, as mentioned above. Subsequently, the same slide was stained with either EGFR, pEGFR, HER2, pPI3K, or pAkt antibodies for 45 minutes. Cells were then incubated with the corresponding secondary antibodies for 45 minutes. Slides were analyzed using a confocal laser scanning microscope module (Leica Lasertechnik, Heidelberg, Germany) and images were analyzed using the respective software.
Immunomagnetic separation of CTCs
A total of 2 × 107 PBMCs, isolated as described above, were placed in 1 ml phosphate-buffered saline/20% fetal calf serum. Fifty microliters of CELLection beads (Dynal, Inc., Wirral, UK; coated with EpCAM monoclonal antibody via a DNA linker to provide a cleavable site for cell detachment) were added to the PBMCs. After 30 minutes of incubation at 4°C, cells were washed three times with RPMI/1% fetal calf serum. Supernatant was removed and 4 μl of releasing buffer in 200 μl RPMI/1% fetal calf serum was added to the beads. After 15 minutes of incubation at room temperature, samples were placed in a magnetic device and the released cells were transferred to a different tube. Isolated cells were centrifuged at 2,000 rpm for 2 minutes on glass slides. Double staining microscopy experiments were performed as described previously.
Results
Detection of circulating EGFR/CK double-positive tumor cells
EGFR-positive CTCs were observed in 38% (six out of 16) and 44% (seven out of 16) patients with early and metastatic breast cancer, respectively (Figure 1; see also Additional file 1). Patient characteristics are presented in Tables 1 and 2. Table 3 presents the frequency of EGFR-positive cells detected per sample. Among a total of 62 CTCs identified in patients with early disease, seven (12%) stained positively for both EGFR and CK, whereas the respective numbers for patients with metastatic disease were 68 (42%) out of 161 cells.
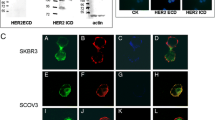
EGFR and HER2 expression in CTCs from breast cancer patients. (a) Representative images of confocal laser scanning microscopic sections of PBMC cytospins stained with pancytokeratins A45 B/B3 (green) and EGFR anti-rabbit (red) antibodies, showing consecutive scanning sections from the upper cytoplasmic region toward the basal attachment site in step sizes of 0.8 μm (magnification: 600×). (b) Representative images of a cytospin, double stained with monoclonal pancytokeratin (A45-B/B3; green) and polyclonal EGFR anti-rabbit (red) antibodies (magnification: 600×). Also shown is quantification of EGFR and CK co-expression in adjuvant and metastatic CTCs. (c) Representative image of cytospin, double stained with polyclonal pancytokeratin and monoclonal HER2 (green) antibodies (magnification: 600×). Quantification of HER2 and CK co-expression in adjuvant and metastatic CTCs. CK, cytokeratin; CTC, circulating tumor cell; EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; PBMC, peripheral blood mononuclear cell.
The distribution of EGFR (red staining) and CK (green staining) in CTCs using immunofluorescence microscopy is demonstrated in Figures 1a,b. Sequential sections of the CTCs with confocal laser scanning microscopy confirmed that EGFR staining was mainly membranous and – to a lesser extent – intracellular, whereas CK was mainly located within the cytoplasm (Figure 1a).
As a negative control, PBMCs from 20 healthy female blood donors were also examined for EGFR/CK expression by double staining experiments. There were no cells that could be stained positive for both EGFR and CK in these samples. However, in two samples, EGFR-positive and CK-negative cells were detected. In order to characterize these cells further, double-staining experiments with EGFR and CD45 antibodies were conducted, which revealed positive staining for both proteins, implying a hematopoietic origin for these cells (data not shown).
The EGFR status of primary tumors and CTCs in early and metastatic breast cancer patients is shown in Table 4. In three out of 12 (25%) and four out of 10 (40%) there was a discrepancy in EGFR expression between the primary tumor and CTCs in early and metastatic breast cancer, respectively.
Detection of circulating HER2/CK double-positive tumor cells
To investigate HER2 expression in CTCs of the same cohort of patients, double-staining experiments were performed using HER2 and CK antibodies. The specificity of these antibodies has been previously reported [26] and no CK-positive/HER2-positive cells could be detected in normal blood donors or in CK-19 mRNA-negative patients with early breast cancer. HER2/CK double-positive cells were identified in CTCs of eight out of 16 patients (50%) with early, and in CTCs from ten out of 16 patients (63%) with metastatic disease (Figure 1c; also see Additional file 1). The frequency of HER2-positive cells detected per sample is presented in Table 3. The intracellular distribution of HER2 using confocal laser scanning microscopy is shown in Figure 1c. Among a total of 51 CTCs identified in patients with early disease, 19 (37%) were CK/HER2-positive, whereas the respective numbers for patients with metastatic disease were 35 out of 103 cells (34%). Three (19%) patients with early and five (31%) patients with metastatic breast cancer had CTCs in their blood expressing both receptors (EGFR and HER2).
As shown in Table 4, there was a discrepancy between the expression of HER2 in primary tumors and CTCs in seven out of 15 (47%) and seven out of 16 (44%) patients with early and metastatic breast cancer, respectively, implying that this is a relatively common phenomenon.
Evaluation of EGFR and downstream PI-3 and Akt kinases activation in CTCs
To investigate whether EGFR is activated in CTCs, double-staining experiments using pEGFR and A45-B/B3 pancytokeratin antibodies were performed. Indeed, pEGFR was observed in patients with EGFR-positive CTCs. The proportion of pEGFR expression in early and metastatic patients was two out of six (33%) and six out of seven (86%), respectively (Figure 2a). Among a total of 20 pEGFR-positive CTCs identified in patients with early disease, three (15%) stained positive for both pEGFR and CK, whereas the respective numbers for patients with metastatic disease were 22 out of 56 cells (39%).
EGFR, PI3K, and Akt activation in CTCs from breast cancer patients. (a) Representative micrograph of cytospin, double-stained with monoclonal pancytokeratin (A45-B/B3; green) and polyclonal pEGFR anti-rabbit (red) antibodies (magnification: 600×). Also shown is quantification of pEGFR and CK co-expression in adjuvant and metastatic CTCs. (b) Representative image of a cytospin, double stained with monoclonal pancytokeratin (A45-B/B3; green) and polyclonal pPI3K (red) antibodies (magnification: 400×). Also shown is quantification of pPI3K and CK co-expression in adjuvant and metastatic CTCs. (c) Representative micrograph of cytospin, double stained with monoclonal pancytokeratin (A45-B/B3; green) and polyclonal pAkt (red) antibodies (magnification: 600 ×). Also shown is quantification of pAkt and CK co-expression in adjuvant and metastatic CTCs. CK, cytokeratin; CTC, circulating tumor cell; EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; PI3K, phosphoinositide-3 kinase.
To evaluate whether pro-survival PI3K and Akt signaling kinases operating downstream of EGFR are activated in CTCs, double-staining experiments using CK and pPI3K or pAkt antibodies were performed [16]. It was observed that the great proportion of patients (88%) with both early and metastatic disease harbored CTCs expressing pPI3K (Figure 2b; also see Additional file 1). The frequency of pPI3K-positive CTCs is shown in Table 3. Interestingly, a large proportion of CTCs of each individual patient were pPI3K positive both in the early (median 100%, range 80% to 100%) and the metastatic (median 90%, range 12.50% to 100%) settings.
Similarly, double-staining experiments using CK and pAkt antibodies revealed the presence of pAkt (Figure 2c) in 13 out of 16 patients (81%) both in early and metastatic breast cancer (see Additional file 1). Moreover, a significant proportion of CTCs of each patient were pAkt positive both in early (median 100%, range 16.6% to 100%) and metastatic (median 83%, range 8.70% to 100%) settings. The frequency of pAkt-positive CTCs is presented in Table 3. Double-staining experiments using blood from 10 normal female donors with A45-B/B3 antibody coupled with either pPI3K or pAkt revealed no double-stained cells, implying the absence of CK-positive cells, which express activated signaling kinases in the blood of healthy women.
Co-expression of EGFR and activated kinases in CTCs
To investigate further whether EGFR and HER2 as well as activated PI3K and Akt signaling kinases are co-expressed in CTCs, we performed immunomagnetic separation of CTCs using EpCAM coated beads in three patients whose CTCs were positive for all of the examined molecules. Subsequently, cytospins of the isolated cells were double stained with EGFR and either HER2, pEGFR, pPI3K, or pAkt. As shown in Figure 3, both EGFR and HER2 were co localized on the same cell. Moreover, EGFR was shown to be co-expressed with pEGFR, pPI3K, or pAkt (Figure 3a), implying the presence of an activated pathway in CTCs downstream of EGFR involving PI3K and Akt.
Immumomagnetic separation of CTCs. Representative confocal laser scanning micrographs of cytospins after immunomagnetic separation of CTCs with EpCAM antibody. Cell cytospins were double stained with EGFR antibody and one of the following: HER2, pEGFR, pPI3K, or pAkt antibody. Cytospin were also double stained with HER2 antibody and either pPI3K or pAkt antibodies (magnification: 400×). EGFR is co-expressed with HER2, pEGFR, phospho-PI3K, and pAkt kinase. CTC, circulating tumor cell; EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; PBMC, peripheral blood mononuclear cell; PI3K, phosphoinositide-3 kinase.
Discussion
The presence of CTCs in the peripheral blood of patients with breast cancer correlates with poor clinical outcome [4–7]. In recent years, significant effort has been made to achieve specific and sensitive detection of CTCs, which poses significant technical challenges because of the rarity of these cells among an abundance of erythrocytes and leukocytes [9, 35, 36]. The rarity of CTCs is also a significant limiting factor for their detailed characterization. It is well known that breast cancers exhibit significant heterogeneity and – at least theoretically – the population of CTCs should include the fraction of tumor cells with the biological potential to form metastatic deposits. Therefore, the genomic and phenotypic analysis of CTCs is of paramount importance for patients with breast cancer, because it provides an insight into the metastatic properties of the primary tumor and permits the exploitation of targeted therapies that are more likely to be effective. A CK-positive cell can be characterized as a CTC if cytomorphological criteria described by Meng and coworkers [33] are satisfied. Furthermore, we and others have previously demonstrated the malignant origin of CK-positive cells, because fluorescence in situ hybridization (FISH) analysis identified HER2 gene amplification and aneusomy [10, 12].
EGFR is frequently over-expressed in breast cancers [19, 20], and EGFR signaling is correlated with poor patient outcome [22, 23]. In the present study, by using immunofluorescence microscopy, we detected EGFR-positive CTCs in 38% and 44% of patients with early and metastatic disease, respectively, harboring CTCs in their blood. In accordance with these findings, EGFR mRNA expression has previously been reported in the blood of 22% to 48% of patients with metastatic breast cancer [37, 38]. However, it should be noted that molecular techniques suffer from a relative lack of specificity, because illegitimate transcription of the EGFR gene in leukocytes cannot be excluded [39]. Conversely, cytological techniques permit evaluation of cell morphology and identification of those cells that have tumor-like appearance. In accordance with this assumption, in the present study we identified EGFR expressing hematopoetic cells in 10% of samples from healthy donors.
Despite the relatively small number of patients for whom the primary tumor was available for EGFR evaluation, it was evident that an EGFR-negative primary tumor does not preclude the presence of EGFR-expressing CTCs. Moreover, a higher proportion of the evaluated CTCs was stained positive for EGFR in patients with metastatic than in patients with early breast cancer. This observation suggests that EGFR is expressed in a predominant clone of cells that contribute to disease progression. Conversely, HER2 expression in the total number of CTCs in early and metastatic disease was similar. This could probably be explained by the observation that human breast cancer is often characterized by a gradual progression to an estrogen-independent, EGFR-positive, and highly metastatic phenotype [40].
In a previous report [26], HER2 expression was reported in cells circulating in the blood of patients with early breast cancer. In another study [15], the presence of HER2-expressing CTCs was found to be suggestive of poor disease-free and overall survival in stage I to III breast cancer. HER2/CK-positive cells were recently characterized as tumor cells using FISH analysis [11]. In the present study, double-staining experiments using pancytokeratin and HER2 antibodies revealed that CK-positive CTCs also co-express HER2. The 50% expression rate in patients with early disease shown here is almost identical to the 48.6% of HER2-positive CTCs in patients with primary breast cancer reported recently by Wulfing and coworkers [15]. It is interesting to note that, in accordance with the above mentioned study [15], we observed a discrepancy between the detection of HER2-positive CTCs and the HER2 staining score of the corresponding primary tumor (Table 4). HER2 positivity in the primary tumor was assessed in accordance with the 2007 criteria proposed by Wolff and coworkers [41], and FISH evaluation was performed in patients with immunohistochemical staining score 2. This discrepancy was also observed in the EGFR expression pattern (Table 4). This could be related to the different methods used for the evaluation of these receptors in the primary tumors and/or the heterogeneity of CTCs. Interestingly, by using immunomagnetic separation of CTCs in three patients, co-localization of both receptors on same cells was demonstrated, thus suggesting that an EGFR and HER2 crosstalk may be operating [42].
Additional experiments revealed that pEGFR is also expressed on the CTCs of both early and metastatic breast cancer patients. The presence of pEGFR on CTCs further supports the hypothesis that EGFR plays a functional role in the biology of these cells. Indeed, EGFR activation was demonstrated in a higher proportion of patients with metastatic disease compared with patients with early disease. Moreover, a higher proportion of the total number of EGFR-expressing CTCs was activated in advanced compared with early disease. Although the small number of patients examined precludes firm conclusions, this finding further suggests that EGFR signaling may be involved in breast cancer progression.
PI3K and Akt kinases operate downstream of EGFR and HER2 in cancer cells, transmitting signals that regulate cell survival and cell migration [16, 43–45]. In a previous report [26], pPI3K was shown to be expressed on the CTCs of patients with early breast cancer. Here we report that pPI3K and pAkt were identified in a significant proportion of CTCs from patients with early and metastatic disease. Their co-localization with EGFR is suggestive of an active pathway involving PI3K and Akt that is initiated upon EGFR activation. Moreover, a statistically significant correlation (P = 0.038) between the number of CTCs expressing pPI3K and pAkt in metastatic patients was observed, suggesting the existence of an active pathway in these cells; conversely, the same correlation was not observed in patients with early breast cancer. This could be due to the heterogeneity of CTCs in these patients [13]. Because the aim of the present study was to evaluate the expression of EGFR, HER2, pAkt and pPI3K in CTCs, the presented data do not have the statistical power to establish clinical correlations because of the small number of enrolled patients. The clinical significance of EGFR, HER2, pAkt, and pPI3K expression in CTCs should be investigated in large prospective clinical trials.
Conclusion
This is the first report to demonstrate expression of activated EGFR and Akt in CTCs in both adjuvant and metastatic settings. The co-expression of HER2 receptor could confer a more aggressive phenotype on EGFR-positive CTCs [46, 47]. Our observations suggest that EGFR-triggered and/or HER2-triggered PI3K/Akt activation may be involved in the regulation of the malignant and metastatic potential of CTCs. Therefore, these molecules could serve as targets for the elimination of micrometastatic disease. In view of the limited capacity of standard systemic treatments to control or eliminate CTCs [48], the data presented in the present study could have important therapeutic implications for novel targeted treatment strategies against micrometastatic disease.
Abbreviations
- CK:
-
cytokeratin
- CTC:
-
circulating tumor cell
- EGFR:
-
epidermal growth factor receptor
- FISH:
-
fluorescence in situ hybridization
- HER:
-
human epidermal growth factor receptor
- PBMC:
-
peripheral blood mononuclear cell
- PI3K:
-
phosphoinositide-3 kinase.
References
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW: Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004, 10: 6897-6904. 10.1158/1078-0432.CCR-04-0378.
Mocellin S, Keilholz U, Rossi CR, Nitti D: Circulating tumor cells: the 'leukemic phase' of solid cancers. Trends Mol Med. 2006, 12: 130-139. 10.1016/j.molmed.2006.01.006.
Kruger W, Krzizanowski C, Holweg M, Stockschlader M, Kroger N, Jung R, Mross K, Jonat W, Zander AR: Reverse transcriptase/polymerase chain reaction detection of cytokeratin-19 mRNA in bone marrow and blood of breast cancer patients. J Cancer Res Clin Oncol. 1996, 122: 679-686. 10.1007/BF01209032.
Stathopoulou A, Vlachonikolis I, Mavroudis D, Perraki M, Kouroussis C, Apostolaki S, Malamos N, Kakolyris S, Kotsakis A, Xenidis N, Reppa D, Georgoulias V: Molecular detection of cytokeratin-19-positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J Clin Oncol. 2002, 20: 3404-3412. 10.1200/JCO.2002.08.135.
Lobodasch K, Frohlich F, Rengsberger M, Schubert R, Dengler R, Pachmann U, Pachmann K: Quantification of circulating tumour cells for the monitoring of adjuvant therapy in breast cancer: an increase in cell number at completion of therapy is a predictor of early relapse. Breast. 2007, 16: 211-218. 10.1016/j.breast.2006.12.005.
Xenidis N, Perraki M, Kafousi M, Apostolaki S, Bolonaki I, Stathopoulou A, Kalbakis K, Androulakis N, Kouroussis C, Pallis T, Christophylakis C, Argyraki K, Lianidou ES, Stathopoulos S, Georgoulias V, Mavroudis D: Predictive and prognostic value of peripheral blood cytokeratin-19 mRNA-positive cells detected by real-time polymerase chain reaction in node-negative breast cancer patients. J Clin Oncol. 2006, 24: 3756-3762. 10.1200/JCO.2005.04.5948.
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF: Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004, 351: 781-791. 10.1056/NEJMoa040766.
Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC, Fritsche HA, Hortobagyi GN, Terstappen LW: Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005, 23: 1420-1430. 10.1200/JCO.2005.08.140.
Brandt B, Meyer-Staeckling S, Schmidt H, Agelopoulos K, Buerger H: Mechanisms of egfr gene transcription modulation: relationship to cancer risk and therapy response. Clin Cancer Res. 2006, 12: 7252-7260. 10.1158/1078-0432.CCR-06-0626.
Fehm T, Sagalowsky A, Clifford E, Beitsch P, Saboorian H, Euhus D, Meng S, Morrison L, Tucker T, Lane N, Ghadimi BM, Heselmeyer-Haddad K, Ried T, Rao C, Uhr J: Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin Cancer Res. 2002, 8: 2073-2084.
Meng S, Tripathy D, Shete S, Ashfaq R, Haley B, Perkins S, Beitsch P, Khan A, Euhus D, Osborne C, Frenkel E, Hoover S, Leitch M, Clifford E, Vitetta E, Morrison L, Herlyn D, Terstappen LW, Fleming T, Fehm T, Tucker T, Lane N, Wang J, Uhr J: HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci USA. 2004, 101: 9393-9398. 10.1073/pnas.0402993101.
Bozionellou V, Mavroudis D, Perraki M, Papadopoulos S, Apostolaki S, Stathopoulos E, Stathopoulou A, Lianidou E, Georgoulias V: Trastuzumab administration can effectively target chemotherapy-resistant cytokeratin-19 messenger RNA-positive tumor cells in the peripheral blood and bone marrow of patients with breast cancer. Clin Cancer Res. 2004, 10: 8185-8194. 10.1158/1078-0432.CCR-03-0094.
Klein CA, Blankenstein TJ, Schmidt-Kittler O, Petronio M, Polzer B, Stoecklein NH, Riethmuller G: Genetic heterogeneity of single disseminated tumour cells in minimal residual cancer. Lancet. 2002, 360: 683-689. 10.1016/S0140-6736(02)09838-0.
Campiglio M, Locatelli A, Olgiati C, Normanno N, Somenzi G, Vigano L, Fumagalli M, Menard S, Gianni L: Inhibition of proliferation and induction of apoptosis in breast cancer cells by the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor ZD1839 ('Iressa') is independent of EGFR expression level. J Cell Physiol. 2004, 198: 259-268. 10.1002/jcp.10411.
Wulfing P, Borchard J, Buerger H, Heidl S, Zanker KS, Kiesel L, Brandt B: HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin Cancer Res. 2006, 12: 1715-1720. 10.1158/1078-0432.CCR-05-2087.
Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A: The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer. 2001, 8: 11-31. 10.1677/erc.0.0080011.
Yarden Y: Biology of HER2 and its importance in breast cancer. Oncology. 2001, 61: 1-13. 10.1159/000055396.
Hendriks BS, Wiley HS, Lauffenburger D: HER2-mediated effects on EGFR endosomal sorting: analysis of biophysical mechanisms. Biophys J. 2003, 85: 2732-2745.
Salomon DS, Brandt R, Ciardiello F, Normanno N: Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995, 19: 183-232. 10.1016/1040-8428(94)00144-I.
Torregrosa D, Bolufer P, Lluch A, Lopez JA, Barragan E, Ruiz A, Guillem V, Munarriz B, Garcia CJ: Prognostic significance of c-erbB-2/neu amplification and epidermal growth factor receptor (EGFR) in primary breast cancer and their relation to estradiol receptor (ER) status. Clin Chim Acta. 1997, 262: 99-119. 10.1016/S0009-8981(97)06542-X.
Woodburn JR: The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther. 1999, 82: 241-250. 10.1016/S0163-7258(98)00045-X.
Kenny PA, Bissell MJ: Targeting TACE-dependent EGFR ligand shedding in breast cancer. J Clin Invest. 2007, 117: 337-345. 10.1172/JCI29518.
Buchholz TA, Tu X, Ang KK, Esteva FJ, Kuerer HM, Pusztai L, Cristofanilli M, Singletary SE, Hortobagyi GN, Sahin AA: Epidermal growth factor receptor expression correlates with poor survival in patients who have breast carcinoma treated with doxorubicin-based neoadjuvant chemotherapy. Cancer. 2005, 104: 676-681. 10.1002/cncr.21217.
de Bono JS, Attard G, Adjei A, Pollak MN, Fong PC, Haluska P, Roberts L, Melvin C, Repollet M, Chianese D, Connely M, Terstappen LW, Gualberto A: Potential applications for circulating tumor cells expressing the insulin-like growth factor-I receptor. Clin Cancer Res. 2007, 13: 3611-3616. 10.1158/1078-0432.CCR-07-0268.
Hayes DF, Walker TM, Singh B, Vitetta ES, Uhr JW, Gross S, Rao C, Doyle GV, Terstappen LW: Monitoring expression of HER-2 on circulating epithelial cells in patients with advanced breast cancer. Int J Oncol. 2002, 21: 1111-1117.
Kallergi G, Mavroudis D, Georgoulias V, Stournaras C: Phosphorylation of FAK, PI-3K, and impaired actin organization in CK-positive micrometastatic breast cancer cells. Mol Med. 2007, 13: 79-88. 10.2119/2006-00083.Kallergi.
Ring A, Smith IE, Dowsett M: Circulating tumour cells in breast cancer. Lancet Oncol. 2004, 5: 79-88. 10.1016/S1470-2045(04)01381-6.
Stathopoulou A, Gizi A, Perraki M, Apostolaki S, Malamos N, Mavroudis D, Georgoulias V, Lianidou ES: Real-time quantification of CK-19 mRNA-positive cells in peripheral blood of breast cancer patients using the lightcycler system. Clin Cancer Res. 2003, 9: 5145-5151.
Anderson NG, Ahmad T, Chan K, Dobson R, Bundred NJ: ZD1839 (Iressa), a novel epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, potently inhibits the growth of EGFR-positive cancer cell lines with or without erbB2 overexpression. Int J Cancer. 2001, 94: 774-782. 10.1002/ijc.1557.
Liang K, Lu Y, Li X, Zeng X, Glazer RI, Mills GB, Fan Z: Differential roles of phosphoinositide-dependent protein kinase-1 and akt1 expression and phosphorylation in breast cancer cell resistance to Paclitaxel, Doxorubicin, and gemcitabine. Mol Pharmacol. 2006, 70: 1045-1052. 10.1124/mol.106.023333.
Kallergi G, Tsapara A, Kampa M, Papakonstanti EA, Krasagakis K, Castanas E, Stournaras C: Distinct signaling pathways regulate differential opioid effects on actin cytoskeleton in malignant MCF7 and nonmalignant MCF12A human breast epithelial cells. Exp Cell Res. 2003, 288: 94-109. 10.1016/S0014-4827(03)00210-6.
Kallergi G, Agelaki S, Markomanolaki H, Georgoulias V, Stournaras C: Activation of FAK/PI3K/Rac1 signaling controls actin reorganization and inhibits cell motility in human cancer cells. Cell Physiol Biochem. 2007, 20: 977-986. 10.1159/000110458.
Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D, Haley B, Morrison L, Fleming TP, Herlyn D, Terstappen LW, Fehm T, Tucker TF, Lane N, Wang J, Uhr JW: Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004, 10: 8152-8162. 10.1158/1078-0432.CCR-04-1110.
Fehm T, Solomayer EF, Meng S, Tucker T, Lane N, Wang J, Gebauer G: Methods for isolating circulating epithelial cells and criteria for their classification as carcinoma cells. Cytotherapy. 2005, 7: 171-185. 10.1080/14653240510027082.
Lacroix M: Significance, detection and markers of disseminated breast cancer cells. Endocr Relat Cancer. 2006, 13: 1033-1067. 10.1677/ERC-06-0001.
Paterlini-Brechot P, Benali NL: Circulating tumor cells (CTC) detection: Clinical impact and future directions. Cancer Lett. 2007
De LA, Pignata S, Casamassimi A, D'Antonio A, Gridelli C, Rossi A, Cremona F, Parisi V, De MA, Normanno N: Detection of circulating tumor cells in carcinoma patients by a novel epidermal growth factor receptor reverse transcription-PCR assay. Clin Cancer Res. 2000, 6: 1439-1444.
Leitzel K, Lieu B, Curley E, Smith J, Chinchilli V, Rychlik W, Lipton A: Detection of cancer cells in peripheral blood of breast cancer patients using reverse transcription-polymerase chain reaction for epidermal growth factor receptor. Clin Cancer Res. 1998, 4: 3037-3043.
Kowalewska M, Chechlinska M, Markowicz S, Kober P, Nowak R: The relevance of RT-PCR detection of disseminated tumour cells is hampered by the expression of markers regarded as tumour-specific in activated lymphocytes. Eur J Cancer. 2006, 42: 2671-2674. 10.1016/j.ejca.2006.05.036.
Safarians S, Sternlicht MD, Yamanishi DT, Love SM, Barsky SH: Human breast cancer progression can be regulated by dominant trans-acting factors in somatic cell hybridization studies. Cancer Res. 1996, 56: 3560-3569.
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van d V, Wheeler TM, Hayes DF: American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007, 25: 118-145. 10.1200/JCO.2006.09.2775.
Nicholson RI, Hutcheson IR, Knowlden JM, Jones HE, Harper ME, Jordan N, Hiscox SE, Barrow D, Gee JM: Nonendocrine pathways and endocrine resistance: observations with antiestrogens and signal transduction inhibitors in combination. Clin Cancer Res. 2004, 10: 346S-354S. 10.1158/1078-0432.CCR-031206.
Braun S, Schlimok G, Heumos I, Schaller G, Riethdorf L, Riethmuller G, Pantel K: ErbB2 overexpression on occult metastatic cells in bone marrow predicts poor clinical outcome of stage I-III breast cancer patients. Cancer Res. 2001, 61: 1890-1895.
Ono M, Kuwano M: Molecular mechanisms of epidermal growth factor receptor (EGFR) activation and response to gefitinib and other EGFR-targeting drugs. Clin Cancer Res. 2006, 12: 7242-7251. 10.1158/1078-0432.CCR-06-0646.
Scaltriti M, Baselga J: The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006, 12: 5268-5272. 10.1158/1078-0432.CCR-05-1554.
Gee JM, Robertson JF, Gutteridge E, Ellis IO, Pinder SE, Rubini M, Nicholson RI: Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer. 2005, 12: S99-S111. 10.1677/erc.1.01005.
Nicholson RI, McClelland RA, Finlay P, Eaton CL, Gullick WJ, Dixon AR, Robertson JF, Ellis IO, Blamey RW: Relationship between EGF-R, c-erbB-2 protein expression and Ki67 immunostaining in breast cancer and hormone sensitivity. Eur J Cancer. 1993, 29A: 1018-1023. 10.1016/S0959-8049(05)80215-1.
Braun S, Kentenich C, Janni W, Hepp F, de WJ, Willgeroth F, Sommer H, Pantel K: Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J Clin Oncol. 2000, 18: 80-86.
Acknowledgements
This work was partly supported by Research Grants from Cretan Association of Biomedical Research (CABR), Phizer Hells, Astra Zeneca Hellas and by the European Social Fund and National Recourses (Pythagoras program).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
GK participated in the design and coordination of the study. She performed the immunofluoresence experiments as well as the immunomagnetic separations and the CTC cultures. She also drafted the manuscript. SA participated in the design coordination of the study and drafted the manuscript. AK collected all the clinicopathological characteristics of the patients. CS participated in the coordination of the study and helped to draft the manuscript. DM participated in the design and coordination of the study, and helped to draft the manuscript. VG provided general support and participated in study design.
Electronic supplementary material
13058_2008_2113_MOESM1_ESM.doc
Additional file 1: File listing the expression levels of CK, EGFR, pEGFR, HER2, pPI3K, and pAkt in CTCs of breast cancer patients. (DOC 27 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kallergi, G., Agelaki, S., Kalykaki, A. et al. Phosphorylated EGFR and PI3K/Akt signaling kinases are expressed in circulating tumor cells of breast cancer patients. Breast Cancer Res 10, R80 (2008). https://doi.org/10.1186/bcr2149
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/bcr2149