Abstract
Massive tissue remodelling occurs within the mammary gland during pregnancy, resulting in the formation of lobuloalveoli that are capable of milk secretion. Endocrine signals generated predominantly by prolactin and progesterone operate the alveolar switch to initiate these developmental events. Here we review the current understanding of the components of the alveolar switch and conclude with an examination of the role of the ets transcription factor Elf5. We propose that Elf5 is a key regulator of the alveolar switch.
Similar content being viewed by others
Introduction: the alveolar switch
Massive tissue remodelling within the mammary gland during pregnancy results in the formation of the secretory lobuloalveolar units in preparation for lactation. The initial proliferative phase of alveolar morphogenesis is instigated by an increase in the level of serum prolactin (Prl) and progesterone (Pg) [1]. These hormones activate the alveolar switch, a genetic program that coordinates changes in mammary epithelial cell proliferation, migration, differentiation and deletion within the many tissue types of the mammary gland. Here we review our current understanding of the genetic program controlling alveolar morphogenesis, using the mouse as a model of the human breast [2]. We then examine the role played by the ets transcription factor Elf5 in coordinating this program in epithelial cells, and propose that Elf5 is a central component of the alveolar switch.
Tissue remodelling during pregnancy
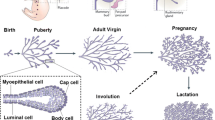
The most striking aspect of mammary development during pregnancy is massive tissue remodelling. During the alveolar morphogenesis phase [3], rapid and global proliferation of the epithelial cells occurs within the ductal branches and developing alveoli. This increases both epithelial cell number and epithelial surface area, actions essential for sufficient milk production during lactation. Cell differentiation becomes dominant from mid-pregnancy as the gland moves into the secretory initiation phase [3]. The developing alveoli cleave and the alveolar cells become polarised and form a sphere-like single layer of epithelial cells that envelopes a circular lumen, connected to the ductal network via a single small duct. Each individual alveolus is surrounded by a basket-like architecture of contractile myo-epithelial cells. The myo-epithelium of the alveoli is discontinuous so that the luminal cells directly contact the underlying basement membrane, which forms part of the extracellular matrix. Some cells of the ductal network also contact the basement membrane. Contact is required for complete lobuloalveolar differentiation [4, 5], seen morphologically by the appearance of lipid droplets [6] and by the initiation of gene expression in a defined order [7]. Nearing parturition, alveolar tight junctions close and milk and colostrum proteins move into the alveolar lumen, in preparation for active milk secretion post-partum, which marks the onset of the secretory activation phase [8] (Figure 1).
Alveolar morphogenesis. Mammary wholemounts (Carmine alum stain top row) and mammary cellular architecture (low power, middle row; high power, bottom row) in virgin, 12 days posts coitus (dpc), 18 dpc and 1 day post partum (1 dpp) murine mammary glands. Ductal epithelial cells (arrow) and myoepithelial cells (arrowhead) arise from a common mammary epithelial stem cell. Massive epithelial cell proliferation occurs at the onset of pregnancy, which is co-ordinated predominantly by prolactin and progesterone. At mid-pregnancy (12 dpc), developing alveoli continue to proliferate and polarise to form a sphere-like single layer of epithelial cells enveloping a circular lumen (indicated by X). This is followed by further cell proliferation and differentiation categorised by the expression of milk genes and the formation of cytoplasmic lipid droplets (indicated by asterisks). At 18 dpc, alveoli have large amounts of lipid and milk protein expression is increased. At parturition, tight junctions between alveolar cells close and milk proteins and lipid are secreted into the alveolar lumen (X). An expansion of the vasculature (open arrows) and reduction in the adipocyte (A) area is also apparent in the stroma.
The epithelial expansion is paralleled by equally dramatic changes in other tissue compartments. Adipocytes lose their lipid content and remain as long projections scattered throughout the alveolar epithelium [9]. A huge expansion of the vasculature also occurs within the stroma, to provide the large quantities of energy, sugars, amino acids and solutes required for milk production [10]. Developmental events are also elicited elsewhere in the animal; for example, the gut and liver enlarge dramatically to cope with the energy needs of gestation and lactation. The brain is programmed for correct maternal behaviour by Prl [11]. Thus, the alveolar switch is part of a larger mechanism controlling all aspects of adaptation to pregnancy and lactation.
Another striking aspect of tissue remodelling during pregnancy is its cyclical nature. Following weaning nearly all of the development induced by the alveolar switch is removed by programmed cell death during the involution phase, only to redevelop with the next pregnancy. This observation first led researchers to hypothesise that mammary tissue must contain persistent self-renewing mammary stem cells (reviewed in [12]). The ability of small epithelial transplants to recapitulate a complete and fully functional epithelial mammary gland reinforced this view [13]. The presence of a single mammary stem cell was indicated by limiting dilution experiments and the existence of committed progenitor cells was demonstrated by transplants that showed limited developmental capacity [14]. This cell was recently isolated and elegantly demonstrated to be capable of producing a renewable and complete mammary epithelium [15]. Thus it is hypothesised, based on a paradigm developed in the hematopoietic system, that a primary mammary epithelial stem cell gives rise to a hierarchy of epithelial progenitor cell lineages to ultimately produce the different cells found in the mammary epithelium [16, 17]. The flux of cells through these lineages is likely to be controlled by, and in turn control, the patterns of gene expression that comprise the alveolar switch. Integrating our knowledge of gene expression patterns with the emerging knowledge regarding stem cell lineages and their interactions offers us an unprecedented opportunity to understand this phase of mammary development.
Prolactin and progesterone initiation of alveolar morphogenesis
The formation of the milk secreting structures during pregnancy is dependent on a synergy between Prl and Pg signalling [6]. These hormones trigger an initial wave of cell proliferation during days two to six of pregnancy [18]. The progesterone receptor (Pgr) knockout mouse demonstrated that Pg is required for alveolar morphogenesis, and epithelial recombination experiments demonstrated that Pgr in the mammary epithelium, not the stroma, was essential for epithelial cell proliferation [19]. Not all mammary epithelial cells express Pgr and, therefore, are unable to respond to Pg directly. Mammary gland chimeras made from Pgr+/+ and Pgr-/- mammary epithelial cells (MECs) demonstrated that Pgr-/- epithelial cells proliferate in response to Pg and, therefore, must respond to a paracrine factor from Pgr+/+ cells [1]. Indeed, in the epithelium, proliferating cells segregate with Pgr positive cells [20]. This is also true for estrogen receptor positive cells [21]. Further, steroid receptor positive cells are in close proximity to proliferating cells, indicating that proliferation is mediated, at least in part, by a paracrine mechanism. This heterogeneous receptor patterning observed in the luminal epithelium is required for complete lobuloalveolar development [22].
Wingless-related MMTV integration site 4 (Wnt4) and receptor activator of nuclear factor (NF)-κB ligand (RankL) are targets of the Pgr signalling pathway and may be the paracrine factors responsible for cellular proliferation in steroid receptor negative cells. Over-expression of the proto-oncogene Wnt1 can rescue pregnancy-induced ductal side branching in Pgr knockout mice, indicating that a Wnt factor may be an important paracrine mediator of Pg-induced ductal side branching during early pregnancy [23]. Mammary transplants of Wnt4-/- epithelium have demonstrated that Wnt4 acts in a paracrine fashion to stimulate epithelial ductal side branching during early pregnancy. In these experiments, normal lobuloalveolar proliferation was observed during the later half of pregnancy, indicating that other factors mediating proliferation in late pregnancy may be involved [23].
The RankL target, NF-κB, is required for cyclin D1 (Ccnd1) activation via the kinase IκB (IKKα) in neighbouring proliferating cells. Germ line deletion of both RankL and its receptor (Rank) in mice resulted in failed alveolar morphogenesis due to reduced proliferation and increased apoptosis of alveolar epithelial cells [24]. These effects were mediated by protein kinase B (PKB/Akt), demonstrating that this pathway is essential for the formation of lobuloalveolar structures [24]. The RankL/NF-κB/Ccnd1 pathway is now known to be crucial for the formation of alveolar structures during pregnancy [25], and NF-κB is essential for Pg driven proliferation within alveoli [20]. RankL also co-localises with Pgrs in response to pregnancy levels of estrogen and Pg, indicating this is an important part of the response. In primary MEC cultures, Pg acts in synergy with estrogen to increase Ccnd1 transcription, resulting in increased proliferation [26]. Together, these data indicate that Pg may drive the proliferation of neighbouring cells via RankL/NF-κB, resulting in Ccnd1 transcription (Figure 2a,b). Pgr consists of two isoforms, PgrA and PgrB, which are expressed from a single gene. The PgrB isoform is essential and sufficient for alveolar morphogenesis during pregnancy. Alveoli in PgrB knockout mice fail to develop due to impaired proliferation of the ductal and alveolar compartment, which is possibly mediated via activation of RankL [27].
Molecular control of alveolar morphogenesis. Signalling from the progesterone receptor (Pgr) and prolactin receptor (Prlr) is essential for alveolar morphogenesis in pregnancy. Increases in serum progesterone (Pg) and prolactin (Prl) result in luminal cell proliferation during early pregnancy, which continues throughout gestation. (a,b) Heterogenous receptor patterning is essential for complete alveolar morphogenesis. (a) Transforming growth factor (Tgf)-β1 signalling via phosphorylation of Smad results in the transcription of target genes, which act to control proliferation in steroid receptor positive cells. Wnt4 and RankL are transcribed in response to Pgr signalling, probably in cooperation with Prl signalling, and appear to stimulate proliferation of neighbouring cells via paracrine mechanisms. (b) RankL binds to its receptor Rank in a neighbouring cell and activates the RankL/nuclear factor (NF)-κB pathway, resulting in cyclin-D1 (Ccnd1) transcription and proliferation. Wnt4 binds and activates its target β-catenin, which has specific roles for both luminal and myo-epithelium for cell fate decisions involving both proliferation and differentiation. (a,c) Prl binds to Prlr and activates the Jak2/Stat5 cascade, resulting in the transcription of genes, including various transcription factors (TF) involved in epithelial morphogenesis and branching (Wnt4), establishment of epithelial polarity and cell-cell interactions (claudins and connexins), stromal epithelial interactions (collagen and laminin), proteins that regulate their own pathway (Socs1/2) and lactation (serotonin and milk proteins). Prl signalling also results in the transcription of cyclin D1 via an insulin growth factor 2 dependent mechanism. The ets transcription factor Elf5, transcribed in response to Prl, can completely compensate for the loss of Prlr signalling. Laminin in the extracellular matrix binds to β1-integrin when contact between the basement membrane and the luminal epithelium is established, and is essential for the maintenance of alveolar cell polarity and differentiation. ErbB4 and its ligands complement Prlr signalling as activation of ErbB4 results in Stat5 phosphorylation and translocation to the nucleus. GJ, gap junction; L, lipid droplet; TJ, tight junction.
Pituitary Prl stimulation of ovarian Pg assists in maintaining the required levels of Pg during early pregnancy [28]. In addition, up-regulation of Pgr expression by Prl, and Prl receptor (Prlr) expression by Pg, suggests that these hormones may interact in a synergistic manner to control alveolar development. Prolactin receptor knockout mice (Prlr-/-) have demonstrated the importance of this receptor during mammary development [29]. Like Pgr, experiments with Prlr-/- mice have shown that the presence of Prlr in the epithelial cells, not the stroma, is essential for normal lobuloalveolar differentiation [30]. Prlr-/- mammary transplants fail to develop lobuloalveoli and produce milk proteins during pregnancy, illustrating that Prlr is essential in the mammary epithelium during alveolar morphogenesis. The downstream targets of prolactin signalling will be discussed in more detail later in this review.
The neuronal peptide galanin (Gal) regulates Prl secretion from the pituitary lactotrophs [31]. In addition, the mammary epithelium is responsive to Gal, as it augments alveolar morphogenesis in mammary explants in the presence of Prl [32]. Gal-/- mice show increased levels of the inhibitory phosphorylated form of Prl [33], and are unable to nurse pups due to failed secretory activation [34]. Therefore, Gal has dual actions: firstly, an indirect role by modulating pituitary Prl and phosphorylated Prl release; and secondly, a direct cell autonomous role in the formation of lobuloalveoli during pregnancy.
Other hormones can influence alveolar morphogenesis. Growth hormone may act in combination with Prl to mediate alveolar proliferation. Growth hormone treatment restores alveolar morphogenesis but inhibits lactation in Prlr+/-mammary glands [35]. Placental lactogen is released from the placenta during pregnancy and can fully compensate for Prl, allowing alveolar morphogenesis in Prl-/- mice [36].
Molecular modulators of Prl induced alveolar morphogenesis
Members of the Prl-signalling pathway are essential for normal alveolar morphogenesis [37]. Prlr dimerization occurs after Prl binding and leads to the phosphorylation of the associated Janus kinase (Jak2) [38, 39], which in turn phosphorylates specific residues on the Prlr [40]. Stat5 is then recruited to the receptor and is phosphorylated by Jak2 [41]. Phosphorylated Stat5 is then translocated to the nucleus where it can activate transcription of multiple genes [42] involved a variety of processes during alveolar morphogenesis, including establishment of epithelial polarity and cell-cell interactions, stromal epithelial interactions and milk protein expression during lactation (Figure 2c). Both isoforms of Stat5, Stat5a and Stat5b, when knocked out in mice, result in lobuloalveolar defects [43–45]. The phenotype is more severe in combined Stat5a/Stat5b knockout animals. One class of genes activated by the prolactin-signalling pathway are the suppressor of cytokine signalling (Socs) members, which act to shut down the Prl-signalling pathway. Socs1 knockout mice show precocious development during pregnancy, and Socs1+/- mice can restore the lobuloalveolar defects present in Prlr+/- mice due to Prlr haplo-insufficiency [46]. Similarly, loss of Socs2 can also rescue lactation in Prlr+/- females [47].
Transcript profiling of Prlr knockout mammary glands identified a panel of genes that require Prlr-mediated signalling for increased expression during early pregnancy [46, 48] (Figure 2c). Two members of the collagen family and laminin were identified. These molecules are cell adhesion components of the extracellular matrix and play an important part in the epithelial-stromal signalling required for full lobuloalveolar differentiation and gene expression [4, 7]. Alveolar morphogenesis induced by Prl involves the establishment of polarity and cell-cell communication. The maintenance of cellular polarity is regulated by the closure of tight junctions, and the expression of tight junction proteins Claudin-3 and Claudin-7 was reduced in Prlr-/- mammary transplants [46]. The gap junction protein Connexin 26 was also identified and is involved in the exchange of small ions and metabolites [49]. Recently, Connexin-26 was shown to be important in full lobuloalveolar development and in the prevention of alveolar cellular apoptosis [50].
Wnt4 was also down-regulated in Prlr-/- transplants, indicating that it is potentially a target of Prlr signalling [46]. The downstream target of Wnt, β-catenin, has specific actions in both the luminal and myoepithelial compartments of the epithelium, and as a component of cell-cell junctions appears to have a role in signalling to luminal epithelial cells [51, 52]. Indeed, activation of β-catenin within the basal epithelial cells results in premature differentiation of the luminal epithelium during pregnancy and persistent proliferation resulting in tumors. These tumors consisted predominantly of undifferentiated basal cells, which were amplified in response to β-catenin activation, thereby implicating this molecule in cell fate decisions in the mammary gland [52].
The gene encoding RankL was also identified as potentially regulated by Prl [46, 53]. Ccnd1 null mutants exhibit significantly delayed alveolar cell proliferation and impaired lactation, which was shown to be epithelial cell autonomous [54]. Interestingly, Prl can induce Ccnd1 expression via induction of insulin growth factor 2, independent of RankL induction [55]. The similarities between Prl- and Pg-mediated effects on both RankL and Wnt signalling is further evidence of the co-operation of these pathways for alveolar cell proliferation during early pregnancy (Figure 2a).
Gene expression profiling of Prl-/- mice has also identified unique targets of mammary development. Expression of tryptophan hydroxylase, the rate-limiting enzyme in serotonin biosynthesis, is increased by Prl during pregnancy and lactation. Accumulation of serotonin due to milk engorgement experienced during weaning or experimentally via teat sealing inhibits milk gene expression and can induce involution, providing a mechanism that is put in place by Prl to stop lactation at weaning [56].
Transcription factors involved in alveolar morphogenesis
Prl and Pg and other factors induce the transcription of genes via activation of target transcription factors. These include Stat5 and the steroid hormone receptors as discussed previously, which bind to DNA and result in the transcription of genes involved in many aspects of alveolar morphogenesis. Further, some of these target genes are transcription factors also, which act to induce the expression of genes or groups of genes involved in lobuloalveolar development. An example is the transcription factor Srebf1, which was identified from transcript profiling experiments on three mouse models of failed secretory activation [33]. Srebf1 controls the expression of a number of key lipid metabolism genes [57] that showed reduced expression concomitantly with decreased Srebf1 expression [33]. Some transcription factors that appear to be involved in alveolar morphogenesis include the homeobox genes, helix-loop-helix genes, Stats, Tcf/Lef family, NF-κB, the Ceb/p family, the nuclear factor family and the Ets transcription factors. The regulation of cellular proliferation during mammary development by the homeobox genes, helix-loop-helix genes, stats and ets transcription factors has been reviewed previously [58].
Pg and Prl are hypothesised to influence the expression of β-catenin via induction of the Wnt pathway, as discussed earlier. β-Catenin regulates the activity of the Tcf/Lef family of transcription factors, which appear to mediate β-catenin signalling and, therefore, may play a role during alveolar morphogenesis [59]. Inhibition of β-catenin results in alveolar apoptosis and greatly reduced milk production capacity. Mice lacking Lef-1 demonstrate a failure to form the alveolar bud at embryonic day 13. The expression of Lef-1 was co-expressed with β-catenin, and shows a similar expression pattern in response to parathyroid hormone-related protein [60]. Thus, Lef-1 may act to mediate the actions of β-catenin, although its effects during alveolar morphogenesis are still unclear.
The NF1 family of transcription factors also play a role in functional differentiation as they regulate the transcription of milk protein genes such as those encoding whey acidic protein, α-lactalbumin and β-lactoglobulin [61]. The NF1-C2 isoform member of this family induces the expression of the milk genes encoding carboxyl ester lipase and whey acidic protein. Prl regulates the protein expression of NF1-C2 in NmuMG cells, and its expression is reduced in the nucleus of Prlr-/- luminal cells at mid-pregnancy, indicating that NF1-C2 may be regulated by Prl signalling during pregnancy and involved in expression of milk genes in preparation for lactation [62].
The helix-loop-helix transcription factors Id1 and Id2 have varying expression in the mammary gland. Id1 expression is increased during early pregnancy, remains low during lactation and rises again at involution. Unlike Id1, Id2 remains high during lactation, indicating that these isoforms have specific functional roles during alveolar morphogenesis [63]. Id1 is specifically expressed by the expanding epithelium during the alveolar proliferative phase and is inversely correlated with the expression of β-casein; it appears, therefore, to be an important factor during early alveolar proliferation. Id1 also regulates Clusterin, which is involved in the regulation of cell-cell interactions. Additionally, lobuloalveolar development is severely impaired in Id2 knockout mice. Reduced proliferation and increased apoptosis has been observed in mammary epithelium lacking Id2, resulting in the failure to form alveolar structures and consequently failure of lactation [64]. Id2 also promotes differentiation in MEC cultures, indicating Id2 is essential for the differentiation of the mammary epithelium [63].
The transcription factor NF-κB discussed earlier in this review is essential for Pg induced alveolar cell proliferation resulting in Ccnd1 transcription [20, 25]. NF-κB can also induce the transcription of many genes involved in the regulation of apoptosis. NF-κB levels are induced during pregnancy, decline during lactation and are re-induced during lactation implying a role in mammary gland remodelling. It is also hypothesised that NF-κB is an essential 'checkpoint' of apoptosis, whose actions are dependent on association with specific transcriptional regulators. Thus NF-κB is an important transcription factor controlling both proliferation and apoptosis in the epithelium during pregnancy [65].
The C/ebp family of proteins appear to be important regulators of alveolar morphogenesis (for a review, see [66]). C/ebpβ and C/ebpδ isoforms are increased during pregnancy and decline during lactation, indicating that they play a critical role in alveolar morphogenesis and early milk gene expression. Transplantation experiments have revealed that C/ebpβ is required in epithelial cells for normal lobuloalveolar development during pregnancy, and C/ebpβ knockout mice display phenotypes similar to Pgr, Prlr, Stat5a/b, Ccnd1, Id2 and RankL knockouts [66]. Interestingly Pgr expression was dramatically increased in the mammary glands of C/ebpβ null mice and, in addition, the expression of Pgr was unusually uniform within the epithelium [67]. These effects were associated with a 10-fold decrease in the rate of proliferation. There was, however, no change in the expression of C/ebpβ in the mammary glands of Pgr knockout mice, indicating that C/ebpβ is upstream of Pgr and possibly controls the spatial distribution of epithelial cells, which influence proliferation in alveolar progenitors [67]. C/ebpβ null epithelium significantly increased Tgf-β and Smad2 signalling, and this pathway is known to inhibit cellular proliferation [68]. Cell cycle progression in C/ebpβ null MECs was blocked at the G1/S transition, preventing these cells from proliferating in response to early pregnancy levels of Pg and estrogen [69]. Therefore, C/ebpβ is essential for controlling cell fate decisions within the mammary gland, including attenuating Pgr expression resulting in mammary epithelial cell differentiation during pregnancy.
The expression of the Ets transcription factor subfamily Pea3 is elevated at the onset of pregnancy but declines during mid-pregnancy to low levels at lactation and involution, suggesting a role in early pregnancy induced ductal outgrowth. Three members of the Pea3 subfamily are expressed by both the myoepithelium and the luminal cells, although their expression varies during pregnancy, suggesting multiple signalling roles during alveolar morphogenesis. The expression of all members of the family remains in the myoepithelium during pregnancy, although the expression of the ER81 member declines in the luminal epithelium seven days after impregnation. Increased numbers of dividing cells were observed in the terminal end buds of Pea3 knockout mice, and mammary gland transplants of Pea3 knockout epithelium displayed reduced mammary branching during pregnancy, suggesting a role for Pea3 in progenitor cell differentiation [70].
Other factors involved in alveolar morphogenesis
The receptor tyrosine kinase ErbB (epidermal growth factor) family and their ligands are important mediators of all aspects of mammary development. There are four receptors: epidermal growth factor receptor/ErbB/Her1, ErbB2/Her2/neu, ErbB3/Her3 and ErbB4/Her4, which are activated by a variety of ligands inducing activation via dimerisation and cross phosphorylation. ErbB ligands share a 50 amino acid domain, which is homologous to epidermal growth factor. Mice expressing a truncated dominant negative allele of ErbB2 did not exhibit a phenotype until late pregnancy, when alveoli failed to expand and distend, indicating that ErbB2 is critical for secretory activation, and will be discussed later in this review series [71]. Conditional deletion of ErbB4 within the mammary gland at pregnancy demonstrated a critical role for this receptor during alveolar morphogenesis [72]. Alveolar expansion was reduced from 13.5 days post coitus in mammary epithelium lacking ErbB4, resulting in incomplete alveolar development and failure to nurse pups due to reduced milk gene expression. Alveolar proliferation was attenuated and Stat5 phosphorylation was abolished. The ErbB4 ligand neuregulin/heregulin-1 (Nrg) promotes lobulo-alveolar development and the expression of milk genes when used in mammary gland explants [73], indicating a role for this ligand in lobuloalveolar development. In addition, mice that lack the alpha form of Nrg show a similar phenotype to ErbB4 knockout, with reduced alveolar proliferation and differentiation, demonstrated by reduced β-casein expression in reduced alveoli expansion [74].
Other ErbB ligands also appear to have overlapping functions for mammary gland development. Amphiregulin null animals have reduced alveolar development, although the phenotype was much more severe in a triple mutant including knockouts of Tgfα and epidermal growth factor (all ligands of the ErbB family), indicating overlapping and compensatory roles for these ligands during alveolar morphogenesis [75]. Triple mutants developed poorly organised and differentiated alveoli, had reduced milk protein expression and often pups born to these mice did not survive. Amphiregulin loss was also associated with reduced Stat5 phosphorylation. Our transcript profiling experiments demonstrated that amphiregulin was down-regulated in Prlr-/- epithelium [46], indicating that amphiregulin may be modulated by Prlr signalling. These data together indicate important roles for the ErbB receptors and ligands during alveolar morphogenesis. The overlapping phenotypes observed in Prlr, Pgr and ErbB knockout mice suggest there may be some cross-talk between these receptors, which is yet to be fully understood.
The cell surface receptor β1 integrin, which is present on luminal epithelial cells, is an essential mediator of extracellular matrix signalling via its ligands collagen and laminin [76]. Mammary epithelium in mice lacking β1 integrin in the luminal cells, displayed reduced proliferation and alveolar disorganisation [77]. The focal adhesion kinase, which is important in protein complexes that connect the extracellular matrix to the actin cytoskeleton, was also reduced in these mice. Conditional deletion of β1 integrin during early pregnancy and late pregnancy demonstrates that this molecule was important for both the formation of lobuloalveolar structures and for functional differentiation [78]. In these mammary glands, luminal epithelium becomes dissociated from the basement membrane, and cellular polarity is compromised as luminal epithelial cells protrude into the alveolar luminal space. In addition, Prl-stimulated milk protein expression via phosphorylation of Stat5 was largely absent in primary mammary epithelial cells lacking β1-integrin, indicating that it is essential for Prl-induced activation of Stat5 [79].
The cytokine Tgf-β1 is an important regulator of mammary cell proliferation during pregnancy [68]. Tgf-β1 is restricted to the luminal epithelial cells and can control cell proliferation via phosphorylation of Smad following Tgf-β receptor activation [80]. Tgf-β1 heterozygote mice display accelerated lobulo-alveolar development due to increased proliferation, indicating that the expression of Tgf-β1 restricts alveolar cell proliferation. Epithelial cell proliferation was increased more than 15-fold in Tgf-β1 null ovariectomised animals treated with estrogen and Pg compared to wild-type mice [81]. In animals treated with estrogen and Pg, Tgf-β1 expression was restricted to the steroid receptor positive epithelial cells, indicating that Tgf-β1 may play an important role in restricting epithelial cell proliferation in these cells [82].
The ets transcription factor Elf5
Our transcript profiling experiments identified a number of transcription factors that showed reduced expression in response to a loss of Prlr, but profiling of a cell based model of positive Prl action identified the ets transcription factor Elf5 [47]. Ets transcription factors are identified by a highly conserved DNA binding domain (the ets domain), which binds to sites containing a central GGA motif [83]. Ets transcription factors regulate gene expression during the differentiation of multiple tissues including vascular, lymphoid, muscle and bone (reviewed in [84]). Elf5 (e74-like factor 5 or ESE-2) is an epithelial specific member of the Elf subfamily of Ets transcription factors, and is closely related to the epithelial specific Elf3 (ESE-1) and Ehf (ESE-3) [85, 86]. The predicted protein products of mouse Elf5 and human ESE-2 are 95% identical and are expressed as two isoforms produced by alternative start sites. Such high conservation of sequence implies similar conservation of function [86].
Elf5 is expressed specifically in the luminal cells of mammary tissue [47], and its expression is increased dramatically during pregnancy, to levels that far exceed those seen in other tissues. Elf5 can also bind to an ets-like domain in the proximal promoter of whey acidic protein and induce its expression independently of lactogenic hormones, indicating that Elf5 may be an important mediator of alveolar differentiation during mid-pregnancy [87]. Elf5-/- mice die in utero due to a placentation defect [88]. Elf5+/- mice did not lactate due to failed alveolar development and, in some mice where alveoli had formed, differentiation into functional secretory units was severely impaired [89]. Mammary epithelial cell proliferation was reduced throughout alveolar morphogenesis and secretory activation, and mammary epithelial transplants demonstrated that this effect was cell autonomous. The levels of Elf5 are reduced in Prlr+/- glands and there is no similar reduction in the expression of Prlr in Elf5+/-, indicating that Elf5 is downstream of the Prlr [89]. MECs from Prlr-/- mammary glands fail to form lobuloalveoli during pregnancy when transplanted into the cleared fat pad of hosts with a normal endocrine milieu. Retroviral re-expression of Elf5 in Prlr-/- MECs followed by transplantation to a cleared fat pad resulted in a rescue of alveolar morphogenesis [47]. MECs expressing high levels of Elf5 proliferated and differentiated into distended, milk filled alveoli [47]. Thus, re-expression of Elf5 in Prlr-/- MECs could completely compensate for the loss of the Prlr signalling cascade. Prlr-/- MECs expressing lower levels of Elf5 showed development that passed alveolar formation but failed during secretory initiation, mimicking the situation seen in Elf5+/- and Prlr+/- mice. Elf5 is a key mediator of structural and functional development of lobuloalveoli [47]. Elf5 would thus appear to be a master-regulator of the alveolar switch required for alveolar morphogenesis.
Conclusion
It is apparent that a large number of genes can influence alveolar morphogenesis during pregnancy, some of which are shown in Figure 2. A better understanding of the components of the alveolar switch, and thus the regulation of mammary cell proliferation and differentiation, has direct application to the regulation of lactation in agricultural species and the prevention and control of breast cancer. The key question is how the expression of these numerous proteins is organised and regulated by the alveolar switch. One potential model is a hierarchy of transcription factors that are each responsible for regulating an aspect of development. A precedent for this model is provided by the action of the transcription factor Srebf1, which regulates the expression of lipogenic enzymes during secretory initiation [33]. In this model, Elf5 would be placed close to the origin of the hierarchy, as a master regulator of the transcriptional cascade controlling alveolar morphogenesis.
Note
This article is part of a review series on Key stages in mammary gland development, edited by Charles Streuli.
Other articles in the series can be found online at http://breast-cancer-research.com/articles/review-series.asp?series=bcr_keystages.
Abbreviations
- Ccnd1:
-
cyclin D1
- Gal:
-
galanin
- MEC:
-
mammary epithelial cell
- NF:
-
nuclear factor
- Pg:
-
progesterone
- Pgr:
-
progesterone receptor
- Prl:
-
prolactin
- Prlr:
-
prolactin receptor
- RankL/Opgl:
-
receptor activator of NF-κB ligand/osteoprotegrin ligand
- Socs:
-
suppressor of cytokine signalling
- Tgf:
-
transforming growth factor.
References
Brisken C: Hormonal control of alveolar development and its implications for breast carcinogenesis. J Mammary Gland Biol Neoplasia. 2002, 7: 39-48. 10.1023/A:1015718406329.
Cardiff RD, Wellings SR: The comparative pathology of human and mouse mammary glands. J Mammary Gland Biol Neoplasia. 1999, 4: 105-122. 10.1023/A:1018712905244.
Richert MM, Schwertfeger KL, Ryder JW, Anderson SM: An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia. 2000, 5: 227-241. 10.1023/A:1026499523505.
Streuli CH, Edwards GM, Delcommenne M, Whitelaw CB, Burdon TG, Schindler C, Watson CJ: Stat5 as a target for regulation by extracellular matrix. J Biol Chem. 1995, 270: 21639-21644. 10.1074/jbc.270.45.26794.
Fata JE, Werb Z, Bissell MJ: Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004, 6: 1-11.
Neville MC, McFadden TB, Forsyth I: Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia. 2002, 7: 49-66. 10.1023/A:1015770423167.
Rudolph MC, McManaman JL, Hunter L, Phang T, Neville MC: Functional development of the mammary gland: use of expression profiling and trajectory clustering to reveal changes in gene expression during pregnancy, lactation, and involution. J Mammary Gland Biol Neoplasia. 2003, 8: 287-307. 10.1023/B:JOMG.0000010030.73983.57.
Nguyen DA, Parlow AF, Neville MC: Hormonal regulation of tight junction closure in the mouse mammary epithelium during the transition from pregnancy to lactation. J Endocrinol. 2001, 170: 347-356. 10.1677/joe.0.1700347.
Neville MC, Medina D, Monks J, Hovey RC: The mammary fat pad. J Mammary Gland Biol Neoplasia. 1998, 3: 109-116. 10.1023/A:1018786604818.
Djonov V, Andres AC, Ziemiecki A: Vascular remodelling during the normal and malignant life cycle of the mammary gland. Microsc Res Tech. 2001, 52: 182-189. 10.1002/1097-0029(20010115)52:2<182::AID-JEMT1004>3.0.CO;2-M.
Lucas BK, Ormandy CJ, Binart N, Bridges RS, Kelly PA: Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology. 1998, 139: 4102-4107. 10.1210/en.139.10.4102.
Smalley M, Ashworth A: Stem cells and breast cancer: A field in transit. Nat Rev Cancer. 2003, 3: 832-844. 10.1038/nrc1212.
DeOme KB, Faulkin LJJ, Bern HA, Blair PB: Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Research. 1959, 19: 515-520.
Smith GH, Boulanger CA: Mammary epithelial stem cells: transplantation and self-renewal analysis. Cell Prolif. 2003, 36: 3-15. 10.1046/j.1365-2184.36.s.1.2.x.
Shackleton M, F V, Simpson KJ, Stingl J, Smythe GK, Asselin-Labat M-L, Wu L, Lindeman GJ, Visvader JE: Generation of a functional mammary gland from a single stem cell. Nature. 2006, 439: 84-88. 10.1038/nature04372.
Smalley MJ, Clarke RB: The mammary gland "side population": a putative stem/progenitor cell marker?. J Mammary Gland Biol Neoplasia. 2005, 10: 37-47. 10.1007/s10911-005-2539-0.
Stingl J, Raouf A, Emerman JT, Eaves CJ: Epithelial progenitors in the normal human mammary gland. J Mammary Gland Biol Neoplasia. 2005, 10: 49-59. 10.1007/s10911-005-2540-7.
Traurig H: Cell Proliferation in the mammary gland during late pregnancy and lactation. Anatomical Record. 1967, 157: 489-503. 10.1002/ar.1091570309.
Brisken C, Park S, Vass T, Lydon JP, O'Malley BW, Weinberg RA: A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA. 1998, 95: 5076-5081. 10.1073/pnas.95.9.5076.
Conneely OM, Jericevic BM, Lydon JP: Progesterone receptors in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2003, 8: 205-214. 10.1023/A:1025952924864.
Clarke RB, Howell A, Potten CS, Anderson E: Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997, 57: 4987-4991.
Grimm SL, Seagroves TN, Kabotyanski EB, Hovey RC, Vonder-haar BK, Lydon JP, Miyoshi K, Hennighausen L, Ormandy CJ, Lee AV, et al: Disruption of steroid and prolactin receptor patterning in the mammary gland correlates with a block in lobulo-alveolar development. Molecular Endocrinology. 2002, 16: 2675-2691. 10.1210/me.2002-0239.
Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA: Essential function of Wnt-4 in mammary gland development downstream of prog-esterone signaling. Genes Dev. 2000, 14: 650-654.
Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, et al: The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000, 103: 41-50. 10.1016/S0092-8674(00)00103-3.
Cao Y, Karin M: NF-kappaB in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2003, 8: 215-223. 10.1023/A:1025905008934.
Said TK, Conneely OM, Medina D, O'Malley BW, Lydon JP: Prog-esterone, in addition to estrogen, induces cyclin D1 expression in the murine mammary epithelial cell, in vivo. Endocrinology. 1997, 138: 3933-3939. 10.1210/en.138.9.3933.
Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM: Defective mammary gland morphogenesis in mice lacking the prog-esterone receptor B isoform. Proc Natl Acad Sci USA. 2003, 100: 9744-9749. 10.1073/pnas.1732707100.
Binart N, Helloco C, Ormandy CJ, Barra J, Clement-Lacroix P, Baran N, Kelly PA: Rescue of preimplantatory egg development and embryo implantation in prolactin receptor-deficient mice after progesterone administration. Endocrinology. 2000, 141: 2691-2697. 10.1210/en.141.7.2691.
Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, et al: Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997, 11: 167-178.
Brisken C, Kaur S, Chavarria TE, Binart N, Sutherland RL, Wein-berg RA, Kelly PA, Ormandy CJ: Prolactin controls mammary gland development via direct and indirect mechanisms. Developmental Biol. 1999, 210: 96-106. 10.1006/dbio.1999.9271.
Wynick D, Hammond PJ, Akinsanya KO, Bloom SR: Galanin regulates basal and oestrogen-stimulated lactotroph function. Nature. 1993, 364: 529-532. 10.1038/364529a0.
Naylor MJ, Ginsburg E, Iismaa TP, Vonderhaar BK, Wynick D, Ormandy CJ: The neuropeptide galanin augments lobuloalve-olar development. J Biol Chem. 2003, 278: 29145-29152. 10.1074/jbc.M303746200.
Naylor MJ, Oakes SR, Gardiner-Garden M, Harris J, Blazek K, Ho TW, Li FC, Wynick D, Walker AM, Ormandy CJ: Transcriptional changes underlying the secretory activation phase of mammary gland development. Mol Endocrinol. 2005, 19: 1868-1883. 10.1210/me.2004-0254.
Wynick D, Small CJ, Bacon A, Holmes FE, Norman M, Ormandy CJ, Kilic E, Kerr NC, Ghatei M, Talamantes F, et al: Galanin regulates prolactin release and lactotroph proliferation. Proc Natl Acad Sci USA. 1998, 95: 12671-12676. 10.1073/pnas.95.21.12671.
Allan GJ, Tonner E, Barber MC, Travers MT, Shand JH, Vernon RG, Kelly PA, Binart N, Flint DJ: Growth hormone, acting in part through the insulin-like growth factor axis, rescues developmental, but not metabolic, activity in the mammary gland of mice expressing a single allele of the prolactin receptor. Endocrinology. 2002, 143: 4310-4319. 10.1210/en.2001-211191.
Horseman ND: Prolactin and mammary gland development. J Mammary Gland Biol Neoplasia. 1999, 4: 79-88. 10.1023/A:1018708704335.
Hennighausen L, Robinson GW: Information networks in the mammary gland. Nature Rev Mol Cell Biol. 2005, 6: 715-725. 10.1038/nrg1677.
Han Y, Watling D, Rogers NC, Stark GR: JAK2 and STAT5, but not JAK1 and STAT1, are required for prolactin-induced beta-lactoglobulin transcription. Mol Endocrinol. 1997, 11: 1180-1188. 10.1210/me.11.8.1180.
Pezet A, Buteau H, Kelly PA, Edery M: The last proline of Box 1 is essential for association with JAK2 and functional activation of the prolactin receptor. Mol Cell Endocrinol. 1997, 129: 199-208. 10.1016/S0303-7207(97)00063-4.
Lebrun JJ, Ali S, Goffin V, Ullrich A, Kelly PA: A single phospho-tyrosine residue of the prolactin receptor is responsible for activation of gene transcription. Proc Natl Acad Sci USA. 1995, 92: 4031-4035. 10.1073/pnas.92.9.4031.
DaSilva L, Rui H, Erwin RA, Howard OM, Kirken RA, Malabarba MG, Hackett RH, Larner AC, Farrar WL: Prolactin recruits STAT1, STAT3 and STAT5 independent of conserved receptor tyrosines TYR402, TYR479, TYR515 and TYR580. Mol Cell Endocrinol. 1996, 117: 131-140. 10.1016/0303-7207(95)03738-1.
Wakao H, Gouilleux F, Groner B: Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1995, 14: 854-855.
Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L: Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997, 11: 179-186.
Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L: Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci USA. 1995, 92: 8831-8835. 10.1073/pnas.92.19.8831.
Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN: Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998, 93: 841-850. 10.1016/S0092-8674(00)81444-0.
Ormandy CJ, Naylor M, Harris J, Robertson F, Horseman ND, Lin-deman GJ, Visvader J, Kelly PA: Investigation of the transcriptional changes underlying functional defects in the mammary glands of prolactin receptor knockout mice. Recent Prog Hormone Res. 2003, 58: 297-323. 10.1210/rp.58.1.297.
Harris J, Stanford PM, Sutherland K, Oakes SR, Naylor MJ, Robertson FG, Blazek KD, Kazlauskas M, Hilton HN, Wittlin S, et al: Socs2 and Elf5 mediate prolactin-induced mammary gland development. Mol Endocrinol. 2006,
Harris J, Stanford PM, Oakes SR, Ormandy CJ: Prolactin and the prolactin receptor: new targets of an old hormone. Ann Med. 2004, 36: 414-425. 10.1080/07853890410033892.
Monaghan P, Moss D: Connexin expression and gap junctions in the mammary gland. Cell Biol Int. 1996, 20: 121-125. 10.1006/cbir.1996.0016.
Bry C, Maass K, Miyoshi K, Willecke K, Ott T, Robinson GW, Hennighausen L: Loss of connexin 26 in mammary epithelium during early but not during late pregnancy results in unscheduled apoptosis and impaired development. Dev Biol. 2004, 267: 418-429. 10.1016/j.ydbio.2003.11.022.
Imbert A, Eelkema R, Jordan S, Feiner H, Cowin P: Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J Cell Biol. 2001, 153: 555-568. 10.1083/jcb.153.3.555.
Teuliere J, Faraldo MM, Deugnier MA, Shtutman M, Ben-Ze'ev A, Thiery JP, Glukhova MA: Targeted activation of beta-catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia. Development. 2005, 132: 267-277. 10.1242/dev.01583.
Srivastava S, Matsuda M, Hou Z, Bailey JP, Kitazawa R, Herbst MP, Horseman ND: Receptor activator of NF-kappaB ligand induction via Jak2 and Stat5a in mammary epithelial cells. J Biol Chem. 2003, 278: 46171-46178. 10.1074/jbc.M308545200.
Fantl V, Edwards PA, Steel JH, Vonderhaar BK, Dickson C: Impaired mammary gland development in Cyl-1(-/-) mice during pregnancy and lactation is epithelial cell autonomous. Dev Biol. 1999, 212: 1-11. 10.1006/dbio.1999.9329.
Brisken C, Ayyannan A, Nguyen C, Heineman A, Reinhardt F, Jian T, Dey SK, Dotto GP, Weinberg RA, Jan T: IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev Cell. 2002, 3: 877-887. 10.1016/S1534-5807(02)00365-9.
Matsuda M, Imaoka T, Vomachka AJ, Gudelsky GA, Hou Z, Mistry M, Bailey JP, Nieport KM, Walther DJ, Bader M, et al: Serotonin regulates mammary gland development via an autocrine-paracrine loop. Dev Cell. 2004, 6: 193-203. 10.1016/S1534-5807(04)00022-X.
Horton JD, Goldstein JL, Brown MS: SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002, 109: 1125-1131. 10.1172/JCI200215593.
Coletta RD, Jedlicka P, Gutierrez-Hartmann A, Ford HL: Transcriptional control of the cell cycle in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2004, 9: 39-53. 10.1023/B:JOMG.0000023587.40966.f6.
Hatsell S, Rowlands T, Hiremath M, Cowin P: Beta-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2003, 8: 145-158. 10.1023/A:1025944723047.
Foley J, Dann P, Hong J, Cosgrove J, Dreyer B, Rimm D, Dunbar M, Philbrick W, Wysolmerski J: Parathyroid hormone-related protein maintains mammary epithelial fate and triggers nipple skin differentiation during embryonic breast development. Development. 2001, 128: 513-525.
Murtagh J, Martin F, Gronostajski RM: The Nuclear Factor I (NFI) gene family in mammary gland development and function. J Mammary Gland Biol Neoplasia. 2003, 8: 241-254. 10.1023/A:1025909109843.
Johansson EM, Kannius-Janson M, Gritli-Linde A, Bjursell G, Nilsson J: Nuclear factor 1-C2 is regulated by prolactin and shows a distinct expression pattern in the mouse mammary epithelial cells during development. Mol Endocrinol. 2005, 19: 992-1003. 10.1210/me.2004-0359.
Desprez PY, Sumida T, Coppe JP: Helix-loop-helix proteins in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2003, 8: 225-239. 10.1023/A:1025957025773.
Mori S, Nishikawa SI, Yokota Y: Lactation defect in mice lacking the helix-loop-helix inhibitor Id2. Embo J. 2000, 19: 5772-5781. 10.1093/emboj/19.21.5772.
Clarkson RW, Watson CJ: NF-kappaB and apoptosis in mammary epithelial cells. J Mammary Gland Biol Neoplasia. 1999, 4: 165-175. 10.1023/A:1018725207969.
Grimm SL, Rosen JM: The role of C/EBPbeta in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2003, 8: 191-204. 10.1023/A:1025900908026.
Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM: C/EBPbeta (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol Endocrinol. 2000, 14: 359-368. 10.1210/me.14.3.359.
Barcellos-Hoff MH, Ewan KB: Transforming growth factor-beta and breast cancer: Mammary gland development. Breast Cancer Res. 2000, 2: 92-99. 10.1186/bcr40.
Grimm SL, Contreras A, Barcellos-Hoff MH, Rosen JM: Cell cycle defects contribute to a block in hormone-induced mammary gland proliferation in CCAAT/enhancer-binding protein (C/EBPbeta)-null mice. J Biol Chem. 2005, 280: 36301-36309. 10.1074/jbc.M508167200.
Kurpios NA, Sabolic NA, Shepherd TG, Fidalgo GM, Hassell JA: Function of PEA3 Ets transcription factors in mammary gland development and oncogenesis. J Mammary Gland Biol Neoplasia. 2003, 8: 177-190. 10.1023/A:1025948823955.
Jones FE, Stern DF: Expression of dominant-negative ErbB2 in the mammary gland of transgenic mice reveals a role in lobuloalveolar development and lactation. Oncogene. 1999, 18: 3481-3490. 10.1038/sj.onc.1202698.
Long W, Wagner KU, Lloyd KC, Binart N, Shillingford JM, Hen-nighausen L, Jones FE: Impaired differentiation and lactational failure of Erbb4-deficient mammary glands identify ERBB4 as an obligate mediator of STAT5. Development. 2003, 130: 5257-5268. 10.1242/dev.00715.
Yang Y, Spitzer E, Meyer D, Sachs M, Niemann C, Hartmann G, Weidner KM, Birchmeier C, Birchmeier W: Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. J Cell Biol. 1995, 131: 215-226. 10.1083/jcb.131.1.215.
Li L, Cleary S, Mandarano MA, Long W, Birchmeier C, Jones FE: The breast proto-oncogene, HRGalpha regulates epithelial proliferation and lobuloalveolar development in the mouse mammary gland. Oncogene. 2002, 21: 4900-4907. 10.1038/sj.onc.1205634.
Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC: Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999, 126: 2739-2750.
Zutter MM, Sun H, Santoro SA: Altered integrin expression and the malignant phenotype: the contribution of multiple integrated integrin receptors. J Mammary Gland Biol Neoplasia. 1998, 3: 191-200. 10.1023/A:1018798907544.
Li N, Zhang Y, Naylor MJ, Schatzmann F, Maurer F, Wintermantel T, Schuetz G, Mueller U, Streuli CH, Hynes NE: Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. EMBO J. 2005, 24: 1942-1953. 10.1038/sj.emboj.7600674.
Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, Wang P, Schatzmann F, Wintermantel T, Schuetz G, et al: Ablation of β1 integrin in mammary epithelium reveals a key role for inte-grin in glandular morphogenesis and differentiation. J Cell Biol. 2005, 171: 717-728. 10.1083/jcb.200503144.
Naylor M, Streuli C: Integrin regulation of mammary gland development. Integrins and Development. Edited by: Danin E. 2006, Georgetown, TX Landes Bioscience, 176-185.
Massague J, Chen YG: Controlling TGF-beta signaling. Genes Dev. 2000, 14: 627-644.
Ewan KB, Shyamala G, Ravani SA, Tang Y, Akhurst R, Wakefield L, Barcellos-Hoff MH: Latent transforming growth factor-beta activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation. Am J Pathol. 2002, 160: 2081-2093.
Ewan KB, Oketch-Rabah HA, Ravani SA, Shyamala G, Moses HL, Barcellos-Hoff MH: Proliferation of estrogen receptor-alpha-positive mammary epithelial cells is restrained by transforming growth factor-beta1 in adult mice. Am J Pathol. 2005, 167: 409-417.
Sharrocks AD, Brown AL, Ling Y, Yates PR: The ETS-domain transcription factor family. Int J Biochem Cell Biol. 1997, 29: 1371-1387. 10.1016/S1357-2725(97)00086-1.
Oikawa T, Yamada T: Molecular biology of the Ets family of transcription factors. Gene. 2003, 303: 11-34. 10.1016/S0378-1119(02)01156-3.
Oettgen P, Kas K, Dube A, Gu X, Grall F, Thamrongsak U, Akbar-ali Y, Finger E, Boltax J, Endress G, et al: Characterization of ESE-2, a novel ESE-1-related Ets transcription factor that is restricted to glandular epithelium and differentiated ker-atinocytes. J Biol Chem. 1999, 274: 29439-29452. 10.1074/jbc.274.41.29439.
Zhou J, Ng AY, Tymms MJ, Jermiin LS, Seth AK, Thomas RS, Kola I: A novel transcription factor, ELF5, belongs to the ELF subfamily of ETS genes and maps to human chromosome 11p13-15, a region subject to LOH and rearrangement in human carcinoma cell lines. Oncogene. 1998, 17: 2719-2732. 10.1038/sj.onc.1202198.
Thomas RS, Ng AN, Zhou J, Tymms MJ, Doppler W, Kola I: The Elf group of Ets-related transcription factors. ELF3 and ELF5. Adv Exp Med Biol. 2000, 480: 123-128.
Donnison M, Beaton A, Davey HW, Broadhurst R, L'Huillier P, Pfeffer PL: Loss of the extraembryonic ectoderm in Elf5 mutants leads to defects in embryonic patterning. Development. 2005, 132: 2299-2308. 10.1242/dev.01819.
Zhou J, Chehab R, Tkalcevic J, Naylor MJ, Harris J, Wilson TJ, Tsao S, Tellis I, Zavarsek S, Xu D, et al: Elf5 is essential for early embryogenesis and mammary gland development during pregnancy and lactation. EMBO J. 2005, 24: 635-644. 10.1038/sj.emboj.7600538.
Acknowledgements
This work is supported by The National Health and Medical Research Council of Australia, the Cancer Council New South Wales, United States DoD BCRP (DAMD17-03-1-0686) (SRO), and the University of New South Wales University Postgraduate Award (HNH).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
Oakes, S.R., Hilton, H.N. & Ormandy, C.J. Key stages in mammary gland development - The alveolar switch: coordinating the proliferative cues and cell fate decisions that drive the formation of lobuloalveoli from ductal epithelium. Breast Cancer Res 8, 207 (2006). https://doi.org/10.1186/bcr1411
Published:
DOI: https://doi.org/10.1186/bcr1411