Abstract
The pathogenesis of rheumatoid arthritis (RA) and osteoarthritis (OA) remains obscure, although angiogenesis appears to play an important role. We recently confirmed an overexpression of two angiogenic factors, namely vascular endothelial growth factor (VEGF) and platelet-derived endothelial cell growth factor (PD-ECGF), by the lining and stromal cells of the synovium in both conditions. Because hypoxia inducible factor (HIF)-1α and HIF-2α are essential in regulating transcription of the VEGF gene, active participation of HIF-α molecules in the pathogenesis of these arthritides is anticipated. We investigated the immunohistochemical expression of HIF-1α and HIF-2α in the synovium of 22 patients with RA, 34 patients with OA and 22 'normal' nonarthritic individuals, in relation to VEGF, VEGF/KDR (kinase insert domain protein receptor) vascular activation, PD-ECGF and bcl-2. A significant cytoplasmic and nuclear overexpression of HIF-1α and HIF-2α was noted in the synovial lining and stromal cells of both diseases relative to normal. Overexpression of HIF-αs was related to high microvessel density, high PD-ECGF expression and high VEGF/KDR receptor activation, suggesting HIF-α-dependent synovial angiogenesis in OA. By contrast, the activation of the angiogenic VEGF/KDR pathway was persistently increased in RA, as indeed was microvessel density and the expression of PD-ECGF, irrespective of the extent of HIF-α expression, indicating a cytokine-dependent angiogenesis. In all cases, the VEGF/KDR vascular activation was significantly lower in OA than in RA, suggesting a relative failure of the HIF-α pathway to effectively produce a viable vasculature for OA, which is consistent with the degenerative nature of the disease. The activation of the HIF-α pathway occurs in both RA and OA, although for unrelated reasons.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA), a polyarticular disease of autoimmune nature [1], and osteoarthritis (OA) a noninflammatory degenerative disease of the articular cartilage [2], have in common an increased tendency for new blood vessel formation [3–5]. This phenomenon, however, may not necessarily proceed in a similar manner in the two conditions. Neoangiogenesis is important in the development of new cartilage and mineralization in OA [6], whereas the same process contributes to synovitis, pannus formation and articular cartilage destruction in RA [7]. In a previous study [8], we showed increased levels of expression of the angiogenic factors vascular endothelial growth factor (VEGF) and platelet-derived endothelial cell growth factor (PD-ECGF; also known as thymidine phosphorylase) and, as a consequence, an increased microvessel density (MVD) of the entire synovial vasculature in both RA and OA, relative to normal. However, the presence of an activated synovial vasculature ('VEGF/kinase insert domain protein receptor [KDR] complex' expression) was high only in the case of RA. This failure of OA to activate the VEGF/KDR pathway, in the presence of increased VEGF expression, is consistent with the degenerative nature of the disease, whereas the profoundly upregulated VEGF/KDR pathway in RA pursues a destructive angiogenesis-related course.
Hypoxia inducible factor (HIF)-1α and HIF-2α are important transcription factors, regulating VEGF gene responses to hypoxic stimuli. Reduction in the degradation rate of HIF-αs, as occurs under hypoxic stress, results in accumulation of HIF-1α and HIF-2α proteins and upregulation of the angiogenic process [9, 10]. The direct link between accumulation of HIF-αs and overexpression of VEGF [11, 12], and the important role of the VEGF angiogenic pathway in arthritides suggest a central role for HIF-αs in the pathogenesis of RA and OA.
In the present study, we investigated the immunohistochemical expression of HIF-1α and HIF-2α in synovial tissues in RA and OA. The results were related to the angiogenic process in the synovial membrane and to the antiapoptotic protein bcl-2. More specifically, the results were analyzed with reference to MVD, VEGF, and the activation of the angiogenic pathways VEGF/KDR and PD-ECGF.
Material and methods
Formalin-fixed paraffin-embedded synovial tissues were retrieved from the files of the Departments of Pathology, Democritus University of Thrace, Alexandroupolis, Greece, and Nuffield Orthopaedic Centre, Oxford, UK. The material was from 22 cases of active RA, 34 cases of OA and 22 nonarthritic cases derived from hip joint replacement following fracture. Table 1 shows the patient characteristics. Histological confirmation of the arthritic pathology was performed on haematoxylin and eosin stained sections.
Immunohistochemistry for HIF-1α and HIF-2α expression
The HIF-1α and HIF-2α proteins were detected using ESEE 122 (IgG1 monoclonal antibody [mAb]; dilution 1:20) and the EP190b (IgG1 mAb; neat), as previously described [13, 14]. Sections were deparaffinized and peroxidase was quenched with methanol and 3% H2O2 for 15 min. Microwaving for antigen retrieval was used (3 × 5 min). The primary antibodies were applied for 90 min. Following washing with tris-buffered saline (TBS), sections were incubated with a secondary antirabbit antimouse antibody (Kwik Biotinylated Secondary, 0.69A Shandon-Upshaw, Pittsburgh, PA, USA) for 15 min and washed in tris-buffered saline. Kwik Streptavidin peroxidase reagent (039A Shandon-Upshaw) was applied for 15 min and sections were again washed in TBS. The colour was developed by 15 min of incubation with diamone benzidine solution, and sections were weakly counterstained with haematoxylin. Breast cancer tissues with strong nuclear HIF-1α and HIF-2α expression were used as positive controls. Normal mouse IgG was substituted for primary antibody as negative control at the same concentration as the test antibody.
Immunohistochemistry for VEGF, VEGF/KDR, PD-ECGF, and endothelial cell expression
VEGF expression and that of the VEGF/KDR complex was assessed using the 11B5 mAb, an IgM isotype produced using the VEGF amino-terminus as an immunogen [15]. VEGF expression was also assessed using the VG1 mAb (VEGF blocking mAb, IgG isotype), which recognizes the 121, 165, and 189 isoforms of VEGF [16]. Assessment of PD-ECGF expression was performed using the P-GF.44C mAb [17]. Paraffin embedded sections, 3 μm thick, were stained using the alkaline phosphatase/antialkaline phosphatase (APAAP) procedure, following microwaving for antigen retrieval. The primary antibodies were applied at room temperature as follows: 11B5 at dilution 1:3 for 120 min; VG1 at dilution 1:3 for 90 min; and P-GF.44C at dilution 1:3 for 30 min. They were subsequently washed in TBS. Rabbit antimouse antibody 1:50 (vol:vol) was applied for 30 min, followed by application of APAAP complex 1:1 (vol:vol) for 30 min. After washing in TBS, the last two steps were repeated for 10 min each. This step was not required for the PGF-44c staining. The colour was developed by 15 min of incubation with new fuchsin solution and sections were weakly counterstained with haematoxylin. Non-specific immunoglobulins were substituted for primary antibody as negative controls at the same concentration as the test antibody.
The JC70 monoclonal antibody (DAKO, Glostrup, Denmark), which recognizes the CD31 pan-endothelial antigen (platelet/endothelial cell adhesion molecule-1) [18], was used for microvessel staining (dilution 1:50 for 30 min) on 3 μm thick paraffin embedded sections. The above-mentioned APAAP technique was applied using protease digestion, rather than microwaving, for antigen retrieval.
Immunohistochemistry for bcl-2 expression
The clone 124 (dilution 1:20; DAKO) was used for bcl-2 assessment. Sections were dewaxed and endogenous peroxidase was quenched by 30 min of incubation in 0.6% H2O2 in methanol. Sections were rehydrated and heated in citrate buffer (pH 6.0) in a microwave oven for 10 min. The primary antibody was applied at a dilution 1:80 and the sections were incubated overnight at 4°C. Thereafter, tissues were treated with the ABC technique using the ABC kit (DAKO). The peroxidase reaction was developed using diaminobenzidine as chromogen and sections were counterstained with haematoxylin. For negative controls we used a nonspecific IgG (normal rabbit IgG) instead of the primary antibody.
Assessment of synovial lining and stromal cell reactivity
The expression levels of HIF-1α, HIF-2α, VEGF, VEGF/KDR, PD-ECGF, and bcl-2 were assessed at the synovial lining and the underlying stromal cells. Morphologic criteria were used to distinguish lymphocytes and macrophages on a background of stromal fibroblasts. The staining was assessed by two independent observers (AG and ES) using a ×200 magnification. The percentage of immunoreactive synovial membrane cells was recorded in all optical fields.
Assessment of standard and activated microvessel densities
The standard MVD, which corresponds to the entire tissue vascular network of the tissue, was detected using mAb CD31. The 'activated MVD' (i.e. the VEGF bound to its receptor KDR [VEGF/KDR complex]) was identified using mAb 11B5.
The method used for microvessel counting was the same for both standard MVD and activated MVD. Sections were scanned at low power (×40 and ×100). The MVD was assessed in all ×200 optical fields by counting vascular structures with clearly defined lumens or a linear shape. The final MVD was the mean score obtained from three fields with the highest individual scores, in order to assess the maximum angiogenic activity in each case.
Statistical analysis
Statistical analysis and graphical presentation were performed using the GraphPad Prism 2.01 package (Graph-Pad, San Diego, CA, USA; http://www.graphpad.com). The Fisher's exact test of the unpaired two-tailed t-test was used for testing relationships between categoric variables as appropriate. Linear regression analysis was used to assess correlation between continuous variables. P < 0.05 was considered statistically significant.
Results
HIF-α expression in synovial lining cells
HIF-1α and HIF-2α expression was both cytoplasmic and nuclear in arthritic synovial tissues (Fig. 1a,1b). The median percentage of synovial lining cells exhibiting cytoplasmic and/or nuclear HIF-1α expression was 50% (range 10–80%; 95% confidence interval [CI] 40–61%) in RA and 60% (range 20–100%; 95% CI 53–73%) in OA. For HIF-2α, the median percentage of positive synovial cells was 60% (range 10–80%; 95% CI 40–63%) in RA and 50% (range 0–100%; 95% CI 43–66%) in OA (Table 2). Normal synovia were, in most cases, unreactive, and only in a small percentage of cases (6/22 and 4/22 for HIF-1α and HIF-2α, respectively) exhibited a weak and focal cytoplasmic reactivity that in no case exceeded 20% of the cells present. This difference in the frequency of HIF-1α and HIF-2α between the arthritides and the normal tissues was statistically significant (Fig. 2; P < 0.0001).
(a) Mixed nuclear and cytoplasmic expression of synovial lining and stromal cells in a case of RA. Note a similar reactivity in the lymphoid and plasma cell component. (b) Mixed nuclear and cytoplasmic expression of synovial lining cells, stromal cells, and endothelial cells in a case of osteoarthritis (OA). (c) Mixed nuclear and cytoplasmic expression of endothelial cells in a case of RA.
The pathologic synovial tissues were grouped into categories of high and low HIF-α reactivity (Table 3), using a 40% cytoplasmic/nuclear reactivity as a cut-off point. This represents the percentage of HIF positive synovial cells corresponding to the lowest 95% CI value of HIF reactivity noted in the arthritic material.
HIF-α expression in synovial stromal cells
Normal synovial stromal cells were, by and large, negative to HIF-1α and HIF-2α proteins, although focal cytoplasmic reactivity was noted in some cases. In contrast, fibroblastic HIF-α positivity was noted in both RA and OA, which, in most cases, was strong and diffuse. Macrophages and blood vessels (Fig. 1c), together with the lymphoid and plasma cell component of RA, were also reactive to HIF-αs.
VEGF and PD-ECGF reactivity
VEGF expression was invariably cytoplasmic, and usually strong and diffuse in the synovial lining cells of RA and OA. Normal synovium reacted only weakly with VEGF antibodies. PD-ECGF expression was mixed cytoplasmic and nuclear, and was noted in varying percentages of synovial lining cells in both RA and OA. PD-ECGF expression was absent or focally weak in the normal synovium.
VEGF expression was diffuse in the fibroblasts of both RA and OA. Normal material was either negative or focally weak. PD-ECGF expression was diffuse in all cases of RA, but such reactivity was either focal or diffuse in OA material. Fibroblasts did not express PD-ECGF in normal tissues. The lymphocytic component of RA showed PD-ECGF and VEGF reactivity. Foamy macrophages exhibited strong VEGF expression.
Analysis of the extent of HIF-α expression
Table 4 shows the association between the extent of HIF-αs expression in RA and OA and the various parameters investigated. The most important findings are as follows. First, In OA a high HIF-1α, but not HIF-2α, synovial lining/stromal cell expression was significantly associated with increased standard MVD, VEGF/KDR activated MVD, and PD-ECGF expression. Second, the activated MVD and synovial stromal cell PD-ECGF reactivity were significantly higher in RA than in OA (P < 0.001), and this was independent of HIF-α expression (Fig. 3a,3b). Finally, extensive bcl-2 expression by synovial membrane cells was noted only in OA, and this was directly associated with HIF-1α expression (Fig. 4).
(a) Relationship of HIF-1α expression in OA and RA with VEGF/KDR vascular activation pathway. Note that the degree of VEGF/KDR microvessel density is directly correlated with the degree of HIF-1α expression only in the case of OA; VEGF/KDR is consistently high in RA, and higher than in OA. (b) Relationship of HIF-1α expression in osteoarthritis (OA) and rheumatoid arthritis (RA) with stromal cell thymidine phosphorylase (TP; referred to in the text as platelet-derived endothelial cell growth factor [PD-ECGF]) reactivity. Note that the degree of TP expression is directly correlated with the degree of HIF-1α expression only in the case of OA; TP expression is consistently high in RA, and higher than in OA.
Discussion
RA and OA, two common conditions with different clinical features [1, 2], have in common an increased tendency for new blood vessel formation [3–5]. The importance of VEGF in the pathogenesis of RA and OA has been emphasized in several recent reports [7, 19]. Following chronic inflammation, an upregulation of VEGF increases vascular permeability [20], resulting in edema, protein leakage, and probably granulation tissue formation (pannus) with erosion of the articular cartilage and progressive destruction of the joint. In a previous study [8], we showed that VEGF is overexpressed in RA and OA, and such pathologic synovia are highly vascularized as compared with normal controls. Similarly, the expression of PD-ECGF, another potent factor for angiogenesis, was considerably enhanced in the arthritic synovial membranes. In the present study, we found a varying degree of expression of HIF-αs in the synovial lining and stromal cells of RA and OA, whereas normal synovium was persistently negative. The lack of HIF-1α expression by the normal synovium was also reported by Hollander and coworkers [21]. In the latter study, however, HIF-1α was more prominent in RA than in OA, which was not confirmed in our study, which included a larger number of specimens. As HIF-αs are directly involved in the upregulation of VEGF, it might be suggested that VEGF overexpression in arthritides is probably a result of HIF pathway activation.
There were differences in the expression of HIF-αs between RA and OA. Thus, although the extent of detection of HIF-αs was more or less similar in both conditions, HIF-1α expression in OA only was significantly associated with standard MVD, VEGF/KDR activated MVD, PD-ECGF expression and with the antiapoptotic protein bcl-2. In the case of RA, the high standard MVD was independent of the extent of HIF-1α expression, whereas the VEGF/KDR activated MVD was persistently higher than that in OA, irrespective of the extent of HIF-1α staining. Similarly, the extent of PD-ECGF expression in the synovial rheumatoid stroma was significantly higher than that in the osteoarthritic, regardless of the magnitude of HIF-1α reactivity.
Hypoxic stimulation is the primary cause for intracellular HIF-1α accumulation, not because of increased mRNA transcription or translation but rather as a result of a redox-sensitive stabilization [22]. Following HIF-α heterodimerization with the HIF-1β unit, the complex enters into the nucleus, binds to DNA at the hypoxia response elements of target genes (i.e. VEGF), and induces transcription. Although upregulation of the HIF pathway may also occur as a result of a genetic alteration [23, 24], the nonmalignant nature of RA and OA suggests that hypoxic signaling may be implicated in the pathogenesis of these diseases. However, a range of growth factor signalling pathways and cytokines can also upregulate HIF-αs (e.g. tumor necrosis factor-α, epidermal growth factor, insulin growth factor-II and thrombin) [25–28].
The direct association of the extent of HIF-1α with MVD and the VEGF/KDR activated MVD in OA is consistent with the notion that VEGF is induced by hypoxia after activation of the HIF-α pathway. This is further reinforced by the direct association between HIF-1α expression and the expression of proteins PD-ECGF and bcl-2. Oxidative stress is probably a major stimulus for the expression of PD-ECGF [29], whereas hypoxic induction of bcl-2 was shown to prevent apoptotic cell death induced by hypoxia [30–33]. It is possible then that within the degenerative context of the osteoarthritic disease, impaired vascular homeostasis results in focal, still progressively expanding, hypoxic regions in the synovium. In these areas, upregulation of HIF-αs leads to overexpression of VEGF and PD-ECGF by the synovial lining and stromal cells, and to the genesis of a defective vascular network with poor survival ability. As previously shown, the activation of the OA vasculature is low, despite the over-production of VEGF [8]. Although a relationship between HIF-1α and VEGF/KDR activated MVD was observed in the present study, the magnitude of the increase was limited. Given that the importance of the VEGF/KDR pathway in mediating endothelial cell survival has repeatedly been confirmed [34–36], the survival ability of the OA vasculature may be hindered despite an upregulated HIF/VEGF system.
In RA, high MVD, high activated VEGF/KDR pathway, and upregulated PD-ECGF expression were constant features, independent of the extent of the activated HIF-α pathway. This may mean that angiogenesis is not exclusively dependent on the extent of HIF reactivity and that hypoxia is not the only factor that upregulates the HIF/VEGF pathway. It is well known that a variety of cytokines are produced by lymphocytes in the context of the rheumatoid pathology (i.e. interleukin-1 and tumor necrosis factor [37, 38]) and that blocking such cytokines induces clinical remission of the disease [39]. Cytokines released by the immune response system may directly stimulate both HIF-α dependent mRNA transcription [40] and HIF-independent VEGF or PD-ECGF overexpression [41–44]. The latter mechanism is less likely to be engaged in OA, where the synovial tissues bear reduced cytokine expression as compared with RA [45]. By contrast, a dense vasculature, characterized by a VEGF/KDR-activated status [8] and pannus formation, is probably a primary event in RA.
Our finding that bcl-2 is predominantly expressed in OA whereas rheumatoid synovium lacks expression of this anti-apoptotic protein is in direct contrast to a previously reported study by Perlman and coworkers [46]. Although this discrepancy is difficult to explain, forced bcl-2 down-regulation failed to induce cell death in rheumatoid fibroblasts, suggesting that bcl-2 is probably of minor importance in the pathology of the synovium [46]. An experimental study from the same group concluded that the expression of bcl-2 is temporally expressed, so that its role in RA may be confined to just a step in the development of rheumatic pathology [47]. In accordance with the diminished role of bcl-2 in RA is a study conducted by Chu and coworkers [48], which showed lack of bcl-2 involvement in apoptosis in RA.
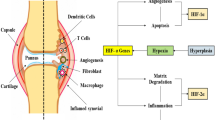
It is concluded that activation of the HIF-α pathway occurs in both RA and OA, although for unrelated reasons. Hypoxia, consistent with an impaired vascular homeostasis, may hinder the angiogenic effect of the upregulated HIF/VEGF pathway in OA. Deranged vascular homeostasis should not be attributed to a defective HIF pathway, but rather to a defective communication between VEGF and vascular receptors. Furthermore, the intact VEGF-dependent angiogenic and vascular survival pathway of RA appears to be cytokine, rather than hypoxia, stimulated. This premise is schematically represented in Fig. 5.
Abbreviations
- APAAP:
-
alkaline phosphatase/antialkaline phosphatase
- CI:
-
confidence interval
- HIF:
-
hypoxia inducible factor
- KDR:
-
kinase insert domain protein receptor
- mAb:
-
monoclonal antibody
- MVD:
-
microvessel density
- OA:
-
osteoarthritis
- PD-ECGF:
-
platelet-derived endothelial cell growth factor
- RA:
-
rheumatoid arthritis
- TBS:
-
tris-buffered saline
- VEGF:
-
vascular endothelial growth factor.
References
Maini RN: Autoimmunity in rheumatoid arthritis. An approach via a study of B lymphocytes. Rheum Dis Clin North Am. 1987, 13: 319.
Hough AJ: Pathology of osteoarthritis. In Arthritis and Allied Conditions. Edited by: McCarty DJ, Koopman WJ. 1993, Philadelphia: Lea and Febiger, 1135-1153.
Brown RA, Weiss JB: Neovascularisation and its role in the osteoarthritic process. Ann Rheum Dis. 1998, 47: 881-885.
Sattar A, Kumar P, Kumar S: Rheumatoid- and osteoarthritis: quantitation of ultrastructural features of capillary endothelial cells. J Pathol. 1986, 148: 45-53.
Semble EL, Turner RA, McCrickard EL: Rheumatoid arthritis and osteoarthritis synovial fluid effects on primary human endothelial cell cultures. J Rheumatol. 1985, 12: 237-241.
Brown RA, Weiss JB, Tomlinson IW, Philip P, Kumar S: Angiogenic factor from synovial fluid resembling that from tumours. Lancet. 1980, 1: 682-685. 10.1016/S0140-6736(80)91173-3.
Walsh DA: Angiogenesis and arthritis. Rheumatology. 1999, 38: 103-112. 10.1093/rheumatology/38.2.103.
Giatromanolaki A, Sivridis E, Athanassou N, Zois E, Thorpe PE, Brekken RA, Gatter KC, Harris AL, Koukourakis MI: The angiogenic pathway 'vascular endothelial growth factor/flk-1(KDR)-receptor' in rheumatoid arthritis and osteoarthritis. J Pathol. 2001, 194: 101-108. 10.1002/path.842.
Blancher C, Harris AL: The molecular basis of the hypoxia response pathway: tumour hypoxia as a therapy target. Cancer Metast Rev. 1998, 17: 187-194. 10.1023/A:1006002419244.
Semenza GL: Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998, 8: 588-594. 10.1016/S0959-437X(98)80016-6.
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL: Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996, 16: 4604-4613.
Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y: A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997, 94: 4273-4278. 10.1073/pnas.94.9.4273.
Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, Ratcliffe PJ, Maxwell PH: Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1α. Blood. 1998, 92: 2260-2268.
Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL: The expression and distribution of the hypoxia inducible factors HIF-1α and HIF-2α in normal human tissues, cancers and tumor associated macrophages. Am J Pathol. 2000, 157: 411-421.
Brekken RA, Huang X, King SW, Thorpe PE: Vascular endothelial growth factor as a marker of tumor endothelium. Cancer Res. 1998, 58: 1952-1959.
Zhang L, Scott P, Turley H, Leek R, Lewis CE, Gatter KC, Harris AL, Mackenzie IZ, Rees MP, Bicknell R: Validation of anti-vascular endothelial growth factor (anti-VEGF) antibodies for immunohistochemical localization of VEGF in tissue sections: expression of VEGF in the human endometrium. J Pathol. 1998, 185: 402-408. 10.1002/(SICI)1096-9896(199808)185:4<402::AID-PATH112>3.3.CO;2-P.
Fox SB, Moghaddam A, Westwood M, Turley H, Bicknell R, Gatter KC, Harris AL: Platelet derived endothelial cell growth factor/thymidine phosphorylase expression in normal tissues an immunohistochemical study. J Pathol. 1995, 176: 183-190.
Parums DV, Cordell JL, Micklem K, Heryet AR, Gatter KC, Mason DY: JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol. 1990, 43: 752-757.
Koch AE, Harlow LA, Haines GK, Amento EP, Unemori EN, Wong PL, Pope RM, Ferrara N: Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol. 1994, 152: 4149-4156.
Ferrara N, Houck K, Jakeman L, Leung DW: Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992, 13: 18-32. 10.1210/er.13.1.18.
Hollander AP, Corke KP, Freemont AJ, Lewis CE: Expression of hypoxia-inducible factor 1alpha by macrophages in the rheumatoid synovium: implications for targeting of therapeutic genes to the inflamed joint. Arthritis Rheum. 2001, 44: 1540-1544. 10.1002/1529-0131(200107)44:7<1540::AID-ART277>3.0.CO;2-7.
Huang LE, Gu J, Schau M, Bunn F: Regulation of hypoxia-inducible factor 1a is mediated by an O2-dependent degradation domain via the ubiquitine-proteasome pathway. Proc Natl Acad Sci USA. 1998, 95: 7987-7992. 10.1073/pnas.95.14.7987.
Mazure NM, Chen EY, Laderoute KR, Giaccia AJ: Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Haras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997, 90: 3322-3331.
Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson AB, Stokoe D, Giaccia AJ: Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000, 14: 391-396.
Albina JE, Mastrofrancesco B, Vessella JA, Louis CA, Henry WL, Reichner JS: HIF-1 expression in healing wounds: HIF-1alpha induction in primary inflammatory cells by TNF-alpha. Am J Physiol Cell Physiol. 2000, 281: 1971-1977.
Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL: Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999, 59: 3915-3918.
Gorlach A, Diebold I, Schini-Kerth VB, Berchner-Pfannschmidt U, Roth U, Brandes RP, Kietzmann T, Busse R: Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: Role of the p22(phox)-containing NADPH oxidase. Circ Res. 2001, 89: 47-54.
Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL: Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growthfactor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000, 60: 1541-1545.
Griffiths L, Dachs GU, Bicknell R, Harris AL, Stratford IJ: The influence of oxygen-tension and pH on the expression of platelet-derived endothelial cell growth factor thymidine phosphorylase in human breast-tumor cells grown in vitro and in vivo. Cancer Res. 1997, 57: 570-572.
Freeland K, Boxer LM, Latchman DS: The cyclic AMP response element in the Bcl-2 promoter confers inducibility by hypoxia in neuronal cells. Brain Res Mol Brain Res. 2001, 92: 98-106. 10.1016/S0169-328X(01)00158-9.
Oehler MK, Norbury C, Hague S, Rees MC, Bicknell R: Adrenomedullin inhibits hypoxic cell death by upregulation of bcl-2 in endometrial cancer cells: a possible promotion mechanism for tumour growth. Oncogene. 2001, 20: 2937-2945. 10.1038/sj.onc.1204422.
Shimizu S, Eguchi Y, Kosaka H, Kamiike W, Matsuda H, Tsujimoto Y: Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature. 1995, 374: 811-813. 10.1038/374811a0.
Yamabe K, Shimizu S, Kamiike W, Waguri S, Eguchi Y, Hasegawa J, Okuno S, Yoshioka Y, Ito T, Sawa Y, Uchiyama Y, Tsujimoto Y, Matsuda H: Prevention of hypoxic liver cell necrosis by in vivo human bcl-2 gene transfection. Biochem Biophys Res Commun. 1998, 243: 217-223. 10.1006/bbrc.1997.7925.
Alon T, Hemo I, Itin A, Peer J, Stone J, Keshet E: Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995, 1: 1024-1018. 10.1038/nm1095-1024.
Gerber HP, Dixit V, Ferrara N: Vascular endothelial growth factor induces expression of the anti-apoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998, 273: 13313-13316. 10.1074/jbc.273.21.13313.
Watanabe Y, Dvorak HF: Vascular permeability factor/vascular endothelial growth factor inhibits anchorage-disruption-induced apoptosis in microvessel endothelial cells by inducing scaffold formation. Exp Cell Res. 1997, 233: 340-349. 10.1006/excr.1997.3583.
Paleolog EM, Young S, Stark AC, McCloskey RV, Feldmann M, Maini RN: Modulation of angiogenic vascular endothelial growth factor by tumor necrosis factor alpha and interleukin-1 in rheumatoid arthritis. Arthritis Rheum. 1998, 41: 1258-1265. 10.1002/1529-0131(199807)41:7<1258::AID-ART17>3.0.CO;2-1.
Szekanecz Z, Kim J, Koch AE: Chemokines and chemokine receptors in rheumatoid arthritis. Semin Immunol. 2003, 15: C15-21. 10.1016/S1044-5323(02)00124-0.
Gorman JD, Sack KE, Davis JC: Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002, 346: 1349-1356. 10.1056/NEJMoa012664.
Thornton RD, Lane P, Borghaei RC, Pease EA, Caro J, Mochan E: Interleukin 1 induces hypoxia-inducible factor 1 in human gingival and synovial fibroblasts. Biochem J. 2000, 15: 307-312. 10.1042/0264-6021:3500307.
Bucht A, Larsson P, Weisbrot L, Thorne C, Pisa P, Smedegard G, Keystone EC, Gronberg A: Expression of interferon-gamma (IFN-gamma), IL-10, IL-12 and transforming growth factor-beta (TGF-beta) mRNA in synovial fluid cells from patients in the early and late phases of rheumatoid arthritis (RA). Clin Exp Immunol. 1996, 103: 357-367.
Hossain MA, Bouton CM, Pevsner J, Laterra J: Induction of vascular endothelial growth factor in human astrocytes by lead. Involvement of a protein kinase C/activator protein-1 complex-dependent and hypoxia-inducible factor 1-independent signaling pathway. J Biol Chem. 2000, 275: 27874-2782.
Matsumoto K, Kanmatsuse K: Interleukin-18 and interleukin-12 synergize to stimulate the production of vascular permeability factor by T lymphocytes in normal subjects and in patients with minimal-change nephrotic syndrome. Nephron. 2000, 85: 127-133. 10.1159/000045645.
Schwartz EL, Hoffman M, O'Connor CJ, Wadler S: Stimulation of 5-fluorouracil metabolic activation by interferon-α in human colon carcinoma cells. Biochem Biophys Res Commun. 1992, 182: 1232-1239. 10.1016/0006-291X(92)91863-L.
Dolhain RJ, ter Haar NT, Hoefakker S, Tak PP, de Ley M, Claassen E, Breedveld FC, Miltenburg AM: Increased expression of interferon (IFN) gamma together with IFN gamma receptor in the rheumatoid synovial membrane compared with synovium of patients with osteoarthritis. Br J Rheumatol. 1996, 35: 24-32.
Perlman H, Liu H, Georganas C, Koch AE, Shamiyeh E, Haines GK, Pope RM: Differential expression pattern of the anti-apoptotic proteins, Bcl-2 and FLIP, in experimental arthritis. Arthritis Rheum. 2001, 44: 2899-2908. 10.1002/1529-0131(200112)44:12<2899::AID-ART478>3.0.CO;2-X.
Perlman H, Georganas C, Pagliari LJ, Koch AE, Haines K, Pope RM: Bcl-2 expression in synovial fibroblasts is essential for maintaining mitochondrial homeostasis and cell viability. J Immunol. 2000, 164: 5227-5235.
Chou CT, Yang JS, Lee MR: Apoptosis in rheumatoid arthritis: expression of Fas, Fas-L, p53, and Bcl-2 in rheumatoid synovial tissues. J Pathol. 2001, 193: 110-116. 10.1002/1096-9896(2000)9999:9999<::AID-PATH746>3.0.CO;2-K.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Rights and permissions
About this article
Cite this article
Giatromanolaki, A., Sivridis, E., Maltezos, E. et al. Upregulated hypoxia inducible factor-1α and -2α pathway in rheumatoid arthritis and osteoarthritis. Arthritis Res Ther 5, R193 (2003). https://doi.org/10.1186/ar756
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/ar756