Abstract
Introduction
Psoriasis and psoriatic arthritis (PsA) increase cardiovascular disease (CVD) risk, but surrogate markers for CVD in these disorders are inadequate. Because the presence of sacroiliitis may portend more severe PsA, we hypothesized that sacroiliitis defined by computed tomography (CT) would be associated with increased vascular inflammation defined by 18-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT), which is an established measure of CVD.
Methods
Participants (n = 65) underwent whole-body FDG-PET/CT. Metabolic activity of the aorta was measured using the maximal standardized uptake value (SUVmax), a measure of atherosclerotic plaque activity. The primary outcome was aortic vascular inflammation. Linear regression (with β-coefficients (β) and P-values reported for PsA and sacroiliitis) was used to adjust for CVD risk factors to determine associations of PsA or sacroiliitis with vascular inflammation. Likelihood ratio testing was performed to evaluate the contribution of sacroiliitis to vascular disease estimation compared to the effects of PsA and traditional CVD risk factors.
Results
Vascular inflammation (measured as SUVmax) was greater (P < 0.001) in patients with sacroiliitis (mean ± SD = 7.33 ± 2.09) defined by CT compared to those without sacroiliitis (6.39 ± 1.49, P = 0.038). There were associations between PsA and aortic inflammation (β = 0.124, P < 0.001) and between sacroiliitis and aortic inflammation (β = 0.270, P < 0.001) after adjusting for CVD risk factors. Sacroiliitis predicted vascular inflammation beyond PsA and CVD risk factors (χ2 = 124.6, P < 0.001).
Conclusions
Sacroiliitis is associated with increased vascular inflammation detected by FDG-PET/CT, suggesting that sacroiliac joint disease may identify patients at greater risk for CVD. Large, ongoing prospective studies are required to confirm these findings.
Similar content being viewed by others
Introduction
Systemic inflammatory disorders, including psoriasis (Pso)[1–3] and psoriatic arthritis (PsA)[3], increase incident cardiovascular events beyond traditional cardiovascular disease (CVD) risk factors[4]. Understanding of this elevated risk has been hampered by the lack of suitable clinical biomarkers linking systemic inflammation and CVD. Prevention of increased CVD morbidity and mortality in Pso and PsA requires identification of those patients at greatest risk for these diseases prior to the development of overt symptoms. 18-Fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) is an imaging modality that may provide this capability, as it delineates enhanced metabolic activity in tissues in vivo, including vascular inflammation[5–9]. Importantly, vascular inflammation seen on FDG-PET/CT scans has been shown to precede the development of atherosclerotic disease[10] and to predict future cardiovascular events[11, 12]. Our prior investigations in which we utilized FDG-PET/CT have shown increased inflammation in multiple tissues, including the skin, liver, joints, entheses and vasculature in Pso and PsA patients compared to healthy controls[6, 13, 14]. We have also previously demonstrated relationships between FDG-PET/CT imaging measurements and both known and novel CVD biomarkers, thereby validating FDG-PET/CT findings as reliable predictors of outcome[15]. However, these pilot studies were underpowered to adequately assess for PsA or axial disease.
Sacroiliitis is a characteristic feature of the spondyloarthropathies, including PsA[16]. Imaging of patients with sacroiliitis is an element of the current classification criteria for these disorders[17, 18]. Imaging of the sacroiliac (SI) joints by CT scan is one modality that is accepted for making the diagnosis of sacroiliitis[19–27]. Further, the presence of sacroiliitis may identify a PsA subgroup at risk for more severe arthropathy[28, 29]. Greater inflammatory burden imposed by severe Pso[1, 30, 31] and the presence of PsA[32, 33] increases the risk of CVD. Thus, we hypothesized that PsA and sacroiliitis diagnosed by CT scan would be associated with increased vascular inflammation by FDG-PET/CT scan in a well-characterized population of Pso patients with versus without a diagnosis of PsA. Here we present a consecutive sample of 65 patients who completed baseline studies in the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative (PACI; ClinicalTrials.gov Identifier: NCT01778569). A major aim of this prospective, longitudinal study is to identify incident biomarkers of vascular and metabolic disease in Pso and PsA.
Methods
Study population
Whole-body FDG-PET/CT was performed in a consecutive sample of patients (n = 65) ages 18 to 70 years with Pso alone (n = 38) or with both Pso and PsA (n = 27). Our study population was recruited to the National Institutes of Health (NIH) Clinical Center using flyers and pamphlets distributed at dermatology clinics and via academic dermatological societies, as well as by using web-driven tools such as[34] and[35]. Diagnostic confirmation of plaque Pso and assessment of body surface area (BSA) and Psoriasis Area and Severity Index (PASI) were performed by a dermatologist. PsA classifications were confirmed by a rheumatologist according to the ClASsification criteria for Psoriatic ARthritis (CASPAR)[36]. A rheumatologist did the swollen and tender joint counts using the American College of Rheumatology 66/68 joint count core set[37]. Clinical assessment of enthesitis (by Leeds Enthesitis Index score[38]), dactylitis (by number of dactylitic digits) and inflammatory back pain (according to Assessment of SpondyloArthritis international Society (ASAS) criteria[39] and Calin criteria[40]) was also performed by a rheumatologist. Exclusion criteria included disease states that can increase systemic or vascular inflammation, such as known history of CVD, uncontrolled hypertension (defined as systolic blood pressure (SBP) >180 mmHg or diastolic blood pressure (DBP) >95 mmHg), nondermatologic malignant disease within the past 5 years, positive HIV status, major surgery within the past 3 months and history of intravenous drug use or active infection within the preceding 72 hours. Hypertension was defined as SBP >140 mmHg, DBP >90 mmHg and/or currently on antihypertensive therapy. Hyperlipidemia was defined as total cholesterol >200 mg/dl, low-density lipoprotein (LDL) ≥160 mg/dl, high-density lipoprotein (HDL) <40 mg/dl, triglycerides >150 mg/dl and/or use of a cholesterol-lowering agent. Diabetes was defined as fasting glucose ≥126 mg/dl, hemoglobin A1c ≥6.5% and/or taking antidiabetic therapy.
FDG-PET/CT
Whole-body FDG-PET/CT scans were obtained using a standardized published protocol[13]. Following an overnight fast, patients were imaged 60 minutes after receiving an injection of 370 MBq of 18-FDG. Images were acquired in three-dimensional mode using a Siemens Biograph TruePoint PET/CT scanner (Siemens Healthcare, Erlangen, Germany). FDG-PET/CT images were reviewed by a reader, blinded to all patient characteristics, using dedicated PET/CT image analysis software (Extended Brilliance Workstation (EBW); Philips Healthcare, Amsterdam, the Netherlands). FDG uptake in the thoracic and abdominal aorta was quantified using published methods[6]. Briefly, circular two-dimensional regions of interest (ROI) circumscribing the external aortic contour were drawn on serial contiguous transaxial 4-mm sections from the level of the aortic root to the iliac bifurcation (Figure 1). Each axial segment provided two measures of tissue metabolic uptake[6]: mean and maximum standardized uptake (SUVmean and SUVmax, respectively). SUVmean and SUVmax values were determined using commercial software (EBW) for each successive slice. SUVmax values were averaged over three consecutive slices to measure the most inflamed aortic region in each patient. A nuclear medicine specialist independently evaluated FDG-PET/CT scans for imaging quality, radiotracer biodistribution, uptake time and clinical findings.
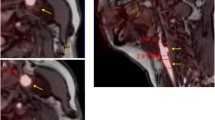
18-fluorodeoxyglucose-positron emission tomography/computed tomography delineates vascular inflammation. Images shown are representative computed tomography (CT) (left panels), 18-fluorodeoxyglucose-positron emission tomography (FDG-PET) (center panels) and fused FDG-PET/CT transaxial (right panels) scans from a patient with an unaffected aortic vasculature (upper panel) and one with an inflamed aortic vasculature (lower panel). Regions of interest outlining the ascending thoracic aorta are depicted in yellow in all images. A ring-shaped focus of increased metabolic activity in the aortic wall is also shown (blue arrows in FDG/PET and fused FDG-PET/CT images).
Imaging evaluation for sacroiliitis
Sacroiliitis diagnosed by CT scan was evaluated using guidelines developed by Geijer and colleagues[41]. Sacroiliitis grade was classified as “no disease” (that is, radiographically normal, degenerative change evident or some blurring of the joint margins), “suspected disease” (that is, unilateral sclerosis with some erosion) or “definite disease” (that is, bilateral sclerosis with erosion, or unilateral or bilateral sclerosis with severe erosion and a change in joint space with or without partial ankylosis, or complete ankylosis). Both axial and coronal CT scan orientations were examined. Sclerosis, erosions, ankylosis and/or joint space changes had to be evident on at least two consecutive slices to be deemed significant.
Serum factor determination
Fasting levels of total, HDL and LDL cholesterol, triglycerides, glucose, erythrocyte sedimentation rate (ESR) and high-sensitivity C-reactive protein (hsCRP) were measured in a clinical laboratory.
Statistical analysis
Normality was assessed by skewness and kurtosis. Normally distributed continuous variables were compared using Student’s t-test. Continuous variables lacking a normal distribution were compared using Mann–Whitney U test. Dichotomous variable comparisons were performed using Fisher’s exact test. Student’s t-test was employed to compare FDG-PET/CT measures (SUVmax) of aortic inflammation between Pso and PsA groups and between patients with or without CT evidence of sacroiliitis. Only those patients with definitive disease defined by CT were considered to have sacroiliitis for all analyses. Patients not meeting the CASPAR criteria[36] for PsA were classified as having PSO. Relationships between variables were determined using Spearman correlation analysis and are reported as Spearman ρ (r) values. Multivariate linear regression analysis was performed using aortic SUVmax as the dependent variable and CVD risk factors (age, sex, body mass index (BMI), diabetes, hypertension, dyslipidemia and pack-years of tobacco use) and either PsA or sacroiliitis evident by CT scan as independent variables, as reported. Sex, diabetes, hypertension, dyslipidemia, PsA and sacroiliitis were adjusted for as dichotomous variables in the models; all other variables were continuous. Similar findings were revealed in independent analyses designating sex, PsA and sacroiliitis as dichotomous variables and age, BMI, fasting glucose, SBP, LDL cholesterol, tobacco use and aortic SUVmax as continuous variables. Both fixed- and random-effects regression analyses were performed on each model to accommodate within-patient correlation of SUVmax values[13]. Because the magnitude of the estimates of PsA and sacroiliitis using vascular measures did not differ when fixed- and random-effects regression analyses were performed, we report β-coefficients (β) and P-values for the fixed effects models after adjustment for CVD risk factors. Further, we examined the outcome variable aortic SUVmax in separate multivariate linear regression models using either slice-by-slice SUVmax measures or collapsed individual patient values. The magnitude of the effect of the covariates on aortic SUVmax was similar in both models; hence, we report the slice-by-slice data. On the basis of prior published results[5] demonstrating that aortic SUV increased 0.1 U (SD ±0.1) per decade of life, we considered this to be a clinically relevant difference. Therefore, with these assumptions, our sample size (n = 65) provided >90% power to detect a difference of 0.1 in aortic vascular inflammation by the presence or absence of SI inflammation. Likelihood-ratio testing was performed in nested Tobit models to determine the incremental value of sacroiliitis to CVD estimation above and beyond PsA and traditional CVD risk factors. STATA 12 statistical software (StataCorp, College Station, TX, USA) was utilized for all analyses. Study approval was obtained from the National Heart, Lung, and Blood Institute Institutional Review Board in accordance with the Declaration of Helsinki. All guidelines for GCP and those set forth by the NIH Radiation Safety Commission and in the Belmont Report (National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research) were followed. All study participants provided written informed consent.
Results
Patient demographics, disease assessments and CVD risk factors
Patient characteristics (n = 65) and laboratory measurements are presented in Table 1 for the whole sample and for the Pso (n = 38) and PsA (n = 27) subgroups. Our study population had a median age of 53 years, was nearly equally distributed by sex (54% male) and had moderate skin disease on average (mean BSA = 9.2, mean PASI score = 7.8) (Table 1). The proportion who had PsA was 41%. Median skin disease duration was 20 years for both the Pso and PsA subgroups, and the median duration of joint disease was 10 years in PsA patients (Table 1). One patient met both the Calin and ASAS criteria for inflammatory back pain. This patient was classified as having Pso, however, due to not meeting the CASPAR criteria for PsA and having CT images showing advanced degenerative and postsurgical changes in the spine without evidence of spondylitis or sacroiliitis. Two additional patients were definitively diagnosed with sacroiliitis by CT without evidence of inflammatory back pain, SI tenderness to palpation or provocative maneuvers, arthritis, enthesitis or dactylitis. Because both of those two patients met the CASPAR criteria for PsA, they were classified as having asymptomatic axial PsA. Sacroiliitis diagnosed by CT scan was confirmed in 44% of PsA patients (Table 1).
We also assessed Medication use and cardiovascular and metabolic comorbidities in our study population (Table 1). Many patients (37%) were using topical steroids, whereas systemic steroid therapy and phototherapy were rare (Table 1). Disease-modifying antirheumatic drug (DMARD) treatment was uncommon and was not statistically different (P = 0.224) between the PsA (15%) and Pso (5%) groups (Table 1). Biologic therapy was more common (P = 0.022) in PsA patients (56%) than in Pso patients (26%). Traditional CVD risk factors, including hypertension, dyslipidemia, diabetes mellitus and tobacco use, were prevalent in our study population, as was treatment for these disorders. Presence and treatment of CVD risk factors were not statistically different between the Pso and PsA subgroups (Table 1).
Vascular inflammation measured by FDG-PET/CT is greater in patients with sacroiliitis defined by CT
We utilized FDG-PET/CT to examine vascular inflammation, as defined by aortic SUVmax, in our study population. Mean (SD) SUVmax values for the aortic region demonstrating the highest vascular inflammation in three contiguous slices were numerically, but not statistically, greater (P = 0.536) in PsA patients (6.72 ± 1.92) compared to Pso patients (6.46 ± 1.43) (Table 2). Because sacroiliitis has been shown to signify worse arthropathy in PsA[28, 29], we hypothesized that the greater inflammatory state in patients with sacroiliitis may lead to increased vascular inflammation visualized by FDG-PET/CT. Indeed, mean aortic SUVmax measures (Table 2) were significantly greater (P = 0.038) in patients with CT evidence of sacroiliitis (7.33 ± 2.09) compared to those without SI joint disease (6.39 ± 1.49). Further, aortic vascular inflammation was greatest in those demonstrating the highest grade (definitive disease) of sacroiliitis by CT scan (Additional file1: Table S1).
Sacroiliitis defined by CT scan correlates with vascular inflammation evaluated by FDG-PET/CT above and beyond CVD risk factors
To determine covariates of vascular inflammation by highest atherosclerotic plaque activity within our cohort, individual Spearman correlation analyses were performed between aortic SUVmax and clinical, laboratory and imaging parameters in the total population and in the Pso and PsA subgroups (Additional file2: Table S2). In unadjusted analyses of the total study population (Additional file2: Table S2), aortic SUVmax was significantly related to traditional CVD risk factors, including age (r = 0.406, P < 0.001), sex (r = 0.146, P < 0.001), BMI (r = 0.507, P < 0.001), hypertension (r = 0.204, P < 0.001), diabetes (r = 0.186, P < 0.001), dyslipidemia (r = 0.226, P < 0.001) and tobacco use (r = -0.021, P = 0.01). Sacroiliitis (r = 0.213, P < 0.001) was more strongly related to vascular inflammation than were the relationships between vascular inflammation and psoriatic arthritis (r = 0.033, P < 0.001), PASI score (r = 0.001, P = 0.919), ESR (r = 0.061, P < 0.001) or hsCRP (r = -0.005, P = 0.513) in the total sample. In general, aortic SUVmax and CVD risk factors were more strongly related in PsA than in Pso patients (Additional file2: Table S2). When these analyses were performed using SUVmean as the outcome variable, similar results were obtained. The aortic SUVmax data are reported, as this measurement indicates the region of most intense vascular inflammation.
In multivariate regression analyses (Table 3), sacroiliitis remained highly associated with vascular inflammation, even after adjusting for age, sex and BMI (β = 0.268, P < 0.001) and with additional adjustment for hypertension, dyslipidemia, diabetes and tobacco use (β = 0.270, P < 0.001). PsA was also associated with vascular inflammation (Table 3) in both partially adjusted (age, sex and BMI; β = 0.117, P < 0.001) and fully adjusted models (age, sex, BMI, hypertension, dyslipidemia, diabetes and tobacco; β = 0.124, P < 0.001). To obtain a crude estimate for systemic inflammatory burden, multivariate regression analyses were further adjusted for ESR and hsCRP levels. Addition of ESR (β = 0.268, P < 0.001) or hsCRP (β = 0.303, P < 0.001) as independent variables did not affect the relationship between sacroiliitis and vascular inflammation in fully adjusted models (Additional file3: Table S3). PsA and aortic SUVmax also remained significantly related after adding ESR (β = 0.110, P < 0.001) or hsCRP (β = 0.133, P < 0.001) into fully adjusted models. The contribution of sacroiliitis to aortic SUVmax estimation beyond PsA and/or traditional CVD risk factors was determined using likelihood-ratio testing (Table 4). Sacroiliitis defined by CT scan predicted vascular inflammation (Table 4) above and beyond PsA in unadjusted models (χ2 = 374.6, P < 0.001) and beyond PsA and CVD risk factors in partially adjusted (χ2 = 132.8, P < 0.001) and fully adjusted models (χ2 = 124.6, P < 0.001). Together, these findings suggest that sacroiliitis defined by CT scan may independently confer risk of higher vascular inflammation in patients with Pso and PsA.
Discussion
To our knowledge, this report is the first to link sacroiliitis defined by CT scan and vascular inflammation in Pso and PsA, an association that persisted after adjustment for traditional CVD risk factors. Aortic inflammation measured by FDG-PET/CT was found to be greater in patients with evidence of sacroiliitis compared to those without SI joint disease. We were able to determine that sacroiliitis impacted vascular inflammation in a dose-dependent fashion, as aortic SUVmax values were greatest in those with sacroiliitis definitively diagnosed by CT scan compared to those with no disease or suspected disease. Further, vascular inflammation measured by FDG-PET/CT correlated with sacroiliitis in both unadjusted models and in models adjusted for CVD risk factors and markers of systemic inflammation. Importantly, sacroiliitis was a strong predictor of CVD above and beyond PsA and traditional risk factors. Taken together, these findings suggest that sacroiliitis not only may identify those at risk for more severe joint disease[28, 29] but may also be an indicator of increased susceptibility to CVD.
Identification of sacroiliitis as a correlate of CVD holds tremendous clinical promise since it is a characteristic feature of PsA[17, 18] and can readily be screened for in this disorder. Since use of FDG-PET/CT for CVD risk stratification in Pso and PsA is unlikely to extend beyond the research realm in the foreseeable future, sacroiliitis could potentially serve as a marker of increased risk of CVD in these populations. Future studies should extend our findings by examining the relationship between sacroiliitis and CVD in other spondyloarthropathies, and by determining whether active inflammation in the SI joint confers even greater risk for CVD. Whether sacroiliitis also relates to other psoriatic comorbidities is a subject of ongoing investigation in our cohort.
It has been suggested that higher inflammatory burden imparts increased CVD risk in the systemic inflammatory diseases[4]. Indeed, greater skin disease severity has been linked to increased CVD mortality in Pso[1, 30, 31, 42], and this observation has been further supported by data demonstrating cardiometabolic and CVD modulation in lesional psoriatic skin[43, 44]. Furthermore, PsA has generally been considered to represent a heightened inflammatory state compared to Pso alone[3], and thus PsA may confer an elevated risk of CVD-associated morbidity and mortality[45]. Although epidemiologic studies have yielded conflicting data regarding all-cause mortality risk in PsA[42, 46–51], the results of several studies demonstrate that PsA increases CVD-attributable mortality compared to the general population[30, 42, 52] and to Pso patients[32, 33, 42, 53]. Our findings suggest that screening for particular features of Pso and PsA rather than global measures of systemic inflammation may be a more viable strategy for identifying patients at increased risk for CVD. In support of this notion, our results presented here demonstrate that sacroiliitis, as defined by CT scan, and vascular inflammation were associated with PsA above and beyond traditional CVD risk factors and independent of levels of systemic inflammatory markers (ESR and hsCRP). Thus, sacroiliitis may be a potentially useful metric of CVD beyond crude measures of systemic inflammation.
We acknowledge that this study has certain limitations and that additional research is required to corroborate and extend our findings. The modest sample size and single-center study design could affect the generalizability of the results. Further, FDG-PET/CT may not be suitable for widespread adoption, owing to its limited availability for nonmalignant conditions and its mild radiation exposure. In addition, evaluation of sacroiliitis by CT scan may overestimate the frequency of patients with sacroiliitis[41, 54], which could have impacted our results. Although analyses were adjusted for CVD risk factors and markers of systemic inflammation, other potential confounding variables, including Pso and PsA disease duration as well as disease activity and therapy, may have decreased the magnitude of the observed associations. Our study design was generally inclusive in order to allow us to understand the impact of Pso and PsA on vascular inflammation across a wide range of disease manifestations and therapies (that is, in a setting closer to the “real world”). Thus, the relationship between sacroiliitis and vascular inflammation may have been underestimated, as no washout medication period was required prior to study enrollment. However, it is remarkable that, though many of the Pso and PsA patients were undergoing treatment to mitigate their inflammatory disease and/or CVD risk factors, we found that sacroiliitis and vascular inflammation were still readily detectible.
Despite these limitations, our data implicate sacroiliitis as a significant and novel marker of increased CVD risk in PsA. Further, FDG-PET/CT may hold promise to improve CVD risk estimation as well as to delineate tissue inflammation in the rheumatic diseases. The PACI study (ClinicalTrials.gov Identifier: NCT01778569) is uniquely poised to address these questions, as it includes a large, ongoing, longitudinal cohort in which serial FDG-PET/CT scans will be utilized to detect incident sacroiliitis and CVD in Pso and PsA patients. Additional studies are needed to substantiate and expand our present findings, to link them to clinical prognoses and to understand the effects of treatment on tissue-specific inflammation in Pso and PsA patients.
Conclusion
Sacroiliitis is associated with increased vascular inflammation beyond traditional CVD risk factors, suggesting that sacroiliitis may identify patients at greater risk for vascular complications.
Authors’ information
SR is an American Board of Internal Medicine–certified rheumatologist, a member of the Group for the Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and a Metzger Clinical Scholar at the National Institutes of Health. SR has extensive expertise in the clinical, basic and translational mechanisms linking inflammatory disorders to cardiovascular and metabolic disease. JD is an Intramural Research Training Awardee (IRTA) at the National Institutes of Health who specializes in multimodal imaging analyses. CM is an American Board of Radiology–certified radiologist and nuclear medicine specialist who works at the National Institutes of Health Clinical Center. HN is an American Board of Dermatology–certified dermatologist with extensive expertise in psoriasis, graft-versus-host disease and the neutrophilic dermatoses, as well as a senior clinical scholar at the National Cancer Institute. ELS is an American Board of Internal Medicine–certified rheumatologist who practices at the Arthritis and Rheumatism Associates, which is the largest rheumatology practice in the Washington, DC, Virginia and Maryland area. He is also a member of the Group for the Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). NNM is an American Board of Internal Medicine–certified cardiologist who specializes in preventative cardiology and cardiovascular imaging. He is the inaugural Lasker Clinical Scholar and section chief in the Section of Inflammation and Cardiometabolic Diseases at the National Heart, Lung, and Blood Institute, National Institutes of Health. He is also a member of Group for the Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the founder of the Psoriasis, Atherosclerosis, and Cardiometabolic Disease Initiative (ClinicalTrials.gov Identifier: NCT01778569).
Abbreviations
- CVD:
-
Cardiovascular disease
- DBP:
-
Diastolic blood pressure
- DMARD:
-
Disease-modifying antirheumatic drug
- ESR:
-
Erythrocyte sedimentation rate
- FDG-PET/CT:
-
18-Fluorodeoxyglucose-positron emission tomography/computed tomography
- HDL:
-
High-density lipoprotein
- hsCRP:
-
High-sensitivity C-reactive protein
- LDL:
-
Low-density lipoprotein
- PASI:
-
Psoriasis Area and Severity Index
- PsA:
-
Psoriatic arthritis
- Pso:
-
Psoriasis
- ROI:
-
Region of interest
- SBP:
-
Systolic blood pressure
- SI:
-
Sacroiliac
- SUV:
-
Standardized uptake value.
References
Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB: Risk of myocardial infarction in patients with psoriasis. JAMA. 2006, 296: 1735-1741.
Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG: Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009, 145: 700-703.
Tobin AM, Veale DJ, Fitzgerald O, Rogers S, Collins P, O’Shea D, Kirby B: Cardiovascular disease and risk factors in patients with psoriasis and psoriatic arthritis. J Rheumatol. 2010, 37: 1386-1394.
Roman MJ, Salmon JE: Cardiovascular manifestations of rheumatologic diseases. Circulation. 2007, 116: 2346-2355.
Bural GG, Torigian DA, Chamroonrat W, Houseni M, Chen W, Basu S, Kumar R, Alavi A: FDG-PET is an effective imaging modality to detect and quantify age-related atherosclerosis in large arteries. Eur J Nucl Med Mol Imaging. 2008, 35: 562-569.
Mehta NN, Torigian DA, Gelfand JM, Saboury B, Alavi A: Quantification of atherosclerotic plaque activity and vascular inflammation using [18-F] fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT). J Vis Exp. 2012, 63: e3777-
Rudd JHF, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, Johnström P, Davenport AP, Kirkpatrick PJ, Arch BN, Pickard JD, Weissberg PL: Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002, 105: 2708-2711.
Tahara N, Kai H, Nakaura H, Mizoguchi M, Ishibashi M, Kaida H, Baba K, Hayabuchi N, Imaizumi T: The prevalence of inflammation in carotid atherosclerosis: analysis with fluorodeoxyglucose-positron emission tomography. Eur Heart J. 2007, 28: 2243-2248.
Tawakol A, Migrino RQ, Hoffmann U, Abbara S, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ: Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol. 2005, 12: 294-301.
Abdelbaky A, Corsini E, Figueroa AL, Fontanez S, Subramanian S, Ferencik M, Brady TJ, Hoffmann U, Tawakol A: Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circ Cardiovasc Imaging. 2013, 6: 747-754.
Paulmier B, Duet M, Khayat R, Pierquet-Ghazzar N, Laissy JP, Maunoury C, Hugonnet F, Sauvaget E, Trinquart L, Faraggi M: Arterial wall uptake of fluorodeoxyglucose on PET imaging in stable cancer disease patients indicates higher risk for cardiovascular events. J Nucl Cardiol. 2008, 15: 209-217.
Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, Nikolaou K, Reiser MF, Bartenstein P, Hacker M: 18 F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009, 50: 1611-1620.
Mehta NN, Yu Y, Saboury B, Foroughi N, Krishnamoorthy P, Raper A, Baer A, Antigua J, Van Voorhees AS, Torigian DA, Alavi A, Gelfand JM: Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18 F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011, 147: 1031-1039.
Rose S, Sheth NH, Baker JF, Ogdie A, Raper A, Saboury B, Werner TJ, Thomas P, Vanvoorhees A, Alavi A, Torigian DA, Gelfand JM, Mehta NN: A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: a pilot study. Am J Cardiovasc Dis. 2013, 3: 273-278.
Yu Y, Sheth N, Krishnamoorthy P, Saboury B, Raper A, Baer A, Ochotony R, Doveikis J, Derohannessian S, Voorhees AS, Torigian DA, Alavi A, Gelfand JM, Mehta NN: Aortic vascular inflammation in psoriasis is associated with HDL particle size and concentration: a pilot study. Am J Cardiovasc Dis. 2012, 2: 285-292.
Helliwell PS: Relationship of psoriatic arthritis with the other spondyloarthropathies. Curr Opin Rheumatol. 2004, 16: 344-349.
Rudwaleit M, Taylor WJ: Classification criteria for psoriatic arthritis and ankylosing spondylitis/axial spondyloarthritis. Best Pract Res Clin Rheumatol. 2010, 24: 589-604.
Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT, Dougados M, Huang F, Gu J, Kirazli Y, Van den Bosch F, Olivieri I, Roussou E, Scarpato S, Sørensen IJ, Valle-Oñate R, Weber U, Wei J, Sieper J: The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011, 70: 25-31.
Cammisa M, Lomuto M, Bonetti MG: Sacroiliitis in seronegative polyarthritis: CT analysis. Clin Exp Rheumatol. 1987, 5: S105-S107.
Devauchelle-Pensec V, D’Agostino MA, Marion J, Lapierre M, Jousse-Joulin S, Colin D, Chary-Valckenaere I, Marcelli C, Loeuille D, Aegerter P, Guis S, Gaudin P, Breban M, Saraux A, Study Group of Spondylarthritis: Computed tomography scanning facilitates the diagnosis of sacroiliitis in patients with suspected spondylarthritis: results of a prospective multicenter French cohort study. Arthritis Rheum. 2012, 64: 1412-1419.
Fewins HE, Whitehouse GH, Bucknall RC: Role of computed tomography in the evaluation of suspected sacroiliac joint disease. J R Soc Med. 1990, 83: 430-432.
Kozin F, Carrera GF, Ryan LM, Foley D, Lawson T: Computed tomography in the diagnosis of sacroiliitis. Arthritis Rheum. 1981, 24: 1479-1485.
Lawson TL, Foley WD, Carrera GF, Berland LL: The sacroiliac joints: anatomic, plain roentgenographic, and computed tomographic analysis. J Comput Assist Tomogr. 1982, 6: 307-314.
Lee YH, Hwang JY, Lee SW, Lee J: The clinical usefulness of multidetector computed tomography of the sacroiliac joint for evaluating spondyloarthropathies. Korean J Intern Med. 2007, 22: 171-177.
Puhakka KB, Jurik AG, Egund N, Schiottz-Christensen B, Stengaard-Pedersen K, van Overeem Hansen G, Christiansen JV: Imaging of sacroiliitis in early seronegative spondylarthropathy: assessment of abnormalities by MR in comparison with radiography and CT. Acta Radiol. 2003, 44: 218-229.
Strobel K, Fischer DR, Tamborrini G, Kyburz D, Stumpe KD, Hesselmann RG, Johayem A, von Schulthess GK, Michel BA, Ciurea A: 18F-fluoride PET/CT for detection of sacroiliitis in ankylosing spondylitis. Eur J Nucl Med Mol Imaging. 2010, 37: 1760-1765.
Geijer M, Sihlbom H, Göthlin JH, Nordborg E: The role of CT in the diagnosis of sacro-iliitis. Acta Radiol. 1998, 39: 265-268.
Chandran V, Tolusso DC, Cook RJ, Gladman DD: Risk factors for axial inflammatory arthritis in patients with psoriatic arthritis. J Rheumatol. 2010, 37: 809-815.
Taccari E, Spadaro A, Riccieri V: Correlations between peripheral and axial radiological changes in patients with psoriatic polyarthritis. Rev Rhum Engl Ed. 1996, 63: 17-23.
Gladman DD, Ang M, Su L, Tom BD, Schentag CT, Farewell VT: Cardiovascular morbidity in psoriatic arthritis. Ann Rheum Dis. 2009, 68: 1131-1135.
Mehta NN, Yu Y, Pinnelas R, Krishnamoorthy P, Shin DB, Troxel AB, Gelfand JM: Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011, 124: 775-e1–775.e6
Husted JA, Thavaneswaran A, Chandran V, Eder L, Rosen CF, Cook RJ, Gladman DD: Cardiovascular and other comorbidities in patients with psoriatic arthritis: a comparison with patients with psoriasis. Arthritis Care Res (Hoboken). 2011, 63: 1729-1735.
Miller IM, Ellervik C, Yazdanyar S, Jemec GB: Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013, 69: 1014-1024.
National Institutes of Health, PACI: The Psoriasis, Atherosclerosis, and Cardiometabolic Disease Initiative. [http://psoriasis.nhlbi.nih.gov] (accessed 7 August 2014)
ResearchMatch. [https://www.researchmatch.org] (accessed 7 August 2014)
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H: Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006, 54: 2665-2673.
Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, Furst D, Goldsmith C, Kieszak S, Lightfoot R, Paulus H, Tugwell P, Weinblatt M, Wildmark R, Williams HJ, Wolfe F, the Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials: The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum. 1993, 36: 729-740.
Healy PJ, Helliwell PS: Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum. 2008, 59: 686-691.
Sieper J, van der Heijde D, Landewé R, Brandt J, Burgos-Vagas R, Collantes-Estevez E, Dijkmans B, Dougados M, Khan MA, Leirisalo-Repo M, van der Linden S, Maksymowych WP, Mielants H, Olivieri I, Rudwaleit M: New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis. 2009, 68: 784-788.
Calin A, Porta J, Fries JF, Schurman DJ: Clinical history as a screening test for ankylosing spondylitis. JAMA. 1977, 237: 2613-2614.
Geijer M, Gadeholt Gothlin G, Gothlin JH: The validity of the New York radiological grading criteria in diagnosing sacroiliitis by computed tomography. Acta Radiol. 2009, 50: 664-673.
Ahlehoff O, Gislason GH, Charlot M, Jørgensen CH, Lindhardsen J, Olesen JB, Abildstrøm SZ, Skov L, Torp-Pedersen C, Hansen PR: Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011, 270: 147-157.
Mehta NN, Li K, Szapary P, Krueger J, Brodmerkel C: Modulation of cardiometabolic pathways in skin and serum from patients with psoriasis. J Transl ‘aMed. 2013, 11: 194-
Suárez-Fariñas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG: Expanding the psoriasis disease profile: interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol. 2012, 132: 2552-2564.
Jamnitski A, Symmons D, Peters MJ, Sattar N, McInnes I, Nurmohamed MT: Cardiovascular comorbidities in patients with psoriatic arthritis: a systematic review. Ann Rheum Dis. 2013, 72: 211-216.
Ali Y, Tom BD, Schentag CT, Farewell VT, Gladman DD: Improved survival in psoriatic arthritis with calendar time. Arthritis Rheum. 2007, 56: 2708-2714.
Buckley C, Cavill C, Taylor G, Kay H, Waldron N, Korendowych E, McHugh N: Mortality in psoriatic arthritis: a single-center study from the UK. J Rheumatol. 2010, 37: 2141-2144.
Gladman DD, Farewell VT, Wong K, Husted J: Mortality studies in psoriatic arthritis: results from a single outpatient center. II. Prognostic indicators for death. Arthritis Rheum. 1998, 41: 1103-1110.
Ogdie A, Haynes K, Troxel AB, Love TJ, Hennessy S, Choi H, Gelfand JM: Risk of mortality in patients with psoriatic arthritis, rheumatoid arthritis and psoriasis: a longitudinal cohort study. Ann Rheum Dis. 2014, 73: 149-153.
Shbeeb M, Uramoto KM, Gibson LE, O’Fallon WM, Gabriel SE: The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, 1982–1991. J Rheumatol. 2000, 27: 1247-1250.
Wong K, Gladman DD, Husted J, Long JA, Farewell VT: Mortality studies in psoriatic arthritis: results from a single outpatient clinic. I Causes and risk of death. Arthritis Rheum. 1997, 40: 1868-1872.
Han C, Robinson DW, Hackett MV, Paramore LC, Fraeman KH, Bala MV: Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006, 33: 2167-2172.
Chin YY, Yu HS, Li WC, Ko YC, Chen GS, Wu CS, Lu YW, Yang YH, Lan CC: Arthritis as an important determinant for psoriatic patients to develop severe vascular events in Taiwan: a nation-wide study. J Eur Acad Dermatol Venereol. 2013, 27: 1262-1268.
Slobodin G, Croitoru S, Starikov N, Younis S, Boulman N, Rimar D, Rosner I, Rozenbaum M, Odeh M: Incidental computed tomography sacroiliitis: clinical significance and inappropriateness of the New York radiological grading criteria for the diagnosis. Clin Rheumatol. 2012, 31: 425-428.
Acknowledgements
The authors would like to thank Daniel Shin for helpful discussions regarding statistical methodologies described in the manuscript and Robert Colbert for critical appraisal of the manuscript. This work was supported by intramural funding from the National Institutes of Health Clinical Center, the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SR and NNM conceived of and designed the study. SR, JD, CM, HN, ELS and NNM acquired, analyzed and interpreted the data. SR, JD, CM, HN, ELS NNM were involved with the drafting and revision of the manuscript. All authors are accountable for the accuracy and integrity of the work, and all authors read and approved the final manuscript.
Electronic supplementary material
13075_2014_4350_MOESM1_ESM.tiff
Additional file 1: Table S1: Aortic vascular inflammation is greater in patients with the highest grade sacroiliitis. These data demonstrate that those patients with definitive sacroiliitis on CT scan demonstrate greater vascular inflammation by FDG-PET/CT compared to patients with suspected or no sacroiliac disease. (TIFF 611 KB)
13075_2014_4350_MOESM2_ESM.tiff
Additional file 2: Table S2: Correlation analysis of aortic vascular inflammation with clinical measures by subgroup. These data demonstrate that cardiovascular risk factors, sacroiliitis by CT scan and psoriatic arthritis are independently associated with vascular inflammation by FDG-PET/CT. (TIFF 608 KB)
13075_2014_4350_MOESM3_ESM.tiff
Additional file 3: Table S3: Sacroiliitis and psoriatic arthritis are positively related to vascular inflammation even after adjustment for cardiovascular disease risk factors and the markers of systemic inflammation ESR and CRP. These data demonstrate that sacroiliitis by CT scan and psoriatic arthritis are associated with vascular inflammation by FDG-PET/CT beyond traditional cardiovascular risk factors and circulating markers of systemic inflammation. (TIFF 932 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Rose, S., Dave, J., Millo, C. et al. Psoriatic arthritis and sacroiliitis are associated with increased vascular inflammation by 18-fluorodeoxyglucose positron emission tomography computed tomography: baseline report from the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative. Arthritis Res Ther 16, R161 (2014). https://doi.org/10.1186/ar4676
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/ar4676