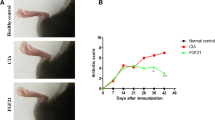
Abstract
Introduction
Hepatocyte growth factor (HGF) is a potent proangiogenic molecule that induces neovascularization. The HGF antagonist, NK4, competitively antagonizes HGF binding to its receptor. In the present study, we determined the inhibitory effect of NK4 in a rheumatoid arthritis (RA) model using SKG mice.
Methods
Arthritis was induced in SKG mice by a single intraperitoneal injection of β-glucan. Recombinant adenovirus containing NK4 cDNA (AdCMV.NK4) was also injected intravenously at the time of or 1 month after β-glucan injection. Ankle bone destruction was examined radiographically. The histopathologic features of joints were examined using hematoxylin and eosin and immunohistochemical staining. Enzyme-linked immunosorbent assays were used to determine the serum levels of HGF, interferon γ (IFN-γ, interleukin 4 (IL-4) and IL-17 production by CD4+ T cells stimulated with allogeneic spleen cells.
Results
The intravenous injection of AdCMV.NK4 into SKG mice suppressed the progression of β-glucan-induced arthritis. Bone destruction was also inhibited by NK4 treatment. The histopathologic findings of the ankles revealed that angiogenesis, inflammatory cytokines and RANKL expression in synovial tissues were significantly inhibited by NK4 treatment. Recombinant NK4 (rNK4) proteins inhibited IFN-γ, IL-4 and IL-17 production by CD4+ T cells stimulated with allogeneic spleen cells.
Conclusions
These results indicate that NK4 inhibits arthritis by inhibition of angiogenesis and inflammatory cytokine production by CD4+ T cells. Therefore, molecular targeting of angiogenic inducers by NK4 can potentially be used as a novel therapeutic approach for the treatment of RA.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease which causes progressive deformity and destruction of the joints [1]. RA is characterized by aggressive synovial expansion and invasion and subsequent destruction of the underlying cartilage and bone. Synovial expansion is dependent on an adequate blood supply for nutrients and oxygen. New blood vessel formation (angiogenesis) is therefore critically important for the delivery of oxygen, nutrients and inflammatory cells to the lesions of RA [2, 3].
There is mounting evidence that angiogenic inducers, such as vascular endothelial growth factor (VEGF), play a pivotal role in RA [4–6]. The intravenous administration of adenovirus expressing sFlt-1, the secreted form of the extracellular domain of the Flt-1 VEGF receptor, in a collagen-induced arthritis (CIA) model results in blocking of VEGF receptor signaling and a reduction in joint swelling [7].
Hepatocyte growth factor (HGF) has angiogenic activity for vascular endothelial cells [8]. HGF has a role in the dynamic construction and reconstruction of normal tissues during organogenesis and tissue regeneration [9]; however, tumor cells utilize the biological actions of HGF for dissociative, invasive and metastatic behavior [10]. The abrogation of HGF receptor (c-Met)-mediated signaling events appears to be a highly promising strategy for the prevention of tumor metastasis [11]. NK4 has been isolated as a competitive antagonist for HGF and the c-Met receptor [12], and subsequent studies have shown that NK4 also inhibits the angiogenic response induced by basic fibroblast growth factor (bFGF) and VEGF [13].
In the present study, we utilized adenovirus expressing the NK4 gene, which has previously been demonstrated to suppress tumor growth and vascularization in mice [14]. Our data demonstrate that adenoviral delivery of NK4 gene significantly suppresses disease activity in a model of RA in SKG mice.
Materials and methods
Mice
Female SKG mice [15–17], 7 to 8 weeks old, were purchased from CLEA Japan (Tokyo, Japan) and maintained under specific pathogen-free conditions in the animal facility of the Hyogo College of Medicine (Nishinomiya, Hyogo, Japan). Female C57BL/6 (B6) mice, 8 to 12 weeks old, were purchased from the Shizuoka Laboratory Animal Center (Shizuoka, Japan). Animal experiments were conducted in accordance with the guidelines of the National Institutes of Health (Bethesda, MD, USA), as specified by the animal care policy of Hyogo College of Medicine. All of the experimental procedures were reviewed and approved by the Animal Care and Use Committee of Hyogo College of Medicine.
Clinical assessment of SKG arthritis
Arthritis was induced by a single intraperitoneal injection of the β-glucan laminarin (45 mg). Joint swelling was monitored by inspection and scored as follows: 0, no swelling; 0.1, swelling of one toe joint; 0.5, mild ankle swelling; and 1.0, severe ankle swelling. The scores for all toes and ankles were totaled for each mouse. The ankle volume was measured with a water replacement plethysmometer (Unicom Japan, Tokyo, Japan).
Preparation and measurement of NK4
AdCMV.NK4 and AdCMV.LacZ are structurally similar replication-deficient recombinant adenovirus type 5 (Ad5)-based vectors with E1 and E3 deletions in which the NK4 gene and LacZ transgene, respectively, are under transcriptional control of the cytomegalovirus (CMV) immediate-early enhancer and promoter. The recombinant virus vectors were grown in HEK-293 cells and twice purified by CsCl gradient centrifugation, and titers were determined by serial dilution end point assay. All vectors were free of replication-competent adenovirus [18]. NK4 in serum was determined using an enzyme-linked immunosorbent assay (ELISA) kit for human HGF (Funakoshi Co, Tokyo, Japan). All work was performed in accordance with an approved protocol from the Institutional Biosafety Committee of Hyogo College of Medicine.
Histopathology and immunohistochemistry
Joints were fixed in 10% formalin, decalcified using 10% ethylenediaminetetraacetic acid in phosphate-buffered saline (PBS) for 3 days, embedded in paraffin, sectioned and stained with hematoxylin and eosin. Microscopic examinations of the joints were conducted with subjective grading of inflammatory cell infiltration, synovial hyperplasia and cartilage and bone destruction. Joint grading was as follows [19]: normal, mild (minimum inflammatory cell infiltration and synovial hyperplasia with some cartilage loss), moderate (more extensive inflammatory cell infiltration and synovial hyperplasia with considerable bone erosion) and severe (complete destruction of joint architecture). All analyses were performed in a blinded fashion by an observer who was unaware of the clinical status of the animal. For immunohistochemical staining, cryostat sections of joints were fixed in cold acetone for 10 min, washed in PBS and depleted of endogenous peroxidase by treatment with 0.3% H2O2 in absolute methanol for 15 min. After blocking nonspecific binding with 10% normal rabbit serum in PBS for 30 min, the sections were incubated with anti-mouse interleukin 6 antibody (IL-6 Ab; goat immunoglobulin G (IgG)), anti-mouse tumor necrosis factor α (TNF-α) Ab (goat IgG), anti-mouse CD31 Ab (goat IgG), anti-RANKL (anti-receptor activator of nuclear factor κB ligand) Ab (goat IgG), anti-mouse IL-17 Ab (goat IgG; all from Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-mouse IL-1 Ab (goat IgG; R&D Systems, Minneapolis, MN, USA), anti-mouse HGF Ab (rabbit IgG) or anti-mouse c-Met Ab (rabbit IgG) at appropriate dilutions for 1 h at room temperature, washed, incubated with biotinylated goat anti-rabbit IgG, washed again and incubated with avidin-biotinylated horseradish peroxidase complex (ABC) and 3,3'-diaminobenzidine tetrahydrochloride (DAB) (VECTASTAIN Elite ABC Kit; Vector Laboratories, Burlingame, CA, USA) and counterstained with Mayer's hematoxylin. MH7A cells and synovial tissue specimens isolated from patients with RA and osteoarthritis (OA) at the time of arthroscopic biopsy or total joint replacement were stained as reported previously [20]. Briefly, synovial tissues were stained with anti-human HGF Ab (rabbit IgG) or anti-human c-Met Ab (rabbit IgG), washed, incubated with biotinylated goat anti-rabbit IgG, washed again, incubated with ABC and DAB and counterstained with Mayer's hematoxylin. All patients gave their informed consent to participate, and the Institutional Medical Ethics Committee approved the study protocol.
Mixed lymphocyte reaction and in vitro cytokine production
The mixed lymphocyte reaction was performed as described previously [21, 22]. Briefly, CD4+ T cells and CD11c+ dendritic cells (DCs) were purified from spleens using immunomagnetic beads (Miltenyi Biotec, Auburn, CA, USA). The purity of the CD4+ and CD11c+ populations was greater than 95% and more than 90%, respectively. DCs from C57BL/6 mice (H-2b; 1 × 106 cells/ml/well) were incubated in the presence or absence of HGF or NK4 for 24 h. After thrice washing with Hanks' balanced salt solution (HBSS), DCs were irradiated (20 Gy) and cocultured with CD4+ T cells from SKG mice (H-2d; 4 × 106 cells/ml/well) in 24-well flat-bottomed plates (Falcon Labware, Lincoln Park, NJ, USA). After 72 h, viable cells were harvested, and, after thrice washing with HBSS, the cells (1 × 105 cells/200 μl/well) were stimulated in 96-well flat-bottomed plates (Falcon Labware) coated with 5 μg/ml anti-mouse CD3 mAb. After 48 h, interferon γ (IFN)-γ, IL-4 and IL-17 concentrations in the culture supernatants were measured by ELISA using anti-mouse IFN-γ, IL-4 and IL-17 mAbs, respectively (Genzyme, Cambridge, MA, USA), according to the manufacturer's protocol.
Serum HGF ELISA
The serum HGF levels in SKG mice were measured by ELISA using anti-mouse HGF mAb (R&D Systems) according to the manufacturer's protocol.
Bone X-ray
The ankles were examined radiographically after the mice were killed by CO2 inhalation on day 60. X-ray images of the ankles were obtained with an X-ray apparatus (μFX-1000; FUJIFILM, Tokyo, Japan) using the following settings: 100 kV, 40 μA and 7-s exposure time.
Statistics
Student's t-test was used for statistical analysis.
Results
AdCMV.NK4 induces NK4 proteins in SKG mice in vivo
To verify the in vivo production of NK4 protein after AdCMV.NK4 injection, AdCMV.NK4 (1 × 109 plaque-forming units (pfu)) was injected intravenously into SKG mice. We observed significantly elevated levels of NK4 protein in the blood 1 day after injection (Figure 1A). Systemically administered adenoviruses are scavenged by the reticuloendothelial system (primarily in the liver) [23]. We also observed the expression of NK4 protein in the liver by immunohistochemistry 1 day after injection (Figure 1B).
AdCMV.NK4 induces NK4 protein in SKG mice. SKG mice were injected intravenously with AdCMV.NK4 (1 × 109 plaque-forming units (pfu)) into the tail vein. One day after injection, serum and liver were obtained and NK4 protein levels in the serum (A) and NK4 protein expression in the liver (B) were measured by hepatocyte growth factor (HGF) enzyme-linked immunosorbent assay (ELISA) or immunohistochemistry, respectively. Data in (A) are represented as the means ± SD (n = 6). The arrows in (B) represent NK4 protein expression in hepatocytes. Original magnification, ×100.
HGF and c-Met expression in the synovia of SKG mice and RA patients
To examine whether the HGF/c-Met pathway is involved in the pathogenesis of RA, we first determined the expression of HGF and c-Met in the synovia of SKG mice. In SKG mice, joint swelling began to develop in a few digits approximately 1 month after β-glucan injection, subsequently progressing to other digits and to larger joints (wrists and ankles) in a symmetric fashion. Immunohistochemical staining of synovial tissues 2 months after β-glucan injection revealed high expression of c-Met in the synovial lining and vascular cells. HGF was also expressed in the synovial lining and fibroblastic cells (Figure 2A). The serum levels of HGF in arthritis-induced SKG mice were higher than in untreated SKG mice (Figure 2B). We next examined the expression of c-Met and HGF in the synovium. c-Met expression in mononuclear cells, blood vessels and synovial lining cells of RA patients was higher than in OA patients. HGF was expressed in the synovial lining and fibroblastic cells of RA and OA synovial tissues (Figure 2C).
Serum levels of hepatocyte growth factor (HGF) and HGF and c-Met expression in the synovium. (A) The histologic feature of swollen joints 60 days after induction of arthritis in SKG mice expressed HGF and c-Met in the synovium. Original magnification, ×200; inset, ×400. (B) The serum levels of HGF in β-glucan-injected SKG mice (60 days after induction) were higher than in untreated SKG mice. Data are represented as the means ± SD (n = 4). *P = 0.06. (C) The histologic feature of joints in RA patients expressed HGF and c-Met in the synovium (BV, blood vessel; F, fibroblastic cell; M, mononuclear cell; OA, osteoarthritis; RA, rheumatoid arthritis; SL, synovial lining cell layer). Original magnification, ×100; inset, ×400. We examined the histologic features of joints in osteoarthritis patients as a control.
AdCMV.NK4 reduces clinical score and joint swelling in SKG mice
To determine whether NK4 inhibited joint swelling, AdCMV.NK4 (1 × 109 pfu), or AdCMV.LacZ (1 × 109 pfu) as a control, was administered intravenously to the mice from the time of β-glucan injection. Joint swelling of the AdCMV.LacZ-treated SKG mice began on day 32 after β-glucan injection and progressed significantly on day 75. In contrast, mice that received AdCMV.NK4 had less joint swelling as determined by the clinical score (Figure 3A) and ankle volume (Figure 3B) 60 days following β-glucan injection. In clinical situations, gene therapy is more likely to be used therapeutically than to prevent disease. To determine the therapeutic effectiveness of this treatment on arthritis, we introduced AdCMV.NK4 (1 × 109 pfu) into SKG mice 1 month after β-glucan injection. SKG mice that received AdCMV.NK4 had less joint swelling than control mice that received AdCMV.LacZ 60 days following β-glucan injection (Figure 3C).
AdCMV.NK4 reduces clinical score and ankle swelling. Arthritis was induced by a single intraperitoneal injection of the β-glucan laminarin (45 mg). AdCMV.NK4 (1 × 109 plaque-forming units (pfu); n = 8; filled squares) or AdCMV.LacZ (1 × 109 pfu; n =8; filled diamonds) was administered intravenously at the time of laminarin injection. (A) Changes in arthritis scores were monitored over the course of 60 days. (B) The ankle swelling in mice treated with AdCMV.NK4 (1 × 109 pfu) or AdCMV.LacZ was examined on day 60 after induction of arthritis. (C) To determine the therapeutic effectiveness of this treatment on arthritis, we introduced AdCMV.NK4 (1 × 109 pfu; n = 8) or AdCMV.LacZ (1 × 109 pfu; n = 8) into SKG mice 1 month after β-glucan injection, and the ankle swelling was examined on day 60 after induction of arthritis (C). Arthritis scores were monitored by inspection as follows: 0, no swelling; 0.1, swelling of one toe joint; 0.5, mild paw swelling; and 1.0, severe paw swelling. The results are expressed as the means ± SD. *P < 0.05 vs SKG mice given AdCMV.NK4. Representative data of two separate experiments are shown.
AdCMV.NK4 reduces histopathologic changes in SKG mice
The histopathologic features of swollen joints in AdCMV.LacZ-treated SKG mice, as shown by hematoxylin and eosin staining, included vigorous proliferation of synovial cells and infiltration of synovial tissues by mononuclear cells and neutrophils, which has been observed in human RA [1]. In contrast, these pathologic changes were significantly inhibited in NK4-treated SKG mice. X-ray examination of the ankle joints 60 days following β-glucan injection of AdCMV.LacZ-treated SKG mice revealed erosion of the cartilage and subchondral bone, whereas these changes were inhibited in NK4-treated SKG mice (Figure 4).
AdCMV.NK4 inhibits histopathologic changes. (A) The histologic features of swollen joints 60 days after induction of arthritis in LacZ-treated SKG mice exhibited severe synovitis accompanying massive subsynovial infiltration of neutrophils, lymphocytes and macrophages with villous proliferation of synoviocytes. These pathologic changes were significantly inhibited in NK4-treated SKG mice. Original magnification, ×100; inset, ×400. (B) X-ray bone examination taken 60 days after induction of arthritis revealed severe destruction of ankle joints in LacZ-treated SKG mice. These bone changes were inhibited in NK4-treated SKG mice. (C) The percentage of joints exhibiting mild (white bar), moderate (gray bar) and severe (black bar) pathologic changes by AdCMV.NK4-treated SKG mice (n = 8) or AdCMV.LacZ-treated SKG mice (n = 8) 60 days after induction of arthritis.
AdCMV.NK4 reduces inflammatory cell infiltration, as well as cytokine and RANKL expression, in synovial tissue
Immunohistochemical staining of synovial tissues from AdCMV.LacZ-treated SKG mice revealed high expression of IL-1, IL-6 and TNF-α. In contrast, AdCMV.NK4-treated SKG mice did not express these cytokines AdCMV.NK4 treatment also inhibited type 17 T-helper (Th17) cell infiltration and RANKL expression in the synovial tissues (Figure 5).
AdCMV.NK4 inhibits inflammatory cell infiltration, as well as cytokine and RANKL expression, in the synovial tissues of SKG mice. Immunohistochemistry of interleukin 1 (IL-1) (A), IL-6 (B), TNF-α (C), CD31 (D), IL-17 (E) and RANKL (receptor activator of nuclear factor κB ligand) (F). High-level expression of IL-1, IL-6, TNF-α, CD31, IL-17 and RANKL in the synovial tissues of LacZ-treated SKG mice was observed (upper). In contrast, the expression was significantly inhibited in the synovial tissues of NK4-treated SKG mice (lower). Original magnification, ×200.
Recombinant NK4 inhibits interferon γ, interleukin 4 and interleukin 17 production by CD4+ T cells in vitro
We examined the effect of recombinant NK4 (rNK4) on the production of IFN-γ, IL-4 and IL-17 by CD4+ T cells stimulated with allogeneic DCs. CD11c+ DCs from C57BL/6 mice were preincubated in the presence or absence of HGF or NK4 for 24 h. CD4+ T cells from SKG mice were cultured with irradiated CD11c+ DCs, and IFN-γ, IL-4 and IL-17 production in culture supernatants was measured by ELISA. Preincubation of rNK4 and HGF inhibited IFN-γ, IL-4 and IL-17 production by CD4+ T cells (Figure 6).
Effects of recombinant NK4 and hepatocyte growth factor on CD4+ T cells. CD11c+ dendritic cells from C57BL/6 mice were incubated in the presence or absence of hepatocyte growth factor or NK4 for 24 h. After thrice washing with Hanks' balanced salt solution (HBSS), dendritic cells (H-2b; 1 × 106 cells/ml/well) were irradiated (20 Gy) and cocultured with CD4+ T cells from SKG mice (H-2d; 4 × 106 cells/ml/well) in 24-well flat-bottomed plates. After 72 h, viable cells were harvested, and, after thrice washing with HBSS, the cells (1 × 105 cells/200 μl/well) were stimulated in 96-well flat-bottomed plates coated with 5 μg/ml anti-mouse CD3 monoclonal antibody. The concentrations of interferon γ (IFN-γ) (A), interleukin 4 (IL-4) (B) and IL-17 (C) in the culture supernatants were measured by enzyme-linked immunosorbent assay. Data represent the means ± SD of three independent experiments. *P < 0.01. NS, not significant.
Discussion
We have demonstrated that the HGF antagonist NK4 significantly suppresses arthritis in a SKG mouse model, as demonstrated by the ankle volume and arthritis score. It has been reported that HGF is expressed in synovial tissues and that vascular endothelial cells express c-Met in patients with RA [24]. Synovial fluid HGF in patients with RA is produced by synovial cells and is related to disease activity [25]. We also demonstrated that HGF is expressed in synovial lining cells, and c-Met is strongly expressed in mononuclear, vascular endothelial and synovial lining cells of RA patients. These results suggest that NK4 inhibits angiogenesis induced by HGF-c-Met signaling in synovial tissues of patients with RA.
NK4 is a proteolytic fragment of HGF, consisting of an N-terminal hairpin domain and four kringle domains of the α chain of HGF [12]. The NK4 fragment appears to be generated by mast cell and neutrophil peptidases under physiologic and pathologic conditions such as inflammation and cancer, thus regulating angiogenesis [26]. In addition to antagonizing HGF by competitively binding to c-Met, NK4 inhibits the angiogenic responses of endothelial cells induced by bFGF and VEGF [13], suggesting that new binding molecules of NK4 other than c-Met may exist, such as perlecan, the major extracellular heparan sulfate proteoglycans associated with blood vessels as previously reported [27].
The systemic administration of AdCMV.NK4 induced the production of significant NK4 protein in the blood and liver 1 day after administration. Adenoviral vectors have particular advantages for use as in vivo gene transfer vehicles, including a broad host range, the ability to infect both dividing and nondividing cells and the ease of high-titer purification [28]. Studies using adenovirus encoding for inflammatory cytokines or their receptors, such as TNF receptor p55, IL-4 and IL-10 in CIA have been reported [29–31]. Administration of adenovirus expressing Flt-1 was also able to suppress clinical scores, ankle swelling and joint destruction [7]. sFlt-1 expression was demonstrated in systemic and effecter regions, although the expression was transient because of antibody responses targeting the adenovirus and human transgene. We also measured NK4 protein in serum by ELISA using anti-human HGF antibody. In mice receiving 1 × 109 pfu of Ad.CMV.NK4 via the tail vein, NK4 protein in serum peaked at more than 1,300 pg/ml 24 h after transduction, then gradually declined to 0 ng/ml 14 days after transduction (data not shown). These results suggest that short-term blockage of angiogenesis in the early phase of arthritis inhibits arthritis in SKG mice. To determine the therapeutic effectiveness of this treatment on arthritis, we introduced AdCMV.NK4 (1 × 109 pfu) into SKG mice 1 month after β-glucan injection. SKG mice that received AdCMV.NK4 had less joint swelling than control mice that received AdCMV.LacZ 60 days following β-glucan injection.
Presentation of antigen by antigen-presenting cells (APCs) to T cells initiates the differentiation of naïve Th cells into effector T cells. The expression of costimulatory molecules on APCs and the cytokine profile produced by APCs play a critical role during the differentiation into each T-cell phenotype, such as Th1, Th2 or regulatory T (Treg) cells [32]. DCs are the most efficient and crucial APCs [33]. Recent studies have reported the effect of HGF on DC function [34, 35]. Rutella et al. [35] reported that, in in vitro experiments, HGF suppresses alloantigen-presenting capacity, modulates costimulatory molecule expression and cytokine production of DCs and generates DCs that induce Treg cells. Okunishi et al. [34] reported that HGF potently suppresses antigen-presenting capacity and IL-12p70 production of DCs, thus inhibiting development of Th1- and Th2-type immune responses induced by ovalbumin. Okunishi et al. [36] also demonstrate that HGF potently inhibits the development of CIA with augmentation of the Th2-type immune response and suppression of IL-17 production. Because NK4 antagonizes HGF, it is thought that NK4 inhibits immune responses induced by HGF. In contrast, AdCMV.NK4 inhibited inflammatory cytokine expression in synovial tissues of SKG mice. In addition, rNK4 inhibited IFN-γ, IL-4 and IL-17 production from the CD4+ T cells stimulated with allogeneic spleen cells. Although the precise mechanisms by which NK4 inhibits inflammatory responses are not clear, it is possible that new binding molecules of NK4 on DCs may exert these functions after binding to NK4.
Conclusions
Our results demonstrate that systemic administration of AdCMV.NK4 inhibits synovial cell proliferation and inflammatory responses in the joints of SKG mice in a RA model. We have also demonstrated that NK4 inhibits inflammatory cytokine production by CD4+ T cells. The data indicate the potential utility of NK4 in the treatment for RA.
Abbreviations
- APC:
-
antigen-presenting cell
- bFGF:
-
basic fibroblast growth factor
- CIA:
-
collagen-induced arthritis
- CMV:
-
cytomegalovirus
- DC:
-
dendritic cell
- HGF:
-
hepatocyte growth factor
- MMP-3:
-
matrix metalloproteinase 3
- OA:
-
osteoarthritis
- RA:
-
rheumatoid arthritis
- VEGF:
-
vascular endothelial growth factor
References
Scott DL, Wolfe F, Huizinga TW: Rheumatoid arthritis. Lancet. 2010, 376: 1094-1108. 10.1016/S0140-6736(10)60826-4.
Szekanecz Z, Koch AE: Vascular involvement in rheumatic diseases: 'vascular rheumatology'. Arthritis Res Ther. 2008, 10: 224-10.1186/ar2515.
Szekanecz Z, Besenyei T, Szentpétery A, Koch AE: Angiogenesis and vasculogenesis in rheumatoid arthritis. Curr Opin Rheumatol. 2010, 22: 299-306. 10.1097/BOR.0b013e328337c95a.
Koch AE, Harlow LA, Haines GK, Amento EP, Unemori EN, Wong WL, Pope RM, Ferrara N: Vascular endothelial growth factor: a cytokine modulating endothelial function in rheumatoid arthritis. J Immunol. 1994, 152: 4149-4156.
Paleolog EM, Young S, Stark AC, McCloskey RV, Feldmann M, Maini RN: Modulation of angiogenic vascular endothelial growth factor by tumor necrosis factor α and interleukin-1 in rheumatoid arthritis. Arthritis Rheum. 1998, 41: 1258-1265. 10.1002/1529-0131(199807)41:7<1258::AID-ART17>3.0.CO;2-1.
Fava RA, Olsen NJ, Spencer-Green G, Yeo KT, Yeo TK, Berse B, Jackman RW, Senger DR, Dvorak HF, Brown LF: Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J Exp Med. 1994, 180: 341-346. 10.1084/jem.180.1.341.
Afuwape AO, Feldmann M, Paleolog EM: Adenoviral delivery of soluble VEGF receptor 1 (sFlt-1) abrogates disease activity in murine collagen-induced arthritis. Gene Ther. 2003, 10: 1950-1960. 10.1038/sj.gt.3302104.
Bussolino F, Di Renzo MF, Ziche M, Bochietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM: Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992, 119: 629-641. 10.1083/jcb.119.3.629.
Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S: Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989, 342: 440-443. 10.1038/342440a0.
Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF: Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003, 4: 915-925. 10.1038/nrm1261.
Matsumoto K, Nakamura T, Sakai K, Nakamura T: Hepatocyte growth factor and Met in tumor biology and therapeutic approach with NK4. Proteomics. 2008, 8: 3360-3370. 10.1002/pmic.200800156.
Date K, Matsumoto K, Shimura H, Tanaka M, Nakamura T: HGF/NK4 is a specific antagonist for pleiotrophic actions of hepatocyte growth factor. FEBS Lett. 1997, 420: 1-6. 10.1016/S0014-5793(97)01475-0.
Kuba K, Matsumoto K, Date K, Shimura H, Tanaka M, Nakamura T: HGF/NK4, a four-kringle antagonist of hepatocyte growth factor, is an angiogenesis inhibitor that suppresses tumor growth and metastasis in mice. Cancer Res. 2000, 60: 6737-6743.
Martin TA, Parr C, Davies G, Watkins G, Lane J, Matsumoto K, Nakamura T, Mansel RE, Jiang WG: Growth and angiogenesis of human breast cancer in a nude mouse tumour model is reduced by NK4, a HGF/SF antagonist. Carcinogenesis. 2003, 24: 1317-1323. 10.1093/carcin/bgg072.
Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, Sakihama T, Matsutani T, Negishi I, Nakatsuru S, Sakaguchi S: Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003, 426: 454-460. 10.1038/nature02119.
Hata H, Sakaguchi N, Yoshitomi H, Iwakura Y, Sekikawa Y, Azuma Y, Kanai C, Moriizumi E, Nomura T, Nakamura T, Sakaguchi S: Distinct contribution of IL-6, TNF-α, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J Clin Invest. 2004, 114: 582-588.
Yoshitomi H, Sakaguchi N, Kobayashi K, Brown GD, Tagami T, Sakihama T, Hirota K, Tanaka S, Nomura T, Miki I, Gordon S, Akira S, Nakamura T, Sakaguchi S: A role for β-glucans and their receptor dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J Exp Med. 2005, 201: 949-960. 10.1084/jem.20041758.
Maemondo M, Narumi K, Saijo Y, Usui K, Tahara M, Tazawa R, Hagiwara K, Matsumoto K, Nakamura T, Nukiwa T: Targeting angiogenesis and HGF function using an adenoviral vector expressing the HGF antagonist NK4 for cancer therapy. Mol Ther. 2002, 5: 177-185. 10.1006/mthe.2002.0533.
Tsunemi S, Iwasaki T, Kitano S, Imado T, Miyazawa K, Sano H: Effects of the novel immunosuppressant FTY720 in a murine rheumatoid arthritis model. Clin Immunol. 2010, 136: 197-204. 10.1016/j.clim.2010.03.428.
Kitano M, Hla T, Sekiguchi M, Kawahito Y, Yoshimura R, Miyazawa K, Iwasaki T, Sano H: Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006, 54: 742-753. 10.1002/art.21668.
Kuroiwa T, Iwasaki T, Imado T, Sekiguchi M, Fujimoto J, Sano H: Hepatocyte growth factor prevents lupus nephritis in a murine lupus model of chronic graft-versus-host disease. Arthritis Res Ther. 2006, 8: R123-10.1186/ar2012.
Iwasaki T, Imado T, Kitano S, Sano H: Hepatocyte growth factor ameliorates dermal sclerosis in the tight-skin mouse model of scleroderma. Arthritis Res Ther. 2006, 8: R161-10.1186/ar2068.
Shayakhmetov DM, Li ZY, Ni S, Liber A: Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J Virol. 2004, 78: 5368-5381. 10.1128/JVI.78.10.5368-5381.2004.
Nagashima M, Hasegawa J, Kato K, Yamazaki J, Nishigai K, Ishiwata T, Asano G, Yoshino S: Hepatocyte growth factor (HGF), HGF activator, and c-Met in synovial tissues in rheumatoid arthritis and osteoarthritis. J Rheumatol. 2001, 28: 1772-1778.
Yukioka K, Inaba M, Furumitsu Y, Yukioka M, Nishino T, Goto H, Nishizawa Y, Morii H: Levels of hepatocyte growth factor in synovial fluid and serum of patients with rheumatoid arthritis and release of hepatocyte growth factor by rheumatoid synovial fluid cells. J Rheumatol. 1994, 21: 2184-2189.
Raymond WW, Cruz AC, Caughey GH: Mast cell and neutrophil peptidases attack an inactivation segment in hepatocyte growth factor to generate NK4-like antagonists. J Biol Chem. 2006, 281: 1489-1494.
Sakai K, Nakamura T, Matsumoto K, Nakamura T: Angioinhibitory action of NK4 involves impaired extracellular assembly of fibronectin mediated by perlecan-NK4 association. J Biol Chem. 2009, 284: 22491-22499. 10.1074/jbc.M109.025148.
Kubo S, Mitani K: A new hybrid system capable of efficient lentiviral vector production and stable gene transfer mediated by a single helper-dependent adenoviral vector. J Virol. 2003, 77: 2964-2971. 10.1128/JVI.77.5.2964-2971.2003.
Kim SH, Evans CH, Kim S, Oligino T, Ghivizzani SC, Robbins PD: Gene therapy for established murine collagen induced arthritis by local and systemic adenovirus-mediated delivery of interleukin-4. Arthritis Res. 2000, 2: 293-302. 10.1186/ar104.
Apparailly F, Verwaerde C, Jacquet C, Auriault C, Sany J, Jorgensen C: Adenovirus-mediated transfer of viral IL-10 gene inhibits murine collagen-induced arthritis. J Immunol. 1998, 160: 5213-5220.
Kim KN, Watanabe S, Ma Y, Thornton S, Giannini EH, Hirsch R: Viral IL-10 and soluble TNF receptor act synergistically to inhibit collagen-induced arthritis following adenovirus-mediated gene transfer. J Immunol. 2000, 164: 1576-1581.
Kapsenberg ML: Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003, 3: 984-993. 10.1038/nri1246.
Steinman RM: Dendritic cells and the control of immunity. Nature. 1998, 392: 245-252. 10.1038/32588.
Okunishi K, Dohi M, Nakagome K, Tanaka R, Mizuno S, Matsumoto K, Miyazaki J, Nakamura T, Yamamoto K: A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J Immunol. 2005, 175: 4745-4753.
Bonanno G, Procoli A, Mariotti A, de Ritis DG, Curti A, Danese S, Pessina G, Pandolfi S, Natoni F, Di Febo A, Scambia G, Manfredini R, Salati S, Ferrari S, Pierelli L, Leone G, Lemoli RM: Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood. 2006, 108: 218-227. 10.1182/blood-2005-08-3141.
Okunishi K, Dohi M, Fujio K, Nakagome K, Tabata Y, Okasora T, Seki M, Shibuya M, Imamura M, Harada H, Tanaka R, Yamamoto K: Hepatocyte growth factor significantly suppresses collagen-induced arthritis in mice. J Immunol. 2007, 179: 5504-5513.
Acknowledgements
The expert technical help of Takehito Imado is gratefully acknowledged. This work was supported by a Grant-in-Aid for Exploratory Research from the Ministry of Education, Science and Culture of Japan (21590187).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All authors were involved in drafting the manuscript or revising the manuscript critically, and all authors approved the final version of the manuscript for publication. ST and SK performed all of the experiments and analyzed the data. TI and HS designed the experiments and take responsibility for the accuracy of the data analysis. KM provided the AdCMV.NK4 and AdCMV.LacZ vectors. TT, SK and MTK prepared the AdCMV.NK4 and AdCMV.LacZ vectors.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tsunemi, S., Iwasaki, T., Kitano, S. et al. Molecular targeting of hepatocyte growth factor by an antagonist, NK4, in the treatment of rheumatoid arthritis. Arthritis Res Ther 15, R75 (2013). https://doi.org/10.1186/ar4252
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/ar4252