Abstract
Introduction
Systemic lupus erythematosus (SLE or lupus) is a chronic autoimmune disease, and kidney involvement with SLE, a.k.a. lupus nephritis (LN), is a frequent and severe complication of SLE that increases patient morbidity and mortality. About 50% of patients with SLE encounter renal abnormalities which, if left untreated, can lead to end-stage renal disease. Kidney biopsy is considered the criterion standard for diagnosis and staging of LN using the International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification, which was developed to help predict renal outcomes and assist with medical decision-making. However, kidney biopsy-based classification of LN is highly invasive and impractical for real-time monitoring of LN status. Here, nuclear magnetic resonance (NMR) spectroscopy-based metabolic profiling was used to identify urinary metabolites that discriminated between proliferative and pure membranous LN as defined by the ISN/RPS classification, and between LN and primary focal segmental glomerulosclerosis (FSGS).
Methods
Metabolic profiling was conducted using urine samples of patients with proliferative LN without membranous features (Class III/IV; n = 7) or pure membranous LN (Class V; n = 7). Patients with primary FSGS and proteinuria (n = 10) served as disease controls. For each patient, demographic information and clinical data was obtained and a random urine sample collected to measure NMR spectra. Data and sample collection for patients with LN occurred around the time of kidney biopsy. Metabolic profiling analysis was done by visual inspection and principal component analysis.
Results
Urinary citrate levels were 8-fold lower in Class V LN compared to Class III/IV patients, who had normal levels of urinary citrate (P < 0.05). Class III/IV LN patients had > 10-fold lower levels of urinary taurine compared to Class V patients, who had mostly normal levels (P < 0.01). Class V LN patients had normal urinary hippurate levels compared to FSGS patients, who completely lacked urinary hippurate (P < 0.001).
Conclusions
This pilot study indicated differences in urinary metabolites between proliferative LN and pure membranous LN patients, and between LN and FSGS patients. If confirmed in larger studies, these urine metabolites may serve as biomarkers to help discriminate between different classes of LN, and between LN and FSGS.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE or lupus) is a chronic autoimmune disease [1]. Kidney involvement with SLE, a.k.a. lupus nephritis (LN), is a frequent and severe complication of SLE that increases patient morbidity and mortality [2]. About 50% of patients with SLE encounter renal abnormalities which, if left untreated, can lead to end-stage renal disease [3, 4].
Kidney biopsy is considered the criterion standard for diagnosis and staging of LN using the International Society of Nephrology/Renal Pathology Society (ISN/RPS) Classification [5]. This classification was developed to help predict renal outcomes and assist with medical decision-making. Treatment of patients diagnosed with ISN/RPS class III or class IV LN requires combination therapy with corticosteroids plus immunosuppressive medications [6], whereas therapeutic choices for class V LN are still under considerable debate [7]. The ISN/RPS class of a patient with LN is not static. Over time, histological features of LN may improve in response to therapy or degenerative changes can accrue. The lack of sensitive and specific non-invasive biomarkers that assist with distinguishing between various LN classes makes it virtually impossible to dynamically monitor changes in LN classes in real time. This impairs the timely initiation of therapy and impairs monitoring of treatment response.
It is particularly difficult to discriminate proliferative LN (class III/IV) from pure membranous LN (class V) clinically, as both are associated with pronounced proteinuria, and changes in blood pressure and renal function. Pronounced proteinuria is also the hallmark of primary focal segmental glomerulosclerosis (FSGS). One of the histological characteristics of FSGS is podocyte injury, which results in different degrees of proteinuria and potentially, hypoalbuminemia, that is, these clinical and histological features can also occur with active LN [8].
In the past, we and others have used proteomics to discover candidate protein biomarkers for LN [9, 10]. Alternative biomarker discovery approaches include metabolomics, that is, the systematic study of small-molecule metabolite profiles or unique chemical fingerprints that are the result of specific cellular processes, and metabonomics, which can be defined as the quantitative measurement of metabolite changes in such metabolic profiles [11–13]. Nuclear magnetic resonance (NMR) finger printing is currently the method of choice for metabonomics because it provides uniform detection of equal sensitivity for all proton-containing small molecules and can provide valuable information on metabolites directly from biofluids with little sample preparation [14–16]. Metabonomics is achieved by maximum data capture through NMR spectroscopy followed by pattern recognition statistics [17].
The objective of this study was to identify urinary metabolites that discriminated between proliferative LN (class III/IV), pure membranous LN (class V), and FSGS, using NMR spectroscopy-based metabonomics. Metabolic profiles of urine samples were investigated using high-field (850 MHz) solution-state NMR spectroscopy. Two urinary metabolites, citrate and taurine, were found to accurately distinguish between class III/IV and class V LN patients. Urinary citrate levels were eight-fold lower than normal in class V compared with class III/IV LN patients, who had normal levels of urinary citrate. Also, class III/IV LN patients had more than 10-fold lower than normal levels of urinary taurine compared with class V patients, who had mostly normal levels of urinary taurine. Finally, urinary hippurate levels accurately distinguished between class V patients, who had normal levels of urinary taurine, in comparison with FSGS patients, who completely lacked urinary hippurate.
Materials and methods
Patients and samples
All research was conducted in compliance with the Helsinki Declaration. Informed consent was obtained from all enrolled patients. The study was approved by the institutional review boards of both the Johns Hopkins Hospital and the Cincinnati Children's Hospital Medical Center. Children and adults diagnosed with SLE [1] who required a kidney biopsy as part of standard of care therapy were eligible for inclusion in this study if a random spot urine sample was available that was collected within 60 days of the kidney biopsy. On the day of the urine sample collection, information about patient demographics, medications, and disease activity was collected. Key laboratory measures were obtained, including complement C3 and C4 levels, anti-dsDNA antibodies (present/absent), amount of proteinuria as estimated by the protein to creatinine ratio (P/C ratio) in a random or 24-hour urine sample, serum creatinine, and glomerular filtration rate (GFR) as estimated by age-appropriate calculation of the creatinine clearance [18, 19]. For SLE patients to be included in the study, they had to have undergone kidney biopsy, found to have either class III or IV LN without membranous features (class III/IV) or pure membranous class V LN as per the ISN/RPS classification [5], had an available stored urine sample collected within 60 days of a kidney biopsy, and signed the informed consent form.
The histological characteristics of each kidney biopsy, as per report from the local pathologists, were reviewed in a blinded fashion by one expert nephropathologist, as per the ISN/RPS classification [5]. The following histological features reflective of active inflammation with LN were recorded: mesangial proliferation, endocapillary karyorrhexis (also: fibrinoid necrosis); cellular crescents; capillary proliferation, subendothelial deposits identifiable by light microscopy (also: wire-loops). We also noted features representing LN chronicity or degenerative damage. These included glomerular sclerosis (segmental or global), fibrosis including fibrous adhesions and fibrous crescents, as well as tubular atrophy. The results of these classifications are summarized in supplementary Table S1 [see Additional data file 1].
Almost all LN studies employ a previously developed scoring system to quantify the amount of overall LN activity and overall LN chronicity present in kidney biopsy specimens [20]. The features of activity and chronicity listed above were categorized as follows: 0 (no lesions), 1 (lesions in up to 25% of glomeruli), 2 (lesions in 25 to 50% of glomeruli) or 3 (lesions in > 50% of glomeruli). Using these numeric values, a Biopsy Activity Index (AI) score (range 0 to 24) and a Biopsy Chronicity Index (CI) score (range 0 to 12) can be calculated, where higher scores represent higher LN activity or chronicity, respectively. The ISN/RPS classification, the AI and the CI have all been validated for use in adults and children with LN [21, 22]. Risk factors for poor LN outcome include AI scores of seven or higher and CI scores of four or higher [21, 23–30]. The AI and CI scores of the patients are also listed in supplementary Table S1 [see Additional data file 1].
Epimembranous deposits, although not included in the AI or the CI scores, were also recorded. Depending on the findings of active inflammation, and chronic changes observed on kidney biopsy, LN is classified in six categories. Pronounced predominance of epimembranous deposits is compatible with class V of LN.
For patients with LN, key laboratory measures were recorded, including complement C3 levels, anti-dsDNA antibodies (present/absent), amount of proteinuria as estimated by the P/C ratio in a random or 24-hour urine sample, serum creatinine, and GFR as estimated by age-appropriate calculation of the creatinine clearance [18, 19]. The renal domain score of the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI-R; range 0 to 16; 0 = inactive LN) served as the clinical measure of LN activity [31]. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index items addressing kidney damage (SDI-R; range 0 to 3; 0 = no LN damage) were recorded as a clinical measure of kidney damage with LN [32]. The results of these laboratory measurements are summarized in supplementary Table S2 [see Additional data file 1].
Ten patients with biopsy-proven primary FSGS and proteinuria served as a disease control group. For controls with FSGS, data and urine samples were collected during visits to the pediatric nephrology clinics.
The demographics and kidney status of all patients included in the study are summarized in Table 1.
Metabolic profiling
Preparation of urine samples for NMR analysis
Urine samples were stored at -80°C after collection and thawed on ice prior to preparation for NMR analysis. A 1 ml aliquot of each sample was centrifuged for 10 minutes at 2655 × g, and then 350 μl of clear urine was pipetted into a 1.5 ml microcentrifuge tube. A volume of 350 ml of buffer (300 mM KH2PO4, 2 mM NaN3, 0.2% trimethylsilyl propionate (TSP) in 20% D2O, pH 7.4) was added to each urine sample. A volume of 600 μl of the urine/buffer mixture was then pipetted into a 5 mm NMR tube (Norell ST500-7, Norell, Inc., Landisville, NJ USA).
NMR data collection and processing
All NMR experiments were carried out on a Bruker Avance™ III spectrometer (Bruker Biospin, Rheinstetten, Germany) operating at 850.10 MHz 1H frequency and equipped with a room temperature 5 mm triple resonance probe with inverse detection and controlled by TopSpin 2.1.4 (Bruker Biospin, Rheinstetten, Germany). All experiments were conducted at 298 K. All data were collected using a spectral width of 20.0 ppm. Three 1H NMR experiments, optimized by Bruker (Bruker BioSpin, Billerica, MA, USA) for use with metabonomic studies, were run on all samples: a standard 1D presaturation (zgpr), a 1D first increment of a nuclear Overhauser effect spectroscopy (NOESY; noesygppr1d) experiment, and a CPMG (cpmgpr1d) experiment. All experiments included presaturation of the water peak. The transmitter offset frequency (O1) was set to 4002.80 Hz to obtain optimal water suppression. The 90° pulse width was determined for every sample using the automatic pulse calculation feature in TopSpin. All pulse widths were between 13 and 16 μs. Water suppression was achieved by irradiation of the water peak during a recycle delay of 4.0s with a pulse power level of 55.92 dB.
One-dimensional zgpr 1H NMR spectra were acquired using two transients and two dummy scans, 65 K points per spectrum giving an acquisition time of 1.92 s, -0.01 Hz of exponential line broadening, and a recycle delay of 4 seconds. Once the zgpr spectrum was determined to be of acceptable quality, based on the line width (< 1 Hz) and line shape (resolved C13 satellites) of the TSP internal standard, the other two experiments were run. The first increment of the 1D NOESY experiment was collected using eight transients with four dummy scans, 65 K points per spectrum giving an acquisition time of 1.92 seconds, a mixing time of 10 ms, and apodized using a Gaussian line broadening parameter of 0.01, and a 4 seconds recycle delay. The CPMG experiment was collected in order to eliminate any broad peaks present in the spectrum. The CPMG experiment used 64 transients with four dummy scans, 65 K points per spectrum giving an acquisition time of 1.87 seconds, a T2 filter loop of 128 with an echo time of 1 ms, apodized using -0.01 Hz of exponential line broadening, and a 4 seconds recycle delay.
All NMR spectra were phased, baseline corrected, and corrected for chemical shift registration relative to TSP in TopSpin 2.1.1 (Bruker Biospin, Billerica, MA, USA).
Box and whisker plot analysis
Box and whisker plots were generated in excel using a template provided by Vertex42 LLC
Principal component analysis
The data were subjected to multivariate statistical analysis using AMIX software version 3.9.7 (Bruker Biospin, Billerica, MA, USA). All NMR spectra were normalized to total intensity prior to principal component analysis (PCA). NMR spectra were binned into 0.03 ppm-wide buckets, using simple rectangular bucketing, over the region of δ10.0 to 0.2 ppm. The region of δ 4.75 to 5.0 was excluded from the analysis to avoid effects of imperfect water suppression. Buckets with variances less than 5% were also excluded from PCA.
Unsupervised PCA was performed without consideration of group information (class III/IV; class V, FSGS). The algorithm employed to calculate the principal components (PC) is discussed by Rousseau et al. [33]. As is commonly done with metabolomic data, visualization of the data was accomplished by inspection of the PC scores plots and loadings plots.
Statistical significance analysis of NMR data
Statistical significance analysis was performed for the comparison of class III/IV and class V LN, as well as the comparison of class V LN and FSGS, using AMIX 3.9.7 (Bruker Biospin, Rheinstetten, Germany), as outlined by Goodpaster et al. [34]. A critical value of alpha of 0.05 was selected to ensure no greater than a 5% false positive rate. In order to correct for multiple simultaneous testing, a Bonferroni correction was applied to the critical value to ensure a constant family-wise false positive rate [34]. The Bonferroni corrected critical value was calculated by dividing alpha by the number of buckets used in the PCA, resulting in a stringent P value threshold for determination of statistically significant changes in resonance intensities between groups being compared. The number of buckets used for statistical significance analysis was determined by the number remaining after omitting buckets that contained less than 5% variance and after omitting buckets in the excluded regions. A change in bucket intensity between groups was determined to be statistically significant if its P value was less than the Bonferroni corrected critical value.
Mahalanobis distance and F-value calculations
Mahalanobis distance calculations and F-value calculations were performed in MatLab as described by Goodpaster et al. [35]. Critical F-values were calculated using a critical F-value calculator [36].
Identification of metabolites
Experimental NMR spectra obtained from study samples were compared with spectra of known metabolites using the ChenomX NMR Suite (ChenomX Inc., Edmonton, Alberta, Canada). The ChenomX database was used to filter for resonance frequencies at chemical shifts corresponding to those identified as outliers by visual comparison as well as in the loadings plot. Spectra present in the ChenomX database were examined to verify if the pattern of peaks matched those observed in the experimental data.
Results and discussion
Patients
A total of seven patients with proliferative LN without membranous features (class III/IV), seven patients with pure membranous LN (class V), and 10 disease controls with primary FSGS were included in the study.
NMR metabonomics data analysis
Due to slight variations in the pH of the urine samples, the NMR peaks of some metabolites experienced pH-dependent chemical shifts, making it difficult to identify variances for these peaks by PCA using standard rectangular bucketing. Therefore, all spectra were also visually inspected to validate NMR resonances that were potentially changing between groups, and then these peaks were locally aligned to enable reliable P score calculations.
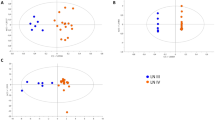
Visual inspection of the NMR spectra of class III/IV and class V LN patients led to the identification of one metabolite, citrate, that had an eight-fold higher urinary concentration (Table 2) (P score = 0.0477) in class III/IV LN patients (1.11 ± 0.97 mM) compared with class V patients (0.14 ± 0.15 mM). The concentration of citrate in the class III/IV group fell into the normal range in human urine [37] whereas the citrate levels in the class V group were at the lower limit of published values [37]. A receiver operator characteristic (ROC) analysis indicated the citrate had 100% specificity at 86% sensitivity and an overall 88% accuracy [see Additional data file 1] for distinguishing between class III/IV and class V LN patients. Urinary citrate levels were also compared with the SLEDAI-R, CI, and P/C ratio (Figure 1). The comparisons shown in Figure 1 further indicated that there was a strong correlation between disease class and citrate levels; however, no correlation was observed between class and SLEDAI, CI, or P/C ratio between class III/IV and class V LN patients. Complete summaries of measured patient indices, including others not presented in Figure 1, are presented in Tables S1 and S2 of the Supplementary Material.
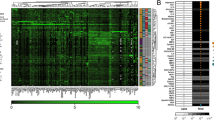
Visual comparison of the NMR spectra of urine samples of patients with class III/IV versus class V LN also revealed higher concentrations (> 10 fold) of taurine in class V LN (2.29 ± 2.47 mM) compared with class III/IV LN patients, which lacked taurine altogether (Table 2) (P score = 0.00141). Taurine is normally present urine at levels in the range from 50 to 750 μM [38]. Therefore, the complete absence of taurine in class III/IV patients indicated a renal pathology that was distinct from class V patients who appeared to have mostly normal levels of taurine, with the exception of one patient who had more than five times the normal amount of taurine in the urine (Table 2). Discrimination of the two groups (class III/IV versus class V) based on urinary taurine levels was confirmed by PCA by restricting the spectral analysis just to the region of the 1H NMR spectra that contained the taurine-specific triplet at δ 3.425 ppm (Figure 2). Inspection of the PC scores plot of these samples showed a significant separation of the class III/IV from the class V populations, primarily based on higher levels of taurine in the urine of patients with class V LN (Figure 2a). The magnitude of the cluster separation was quantified by calculating the Mahalanobis distance between the cluster centroids and the statistical significance of the cluster separation was evaluated by calculating the F-value and comparing it with the critical F-value (Figure 2a). The Mahalanobis distance of 2.282 between class III/IV and class V LN group centroids and the corresponding F-value of 8.353 (critical F-value 3.982) indicated a statistically significant separation of the two groups. The PC loadings plot corresponding to the PC scores plot shown in Figure 2a is shown in Figure 2b. The difference in the mean intensities of the bucket at δ 3.425 ppm, which corresponded to taurine, was found to be statistically significant with a P value of 0.00151.
Principal component analysis of urine samples from patients with class III/IV LN and class V LN. (a) Two-dimensional principal component analysis scores plot of urine samples from patients with class III/IV LN (green) and class V LN (black) for peaks in the region from δ 3.40 to 4.50 ppm calculated using the first two principal components. Each point in the scores plot represents the NMR spectrum of an individual patient projected onto the two-dimensional space defined by the first two principal components. The dashed lines encircling the points define the 95% confidence intervals for each group. The color-matched stars indicate the centroid of each group and the line connecting the stars represents the Mahalanobis distance between the group centroids. (b) The loadings plot corresponding to the scores plot shown in Figure 1a. The labeled bucket (point) corresponds to the triplet belonging to taurine in the 1H NMR spectra. The coordinates of each point indicate the PC loadings for that bucket, and represent how strongly that bucket is weighted in the eigenvector defining either the first or second principal component. The loadings plot points are heat map color-coded according to bucket P value: Black (> 1.25 × 10-2), Blue (1.25 × 10-2-10-5). The Bonferroni corrected α-value was 0.0125.
ROC curve analysis indicated that taurine was a perfect predictor for discrimination between class III/IV and class V LN patients with 100% specificity, 100% sensitivity and 100% accuracy [see Additional data file 1]. Urinary taurine levels were also compared to SLEDAI, CI, and P/C ratio (Figure 1). As indicated above, the data failed to reveal any correlation between LN class and SLEDAI, CI, or urinary P/C ratios; however, a strong correlation between class III/IV and class V LN and was observed with a complete absence of urinary taurine in class III/IV patients compared with mostly normal levels of taurine in class V patients.
Unsupervised PCA was also carried out on normalized 1H NMR spectra obtained from urine samples from Class V LN and FSGS patients. Inspection of the PC scores plot showed separation of the class V from the FSGS group based on the levels of hippurate, which were in the normal range found in human urine [37] in the class V LN group (1.22 ± 0.62 mM), compared with the FSGS group, which completely lacked hippurate (Figure 3a; Table 2). The Mahalanobis distance between the cluster centroids of the class V and FSGS groups was 1.781, with a corresponding F-value of 6.096 (critical F-value 3.739) indicating a statistically significant separation of these groups. NMR spectra of patients with class V LN or FSGS were visually compared to validate the differences in hippurate concentrations indicated by PCA. Figure 4 shows a region of the NMR spectrum that contains two triplets unique to hippurate located at δ 7.64 and δ 7.55 ppm confirming that hippurate was present in the class V group but completely absent in the FSGS group. The differences in the mean bucket intensities at δ 7.55 ppm and δ 7.64 between the two groups (> 10-fold change) were found to be significant: δ 7.55 P value = 0.000171, and δ 7.64 P value = 0.000379, respectively. ROC curve analysis indicated that hippurate was a perfect discriminator for distinguishing between class V LN and FSGS patients with 100% specificity, 100% sensitivity, and 100% accuracy [see Additional data file 1]. Interestingly, the complete lack of hippurate in the FSGS group indicated a unique pathology in FSGS compared with LN that leads to depletion of the normal urinary levels of hippurate.
Principal component analysis of urine samples from class V LN patients and focal segmental glomerulosclerosis patients. (a) Two-dimensional principal component analysis scores plot of urine samples from patients with class V LN patients (black) and focal segmental glomerulosclerosis patients (red) using the first two principal components. The dashed lines encircling the points define the 95% confidence intervals for each group. The color-matched stars indicate the centroid of each group and the line connecting the stars represents the Mahalanobis distance between the group centroids. (b) The loadings plot corresponding to the scores plot in Figure 2a. The buckets shown are in the region from δ 0.02 to 10.0 ppm. The loadings plot is heat map color-coded according to bucket P values: Black (> 1.730 × 10-4), Blue 1.730 × 10-4-10-5). The Bonferroni corrected α-value was 1.730 × 10-4.
Kidney biopsies are currently required to distinguish between different classes of LN, and between LN and other glomerular disorders, based on characteristic histological features. Obtaining kidney biopsies is invasive, and repetitive performance to guide day-to-day medical decisions is not practical. Although diagnostic, kidney biopsies are not suited to pinpoint altered metabolic processes or biological pathways involved in LN, which if detected, could lead to the identification of novel therapeutic targets. As the kidneys filter and reabsorb metabolites to maintain a metabolic equilibrium, existence of renal pathologies can impair the filtration of small metabolites through the glomerulus and their subsequent re-absorption in the renal tubules leading to changes in metabolic profiles [39]. Using NMR-spectroscopy, we found the metabolites taurine, citrate, and hippurate differentially excreted in the urine of patients with proliferative LN, membranous LN, and FSGS.
Comparison of the metabolic profiles of class III/IV LN versus class V LN patients indicated that class III/IV patients had normal urinary citrate levels but low urinary taurine levels whereas class V LN patients exhibited low urinary citrate levels but elevated urinary taurine levels. Based on previous reports, citrate and taurine are both measures of tubular cell function [40]. A possible explanation for the reduced excretion of urinary citrate in the class V LN group could be the presence of metabolic acidosis, which is known to cause decreased urinary excretion of citrate in humans [41]. It is believed that patients experiencing low urinary citrate output may have renal tubular cells that are more acidotic than in the healthy normal populations [42]. The proximal tubules are responsible for the regulation of re-absorption and excretion of citrate [43, 44]. The body's response to metabolic acidosis includes an increase of sodium/citrate co-transporter activity in the kidney, which causes increased citrate transport across the apical membrane into the tubule lumen [44]. Up-regulation of the co-transporter activity results in the increased re-absorption of citrate and reduced excretion of the metabolite into the urine. The cytosolic citrate metabolism also plays a key role in regulating the amount of citrate excreted into the urine. During metabolic acidosis, alterations in the enzyme ATP citrate lyase also results in decreased urinary citrate excretion [44].
Class III/IV LN patients had a striking absence of urinary taurine. Although the pathology leading to a complete absence of urinary taurine is not obvious, the body's store of taurine is known to be regulated by the kidneys and taurine is known to act as an anti-oxidant in a variety of in vitro and in vivo systems, and is used to treat renal dysfunction [45]. Therefore, it is possible that under the conditions of the most severe LN in class III/IV patients the kidneys utilize all available taurine in an attempt to manage or repair the kidney pathology.
Although the majority of patients with class V LN have normal levels of taurine, one patient had more than five times the normal amount of taurine. A possible explanation for the elevated level of taurine in the urine of this exceptional class V LN patient may be a consequence of inadequate re-uptake of taurine into the cells [46]. Taurine is excreted through both bile and urine, but its total body pool is primarily controlled by the kidneys via the renal tubules [47]. Previous studies suggest that tubular dysfunction is a risk factor of taurine deficiency [48]. Patients in renal failure often have low muscle and plasma concentrations of taurine. Although it has been suggested in the past that this was due to reduced taurine synthesis [48], our results suggest that low taurine levels are actually the result of increased urinary taurine excretion.
Increased urinary taurine may also be a result of changes in cysteine metabolism. Hypercysteinemia is associated with alterations of the sulfur metabolism and/or sulfate transport [49]. Taurine is known to play a critical role in these processes [49], and patients with class V LN may be unable to adequately cope with oxidative stress and the elimination of free radicals. Interestingly, based on animal studies, the acquisition of age-related renal fibrosis can be decreased by taurine supplementation [50], and taurine also has anti-hypertensive effects [51].
Histological scoring and quantification of proteinuria are key methods used to survey disease activity and severity in patients with LN [52, 53]. Pirani et al. created a scoring system that is semi-quantitative [54], which was later adapted by Austin et al. [20] This system was developed to calculate the activity of LN (SLEDAI) by assessing six histological factors focusing on the severity of active lesions in the glomeruli, and the chronicity of the disease by evaluating four histological parameters focusing on the reversibility of LN [53]. Wallace et al. provides a table (Table 55-5 in the original article) that outlines the scoring strategies for both the AI and CI [55]. The degree of proteinuria is determined by measuring the P/C ratio in a 24-hour urine collection. This ratio has previously proven to be a reliable predictor of proteinuria in a study of LN patients [56]. The changes in citrate and taurine levels in the patients included in this study were plotted against the SLEDAI, CI, and P/C ratios in order to determine whether a correlation existed between any of these conventionally measured indices and biopsy-determined LN class (Figure 1). The data showed that no clear correlation existed between these conventional indices or P/C ratios and the LN class; however, our data indicated strong correlations between citrate and taurine levels and LN class. The lack of correlation between renal activity and chronicity with disease class is not surprising given that other studies have shown that when applying these indices to all World Health Organization classes of LN, rather than just diffuse proliferative LN, these indices lack an association with long-term prognosis [57, 58]. The inability to relate P/C ratio to disease class is also expected because differing degrees of proteinuria are present throughout the patient population. Visual comparison of the above indices as they related to LN class further demonstrated a strong need for continued development of reliable biomarkers that allow for LN class differentiation, as was seen in the changes in the metabolites identified in this study.
Our pilot study also identified one urinary metabolite, hippurate, whose levels differentiated class V LN and FSGS patients. Specifically, class V LN patients had normal levels of urinary hippurate whereas FSGS patients completely lacked hippurate. Although the pathological link associated with a complete lack of urinary hippurate is not evident, the complete lack of urinary hippurate in FSGS patients is striking. A possible pathological cause could be related to having a distinct gut microbial biota, which has been linked to depleted excretion of hippurate in patients with Crohn's disease [59].
Conclusions
Currently, it is difficult to distinguish between class III/IV and class V LN, and FSGS using conventionally measured indices. Diagnoses require invasive and time-consuming procedures involving biopsies and histological analyses, which make monitoring of real-time changes in the disease pathology impossible using current technologies. Ideally, one would like to develop a non-invasive and rapid biomarker-based methodology to distinguish between class III/IV and class V LN, and FSGS. Here we report putative urinary biomarkers for this purpose obtained from a pilot study. Using NMR spectroscopy, we found that the metabolites taurine, citrate, and hippurate were differentially excreted in the urine of patients with proliferative LN, membranous LN, and FSGS. Not only do these metabolites represent potential biomarkers for distinguishing classes of LN and FSGS, but consideration of the metabolic pathways involving these metabolites should lead to a better understanding of the pathology of the respective disease states. Although the small size of this pilot study limits its statistical power, it is in the realm of similar pilot studies for other diseases [60]. Nonetheless, our study has generated several hypotheses regarding the etiology of LN and FSGS and further validation of our findings is planned using an independent cohort of patients.
Abbreviations
- AI:
-
activity index
- CI:
-
chronicity index
- FSGS:
-
focal segmental glomerulosclerosis
- GFR:
-
glomerular filtration rate
- ISN/RPS:
-
International Society of Nephrology/Renal Pathology Society
- LN:
-
lupus nephritis
- NMR:
-
nuclear magnetic resonance
- NOESY:
-
nuclear Overhauser effect spectroscopy
- P/C ratio:
-
protein to creatinine ratio
- PC:
-
principal component
- PCA:
-
principal component analysis
- ROC:
-
receiver operator curve
- SLE:
-
systemic lupus erythematosus
- SLEDAI-R:
-
Systemic Lupus Erythematosus Disease Activity Index-Range
- SDI-R:
-
Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index-Range
- TSP:
-
trimethylsilyl propionate.
References
Hochberg MC: Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40: 1725-
Chambers SA, Allen E, Rahman A, Isenberg D: Damage and mortality in a group of British patients with systemic lupus erythematosus followed up for over 10 years. Rheumatology (Oxford). 2009, 48: 673-675. 10.1093/rheumatology/kep062.
Austin HA, Illei GG: Membranous lupus nephritis. Lupus. 2005, 14: 65-71. 10.1191/0961203305lu2062oa.
Navaneethan SD, Viswanathan G, Strippoli GF: Treatment options for proliferative lupus nephritis: an update of clinical trial evidence. Drugs. 2008, 68: 2095-2104. 10.2165/00003495-200868150-00002.
Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M: The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004, 15: 241-250. 10.1097/01.ASN.0000108969.21691.5D.
Rivera TL, Belmont HM, Malani S, Latorre M, Benton L, Weisstuch J, Barisoni L, Tseng CE, Izmirly PM, Buyon JP, Askanase AD: Current therapies for lupus nephritis in an ethnically heterogeneous cohort. J Rheumatol. 2009, 36: 298-305.
Mok CC: Membranous nephropathy in systemic lupus erythematosus: a therapeutic enigma. Nat Rev Nephrol. 2009, 5: 212-220. 10.1038/nrneph.2009.14.
Mundel P, Shankland SJ: Podocyte biology and response to injury. J Am Soc Nephrol. 2002, 13: 3005-3015. 10.1097/01.ASN.0000039661.06947.FD.
Das L, Brunner HI: Biomarkers for renal disease in childhood. Curr Rheumatol Rep. 2009, 11: 218-225. 10.1007/s11926-009-0030-4.
Rovin BH, Birmingham DJ, Nagaraja HN, Yu CY, Hebert LA: Biomarker discovery in human SLE nephritis. Bull NYU Hosp Jt Dis. 2007, 65: 187-193.
Boudonck KJ, Rose DJ, Karoly ED, Lee DP, Lawton KA, Lapinskas PJ: Metabolomics for early detection of drug-induced kidney injury: review of the current status. Bioanalysis. 2009, 1: 1645-1663. 10.4155/bio.09.142.
Kraly JR, Holcomb RE, Guan Q, Henry CS: Review: Microfluidic applications in metabolomics and metabolic profiling. Anal Chim Acta. 2009, 653: 23-35. 10.1016/j.aca.2009.08.037.
Nicholson JK, Wilson ID: High resolution proton magnetic resonance spectroscopy of biological fluids. Prog Nucl Magn Reson Spectrosc. 1989, 21: 449-501. 10.1016/0079-6565(89)80008-1.
Lindon JC, Holmes E, Nicholson JK: Pattern recognition methods and applications in biomedical magnetic resonance. Prog Nucl Magn Reson Spectrosc. 2001, 39: 1-40. 10.1016/S0079-6565(00)00036-4.
Lindon JC, Nicholson JK, Holmes E, Everett JR: Metabonomics: Metabolic processes studied by NMR spectroscopy of biofluids. Concepts Magn Reson. 2000, 12: 289-320. 10.1002/1099-0534(2000)12:5<289::AID-CMR3>3.0.CO;2-W.
Park EM, Lee E, Joo HJ, Oh E, Lee J, Lee JS: Inter- and intra-individual variations of urinary endogenous metabolites in healthy male college students using H-1 NMR spectroscopy. Clin Chem Lab Med. 2009, 47: 188-194. 10.1515/CCLM.2009.040.
Nicholson JK, Lindon JC, Holmes E: 'Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999, 29: 1181-1189. 10.1080/004982599238047.
Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976, 58: 259-263.
Kasitanon N, Fine DM, Haas M, Magder LS, Petri M: Estimating renal function in lupus nephritis: comparison of the Modification of Diet in Renal Disease and Cockcroft Gault equations. Lupus. 2007, 16: 887-895. 10.1177/0961203307084167.
Austin HA, Muenz LR, Joyce KM, Antonovych TT, Balow JE: Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 1984, 25: 689-695. 10.1038/ki.1984.75.
Zappitelli M, Duffy CM, Bernard C, Gupta IR: Evaluation of activity, chronicity and tubulointerstitial indices for childhood lupus nephritis. Pediatr Nephrol. 2008, 23: 83-91.
Hiramatsu N, Kuroiwa T, Ikeuchi H, Maeshima A, Kaneko Y, Hiromura K, Ueki K, Nojima Y: Revised classification of lupus nephritis is valuable in predicting renal outcome with an indication of the proportion of glomeruli affected by chronic lesions. Rheumatology (Oxford). 2008, 47: 702-707. 10.1093/rheumatology/ken019.
Cortés-Hernández J, Ordi-Ros J, Labrador M, Segarra A, Tovar JL, Balada E, Vilardell-Tarres M: Predictors of poor renal outcome in patients with lupus nephritis treated with combined pulses of cyclophosphamide and methylprednisolone. Lupus. 2003, 12: 287-296. 10.1191/0961203303lu340oa.
Marks SD, Sebire NJ, Pilkington C, Tullus K: Clinicopathological correlations of paediatric lupus nephritis. Pediatr Nephrol. 2007, 22: 77-83. 10.1007/s00467-006-0296-y.
Zappitelli M, Duffy C, Bernard C, Scuccimarri R, Watanabe Duffy K, Kagan R, Gupta IR: Clinicopathological study of the WHO classification in childhood lupus nephritis. Pediatr Nephrol. 2004, 19: 503-510. 10.1007/s00467-004-1419-y.
Lee BS, Cho HY, Kim EJ, Kang HG, Ha IS, Cheong HI, Kim JG, Lee HS, Choi Y: Clinical outcomes of childhood lupus nephritis: a single center's experience. Pediatr Nephrol. 2007, 22: 222-231.
Demircin G, Oner A, Erdoğan O, Delibaş A, Baysun S, Bülbül M, Bek K, Oksal A: Long-term efficacy and safety of quadruple therapy in childhood diffuse proliferative lupus nephritis. Ren Fail. 2008, 30: 603-609. 10.1080/08860220802132171.
Vachvanichsanong P, Dissaneewate P, McNeil E: Diffuse proliferative glomerulonephritis does not determine the worst outcome in childhood-onset lupus nephritis: a 23-year experience in a single centre. Nephrol Dial Transplant. 2009, 24: 2729-2734. 10.1093/ndt/gfp173.
Hagelberg S, Lee Y, Bargman J, Mah G, Schneider R, Laskin C, Eddy A, Gladman D, Urowitz M, Hebert D, Silverman E: Longterm followup of childhood lupus nephritis. J Rheumatol. 2002, 29: 2635-2642.
Hersh AO, von Scheven E, Yazdany J, Panopalis P, Trupin L, Julian L, Katz P, Criswell LA, Yelin E: Differences in long-term disease activity and treatment of adult patients with childhood- and adult-onset systemic lupus erythematosus. Arthritis Rheum. 2009, 61: 13-20.
Ibañez D, Gladman DD, Urowitz MB: Adjusted mean Systemic Lupus Erythematosus Disease Activity Index-2K is a predictor of outcome in SLE. J Rheumatol. 2005, 32: 824-827.
Gladman DD, Goldsmith CH, Urowitz MB, Bacon P, Fortin P, Ginzler E, Gordon C, Hanly JG, Isenberg DA, Petri M, Nived O, Snaith M, Sturfelt G: The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index for Systemic Lupus Erythematosus International Comparison. J Rheumatol. 2000, 27: 373-376.
Rousseau R, Govaerts B, Verleysen M, Boulanger B: Comparison of some chemometric tools for metabonomics biomarker identification. Selected papers presented at the Chemometrics Congress "CHIMIOMETRIE 2006" Paris, France, 30 November - 1 December. 2006, 91: 54-66.
Goodpaster AM, Romick-Rosendale LE, Kennedy MA: Statistical significance analysis of nuclear magnetic resonance-based metabonomics data. Anal Biochem. 2010, 401: 134-143. 10.1016/j.ab.2010.02.005.
Goodpaster AM, Kennedy MA: Quantification and statistical significance analysis of group separation in NMR-based metabonomics studies. Chemometr Intell Lab. 2011, 109: 162-170. 10.1016/j.chemolab.2011.08.009.
Statistics Calculator (version 3.0). [http://www.danielsoper.com/statcalc3/calc.aspx?id=4]
Saude E, Adamko D, Rowe B, Marrie T, Sykes B: Variation of metabolites in normal human urine. Metabolomics. 2007, 3: 439-451. 10.1007/s11306-007-0091-1.
Laidlaw SA, Shultz TD, Cecchino JT, Kopple JD: Plasma and urine taurine levels in vegans. Am J Clin Nutr. 1988, 47: 660-663.
Quaggin SE, Kreidberg JA: Development of the renal glomerulus: good neighbors and good fences. Development. 2008, 135: 609-620. 10.1242/dev.001081.
Christians U, Schmitz V, Schöning W, Bendrick-Peart J, Klawitter J, Haschke M: Toxicodynamic therapeutic drug monitoring of immunosuppressants: promises, reality, and challenges. Ther Drug Monit. 2008, 30: 151-158. 10.1097/FTD.0b013e31816b9063.
Clarke E, Evans BM, MacIntyre I, Milne MD: Acidosis in experimental electrolyte depletion. Clin Sci. 1955, 14: 421-
Morrissey JF, Ochoa M, Lotspeich WD, Waterhouse C: Citrate Excretion in Renal Tubular Acidosis. Ann Intern Med. 1962, 56: 697-698.
Unwin RJ, Capasso G, Shirley DG: An overview of divalent cation and citrate handling by the kidney. Nephron Physiol. 2004, 98: 15-20. 10.1159/000080259.
Melnick JZ, Preisig PA, Alpern RJ, Baum M: Renal citrate metabolism and urinary citrate excretion in the infant rat. Kidney Int. 2000, 57: 891-897. 10.1046/j.1523-1755.2000.057003891.x.
Trachtman H, Sturman JA: Taurine: A therapeutic agent in experimental kidney disease. Amino Acids. 1996, 11: 1-13. 10.1007/BF00805717.
Sturman JA, Hepner GW, Hofmann AF, Thomas PJ: Metabolism of [35S]taurine in man. J Nutr. 1975, 105: 1206-1214.
Paauw JD, Davis AT: Taurine concentrations in serum of critically injured patients and age- and sex- matched healthy control subjects. Am J Clin Nutr. 1990, 52: 657-660.
Lourenco R, Camilo ME: Taurine: a conditionally essential amino acid in humans? An overview in health and disease. Nutr Hosp. 2002, 17: 262-270.
Yap IK, Angley M, Veselkov KA, Holmes E, Lindon JC, Nicholson JK: Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. J Proteome Res. 2010, 9: 2996-3004. 10.1021/pr901188e.
Cruz CI, Ruiz-Torres P, del Moral RG, Rodriguez-Puyol M, Rodriguez-Puyol D: Age-related progressive renal fibrosis in rats and its prevention with ACE inhibitors and taurine. Am J Physiol Renal Physiol. 2000, 278: F122-129.
Yamori Y, Murakami S, Ikeda K, Nara Y: Fish and lifestyle-related disease prevention: experimental and epidemiological evidence for anti-atherogenic potential of taurine. Clin Exp Pharmacol Physiol. 2004, 31 (Suppl 2): S20-23.
Austin Iii HA, Muenz LR, Joyce KM, Antonovych TA, Kullick ME, Klippel JH, Decker JL, Balow JE: Prognostic factors in lupus nephritis: contribution of renal histologic data. Am J Med. 1983, 75: 382-391. 10.1016/0002-9343(83)90338-8.
Wernick RM, Smith DL, Houghton DC, Phillips DS, Booth JL, Runckel DN, Johnson DS, Brown KK, Gaboury CL: Reliability of histologic scoring for lupus nephritis: a community-based evaluation. Ann Intern Med. 1993, 119: 805-811.
Pirani CL, Pollack VE, Schwartz FD: The reproducibility of semiquantitative analyses of renal histology. Nephron. 1964, 1: 230-237. 10.1159/000179336.
Wallace DJ, Hahn B, Dubois EL: Dubois' lupus erythematosus. 2007, Philadelphia: Lippincott Williams & Wilkin, 7
Christopher-Stine L, Petri M, Astor BC, Fine D: Urine protein-to-creatinine ratio is a reliable measure of proteinuria in lupus nephritis. J Rheumatol. 2004, 31: 1557-1559.
Appel GB, Cohen DJ, Pirani CL, Meltzer JI, Estes D: Long-term follow-up of patients with lupus nephritis: A study based on the classification of the World Health Organization. Am J Med. 1987, 83: 877-885. 10.1016/0002-9343(87)90645-0.
Schwartz MM, Bernstein J, Hill GS, Holley K, Phillips EA: Predictive value of renal pathology in diffuse proliferative lupus glomerulonephritis. Lupus Nephritis Collaborative Study Group. Kidney Int. 1989, 36: 891-896. 10.1038/ki.1989.276.
Williams H, Cox IJ, Walker D, Cobbold J, Taylor-Robinson S, Marshall S, Orchard T: Differences in gut microbial metabolism are responsible for reduced hippurate synthesis in Crohn's disease. BMC Gastroenterology. 2010, 10: 108-10.1186/1471-230X-10-108.
Barba I, de León G, Martín E, Cuevas A, Aguade S, Candell-Riera J, Barrabés JA, Garcia-Dorado D: Nuclear magnetic resonance-based metabolomics predicts exercise-induced ischemia in patients with suspected coronary artery disease. Magn Reson Med. 2008, 60: 27-32. 10.1002/mrm.21632.
Acknowledgements
We would like to acknowledge Dr. David Witte for the interpretation of the kidney biopsies and Drs. Lena Das and Michiko Suzuki for their participation in sample selection. We appreciate the support of the NIAMS Tissue Bank, directed by Dr. Susan Thompson. We would like to thank Mr. Pendl, Mrs. Tsoras, and Mrs. Lurick for the collection and management of the urine samples. This work was supported in part by the Department of Defense (W81XWH-07-1-0322). Dr. Brunner is supported by NIH/NIAMS U01AR059509 and P60AR47784. Sample storage was supported by P30AR047363. Drs. Petri and Kiani are supported by Hopkins Lupus Cohort (NIH AR 43727) and by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR). This work was supported in part by the Department of Defense (W81XWH-07-1-0322). Dr. Kennedy is supported by a grant from the NIH/NCI (1R15CA152985). Dr. Kennedy would like to acknowledge support of Miami University and the Ohio Board of Regents for funding to establish the Ohio Eminent Scholar Laboratory where the metabonomics work was performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Drs. Prasad Devarajan and Brunner are holding a patent on the use of some of the renal biomarkers evaluated for this study.
Authors' contributions
HIB was involved in the study design, data collection, and sample management as well as the interpretation of the data and the development of the manuscript. MRB, RM, SN, MP, and AK were all involved in the data collection, and sample collection and management and the development of the manuscript. PD was responsible for the study design, the interpretation of the data, and the development of the manuscript. LRR was responsible for NMR sample preparation, NMR data collection and analysis, investigation of biological interpretation of data and development of the manuscript. MAK was involved in NMR data analysis, interpretation of data and development of the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
13075_2011_3429_MOESM1_ESM.DOC
Additional file 1: There are two additional tables included in this file, including Table S1, which provides a summary of classifications from histological analyses of kidney biopsy samples, and Table S2, which is a summary of laboratory test scores for LN patients. The file also contains Figure S1, which includes the receiver operator characteristic curves for citrate, hippurate and taurine. (DOC 197 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Romick-Rosendale, L.E., Brunner, H.I., Bennett, M.R. et al. Identification of urinary metabolites that distinguish membranous lupus nephritis from proliferative lupus nephritis and focal segmental glomerulosclerosis. Arthritis Res Ther 13, R199 (2011). https://doi.org/10.1186/ar3530
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/ar3530