Abstract
The presence or absence of antibodies to citrullinated peptides/proteins (ACPA) is an important parameter that helps a clinician set a diagnosis of early rheumatoid arthritis and, hence, initiate treatment. There are several commercial tests available to measure ACPA levels, although it can be difficult to decide what the best test for a given clinical question is. We analyzed literature data in which the diagnostic and other properties of various ACPA tests are compared. The results show that for diagnostic purposes the CCP2 test has the highest specificity, the highest sensitivity in stratified studies and the highest positive predictive value. For the prediction of future joint destruction the CCP2, MCV, and CCP3 tests may be used. The ability to predict the likelihood of not achieving sustained disease-modifying antirheumatic drug-free remission was highest for the CCP2 test. Finally, the levels of anti-CCP2 and anti-CCP3 (and possibly anti-mutated citrullinated vimentin) in rheumatoid arthritis patients are not significantly influenced by TNFα blocking agents.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a common autoimmune disease characterized by chronic inflammation of the joints, which can ultimately lead to cartilage and bone destruction. In the past decade it has become apparent that citrullinated proteins/peptides, and in particular autoantibodies directed to them (anti-citrullinated protein antibodies (ACPA)), are likely to be involved in the development of this disease in at least 70% of the patients (reviewed in [1]). In the clinical setting, ACPA have mostly been detected using the anti-cyclic citrullinated peptide (anti-CCP) test, although more recently other tests using various citrullinated proteins have also been employed.
Recently it became clear that RA patients can be classified into two major subsets; namely, those who have ACPA (anti-CCP(+)) and those who do not (anti-CCP(-)) [2]. Whilst in the early phase of the disease these two groups of patients show a very similar clinical presentation, the picture changes considerably as the disease develops further. The presence of ACPA at early diagnosis predicts more pronounced radiographic progression, as demonstrated by many studies showing a strong association between anti-CCP positivity and the development of bone erosions. Importantly, environmental risk factors (for exam ple, tobacco smoking) differ to a large extent between these two populations [3], and the risk of developing ischemic heart disease is clearly higher in anti-CCP(+) patients compared with anti- CCP(-) RA patients [4]. Furthermore, treatment response to, for example, synthetic disease-modifying antirheumatic drugs such as methotrexate may differ between these groups of patients [5]. It is therefore important for a clinician to be able to accurately separate anti-CCP(+) patients from anti-CCP(-) patients. During such a decision-making process it is important that both clinicians and laboratory specialists are fully aware of the advantages and disadvantages of the various ACPA tests that are commercially available.
The present review intends to critically review the literature on comparisons between the most frequently applied commercial tests in terms of specificity and sensitivity of ACPA detection.
Anti-citrullinated protein antibodies and cyclic citrullinated peptide
Several lines of evidence indicate that the ACPA response in RA patients is polyclonal and heterogeneous [6]. Antibodies to a variety of citrullinated epitopes on different proteins can thus be detected and their production is likely to vary between individual patients. The commercial ACPA tests are all aimed at detecting most, if not all, ACPA epitope reactivities found in RA patients. The majority of published studies in which the presence of ACPA in RA patients was investigated have used the second-generation CCP test (termed the CCP2 test). Using this CCP2 test, about 75% of RA patients with a long-term established diagnosis and 61% of patients with established early RA were anti-CCP(+) (Table 1).
Anti-CCP antibodies of these patients can be eluted from the CCP2 ELISA plate (by low pH or high salt) and the eluate can subsequently be used to stain western blots containing different citrullinated proteins, such as fibrinogen, histones or vimentin. The eluted antibodies react with all of these citrullinated proteins, indicating broad cross-reactivity between anti-CCP and these various antigens (R Toes, personal communication). These data have been complemented by studies of synovial exosomes from RA patients, which were shown to contain ACPA as well as a number of citrullinated constituents - for example, citrullinated fibrinogen peptides and citrullinated Spα (a CD5 antigen-like protein) [7]. Moreover, the number of anti-CCP(-) sera that show reactivity with other citrullinated antigens is very small [8, 9]. Taken together, these data indicate that the vast majority of ACPA can be detected by the CCP2 test.
Anti-CCP(+) RA and anti-CCP(-) RA
Early diagnosis of RA coupled with rational use of disease-modifying antirheumatic drugs has been shown to have a favorable effect on the course of the disease. Early and accurate diagnosis has therefore become increasingly important. For several reasons, the presence of anti-CCP antibodies is a great help to clinicians in deciding which patient needs early treatment.
First, anti-CCP antibodies are very specific for RA, and they are produced at significant levels very early in disease. The specificity of anti-CCP antibodies for the diagnosis of RA is high, as shown in Table 1. In addition, it is known that anti-CCP antibodies can be present many years before the first visit to the clinic (up to 18 years) [10–12].
Second, studies of early arthritis cohorts have shown that a large number of these early arthritis patients cannot be accurately diagnosed at their first visit, and hence are often referred as undifferentiated arthritis patients. If patients are found to be anti-CCP(+) when referred to the clinician, however, more than 90% develop RA within 3 years - in contrast to only 30% of the anti- CCP(-) patients. The presence of anti-CCP antibodies in undifferentiated arthritis therefore accurately predicts development of RA [13, 14].
Third, the presence of anti-CCP antibodies at the first visit to the clinician predicts radiographic progression, as demonstrated by many studies that have shown a strong association of anti-CCP positivity with the development of bone erosions [1, 15, 16]. In the past, IgM rheumatoid factor (RF) positivity was assumed to predict radiographic progression, but a recent report clearly indicates that the radiographic progression seen is actually associated with ACPA(+)/RF(+) and ACPA(+)/RF(-) RA, but not with ACPA(-)/RF(+) and ACPA(-)/RF(-) RA [17]. The conclusion therefore seems to be that the presence of ACPA as such is associated with an erosive course and the presence of IgM RF is just a co-expressed autoantibody, as has been known for a long time.
Fourth, more germinal centers in synovial tissue infiltrates are found in anti-CCP(+) RA patients [18]. It is known that germinal centers contribute to RA pathogenesis by supporting autoantibody production [19]. In the same report, distinct synovial features such as increased fibrosis in the synovial tissue and a thicker synovial lining layer were found in anti-CCP(-) RA patients [18].
In conclusion, although at baseline the clinical features of both RA subsets are very similar, anti-CCP(+) RA is more strongly associated with poor outcome than anti- CCP(-) RA (reviewed in [20]).
This conclusion may also have important implications for treatment. Synthetic disease-modifying antirheumatic drugs, such as methotrexate, are often used in treating RA, frequently in combination with TNFα blockers to enhance the treatment response. In a large Dutch study - the so-called PROMPT study (methotrexate versus placebo treatment) - Van Dongen and coworkers found that anti-CCP(+) patients responded well to methotrexate treatment, while a parallel anti-CCP(-) patient group did not [5]. In a subsequent study it was shown that methotrexate treatment resulted in a more favorable response in patients with a low or intermediate pretreatment level of ACPA [21]. These data not only suggest that the effectiveness of a drug can be different in anti-CCP(+) as compared with anti-CCP(-) arthritis, but also that very early treatment, even in patients not yet fulfilling American College of Rheumatology (ACR) criteria for RA, can be beneficial if used in a selective way [20].
Cyclic citrullinated peptide and other anticitrullinated protein antibody tests
In the past 3 years about 25 articles have appeared in which performances of different ACPA tests were compared. Only nine of these studies, however, followed the essential principle that any such comparisons can only be made when the test results are properly stratified. Stratification means that sensitivity values have to be calculated at a predefined specificity (mostly 98% or more) using the same cohort of RA patients and disease control sera. The chosen specificity should be as high as possible without compromising essential sensitivity, and thus the overall diagnostic efficiency. Another important point is the cohort of patients selected for such studies.
The main advantage of ACPA lies in their proven ability to predict the development of RA and their potential use as a criterion for RA even at baseline [22]. The ideal cohort to evaluate the clinical value of ACPA tests is therefore the mixed population of patients visiting an early arthritis clinic where some patients eventually will develop RA [6]. The use of a cohort of established RA patients with longstanding disease is clearly less useful. In the studies reviewed in the present article, all sorts of RA patient cohorts and control groups have been used, and this, at least in part, explains some of the differences in sensitivity and specificity of ACPA tests between different studies.
The tests
It is primarily the antigen (substrate) that decides how specific or sensitive a test will be. In 2002 the CCP2 test was launched, and this test is still the golden standard and most frequently used test in clinical practice. Table 1 presents the accumulated data from 154 publications using the CCP2 test.
There are at least six tests available using the CCP2 peptides as the antigen (supplied by Axis-Shield, Euro- Diagnostica, Euroimmun, Inova, Phadia, and Abbott). Despite using the same set of CCP2 peptides, these assays tend to show small differences in their diagnostic profiles [23, 24]. The main reason for these differences is that although the antigen is the same, the solid support materials might be different - and the added variables (conjugate, buffers, incubation time, and so forth) are also different, and these may also contribute to the small differences reported.
Aside from the CCP2 test, several other ACPA tests using different substrates have more recently been made commercially available. Some of these newer tests have rarely been used in published studies and are therefore not included in our calculations.
Assays that have been used in published data include a test based on in vitro citrullinated mutated human vimentin as antigen (MCV; Orgentec, Mainz, Germany), the Inova CCP3 test (cyclic citrullinated peptides) and its variant Inova CCP3.1 (Inova, San Diego, CA, USA), the Genesis citrullinated recombinant rat filaggrin (cFil; Genesis, Littleport, UK), the Aesku citrullinated IgG peptide (cIgG; Aesku, Wendelsheim, Germany) and the Astra citrullinated Epstein-Barr virus nuclear antigenderived peptide (Astra, Hamburg, Germany). In publications in which (some of) these tests are compared, the RF-IgM test is also often included. The RF tests are mostly used without a clinically useful cut-off point based on predefined specificity, however, and thus true comparisons of results obtained with this test in different studies are not possible.
Diagnostic performance of anti-citrullinated protein antibody tests
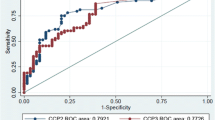
The data extracted from recent literature and tabulated in Tables 2 and 3 show that the CCP2 test still performs best when compared with other ACPA tests. Comparison with the classical IgM-RF test confirms previous reports that the CCP2 test has a superior specificity and, in stratified studies, a much higher sensitivity (Table 2). Recent data from Van der Linden and coworkers [17] - who showed that the rate of joint destruction in RA was not affected by the presence or absence of IgM RF, but rather by the presence or absence of ACPA - corroborate the idea that ACPA positivity should be included as a criterion for the diagnosis of RA in clinical studies [25]. These authors also advocated the inclusion of ACPA into any revision of the current ACR criteria for RA. Interestingly, the European League against Rheumatism has already recommended that the measurement of anti- CCP should be considered in all new cases of RA [26], and at the latest ACR meeting in Philadelphia an ACR/European League against Rheumatism panel of specialists included ACPA testing in the New Rheumatoid Arthritis Criteria.
Several studies have addressed the diagnostic performance of the MCV assay. In stratified studies this test shows a lower sensitivity (see Table 2), and in nonstratified studies a lower specificity, than the CCP2 test [27–30]. A similar conclusion was reached in a recent review on the diagnostic and prognostic properties of the MCV assay [31]. There are a few reports indicating that anti-MCV is present in a significant number of anti-CCP(-) sera [21, 32–34], and this subgroup of patients appears to have a higher rate of radiographic destruction than seronegative patients. Anti-MCV positivity therefore seems to indicate poor radiographic prognosis in a larger group of RA patients than anti-CCP positivity does [34]. Data from the study of Van der Linden and collaborators, however, indicate that the presence of anti-MCV antibody with negative anti-CCP does not strongly affect the level of joint damage in RA [17]. It is clear that further evaluation of the MCV antibody in clinically wellcharacterized cohorts is needed. It would also be useful to introduce a control mutated vimentin antigen (not citrullinated) in order to test whether this CCP-negative population of anti-MCV antibody is directed to the vimentin protein or to the citrulline moiety of mutated vimentin [9, 35].
Only the CCP3 test appears in some studies to be comparable with the CCP2 test [36–38], although in the majority of published studies the CCP3 test shows a somewhat lower specificity and/or sensitivity [23, 24, 39–43]. Based upon the combined results of these studies it can be concluded that the CCP3 test has no apparent diagnostic advantage compared with the CCP2 test. Inova has also recently introduced the CCP 3.1 test, which additionally includes measurement of IgA antibodies. In general this test does not appear to be better than the CCP3 test [23, 41], and the CP 3.1 test would appear to have a limited usage in a routine laboratory setup.
Citrullinated peptide antigens have also been derived from proteins like Epstein-Barr virus nuclear antigen (Astra) and IgG (Aesku). The tests with these peptides were included in the stratified study of Bizzaro and collaborators [23], but scored rather low in sensitivity (Aesku 44%, Astra 47%). In a single study, the Genesis cFil test shows a positive predictive value that is lower than that of the CCP2 test, but higher than the positive predictive value of the CCP3 and MCV tests [44]. Finally, Lutteri and coworkers also measured the diagnostic abilities of a new test using synthetic citrullinated peptides (RA/CP; Triturus, Bad Kreuznach, Germany). The specificity, sensitivity, positive predictive value and negative predictive value of this test were all much lower than those of the CCP2 and CCP3 tests [24].
What test is best for the clinician?
As outlined above, ACPA are not only important predictors of RA development but are also among the most potent predictors of the outcome of RA, as measured by the rate of radiographic joint destruction. There are at least four clinically important reasons to perform and compare ACPA tests: the ability to confirm or predict the development of RA with the highest reliability; the ability to predict radiographic progression; the ability to predict remission; and the ability to predict response to anti- TNFα treatment.
First, from Tables 1 to 3 it is clear that although most ACPA tests are perfectly able to predict or confirm a diagnosis of RA, none of the tests has a better diagnostic record than the CCP2 test. In addition, it was reported recently that the positive predictive value for predicting progression from undifferentiated arthritis to RA was highest for CCP2 (67.1%). Combinations of two or more ACPA tests appear to give no additive value [17]. A somewhat different situation may exist for the additional testing of RF, since RF testing may carry additional clinical value beyond testing for anti-CCP alone [45]. It should be noted that RF positivity still is a criterion for RA, and that RF-positive/anti-CCP(-) RA patients display different environmental risk factors than those that are only anti-CCP(+) [3].
Second, a positive test for anti-CCP2, anti-CCP3, or anti-MCV was associated with a higher Sharp/van der Heijde score at all time points except baseline and was also associated with a higher rate of joint destruction over a period of 7 years [17]. There was no difference between these tests with regard to their ability to predict radiographic progression. The use of a second or third autoantibody test did not increase the predictive accuracy for the rate of joint destruction [17]. Similar results were reported by Dejaco and colleagues [36], Majka and colleagues [46], and Syversen and colleagues [47]. It is also interesting to note that not only in the presence of ACPA, but also in the absence of these antibodies, RF did not significantly correlate with increased rates of joint destruction. This indicates that RF, in contrast to ACPA, does not by itself contribute to disease progression. In some studies MCV was reported to have a somewhat higher sensitivity (mostly accompanied by a lower specificity) than CCP2 (for example [27, 32–34]). These studies, however, also showed that the rate of joint destruction in MCV-positive/CCP(-) patients was comparable with that in patients lacking ACPA, indicating that the presence of anti-MCV antibody alone does not affect the level of joint damage in RA [17, 33, 48].
Third, the test's ability to predict the likelihood of not achieving sustained disease-modifying antirheumatic drug-free remission was highest (11.6%) for anti-CCP2 and varied between 4.7 and 6.0% for anti-CCP3, anti- MCV and RF [17]. Again, performing two ACPA tests had no additional value compared with the anti-CCP2 test alone. It is clear, however, that we need more data on this aspect of ACPA testing.
Finally, treatment of RA is mostly assumed to combat important disease mechanisms and thus lower the inflammation. As a consequence one might also expect a reduction of the activation of autoreactive B cells followed by reduced ACPA levels, which would allow monitoring of the effect of treatment. In the initial studies no significant effect of infliximab on anti-CCP levels was observed (reviewed by Zendman and collaborators [49]). Also in later studies neither anti-CCP3 nor anti-CCP2 levels were found to be influenced by TNFα blocking agents, and the test results failed to predict responses to anti-TNFα treatment [36]. A possible reason for these observations may be that although the inflammation may decrease, the citrullinated antigens (and consequently the production of autoantibodies and immune complexes) are still there, and this is reflected in the antibody levels (see [1]). In other studies, however, significant decreases of anti-CCP2 and anti-MCV titers at 18 months and/or 24 months of infliximab treatment have been reported [27]. At the moment we have to conclude that none of the available ACPA tests unequivocally shows the ability to predict response to treatment.
Perspectives
Reference serum
In most of the commercially available tests the cut-off values used to define a positive result vary significantly, even when the antigenic substrate is provided by the same manufacturer [23]. There is therefore an urgent need for reference material that can help investigators to harmonize data obtained with the various commercially available tests. The use of International Units, based on the reactivity of a reference serum or antibody, will hopefully help laboratory experts and clinicians to decide which serum is ACPA-positive and which is not.
At the request of the Committee for the Standardization of Autoantibodies in Rheumatic and Related Diseases, the Center for Disease Control and Prevention (Atlanta, GA, USA) has prepared a lyophilized reference material, obtained from an RF-positive and ACPA-positive patient, which is available on request as an international ACPA reference reagent. This reference reagent has already been tested by some laboratories using several commercial tests, and was found to improve considerably the comparison of quantitative results between different commercial tests (N Bizzaro, personal communication). The reference serum has also been tested by laboratories of several members of the Committee for the Standardization of Autoantibodies in Rheumatic and Related Diseases, using the same substrate and using kits commercially available in Europe, and is now available at the Center for Disease Control and Prevention for the scientific community (PL Meroni, personal communication).
Universal serum collection
A second important development that will help to compare the specificity and sensitivity of ACPA (and other) tests is the initiative of the European AutoCure consortium to generate a large depository of sera from patients with RA and other rheumatic diseases that will become available for comparative diagnostic studies. The use of a universal set of RA patients and control sera will allow a direct comparison of the diagnostic performance of current tests and those yet to be developed.
Potential test improvements
From several recent studies it became clear that a positive reaction in an ACPA test for non-RA sera is frequently due to the presence of antibodies that recognize the target molecule in a noncitrulline-dependent fashion, because the same molecule containing arginine instead of citrulline was bound by the antibodies at least as efficiently as the citrullinated antigen [9, 35]. This observation indicates that the inclusion of a noncitrullinated control antigen in the test is likely to improve the specificity of the test for RA. Currently it is not clear whether the manufacturers of ACPA tests are considering such a modification of the test.
Finally, since each of the target molecules used for ACPA detection might have its specific utility in the identification of a particular subset of RA patients, the development of multiplex tests combining all of these target molecules in a single analysis may be a significant step forward in the detailed analysis of autoantibody reactivities in sera of this heterogeneous disease. Several experimental platforms can be envisaged to achieve this, including fluorescent secondary antibody-based microarrays, imaging surface plasmon resonance-based microarrays and Luminex addressable beads or nanotechnologybased systems.
Abbreviations
- ACPA:
-
anti-citrullinated protein antibodies
- ACR:
-
American College of Rheumatology
- CCP:
-
cyclic citrullinated peptide
- cFil:
-
citrullinated recombinant rat filaggrin
- cIgG:
-
citrullinated immunoglobulin
- ELISA:
-
enzyme-linked immunosorbent assay
- MCV:
-
mutated citrullinated vimentin
- RA:
-
rheumatoid arthritis
- RF:
-
rheumatoid factor
- TNF:
-
tumor necrosis factor.
References
Van Venrooij WJ, Van Beers JJBC, Pruijn GJM: Anti-CCP antibody, a marker for the early detection of rheumatoid arthritis. Ann NY Acad Sci. 2008, 1143: 268-285. 10.1196/annals.1443.013.
Helm-van Mil Van der AH, Verpoort KN, Breedveld FC, Toes REM, Huizinga TWJ: Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther. 2005, 7: R949-R958. 10.1186/ar1767.
Pedersen M, Jacobsen S, Klarlund M, Pedersen BV, Wiik A, Wohlfahrt J, Frisch M: Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res Ther. 2006, 8: R133-10.1186/ar2022.
Lopez-Longo FJ, Oliver-Minarro D, de la Torre I, Gonzalez-Diaz dR, Sanchez-Ramon S, Rodriguez-Mahou M, Paravisini A, Monteagudo I, Gonzalez CM, Garcia-Castro M, Casas MD, Carreno L: Association between anti-cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Rheum. 2009, 61: 419-424. 10.1002/art.24390.
van Dongen H, van Aken J, Lard LR, Visser K, Ronday HK, Hulsmans HM, Speyer I, Westedt ML, Peeters AJ, Allaart CF, Toes RE, Breedveld FC, Huizinga TW: Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2007, 56: 1424-1432. 10.1002/art.22525.
Verpoort KN, Jol-van der Zijde CM, Papendrecht-van der Voort EA, Ioan-Facsinay A, Drijfhout JW, van Tol MJ, Breedveld FC, Huizinga TW, Toes RE: Isotype distribution of anti-cyclic citrullinated peptide antibodies in undifferentiated arthritis and rheumatoid arthritis reflects an ongoing immune response. Arthritis Rheum. 2006, 54: 3799-3808. 10.1002/art.22279.
Skriner K, Adolph K, Jungblut PR, Burmester GR: Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 2006, 54: 3809-3814. 10.1002/art.22276.
Koga T, Migita K, Miyashita T, Maeda Y, Nakamura M, Abiru S, Myoji M, Komori A, Yano K, Yatsuhashi H, Eguchi K, Ishibashi H: Determination of anti-cyclic citrullinated peptide antibodies in the sera of patients with liver diseases. Clin Exp Rheumatol. 2008, 26: 121-124.
Vannini A, Cheung K, Fusconi M, Stammen-Vogelzangs J, Drenth JP, Dall'Aglio AC, Bianchi FB, Bakker-Jonges LE, van Venrooij WJ, Pruijn GJ, Zendman AJ: Anti-cyclic citrullinated peptide positivity in non-rheumatoid arthritis disease samples: citrulline-dependent or not?. Ann Rheum Dis. 2007, 66: 511-516. 10.1136/ard.2006.058933.
Jorgensen KT, Wiik A, Pedersen M, Hedegaard CJ, Vestergaard BF, Gislefoss RE, Kvien TK, Wohlfahrt J, Bendtzen K, Frisch M: Cytokines, autoantibodies and viral antibodies in premorbid and postdiagnostic sera from patients with rheumatoid arthritis: case-control study nested in a cohort of Norwegian blood donors. Ann Rheum Dis. 2008, 67: 860-866. 10.1136/ard.2007.073825.
Nielen MM, van Schaardenburg D, Reesink HW, Stadt van de RJ, Horst-Bruinsma van der IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA: Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004, 50: 380-386. 10.1002/art.20018.
Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, Sundin U, van Venrooij WJ: Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003, 48: 2741-2749. 10.1002/art.11223.
Helm-van Mil Van der AH, Le Cessie S, van Dongen H, Breedveld FC, Toes RE, Huizinga TW: A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: how to guide individual treatment decisions. Arthritis Rheum. 2007, 56: 433-440. 10.1002/art.22380.
van Gaalen FA, Linn-Rasker SP, van Venrooij WJ, de Jong BA, Breedveld FC, Verweij CL, Toes RE, Huizinga TW: Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum. 2004, 50: 709-715. 10.1002/art.20044.
Machold KP, Stamm TA, Nell VP, Pflugbeil S, Aletaha D, Steiner G, Uffmann M, Smolen JS: Very recent onset rheumatoid arthritis: clinical and serological patient characteristics associated with radiographic progression over the first years of disease. Rheumatology (Oxford). 2007, 46: 342-349. 10.1093/rheumatology/kel237.
Turesson C, Jacobsson LT, Sturfelt G, Matteson EL, Mathsson L, Ronnelid J: Rheumatoid factor and antibodies to cyclic citrullinated peptides are associated with severe extra-articular manifestations in rheumatoid arthritis. Ann Rheum Dis. 2007, 66: 59-64. 10.1136/ard.2006.054445.
Linden van der MP, van der WD, Ioan-Facsinay A, Levarht EW, Stoeken-Rijsbergen G, Huizinga TW, Toes RE, Helm-van Mil van der AH: Value of antimodified citrullinated vimentin and third-generation anti-cyclic citrullinated peptide compared with second-generation anti-cyclic citrullinated peptide and rheumatoid factor in predicting disease outcome in undifferentiated arthritis and rheumatoid arthritis. Arthritis Rheum. 2009, 60: 2232-2241. 10.1002/art.24716.
Van Oosterhout M, Bajema I, Levarht EW, Toes RE, Huizinga TW, Van Laar JM: Differences in synovial tissue infiltrates between anti-cyclic citrullinated peptide-positive rheumatoid arthritis and anti-cyclic citrullinated peptide-negative rheumatoid arthritis. Arthritis Rheum. 2008, 58: 53-60. 10.1002/art.23148.
Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B, Spencer J, Pitzalis C: Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009, 6: e1-10.1371/journal.pmed.0060001.
Klareskog L, Catrina AI, Paget S: Rheumatoid arthritis. Lancet. 2009, 373: 659-672. 10.1016/S0140-6736(09)60008-8.
Visser K, Verpoort KN, van Dongen H, Kooij van der SM, Allaart CF, Toes REM, Huizinga TWJ, Mil AHMV: Pretreatment serum levels of anti-cyclic citrullinated peptide antibodies are associated with the response to methotrexate in recent-onset arthritis. Ann Rheum Dis. 2008, 67: 1194-1195. 10.1136/ard.2008.088070.
Visser H, Le Cessie S, Vos K, Breedveld FC, Hazes JM: How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum. 2002, 46: 357-365. 10.1002/art.10117.
Bizzaro N, Tonutti E, Tozzoli R, Villalta D: Analytical and diagnostic characteristics of 11 2nd- and 3rd-generation immunoenzymatic methods for the detection of antibodies to citrullinated proteins. Clin Chem. 2007, 53: 1527-1533. 10.1373/clinchem.2007.087569.
Lutteri L, Malaise M, Chapelle JP: Comparison of second- and third generation anti-cyclic citrullinated peptide antibodies assays for detecting rheumatoid arthritis. Clin Chim Acta. 2007, 386: 76-81. 10.1016/j.cca.2007.08.002.
Liao KP, Batra KL, Chibnik L, Schur PH, Costenbader KH: Anti-cyclic citrullinated peptide revised criteria for the classification of rheumatoid arthritis. Ann Rheum Dis. 2008, 67: 1557-1561. 10.1136/ard.2007.082339.
Combe B, Landewe R, Lukas C, Bolosiu HD, Breedveld F, Dougados M, Emery P, Ferraccioli G, Hazes JM, Klareskog L, Machold K, Martin-Mola E, Nielsen H, Silman A, Smolen J, Yazici H: EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2007, 66: 34-45. 10.1136/ard.2005.044354.
Nicaise RP, Grootenboer MS, Bruns A, Hurtado M, Palazzo E, Hayem G, Dieude P, Meyer O, Chollet MS: Antibodies to mutated citrullinated vimentin for diagnosing rheumatoid arthritis in anti-CCP-negative patients and for monitoring infliximab therapy. Arthritis Res Ther. 2008, 10: R142-10.1186/ar2570.
Poulsom H, Charles PJ: Antibodies to citrullinated vimentin are a specific and sensitive marker for the diagnosis of rheumatoid arthritis. Clin Rev Allergy Immunol. 2008, 34: 4-10. 10.1007/s12016-007-8016-3.
Sghiri R, Bouajina E, Bargaoui D, Harzallah L, Fredj HB, Sammoud S, Ghedira I: Value of anti-mutated citrullinated vimentin antibodies in diagnosing rheumatoid arthritis. Rheumatol Int. 2008, 29: 59-62. 10.1007/s00296-008-0614-8.
Wagner E, Skoumal M, Bayer PM, Klaushofer K: Antibody against mutated citrullinated vimentin: a new sensitive marker in the diagnosis of rheumatoid arthritis. Rheumatol Int. 2009, 29: 1315-1321. 10.1007/s00296-009-0854-2.
Luime JJ, Colin EM, Hazes JM, Lubberts E: Does anti-MCV has additional value as serological marker in the diagnostic and prognostic work-up of patients with rheumatoid arthritis? A systematic review. Ann Rheum Dis. 2010, 69: 337-344. 10.1136/ard.2008.103283.
Bang H, Egerer K, Gauliard A, Luthke K, Rudolph PE, Fredenhagen G, Berg W, Feist E, Burmester GR: Mutation and citrullination modifies vimentin to a novel autoantigen for rheumatoid arthritis. Arthritis Rheum. 2007, 56: 2503-2511. 10.1002/art.22817.
Liu X, Jia R, Zhao J, Li Z: The role of anti-mutated citrullinated vimentin antibodies in the diagnosis of early rheumatoid arthritis. J Rheumatol. 2009, 36: 1136-1142. 10.3899/jrheum.080796.
Mathsson L, Mullazehi M, Wick MC, Sjoberg O, van Vollenhoven R, Klareskog L, Ronnelid J: Antibodies against citrullinated vimentin in rheumatoid arthritis: higher sensitivity and extended prognostic value concerning future radiographic progression as compared with antibodies against cyclic citrullinated peptides. Arthritis Rheum. 2008, 58: 36-45. 10.1002/art.23188.
Kakumanu P, Yamagata H, Sobel ES, Reeves WH, Chan EK, Satoh M: Patients with pulmonary tuberculosis are frequently positive for anti-cyclic citrullinated peptide antibodies, but their sera also react with unmodified arginine-containing peptide. Arthritis Rheum. 2008, 58: 1576-1581. 10.1002/art.23514.
Dejaco C, Duftner C, Klotz W, Schirmer M, Herold M: Third generation anticyclic citrullinated peptide antibodies do not predict anti-TNF-alpha treatment response in rheumatoid arthritis. Rheumatol Int. 2010, 30: 451-454. 10.1007/s00296-009-0978-4.
Mutlu N, Bicakcigil M, Tasan DA, Kaya A, Yavuz S, Ozden AI: Comparative performance analysis of 4 different anti-citrullinated protein assays in the diagnosis of rheumatoid arthritis. J Rheumatol. 2009, 36: 491-500. 10.3899/jrheum.080656.
Santiago M, Baron M, Miyachi K, Fritzler MJ, Abu-Hakima M, Leclercq S, Bell M, Hudson M, Mathieu JP, Taillefer S, Jones N, Docherty P, Khraishi M, Markland J, Pope J, Robinson D, Smith D, Sutton E: A comparison of the frequency of antibodies to cyclic citrullinated peptides using a third generation anti- CCP assay (CCP3) in systemic sclerosis, primary biliary cirrhosis and rheumatoid arthritis. Clin Rheumatol. 2008, 27: 77-83. 10.1007/s10067-007-0656-4.
Correia ML, Carvalho S, Fortuna J, Pereira MH: Comparison of three anti-CCP antibody tests and rheumatoid factor in RA and control patients. Clin Rev Allergy Immunol. 2008, 34: 21-25. 10.1007/s12016-007-8030-5.
Dos Anjos LM, Pereira IA, 'Orsi E, Seaman AP, Burlingame RW, Morato EF: A comparative study of IgG second- and third-generation anti-cyclic citrullinated peptide (CCP) ELISAs and their combination with IgA third-generation CCP ELISA for the diagnosis of rheumatoid arthritis. Clin Rheumatol. 2009, 28: 153-158. 10.1007/s10067-008-0999-5.
Innala L, Kokkonen H, Eriksson C, Jidell E, Berglin E, Dahlqvist SR: Antibodies against mutated citrullinated vimentin are a better predictor of disease activity at 24 months in early rheumatoid arthritis than antibodies against cyclic citrullinated peptides. J Rheumatol. 2008, 35: 1002-1008.
Cruyssen Vander B, Nogueira L, Van Praet J, Deforce D, Elewaut D, Serre G, De Keyser F: Do all anti-citrullinated protein/peptide antibody tests measure the same? Evaluation of discrepancy between anti-citrullinated protein/peptide antibody tests in patients with and without rheumatoid arthritis. Ann Rheum Dis. 2008, 67: 542-546. 10.1136/ard.2007.071654.
Wu R, Shovman O, Zhang Y, Gilburd B, Zandman-Goddard G, Shoenfeld Y: Increased prevalence of anti-third generation cyclic citrullinated peptide antibodies in patients with rheumatoid arthritis and CREST syndrome. Clin Rev Allergy Immunol. 2007, 32: 47-56. 10.1007/BF02686081.
Coenen D, Verschueren P, Westhovens R, Bossuyt X: Technical and diagnostic performance of 6 assays for the measurement of citrullinated protein/peptide antibodies in the diagnosis of rheumatoid arthritis. Clin Chem. 2007, 53: 498-504. 10.1373/clinchem.2006.078063.
Levesque MC, Zhou ZJ, Moreland LW: Anti-cyclic citrullinated peptide testing for the diagnosis of rheumatoid arthritis and the quest for improved sensitivity and predictive value. Arthritis Rheum. 2009, 60: 2211-2215. 10.1002/art.24720.
Majka DS, Deane KD, Parrish LA, Lazar AA, Baron AE, Walker CW, Rubertone MV, Gilliland WR, Norris JM, Holers VM: Duration of preclinical rheumatoid arthritis-related autoantibody positivity increases in subjects with older age at time of disease diagnosis. Ann Rheum Dis. 2008, 67: 801-807. 10.1136/ard.2007.076679.
Syversen SW, Goll GL, van der HD, Landewe R, Lie BA, Odegard S, Uhlig T, Gaarder PI, Kvien TK: Prediction of radiographic progression in rheumatoid arthritis and the role of antibodies against mutated citrullinated vimentin: results from a ten-year prospective study. Ann Rheum Dis. 2010, 69: 345-351. 10.1136/ard.2009.113092.
Ursum J, Nielen MM, van Schaardenburg D, Horst van der AR, Stadt van de RJ, Dijkmans BA, Hamann D: Antibodies to mutated citrullinated vimentin and disease activity score in early arthritis: a cohort study. Arthritis Res Ther. 2008, 10: R12-10.1186/ar2362.
Zendman AJ, Van Venrooij WJ, Pruijn GJM: Use and significance of anti-CCP autoantibodies in rheumatoid arthritis. Rheumatology (Oxford). 2006, 45: 20-25. 10.1093/rheumatology/kei111.
Damjanovska L, Thabet MM, Levarht EW, Stoeken-Rijsbergen G, Voort van der EI, Toes RE, Huizinga TW, Helm-van Mil van der AH: The diagnostic value of anti-MCV antibodies in differentiating early inflammatory arthritis. Ann Rheum Dis. 2009, doi:10.1136/ard.2009.108456.,
Dejaco C, Klotz W, Larcher H, Duftner C, Schirmer M, Herold M: Diagnostic value of antibodies against a modified citrullinated vimentin in rheumatoid arthritis. Arthritis Res Ther. 2006, 8: R119-10.1186/ar2008.
Soos L, Szekanecz Z, Szabo Z, Fekete A, Zeher M, Horvath IF, Danko K, Kapitany A, Vegvari A, Sipka S, Szegedi G, Lakos G: Clinical evaluation of anti-mutated citrullinated vimentin by ELISA in rheumatoid arthritis. J Rheumatol. 2007, 34: 1658-1663.
Cruyssen Vander B, Cantaert T, Nogueira L, Clavel C, De Rycke L, Dendoven A, Sebag M, Deforce D, Vincent C, Elewaut D, Serre G, De Keyser F: Diagnostic value of anti-human citrullinated fibrinogen ELISA and comparison with four other anti-citrullinated protein assays. Arthritis Res Ther. 2006, 8: R122-10.1186/ar2011.
Luis Caro-Oleas J, Fernandez-Suarez A, Reneses CS, Porrino C, Nunez-Roldan A, Wichmann Schlipf I: Diagnostic usefulness of a third-generation anti-cyclic citrulline antibody test in patients with recent-onset polyarthritis. Clin Chem Lab Med. 2007, 45: 1396-1401. 10.1515/CCLM.2007.294.
Acknowledgements
The authors thank Dr N Bizzaro, Dr P-L Meroni, Dr M Thabet, Dr R Toes and Dr X Bossuyt for helpful comments and are grateful to Dr H Zendman and Dr J van Beers for collecting data for Table 1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
GJMP and WJvV are scientific advisors for Axis-Shield Diagnostics Ltd and Euro-Diagnostica AB. AW declares that he has no competing interests.
Rights and permissions
About this article
Cite this article
Pruijn, G.J., Wiik, A. & van Venrooij, W.J. The use of citrullinated peptides and proteins for the diagnosis of rheumatoid arthritis. Arthritis Res Ther 12, 203 (2010). https://doi.org/10.1186/ar2903
Published:
DOI: https://doi.org/10.1186/ar2903