Abstract
Introduction
5-Loxin® is a novel Boswellia serrata extract enriched with 30% 3-O-acetyl-11-keto-beta-boswellic acid (AKBA), which exhibits potential anti-inflammatory properties by inhibiting the 5-lipoxygenase enzyme. A 90-day, double-blind, randomized, placebo-controlled study was conducted to evaluate the efficacy and safety of 5-Loxin® in the treatment of osteoarthritis (OA) of the knee.
Methods
Seventy-five OA patients were included in the study. The patients received either 100 mg (n = 25) or 250 mg (n = 25) of 5-Loxin® daily or a placebo (n = 25) for 90 days. Each patient was evaluated for pain and physical functions by using the standard tools (visual analog scale, Lequesne's Functional Index, and Western Ontario and McMaster Universities Osteoarthritis Index) at the baseline (day 0), and at days 7, 30, 60 and 90. Additionally, the cartilage degrading enzyme matrix metalloproteinase-3 was also evaluated in synovial fluid from OA patients. Measurement of a battery of biochemical parameters in serum and haematological parameters, and urine analysis were performed to evaluate the safety of 5-Loxin® in OA patients.
Results
Seventy patients completed the study. At the end of the study, both doses of 5-Loxin® conferred clinically and statistically significant improvements in pain scores and physical function scores in OA patients. Interestingly, significant improvements in pain score and functional ability were recorded in the treatment group supplemented with 250 mg 5-Loxin® as early as 7 days after the start of treatment. Corroborating the improvements in pain scores in treatment groups, we also noted significant reduction in synovial fluid matrix metalloproteinase-3. In comparison with placebo, the safety parameters were almost unchanged in the treatment groups.
Conclusion
5-Loxin® reduces pain and improves physical functioning significantly in OA patients; and it is safe for human consumption. 5-Loxin® may exert its beneficial effects by controlling inflammatory responses through reducing proinflammatory modulators, and it may improve joint health by reducing the enzymatic degradation of cartilage in OA patients.
Trail Registration
(Clinical trial registration number: ISRCTN05212803.)
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is the commonest form of inflammatory joint disease, characterized by articular cartilage degradation with an accompanying peri-articular bone response [1, 2]. OA affects nearly 21 million people in the USA, accounting for 25% of visits to primary care physicians. It is estimated that 80% of the population will have radiographic evidence of OA by age 65 years, although only 60% of those will be symptomatic [3]. Clinical manifestations of OA of the knee include pain in and around the joint, stiffness of the joint after rest, crepitating on motion and limited joint motion, among others [4]. Current recommendations for managing OA focus on relieving pain and stiffness and improving physical function as important goals of therapy [5, 6]. Currently available medication regimens for most cases include nonopioid analgesics such as acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), including cyclo-oxygenase II inhibitors. These pharmaceutical agents can reduce both pain and inflammation quite effectively, but long-term use of NSAIDs has been found to be associated with enhanced risk for gastrointestinal bleeding, hypertension, congestive heart failure and renal insufficiency, among other adverse effects [7–9]. Because of the high incidence of adverse events associated with both nonselective and cyclo-oxygenase II selective NSAID therapy, effective and safer alternative treatments for OA are urgently needed.
In recent years, the gum resin extracted from the ancient herb Boswellia serrata has gained much attention as a potent anti-inflammatory, anti-arthritic and analgesic agent [10, 11]. 3-O-acetyl-11-keto-beta-boswellic acid (AKBA) is the most active component of Boswellia extract and has been demonstrated to be a potent inhibitor of 5-lipoxygenase (5-LOX), which is a key enzyme in the biosynthesis of leukotrienes from arachidonic acid in the cellular inflammatory cascade [12, 13].
5-Loxin® is a novel B. serrata extract enriched to 30% AKBA (US Patent publication no.: 2004/0073060A1). In the carrageenan-induced inflammation model, 5-Loxin® treatment yielded significant improvement in paw inflammation in albino Wister rats [14]. Cell based in vitro studies and in vivo experiments conducted in Sprague-Dawley rats suggest that 5-Loxin® can inhibit proinflammatory cytokines such as tumour necrosis factor-α, interleukin-1β (unpublished data, Sengupta K, Alluri KV, and Golakoti T). Furthermore, Affimatrix gene chip analysis demonstrates 5-Loxin® can potentially inhibit the tumour necrosis factor-α induced gene expression of matrix metalloproteinases (MMPs), adhesion molecules such as intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and mediators of apoptosis in human microvascular endothelial cells [14]. Importantly, extensive acute and dose-dependent subchronic safety experiments on rats demonstrate that 5-Loxin® does not exhibit toxic manifestations, even at a dose 2,000 to 3,000 times higher than the human equivalence dose [15]. In addition, 5-Loxin® does not exhibit genotoxicity in the standard AMES bacterial reverse mutation assay (INTOX, 375, Urawade, Pirangut-Urawade Road, Tal. Mulshi, Pune – 412108, India; study no. 4477/05).
Although a significant number of clinical study reports support the anti-inflammatory and anti-arthritic properties of Boswellia extract [16–19], to the best of our knowledge no reports on the efficacy of AKBA-enriched 5-Loxin® in OA in humans have been published. Therefore, in the present double-blind and placebo-controlled clinical study, we sought to evaluate the efficacy and safety of 5-Loxin® in treatment of OA of the knee. We assessed the effectiveness of 100 mg/day and 250 mg/day 5-Loxin® on pain, joint stiffness and mobility in OA patients. We also explored the effect of 5-Loxin® on the cartilage degrading enzyme MMP-3 in OA patients treated with 5-Loxin®.
Materials and methods
Recruitment of patients
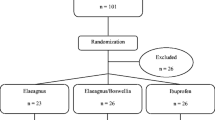
This trial was performed at Alluri Sitarama Raju Academy of Medical Sciences (ASRAM), Eluru, Andhra Pradesh, India from July 2006 to October 2006 (clinical trial registration number: ISRCTN05212803). The study protocol was evaluated and approved by the ASRAM Institutional Review Board. An overview of the clinical study is provided in Figure 1. Briefly, 236 patients out of 823 attending the orthopaedic Outpatients Department of the ASRAM Hospital were selected, based on the signs, symptoms and radiological changes consistent with OA in the first phase of the screening procedure. A total of 75 patients suffering for more than 3 months with medial tibiofemoral OA were selected using inclusion/exclusion criteria summarized in Table 1. All patients signed the Institutional Review Board approved consent form. Patients were otherwise healthy, were aged 40 years or older, and had a diagnosis of OA, fulfilling the American College of Rheumatology classification criteria [4]. After recruitment, the patients were randomly distributed into three groups; demographic data and baseline characteristics are summarized in Table 2.
Flow chart of the patients who participated in the clinical trial. Evaluations of physical activity and pain scores, serum biochemistry, haematology, urine biochemistry and proinflammatory cytokines were done at baseline (day 0) and on days 7, 30, 60 and 90 during follow up. Assessments of matrix metalloproteinase-3 were done on days 0 and 90 only.
Before study enrollment, patients were required to be taking an NSAID at prescription strength for at least 30 days or acetaminophen 1,200 to 4,000 mg/day on a regular basis (at least 25 of the preceding 30 days) with a history of therapeutic benefit. Eligibility required patients to meet specific flare criteria upon medication washout. At screening, patients had to demonstrate a visual analog scale (VAS) score between 40 and 70 mm during the most painful knee movement, and Lequesne's Functional Index (LFI) score greater than 7 points after 7-day withdrawal of usual medication.
Study design
A total of 75 selected patients with symptoms of moderate to mild OA were recruited into the study. Each patient was randomly assigned to a treatment group using a randomization table generated using validated computer software (RANCODE; IDV, Gauting, Germany). Treatment allocation depended only on the time sequence in which patients entered the study, thus minimizing selection bias. The clinical trial pharmacist and statistician ensured that treatment codes remained confidential. The patients were distributed into three groups: placebo (n = 25); 30% AKBA enriched B. serrata extract (5-Loxin®) low-dose group (100 mg/day), in which patients received 50 mg encapsulated 5-Loxin® twice daily (n = 25); and 5-Loxin® high-dose group (250 mg/day), in which patients received 125 mg encapsulated 5-Loxin® twice daily (n = 25). Patients in the placebo group received two capsules of similar color, taste and appearance but filled only with rice bran.
Each patient completed a questionnaire, providing details regarding demographics, medical history and nutritional status, at the baseline evaluation and during the follow-up evaluations on days 7, 30, 60 and 90. At the baseline evaluation, and at each visit during the 90-day follow up period, all patients were assessed for pain scores and physical ability. Various parameters of serum biochemistry, haematology and urine analysis were carried out on each evaluation day. Serum samples were collected at all evaluation days for proinflammatory modulators. Knee joint synovial fluid was aseptically collected at baseline and at day 90 for evaluation of MMP-3 concentration. Safety was monitored by clinical and laboratory assessments conducted at study visits and patient-reported adverse experiences.
Functional disability and pain score evaluation
The investigators assessed the functional disability reported by the patients at baseline and on each follow-up visit (days 7, 30, 60 and 90). Questionnaire-based assessment of pain, stiffness and physical function were done using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) index [20], LFI [21] and VAS [22]. The WOMAC index produces scores for three subscales: pain, stiffness and physical function. The pain, stiffness and function subscales of the WOMAC were converted to a 0 to 100 normalized units (NU) scale [23]. The pain subscale was the average of the first five questions of WOMAC and measured using the NU scale from 0 mm ('no pain') to 100 mm ('extreme pain') for each question. The stiffness subscale was the average of questions 6 and 7, measured using the NU scale from 0 mm ('no stiffness') to 100 mm ('extreme stiffness') for each question. The physical function subscale was the average of questions 8 through 24 of the WOMAC and measured by NU scale from 0 mm ('no difficulty') to 100 mm ('extreme difficulty') for each question. Analyses of these end-points were based upon the time-weighted average change from baseline over 90 days.
Haematological and biochemical evaluations
For assessment of safety of 5-Loxin®, several parameters were evaluated in serum, urine and whole blood of all patients at each visit of the study duration (Table 3). Serum biochemical parameters and haematological parameters were measured using the automated analyzer HumaStar 300 (Human, Wiesbaden, Germany) and the haematological counter Humacount (Human), respectively. The urine analysis was carried out by microscopy and by using UroColor™10 Dip Sticks (Standard Diagnostics, Kyonggi-do, Korea).
Assessment of matrix metalloproteinase-3 in synovial fluids
MMP-3 (R&D Systems, Minneapolis, USA) were quantitatively measured by ultrasensitive ELISA method. Assay procedures adhered to the protocol supplied by the manufacturers. Briefly, synovial fluid samples were incubated on capture antibody coated 96-well microplates. Specifically bound antigen was detected by appropriate biotinylated detection antibody and was probed with horseradish peroxidase enzyme. The specific immune reaction was detected by substrate solution and the colour development was read with the help of micro-plate reader (Bio-Rad, Hercules, CA, USA). A standard curve was generated by plotting the optical density at respective known concentration of MMP3. The sensitivity of MMP-3 detection ELISA kit is 9 pg/ml.
Rescue medication
Patients were prescribed ibuprophen 400 mg tablets (maximum 400 mg thrice daily; total 1,200 mg) as rescue analgesia on days 7, 30 and 60, based on pain intensity reported to the study physician by the patient. However, the patients were instructed not to take medicine at least 3 days before each evaluation. No other OA interventions were allowed during the study period.
Statistical analysis
We performed detailed statistical analyses using SAS software to evaluate the efficacy of two doses of 5-Loxin® in comparison with the placebo group in terms of improvement in pain and physical ability scores, and to assess biomolecular markers at baseline and days 7, 30, 60 and 90 of treatment. Pair-wise changes were examined by carrying out a least significant difference test for all possible pairs. The significance of the effects of the treatment groups was compared by using one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison tests. Results with P < 0.05 are considered statistically significant.
This is a three-arm (two doses of 5-Loxin® and placebo), randomized, double-blind, placebo-controlled, single-centre trial conducted over 90 days. The trial's primary objective was to determine the effects of 5-Loxin® on pain, physical function and joint stiffness. For power calculations, the estimates for variability and assumed mean changes for each treatment group were based on results from previous placebo-controlled studies of celecoxib, etoricoxib and rofecoxib conducted in patients with OA [24–27]. We believe that an intervention that gives an average improvement of mean change + 1 standard deviation, rather than mean change only, will provide results of greater significance [28]. Our trial is designed to have more than 80% power to detect a situation in which either active drug dosage yields an improvement to at least mean change + 0.9 standard deviation, under a conservative assumption, and we tested differences between groups in mean improvement using ANOVA (α = 0.05, two-sided). With 25 patients per group, we would have a 93% chance of observing at least one example of any side effect occurring in 10% or more of the patient population at a specific dosage.
Results
Baseline characteristics
Descriptive statistics comparing demographic variables, baseline disease characteristics and baseline outcome measures (that is, WOMAC pain, function and stiffness subscores) are provided in Table 2. Overall, the treatment groups receiving 5-Loxin® low dose (100 mg/day, n = 25), 5-Loxin® high dose (250 mg/day, n = 25) and placebo (n = 25), were similar with respect to sex, age, Body Mass Index and pain severity (Table 2). The patients were randomly distributed into three groups. Although there are some differences in baseline characteristics of gender, body mass index and WOMAC scores, those are statistically not significant.
Clinical efficacy
We compared the scores between the treatment groups obtained at day 90. Both doses of 5-Loxin® conferred clinically and statistically significant improvements in pain scores and physical ability scores in OA patients between baseline and day 90 (Table 4).
Tukey's multiple comparison test revealed statistically significant improvements by 48.83% (P < 0.001), 23.79% (P < 0.036) and 39.61% (P = 0.009) in VAS, LFI and WOMAC pain scores, respectively, in the low-dose (100 mg 5-Loxin®) group versus the placebo group (Table 4). Improvements by 42.5% (P = 0.120) and 28.62% (P = 0.100) score in WOMAC stiffness and WOMAC functional ability, respectively were also achieved in the low-dose group (Table 4).
In comparison with the placebo group, the high-dose (250 mg 5-Loxin®) group also exhibited statistically significant improvements in all parameters (Table 4). The high-dose group showed improvements by 65.94% (P < 0.001), 31.34% (P < 0.017), 52.05% (P < 0.001), 62.22% (P = 0.014) and 49.34% (P = 0.002) in VAS, LFI, WOMAC pain, WOMAC stiffness and WOMAC functional ability scores, respectively.
Student's t-test analyses revealed that MMP-3 concentration (P < 0.0001) in synovial fluids and VAS pain scores (P = 0.001) were significantly lower in the high-dose group than in the low-dose group. It is worth noting that both low-dose and high-dose treatment groups exhibited improvement in pain scores and physical ability scores as early as 7 days after the start of treatment, and these indices continued to improve throughout the 90 days of treatment (Figure 2). After 7 days, the low-dose and high-dose treatment groups exhibited 10.09% (P = 0.05) and 12.18% (P = 0.02) reductions in VAS, respectively, compared with the placebo group. In addition, WOMAC physical ability also improved by 14.38% (P < 0.01) after 7 days of treatment with high-dose 5-Loxin® (Figure 2).
Function, pain and stiffness scores. Presented are the mean scores for (a) visual analog scale, (b) Lequesne's Functional Index, (c) Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)-pain, (d) WOMAC-stiffness, and (e) WOMAC-functional ability in the low-dose (100 mg/day 5-Loxin®) and high-dose (250 mg/day 5-Loxin®) groups and placebo group at different time points, as indicated. Each bar represents mean concentration ± standard deviation. In comparison with placebo, the change in scores in the treatment groups was tested for significance using Tukey's multiple comparison test; asterisk indicates statistical significance.
Assessment of MMP-3 concentration
OA is a degenerative joint disorder; in molecular pathogenesis of OA, proteolytic enzymes such as MMPs are highly elevated in body fluids such as serum and synovial fluids, which cause potential damage in cartilage tissues [29]. Therefore, in order to determine whether 5-Loxin® treatment can normalize the MMP level, we evaluated the concentration of MMP-3 in synovial fluids collected from the patients. Figure 3 illustrates changes in MMP-3 concentration in synovial fluid samples collected from patients of all groups included in the study. Pair-wise comparisons indicated that at the end of the study both treatment groups exhibited highly significant reductions in MMP-3 in synovial fluid. Compared with the placebo group, the low-dose (100 mg) and high-dose (250 mg) 5-Loxin® groups showed 31.37% (P = 0.002) and 46.4% (P < 0.001) reductions in MMP-3 concentration, respectively. Regular t-tests revealed that high-dose 5-Loxin® treatment significantly reduced (P < 0.0001) synovial MMP-3 concentration when compared with the low-dose group (Table 4). Compared with baseline, the Wilcoxon sign-rank-sum test revealed that the low-dose and high-dose groups conferred 28.69% (P = 0.0013) and 46.33% (P < 0.0001) reductions in synovial fluid MMP-3 concentration at day 90. The MMP-3 level in the placebo group remained virtually unchanged at day 90 compared with baseline.
Reduction of Synovial MMP-3 levels. Presented are the matrix metalloproteinase (MMP)-3 levels in synovial fluid collected from 5-Loxin® treated and placebo patients with osteoarthritis. At day 90 there was no significant change in MMP-3 concentration in the placebo group compared with baseline. In comparison with the placebo group, at the end of the study the groups receiving100 mg/day and 250 mg/day 5-Loxin® showed 31.37% (P = 0.002) and 46.4% (P < 0.001) reductions in MMP-3 concentration, respectively. Change in MMP-3 concentration between the active treatment groups was not significant (P = 0.213). Each bar represents mean concentration of MMP-3 (ng/ml synovial fluid) ± standard deviation.
Biochemical evaluations
As a part of the safety evaluation, laboratory tests were performed to evaluate different biochemical parameters in serum and urine, and haematological parameters. The tested parameters in serum biochemistry, and haematological and urine analysis are summarized in Table 3. The significance of the differences between baseline and 90 days was tested by using repeated measures ANOVA. The F ratio is considered significant if P < 0.05. Although minor changes were observed in some of the parameters, they remained within the normal laboratory range. Statistical analyses of these parameters did not identify any statstically significant changes. Similarly, haematological and urinary parameters also exhibited no significant changes in the active treatment groups compared with placebo (data not shown). These findings further demonstrate the safety of 5-Loxin® in humans.
Adverse events
During the course of the 90-day study period, some minor adverse events were noted: diarrhoea, nausea, abdominal pain, mild fever (up to 37.5°C [99.5°F]) and general weakness. The patients who reported these minor events were distributed evenly throughout the placebo and active treatment groups. The numbers of minor adverse events reported by the patients during the study are summarized in Table 5.
Dropouts
Five patients (one from the low-dose [100 mg 5-Loxin®] group, and two each from placebo and high-dose [250 mg 5-Loxin®] group) were excluded from the study because they were suffering from a nonfatal viral infection during the course of study.
Discussion
To the best of our knowledge, this is the first clinical study to evaluate the efficacy of 5-Loxin® in OA. This study also provides important information regarding the possible molecular mechanisms of action of an anti-inflammatory compound of herbal origin in the treatment of OA. We demonstrated that 5-Loxin® has potential efficacy in terms of reducing pain and improving the physical ability of OA patients. A novel aspect of the present study is its evaluation of the effect of 5-Loxin® treatment on the cartilage degrading enzyme MMP-3 in synovial fluid from OA patients. In this 90-day clinical study, we also assessed the safety of 5-Loxin® in OA patients.
Pain, stiffness of joints, reduced joint movement and physical disability are the major clinical manifestations of OA [1, 30]. Our study demonstrates that 5-Loxin® potentially improves pain, joint stiffness and physical function in OA patients (Figure 2). The statistical analyses revealed that the improvements in physical parameters and reductions in synovial MMP-3 levels were significantly decreased in the treatment groups as compared with the placebo group (Figures 2 and 3). In addition, in order to check improvements in the treatment groups, we compared the data for all parameters between the baseline and day 90. Paired t-test revealed that both treatment groups had highly statistically significant improvements in all parameters. Comparing the high-dose versus the low-dose groups at day 90, significant differences were observed only for VAS for pain and synovial fluid MMP3 concentration (Table 4). This finding suggests that the higher dose of 5-Loxin® has better therapeutic efficacy against OA. We observed that, in comparison with baseline, there were downward trends in VAS score, LFI and WOMAC scores in the placebo group. We believe that this might be partly attributable to the placebo effect [31, 32] manifesting while patients completed the questionnaires, and partly due to the consumption of ibuprophen as rescue medication by more patients in the placebo group during the study. Interestingly, at the end of the study we found that the total number of participants requesting rescue medication was 16.7% and 72.2% higher in the placebo group than in the groups receiving 100 mg and 250 mg 5-Loxin®, respectively.
An important observation in the present study is that 250 mg/day 5-Loxin® had a significant effect in lowering VAS score by 12.18% (P = 0.02) and WOMAC function score by 14.38% (P < 0.01) in OA patients as early as 7 days after the start of treatment. These findings therefore indicate that 5-Loxin® confers prompt and significant pain relief and improvement in physical ability in OA patients. Existing information reveals that glucosamine usually takes 6 weeks to achieve significant beneficial effect in terms of pain relief in OA [33]. In addition, a randomized, double-blind, placebo-controlled trial [34] showed that 4,000 mg milk protein concentrate per day (Microlactin™; Stolle Milk Biologics Inc., Cincinnati, OH, USA) yields significant improvement in WOMAC score after 2 weeks of treatment on OA patients.
In OA patients, MMPs such as MMP-3 are over-expressed and abundant in fluids of the synovial cavity, and cause degeneration of cartilage tissue [35, 36]. 5-Loxin® was able to reduce the elevated MMP-3 level in synovial fluid. This finding indicates that reduction in synovial fluid MMP-3 level by 5-Loxin® is consistent with improvements in abnormal joint physiology in OA. Therefore, these data together demonstrate that 5-Loxin® potentially has effects in terms of reducing the pain and improving physical ability and joint health; it is most likely that these improvements occur through downregulation of cartilage degrading enzymes such as MMP-3.
Earlier, in acute and subchronic toxicity studies we demonstrated that 5-Loxin® (a 30% enriched product of AKBA, which is the active component of Boswellia extract) is safe and nontoxic in rats [15]. Additionally, 5-Loxin® did not exhibit mutagenicity in the standard AMES test (INTOX; study no. 4477/05). In the present study biochemical parameters in serum, haematological parameters and urine analysis (Table 3) also did not reveal any major adverse effect of the test compound in OA patients. Taken together, these observations further demonstrate that 5-Loxin® is potentially safe in the treatment of OA in humans.
Conclusion
In summary, the present study provides the evidence in support of the potential efficacy and safety of 5-Loxin® in patients with OA: 5-Loxin® significantly improved joint function and exhibited better therapeutic efficacy at 250 mg/day than at 100 mg/day; it reduces pain rapidly, as early as after 1 week of treatment; it reduces levels of the cartilage degrading enzyme MMP-3 in synovial fluid; and, most importantly, 5-Loxin® is safe for human consumption, even long term. This study provides important information about the efficacy and safety of 5-Loxin® in the treatment of OA, which may be useful in promoting 5-Loxin® as a promising alternative therapeutic strategy that may be used as a nutritional supplement against OA.
Abbreviations
- AKBA:
-
= 3-O-acetyl-11-keto-beta-boswellic acid
- ANOVA:
-
= analysis of variance
- ASRAM:
-
= Alluri Sitarama Raju Academy of Medical Sciences
- BMI:
-
= Body Mass Index
- ELISA:
-
= enzyme-linked immunosorbent assay
- LFI:
-
= Lequesne's Functional Index
- MMP:
-
= matrix metalloproteinase
- NSAID:
-
= nonsteroidal anti-inflammatory drug
- NU:
-
= normalized units
- OA:
-
= osteoarthritis
- VAS:
-
= visual analog scale
- WOMAC:
-
= Western Ontario and McMaster Universities Osteoarthritis Index.
References
Felson DT: An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004, 42: 1-9. 10.1016/S0033-8389(03)00161-1.
Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, Sowers M, McAlindon T, Spector TD, Poole AR, Yanovski SZ, Ateshian G, Sharma L, Buckwalter JA, Brandt KD, Fries JF: Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000, 133: 635-646.
Green GA: Understanding NSAIDs: from aspirin to COX-2. Clin Cornerstone. 2001, 3: 50-60. 10.1016/S1098-3597(01)90069-9.
Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, Moskowitz RW, Schnitzer TJ: Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum. 1995, 38: 1541-1546. 10.1002/art.1780381104.
Anonymous: Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update: American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000, 43: 1905-1915. 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P.
Pendleton A, Arden N, Dougados M, Doherty M, Bannwarth B, Bijlsma JW, Cluzeau F, Cooper C, Dieppe PA, Günther KP, Hauselmann HJ, Herrero-Beaumont G, Kaklamanis PM, Leeb B, Lequesne M, Lohmander S, Mazieres B, Mola EM, Pavelka K, Serni U, Swoboda B, Verbruggen AA, Weseloh G, Zimmermann-Gorska I: EULAR recommendations for the management of osteoarthritis: report of task force standing committee for International Clinical Studies including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2000, 59: 936-944. 10.1136/ard.59.12.936.
Singh G: Recent considerations in nonsteroidal anti-inflammatory drug gastropathy. Am J Med. 1998, 105:
Griffin MR: Epidemiology of nonsteroidal anti-inflammatory drug associated gastrointestinal injury. Am J Med. 1998, 104:
Wright JM: Double-edged sword of COX-2 selective NSAIDs. CMAJ. 2002, 167: 1131-1137.
Singh GB, Atal CK: Pharmacology of an extract of salai guggal ex-Boswellia serrata, a new non-steroidal anti-inflammatory agent. Agents Actions. 1986, 18: 407-412. 10.1007/BF01965005.
Ethan B, Heather B, Theresa DH, Ivo F, Sadaf H, Jens H, David S, Catherine U: Boswellia: An evidence-based systematic review by the natural standard research collaboration. J Herbal Pharmacother. 2004, 4: 63-83. 10.1300/J157v04n02_07.
Safayhi H, Mack T, Sabieraj J, Anazodo MI, Subramanian LR, Ammon HPT: Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. J Pharmacol Exp Ther. 1992, 26: 1143-1146.
Sailer ER, Subramanian LR, Rall B, Hoernlein RF, Ammon HPT, Safayhi H: Acetyl-11-keto-β-boswellic acid (AKBA): structure requirements or binding and 5-lipoxygenase inhibitory activity. Br J Pharmacol. 1996, 117: 615-618.
Roy S, Khanna S, Shah H, Rink C, Phillips C, Preuss H, Subbaraju GV, Trimurtulu G, Krishnaraju AV, Bagchi M, Bagchi D, Sen CK: Human genome screen to identify the genetic basis of the anti-inflammatory effects of Boswellia in microvascular endothelial cells. DNA Cell Biol. 2005, 24: 244-255. 10.1089/dna.2005.24.244.
Lalithakumari K, Krishnaraju AV, Sengupta K, Subbaraju GV, Chatterjee A: Safety and toxicological evaluation of a novel, standardized 3-O-acetyl-11-keto-β-boswellic acid (AKBA)-enriched Boswellia serrata extract (5-Loxin). Toxicol Mechanisms Methods. 2006, 16: 199-226. 10.1080/15376520600620232.
Joos S, Rosemann T, Szecsenyi J, Hahn EG, Willich SN, Brinkhaus B: Use of complementary and alternative medicine in Germany – a survey of patients with inflammatory bowel disease. BMC Complement Altern Med. 2006, 6: 19-10.1186/1472-6882-6-19.
Anthoni C, Laukoetter MG, Rijcken E, Vowinkel T, Mennigen R, Muller S, Senninger N, Russell J, Jauch J, Bergmann J, Granger DN, Krieglstein CF: Mechanisms underlying the anti-inflammatory actions of boswellic acid derivatives in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2006, 290: G1131-G1137. 10.1152/ajpgi.00562.2005.
Gupta I, Parihar A, Malhotra P, Gupta S, Ludtke R, Safayhi H, Ammon HP: Effects of gum resin of Boswellia serrata in patients with chronic colitis. Planta Med. 2001, 67: 391-395. 10.1055/s-2001-15802.
Kimmatkar N, Thawani V, Hingorani L, Khiyani R: Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee: a randomized double blind placebo controlled trial. Phytomedicine. 2003, 10: 3-7. 10.1078/094471103321648593.
Bellamy N, Buchnan WW, Goldsmith CH, Campbell J, Stitt LW: Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to anti-rheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988, 15: 1833-1840.
Lequesne MG, Mery C, Samson M, Gerard P: Indexes of severity for osteoarthritis of the hip and knee validation-value in comparison with other assessment tests. Scand J Rheumatology. 1987, 65: 85-89. 10.3109/03009748709102182.
Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE: Pain measurement: an overview. Pain. 1985, 22: 1-31. 10.1016/0304-3959(85)90145-9.
Bellamy N, Bell MJ, Goldsmith CH, Pericak D, Walker V, Raynauld JP, Torrance GW, Tugwell P, Polisson R: The effectiveness of hylan G-F 20 in patients with knee osteoarthritis: an application of two sets of response criteria developed by the OARSI and one set developed by OMERACT-OARSI. Osteoarthritis Cartilage. 2005, 13: 104-110. 10.1016/j.joca.2004.10.016.
Day R, Morrison B, Luza A, Castaneda O, Strusberg A, Nahir M, Helgetveit KB, Kress B, Daniels B, Bolognese J, Krupa D, Seidenberg B, Ehrich E: A randomized trial of the efficacy and tolerability of the COX-2 inhibitor rofecoxib vs ibuprofen in patients with osteoarthritis. Arch Intern Med. 2000, 160: 1781-1787. 10.1001/archinte.160.12.1781.
Gottesdiener K, Schnitzer T, Fisher C, Bockow B, Markenson J, Ko A, DeTora L, Curtis S, Geissler L, Gertz BJ: Results of a randomized, dose-ranging trial of etoricoxib in patients with osteoarthritis. Rheumatology (Oxford). 2002, 41: 1052-1061. 10.1093/rheumatology/41.9.1052.
Bingham CO, Sebba AI, Rubin BR, Ruoff GE, Kremer J, Bird S, Smugar SS, Fitzgerald BJ, O'Brien K, Tershakovec AM: Efficacy and safety of etoricoxib 30 mg and celecoxib 200 mg in the treatment of osteoarthritis in two identically designed, randomized, placebo-controlled, non-inferiority studies. Rheumatology (Oxford). 2007, 46: 496-507. 10.1093/rheumatology/kel296.
Reginster JY, Malmstrom K, Mehta A, Bergman G, Ko AT, Curtis SP, Reicin AS: Evaluation of the efficacy and safety of etoricoxib compared with naproxen in two, 138-week randomized studies of osteoarthritis patients. Ann Rheum Dis. 2007, 66: 945-951. 10.1136/ard.2006.059162.
Power analysis for ANOVA designs. [http://www.math.yorku.cal/SCS/Online/power/]
Garnero P, Rousseau J, Delmas PD: Molecular basis and clinical use of biochemical markers of bone, cartilage and synovium in joint diseases. Arthritis Rheum. 2000, 43: 953-968. 10.1002/1529-0131(200005)43:5<953::AID-ANR1>3.0.CO;2-Q.
Felson DT: Osteoarthritis of the knee. N Engl J Med. 2006, 354: 841-848. 10.1056/NEJMcp051726.
Turner JA, Deyo RA, Loeser JD, Von Korff M, Fordyce WE: The importance of placebo effects in pain treatment and research. JAMA. 1994, 271: 1609-1614. 10.1001/jama.271.20.1609.
Walach H, Sadaghiani C, Dehm C, Bierman D: The therapeutic effect of clinical trials: understanding placebo response rates in clinical trials: a secondary analysis. BMC Med Res Methodol. 2005, 5: 26-37. 10.1186/1471-2288-5-26.
McAlindon TE, LaValley MP, Gulin JP, Felson DT: Glucosamine and chondroitin for treatment of osteoarthritis: a systematic quality assessment and meta-analysis. JAMA. 2000, 283: 1469-1475. 10.1001/jama.283.11.1469.
Zenk JL, Helmer TR, Kuskowski MA: The effects of milk protein concentrate on the symptoms of osteoarthritis in adults: an exploratory, randomized, double blind, placebo-controlled trial. Curr Ther Res. 2002, 63: 430-442. 10.1016/S0011-393X(02)80049-2.
Caterson B, Flannery CR, Hughes CE, Little CB: Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000, 19: 333-344. 10.1016/S0945-053X(00)00078-0.
Little CB, Hughes CE, Curtis CL, Janusz MJ, Bohne R, Wang-Weigand S, Taiwo YO, Mitchell PG, Otterness IG, Flannery CR, Caterson B: Matrix metalloproteinases are involved in C-terminal and interglobular domain processing of cartilage aggrecan in late stage cartilage degradation. Matrix Biol. 2002, 21: 271-288. 10.1016/S0945-053X(02)00004-5.
Acknowledgements
This study is funded by Laila Impex R&D Center, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
This study is funded by Laila Impex R&D Center, India. KS, TG and KVA are employee of Laila Impex Research Centre, Vijayawada, India. ARS and SM are employee of ASRAM, Eluru, India. KVSS (SV University, Tirupati, India), DD (University of Connecticut, Storrs, CT, USA) and SPR (University of California Davis Medical Center, Davis, CA and VA Medical Center Sacramento, CA, USA) are consultants for the Laila Impex Research Center.
Authors' contributions
KS contributed to the design of the project and data analysis, and was primarily responsible for writing the manuscript. KVA contributed to the design of the project, patient recruitment and management, and data collection. ARS and AM worked with patients to obtain informed consent, conducted clinical evaluations, took samples and evaluated therapeutic response of 5-Loxin®. TG contributed as the study coordinator and helped to review the manuscript. KVSS and DD helped in clinical data analysis. SPR helped in designing the study, conducting data analysis and writing the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Sengupta, K., Alluri, K.V., Satish, A.R. et al. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin®for treatment of osteoarthritis of the knee. Arthritis Res Ther 10, R85 (2008). https://doi.org/10.1186/ar2461
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/ar2461