Abstract
MRI bone oedema occurs in various forms of inflammatory and non-inflammatory arthritis and probably represents a cellular infiltrate within bone. It is common in early rheumatoid arthritis and is associated with erosive progression and poor functional outcome. Histopathological studies suggest that a cellular infiltrate comprising lymphocytes and osteoclasts may be detected in subchondral bone and could mediate the development of erosions from the marrow towards the joint surface. There is emerging evidence from animal models that such an infiltrate corresponds with MRI bone oedema, pointing towards the bone marrow as a site for important pathology driving joint damage in rheumatoid arthritis.
Similar content being viewed by others
In the mid-17th century, a Dutch apprentice to a textile merchant, Anton van Leeuwenhoek, was the first to see and describe bacteria, yeasts and the circulation of blood corpuscles in capillaries using a new tool, the light microscope [1]. The subsequent elucidation of the microbiological basis of infectious disease can be traced back, in part, to his pioneering work in imaging. A parallel exists between the invention of the microscope and the development of magnetic resonance imaging (MRI), which allows new ways to explore biological systems. In rheumatoid arthritis (RA), MRI provides information about synovitis and erosion in early disease [2, 3] when inflammatory and destructive articular change is typically subradiographic. In addition, it has revealed something new and unexpected; the appearance referred to as bone oedema. This MRI finding has been reported in other conditions, such as osteonecrosis [4], osteoarthritis [5], and ankylosing spondylitis [6], and in the sports medicine setting where it appears associated with mechanical stress [7]. However, in RA there is evidence to suggest that bone oedema represents a pivotal change occurring within subchondral bone that may be associated with early events in disease pathogenesis, which have not previously been accessible to any form of imaging.
Just as the existence of micro-organisms was not expected prior to the invention of the microscope, so the presence and importance of bone oedema could not have been predicted using the other sonographic and radiographic imaging techniques used to investigate RA. MRI is unique in that it images protons, which are usually contained within water molecules (hence 'oedema'), these in turn frequently being contained within cells [8]. Although ultrasound can be used to image synovitis by detecting thickening of the synovial membrane [9] and can reveal increased synovial blood flow using Doppler imaging [10], cellular infiltration within bone remains invisible. Radiography, while an excellent technique for imaging cortical bone, also cannot detect subcortical cellular infiltrates, which are not necessarily associated with periarticular osteopenia [11]. Histology could be used to examine subchondral bone but resection of this tissue is almost never done in early RA and the primary focus for tissue immunohistochemistry has been the accessible synovium. There are currently no published studies comparing the histopathology of subchondral bone in RA with MRI appearances (specifically bone oedema) but these are underway. Unfortunately, they are likely to include patients with longstanding disease where erosive and secondary degenerative change could complicate the picture. In ankylosing spondylitis, such a study has recently been published, describing preoperative bone oedema in three of eight ankylosing spondylitis patients with longstanding disease who underwent spinal surgery involving resection of zygapophyseal joints [12]. Concordance was observed between bone oedema and a mononuclear inflammatory infiltrate in bone marrow, but only when the latter was relatively intense, suggesting that the MRI feature is only apparent above a certain threshold.
Until recently, it was necessary to go back to literature published in the early 1980s for a description of the histology of subchondral bone in RA. Barrie [13] in 1981 described "diffuse osteitis" within subchondral bone in 35% of patients undergoing metatarsal head resection. In the November 2005 issue of Arthritis and Rheumatism, Bugatti and colleagues [14] published a similar immunohistochemical study of RA subchondral bone (from specimens obtained at the time of joint replacement), using contemporary techniques. They found lymphoid aggregates on the subchondral side of the joint in established RA, often associated with osteoclasts within the bone marrow abutting the cortex. They concluded that "an inflammatory lymphoid infiltrate ... is a characteristic feature of RA subchondral bone marrow... raising the hypothesis that subchondral bone marrow inflammation might develop independent of the propagation of synovial tissue."
The MRI finding of bone oedema has been an important driver in refocusing interest towards the subchondral bone in early RA. A cohort study published in 1998 [2] revealed bone oedema to be present at the carpus in 64% of RA patients within 6 months of disease onset and in 45% after 6 years [15]. There was clear evidence at one and six years after disease onset [15, 16] that bone oedema was a pre-erosive lesion. The bone oedema score at presentation and one year later was correlated with radiographic erosion and joint space narrowing scores six years later [15] and, interestingly, even with function, as measured by the physical function component of the short-form-36 score [17]. A later study also showed a link between bone oedema scores and tendon function at eight years in these patients [18]. Others have also found bone oedema to be common in RA [19], and it was described by Ostendorf and colleagues [20] at the metatarsal heads within only two months of the onset of symptoms. Tamai and colleagues [21] recently confirmed its association with disease severity as indicated by inflammatory markers such as C-reactive protein and interleukin-6 levels in early RA. At the other end of the spectrum of disease duration, we have recently described florid bone oedema, at the site of intended surgery, in RA patients awaiting joint replacement or fusion. These data suggested that bone oedema may be especially associated with painful and aggressive disease [22]. Taken together, these lines of evidence suggest that the process we recognize as MRI bone oedema is widespread and relatively common in early and late disease and tied to the development of long term joint damage. Before the advent of MRI, this process sited in the subchondral bone was unsuspected and certainly not accorded any significance in terms of disease pathogenesis.
New work is now emerging to link the entity of bone oedema with current theories of the immunopathogenesis of RA. Hirohata and colleagues [23], in a highly accessed article published in Arthritis Research and Therapy in early 2006, described a study of bone marrow cells aspirated from the iliac crests of RA patients. CD34+ stem cells that were abnormally sensitive to tumor necrosis factor (TNF)α [24] were found to express high levels of the nuclear factor kappa B (NFκB) transcription factor, contrasting with cells from osteoarthritis patients where NFκB expression was normal and TNF sensitivity not observed. These authors suggested that a bone marrow stem cell abnormality could underlie RA and proposed a disease model where such cells could, under the influence of TNF, differentiate into fibroblast-like cells, and travel to the synovial membrane where they might appear as type B synoviocytes and promote synovitis [23]. Alternatively, they could travel via the systemic circulation to the subchondral bone marrow and initiate inflammatory and pre-erosive changes from there, possibly including activation of osteoclasts as described by Schwarz and colleagues [25].
Angiogenesis is known to accompany cellular proliferation in rheumatoid synovial membrane via mediators such as vascular endothelial growth factor and platelet derived growth factor [26]. Ostendorf and colleagues [27] investigated rheumatoid finger joints using miniarthroscopy and found that macroscopic vascularization of the synovial membrane correlated with histological features of angiogenesis and clinical signs of disease activity. If the subchondral bone is proposed as another site of cellular proliferation in RA, one would also expect to find angiogenesis there. Interestingly, there is a suggestion from MRI data that this may occur as regions of bone oedema which are typically recognized as areas of hyperintense signal on T2w images, also exhibit increased signal after intravenous injection of gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA). This contrast agent travels within blood vessels and causes hyperintensity in vascular tissue [28]. An example from a patient with a 1 year history of RA is shown in Figure 1.
MRI scans from a 65 year old female rheumatoid arthritis patient with disease duration of one year. (a) Coronal T1 weighted image of the dominant wrist with reduced signal indicating florid bone oedema involving the entire lunate bone (circle). (b) Equivalent image following the injection of contrast (gadolinium diethylenetriamine pentaacetic acid (GdDPTA)) shows very bright signal within the lunate, suggesting the presence of vascularized tissue (slice does not exactly correspond with pre-GdDPTA image). (c) Axial T2w image with bright signal confirming bone oedema at the lunate.
Finally, animal studies are emerging to clarify the role of the bone marrow as a site of pathology in RA. Marinova-Mutafchieva and colleagues [29] described an inflammatory infiltrate in the subchondral bone of TNF-transgenic mice where TNF-responsive mesenchymal cells were identified within enlarged bony canals connecting bone marrow to synovium. Most recently, Proulx and colleagues [30] examined TNF-transgenic mice using a high-resolution 7 Tesla MRI scanner. They described the presence of bone oedema and correlated this histologically with a highly cellular infiltrate within the bone marrow. Another form of imaging, high-resolution multipinhole single-photon-emission computed tomography (MPH-SPECT), has revealed accelerated bone turnover within the joints of interleukin-1 receptor antagonist deficient mice [31]. In a single patient with early RA, increased uptake in a central, interarticular distribution was detected by MPH-SPECT when the MRI signal for bone marrow on short tau inversion recovery (STIR) images was normal, raising the possibility that even earlier changes in the subchondral bone could be apparent using this sensitive, high-resolution technique [32].
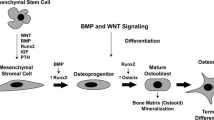
Figure 2 combines evidence from several imaging and histological studies to suggest a disease model for RA, where cells originating from bone marrow travel to the joint and either mediate erosion from synovial membrane inwards or from the subchondral bone outwards towards the joint surface. This bone-marrow-centered model would be consistent with the therapeutic success of drugs such as rituximab [33], aimed at B cells, which may reside in the synovium but originate from the bone marrow. It also predicts that repopulation of the bone marrow with allotypically different cells might effect remission of RA and this has been described in recipients of allogeneic bone marrow transplants performed in the 1980s [34]. It seems we are now on the road to unraveling the mystery of what MRI bone oedema actually means in RA. The implications are exciting and suggest a new focus for understanding disease pathology and influencing disease progression; moving away from the synovium and towards the bone marrow.
Potential role of bone marrow-derived stem cells in trafficking to the subchondral bone and synovial membrane in rheumatoid arthritis joints, resulting in a subchondral cellular infiltrate (seen as bone oedema on MRI) followed by erosion. (a) CD34+ stem cells from bone marrow express high levels of NFkB, which leads to unusual sensitivity to TNFα. (b) Stem cells differentiate into fibroblast-like cells and travel via the circulation to synovial membrane to become type B synoviocytes – here they mediate formation of erosions via production of proinflammatory cytokines and matrix metalloproteinases [23,25]. (c) Stem cells may also traffic to the subchondral bone marrow where they differentiate into mesenchymal cells. These cells could then travel via bony canals from bone marrow to synovium [29] to excite an inflammatory response. (d) Alternatively, stem cells could travel to subchondral bone and at this site could mediate an inflammatory response via T/B cell interactions associated with angiogenesis [26] and osteoclast activation. This could lead to erosions originating from inside the bone, directed outwards towards joint surface [14]. (e) Coronal T2 weighted MRI scan of the wrist in early rheumatoid arthritis reveals bone oedema at the bases of the 2nd and 3rd metacarpals and adjacent regions of trapezoid and capitate carpal bones. Small intraosseous erosions are also apparent.
Abbreviations
- MPH-SPECT:
-
= high-resolution multipinhole single-photon-emission computed tomography
- MRI:
-
= magnetic resonance imaging
- NFκB:
-
= nuclear factor kappa B
- RA:
-
= rheumatoid arthritis
- TNF:
-
= tumor necrosis factor.
References
The Collected Letters of Antoni van Leeuwenhoek 1701–1704. 1996, ISBN: 9026514506. Lisse, The Netherlands: Swets and Zeitlinger, 14:
McQueen FM, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan PLJ, McLean L: Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals a high prevalence of erosion at four months after symptom onset. Ann Rheum Dis. 1998, 57: 350-356.
Klarlund M, Østergaard M, Jensen KE, Madsen JL, Skjødt H, the TIRA group: Magnetic resonance imaging, radiography, and scintigraphy of the finger joints: one year follow up of patients with early arthritis. Ann Rheum Dis. 2000, 59: 521-528. 10.1136/ard.59.7.521.
Lecouvet FE, van de Berg BC, Maldague BE, Lebon CJ, Jamart J, Saleh M, Noel H, Malghem J: Early irreversible osteonecrosis versus transient lesions of the femoral condyles: prognostic value of subchondral bone and marrow changes on MR imaging. AJR. 1998, 170: 71-77.
Carrino JA, Blum J, Parellada JA, Schweitzer ME, Morrison WB: MRI of bone marrow edema-like signal in the pathogenesis of subchondral cysts. Osteoarthritis Cartilage. 2006, 14: 1081-1085. 10.1016/j.joca.2006.05.011.
Braun J, Baraliakos X, Golder W, Brandt J, Rudwaleit M, Listing J, Bollow M, Sieper J, van der Heijde D: Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: Evaluation of a new scoring system. Arthritis Rheum. 2003, 48: 1126-1136. 10.1002/art.10883.
Hoy G, Wood T, Phillips N, Connell D: When physiology becomes pathology: the role of magnetic resonance imaging in evaluating bone marrow oedema in the humerus in elite tennis players with an upper limb pain syndrome. Br J Sports Med. 2006, 40: 710-713. 10.1136/bjsm.2005.021386.
Peterfy CG: New developments in imaging in rheumatoid arthritis. Curr Opin Rheumatol. 2003, 15: 288-295. 10.1097/00002281-200305000-00017.
Grassi W, Cervini C: Ultrasonography in rheumatology: an evolving technique. Ann Rheum Dis. 1998, 57: 268-271.
Szkudlarek M, Court-Payen , Strandberg C, Klarlund M, Klausen T, Østergaard M: Power Doppler ultrasonography for assessment of synovitis in the metacarpophalangeal joints of patients with rheumatoid arthritis: a comparison with dynamic magnetic resonance imaging. Arthritis Rheum. 2001, 44: 2018-2023. 10.1002/1529-0131(200109)44:9<2018::AID-ART350>3.0.CO;2-C.
Peterfy CG: MR imaging. Baillieres Clin Rheumatol. 1996, 10: 635-678. 10.1016/S0950-3579(96)80055-0.
Appel H, Loddenkemper C, Grozdanowicz Z, Ebhardt H, Dreimann M, Hempfing A, Stein H, Metz-Stavenhagen P, Rudwaleit M, Sieper J: Correlation of histopathological findings and magnetic resonance imaging (MRI) in the spine of patients with ankylosing spondylitis. Arthritis Res Ther. 2006, 8: R143-10.1186/ar2035.
Barrie HJ: Histologic changes in rheumatoid disease of the metacarpal and metatarsal heads as seen in surgical material. J Rheumatol. 1981, 8: 246-257.
Bugatti S, Caporali R, Manzo A, Vitolo B, Pitzalis C, Montecucco C: Involvement of subchondral bone marrow in rheumatoid arthritis. Lymphoid neogenesis and in situ relationship to subchondral bone marrow osteoclast recruitment. Arthritis Rheum. 2005, 52: 3448-3459. 10.1002/art.21377.
McQueen FM, Benton N, Perry D, Crabbe J, Robinson E, Yeoman S, McLean L, Stewart N: Bone oedema scored on magnetic resonance scans of the dominant carpus at presentation predicts radiographic joint damage at the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum. 2003, 48: 1814-1827. 10.1002/art.11162.
McQueen FM, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan PLJ, McLean L: Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Ann Rheum Dis. 1999, 58: 156-163.
Benton N, Stewart N, Crabbe J, Robinson E, Yeoman S, McQueen FM: MRI of the wrist in early rheumatoid arthritis can be used to predict functional outcome at 6 years. Ann Rheum Dis. 2004, 63: 555-561. 10.1136/ard.2003.011544.
Zheng S, Yeoman S, Robinson E, Crabbe J, Stewart N, Rouse J, McQueen FM: Magnetic resonance imaging (MRI) bone oedema predicts 8 year tendon function at the wrist but not the requirement for orthopaedic surgery in rheumatoid arthritis patients. Ann Rheum Dis. 2006, 65: 607-611. 10.1136/ard.2005.043323.
Savnik A, Malmskov H, Thomsen HS, Graff LB, Nielsen H, Danneskiold-Samsoe B, Boesen J, Bliddal H: MRI of the wrist and finger joints in inflammatory joint diseases at 1-yr interval: MRI features to predict bone erosions. Eur Radiol. 2002, 12: 1203-1210. 10.1007/s003300101114.
Ostendorf B, Scherer A, Modder U, Schneider M: Diagnostic value of magnetic resonance imaging of the forefeet in early rheumatoid arthritis when findings on imaging of the metacarpophalageal joints of the hands remain normal. Arthritis Rheum. 2004, 50: 2094-2102. 10.1002/art.20314.
Tamai M, Kawakami A, Takao S, Uetani M, Arima K, Tanaka F, Fujikawa K, Aramaki T, Iwanaga N, Izumi Y, et al: Bone marrow oedema determined by MRI reflects severe disease status in patients with early-stage rheumatoid arthritis. Ann Rheum Dis. 2006, 65 (Suppl II): 629-
Gao A, Østergaard M, Robinson E, Dalbeth N, Doyle A, Shalley G, McQueen F: Unexpected finding of frequent high grade MRI bone oedema within the field of surgery in RA patients awaiting joint replacement/fusion at the hands or feet. Arthritis Rheum. 2006, 54 (Suppl): S625-
Hirohata S, Miura Y, Tmita T, Yoshikawa H, Ochi T, Chiorazzi N: Enhanced expression of mRNA for nuclear factor kB1 (p50) in CD34+ cells of the bone marrow in rheumatoid arthritis. Arthritis Res Ther. 2006, 8: R54-10.1186/ar1915.
Hirohata S, Yanagida T, Nagai T, Sawada T, Nakamura H, Yoshino S, Tomita T, Ochi T: Induction of fibroblast-like cells from CD34(+) progenitor cells of the bone marrow in rheumatoid arthritis. J Leukoc Biol. 2001, 70: 413-421.
Schwarz EM, Looney RJ, Drissi MH, O'Keefe RJ, Boyce BF, Xing L, Ritchlin CT: Autoimmunity and bone. Ann NY Acad Sci. 2006, 1068: 275-283. 10.1196/annals.1346.031.
Maruotti N, Cantatore FP, Crivellato E, Vacca A, Ribatti D: Angiogenesis in rheumatoid arthritis. Review. Histol Histopathol. 2006, 21: 557-566.
Ostendorf B, Iking-Konert C, Bleck E, Dann P, Pauly Th, Friemann J, Schneider M: Vascular imaging of rheumatoid synovium: Macroscopic and microscopic analysis of synovial tissue obtained by miniarthroscopy from finger joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2006, 65 (Suppl II): 66-
Stewart N, McQueen FM, Crabbe JC: MRI of the wrist: a pictorial essay. Australas Radiol. 2001, 45: 268-273. 10.1046/j.1440-1673.2001.00919.x.
Marinova-Mutafchieva L, Williams RO, Funa K, Maini RN, Zvaifler NJ: Inflammation is preceded by tumour necrosis factor-dependent infiltration of mesenchymal cells in experimental arthritis. Arthritis Rheum. 2002, 46: 507-513. 10.1002/art.10126.
Proulx S, Kwok E, Shealy DJ, Ritchlin CT, Schwarz EM: Understanding bone marrow edema in arthritis: 3D-MRI and histology analyses of TNF-Tg mice. Arthritis Rheum. 2006, 54 (Suppl): S798-S799.
Ostendorf B, Scherer A, Wirrwar A, Hoppin JW, Lackas C, Schramm NU, Cohnen M, Mödder U, van den Berg WB, Müller HW, et al: High-resolution multi-pinhole single photon emission computed tomography in imaging experimental and human arthritis. Arthritis Rheum. 2006, 54: 1096-1104. 10.1002/art.21732.
Ostendorf B, Scherer A, Wirrwar A, Hoppin JW, Schramm NU, Cohnen M, Moedder U, Müller HW, Schneider M: Precise detection of bony changes in arthritis and osteoarthritis with high-resolution scintigraphy. Ann Rheum Dis. 2006, 65 (Suppl II): 590-
Edwards JCW, Cambridge G: B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006, 6: 394-403. 10.1038/nri1838.
Lowenthal RM, Francis H, Gill DS: Twenty-year remission of rheumatoid arthritis in 2 patients after allogeneic bone marrow transplant. J Rheumatol. 2006, 33: 812-813.
Acknowledgements
The authors wish to thank Professor P Conaghan for use of the MRI scan in Figure 2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
McQueen, F.M., Ostendorf, B. What is MRI bone oedema in rheumatoid arthritis and why does it matter?. Arthritis Res Ther 8, 222 (2006). https://doi.org/10.1186/ar2075
Published:
DOI: https://doi.org/10.1186/ar2075