Abstract
Endothelial cells are active participants in chronic inflammatory diseases. These cells undergo phenotypic changes that can be characterised as activated, angiogenic, apoptotic and leaky. In the present review, these phenotypes are described in the context of human rheumatoid arthritis as the disease example. Endothelial cells become activated in rheumatoid arthritis pathophysiology, expressing adhesion molecules and presenting chemokines, leading to leukocyte migration from the blood into the tissue. Endothelial cell permeability increases, leading to oedema formation and swelling of the joints. These cells proliferate as part of the angiogenic response and there is also a net increase in the turnover of endothelial cells since the number of apoptotic endothelial cells increases. The endothelium expresses various cytokines, cytokine receptors and proteases that are involved in angiogenesis, proliferation and tissue degradation. Associated with these mechanisms is a change in the spectrum of genes expressed, some of which are relatively endothelial specific and others are widely expressed by other cells in the synovium. Better knowledge of molecular and functional changes occurring in endothelial cells during chronic inflammation may lead to the development of endothelium-targeted therapies for rheumatoid arthritis and other chronic inflammatory diseases.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic inflammatory disease affecting the joints, and is associated with increased morbidity and mortality [1–3]. The synovium or synovial membrane, which surrounds the joint cavity, becomes massively hypertrophied in RA. This tissue, known as pannus, can become invasive, penetrating and degrading the cartilage and bone, resulting in joint deformities, in functional deterioration and in profound disability.
The lining layer, or intima, of the synovium is normally one to three cells thick and it comprises macrophage-like cells and fibroblast-like cells [4]. This layer undergoes thickening and hypertrophy in RA, largely due to the increased recruitment of monocytes from the blood supply in the deeper layer, or subintima, of the tissue [5, 6]. Other inflammatory cells such as T cells (mainly CD45RO) and B lymphocytes migrate from the blood into the synovium and can form ectopic lymphoid follicles around blood vessels. These structures resemble the lymphoid follicles of lymph nodes. In addition, neutrophils migrate into the synovium and end up in large numbers in the synovial joint fluid.
The role of endothelial cells in RA
Endothelial cells are active participants in the inflammatory process. They are involved in diverse activities including the regulation of leukocyte extravasation, angiogenesis, cytokine production, protease and extracellular matrix synthesis, vasodilation and blood vessel permeability, and antigen presentation [7].
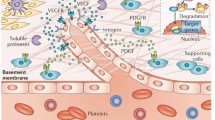
In RA, endothelial cells in the synovium are generally held to play a central role in the pathophysiology. The cells achieve this in several ways. First, as a component of blood vessels in the subintima, endothelial cells allow the migration of leukocytes such as T cells, B cells, monocytes, neutrophils and dendritic cells into the joint tissues and fluid. Endothelial cells undergo activation, expressing adhesion molecules and presenting chemokines, leading to leukocyte migration from the blood into the tissue. Second, the permeability of endothelial cells increases, leading to plasma extravasation, to oedema formation and to swelling of the joint [8]. Third, endothelial cells proliferate as part of the angiogenic process, which allows a supply of oxygen and nutrients to the growing pannus. There is also a net increase in the turnover of endothelial cells since the number of apoptotic endothelial cells increases as well as the number of proliferative cells [9]. Finally, endothelial cells express various cytokines, cytokine receptors and proteases that are involved in angiogenesis, in proliferation and in tissue degradation.
As part of this spectrum of biological activities, synovial endothelial cells in RA express a variety of phenotypes that can be characterised as being activated, angiogenic, apoptotic and leaky. The intent of the present review is to examine the pattern of human endothelial cell gene expression associated with these phenotypic alterations and to examine whether certain genes are selectively regulated in endothelial cells and not in other cell types. (See Table 1 for a summary of genes.)
Morphological and ultrastructural activation
Changes to the endothelium are among the first pathophysiological events that occur in the human RA synovium, and these changes occur in venules and capillaries rather than in arterioles [8, 10]. During the first month of synovitis, these changes include hypertrophy with the cells becoming cuboidal in morphology, the development of gaps between endothelial cells and the presence of multiple concentric layers in the basement membrane. Associated with these changes is the transendothelial migration of numerous mononuclear and polymorphonuclear inflammatory cells.
In another study, synovial biopsies from one patient with monoarthritis who was subsequently found to have RA showed endothelial cell proliferation with no detectable inflammatory infiltrate [8, 10]. Endothelial cell proliferation may thus be the initial event in RA. However, another study suggested that the recruitment of mononuclear cells from the blood into the perivascular areas and the lining layer occurs before endothelial morphological alteration and proliferation [11].
The cuboidal morphology of the endothelial cells of synovial venules resembles that of high endothelial venules (HEV), which are the postcapillary venules of lymphoid tissues specialised in lymphocyte migration [12]. This change presumably represents a response to cytokines and other factors that occur in the synovial milieu, and relates to the increased leukocyte trafficking into the tissue [13]. The postcapillary venules of the rheumatoid synovium in patients with active, untreated disease exhibit HEV-like morphology, especially in regions near lymphocyte aggregates, whereas tissue samples from patients whose disease has been modified by treatment exhibit a flatter endothelium [13, 14].
In the skin of primates, HEV-like blood vessels are induced by stimulation with tumour necrosis factor (TNF)-α and IFN-γ, which also elicit the adhesion and extravasation of inflammatory cells [15]. Other studies have shown that HEV are not absolutely necessary for transendothelial migration of T cells as migration also occurs through flat endothelium, although the transit time is considerably longer [16]. The HEV-like morphology may thus be associated with the increased leukocyte recruitment that occurs in synovial inflammation, due to the enhanced or selective presentation of chemokines and adhesion molecules, whereas the flatter endothelium may be more associated with basal leukocyte trafficking.
Many of these changes are not specific to RA, since cuboidal endothelial cells have been demonstrated in a variety of inflammatory diseases [12]. In addition, multiple concentric layers in the basement membrane of capillaries have been observed in muscle capillaries in other rheumatic diseases such as systemic lupus erythematosis and systemic sclerosis [17, 18], and in nonrheumatic diseases such as Duchenne muscular dystrophy [19].
Gene expression associated with leukocyte migration
Adhesion molecules
As part of leukocyte migration occurring at sites of inflammation, circulating leukocytes adhere to the luminal surface of the endothelium. This interaction, according to the current paradigm, involves the sequential engagement of leukocyte and endothelial adhesion molecules. First, selectins and their carbohydrate counterligands mediate leukocyte tethering and rolling. Leukocyte integrins and their ligands on endothelial cells, including intercellular adhesion molecules (ICAMs), then mediate firm leukocyte adhesion [20].
Endothelial cells have been shown to express a variety of adhesion molecules in the RA synovium. As a part of endothelial activation, several of these molecules are induced or upregulated by cytokines such as TNF-α, IL-1 and IFN-γ [21]. There is good evidence for the presence of selectins and their counterligands that could promote leukocyte tethering and rolling [13, 22]. E-selectin shows an endothelial-selective distribution in synovia where its expression is upregulated in RA compared with osteoarthritic (OA) tissue [23]. This adhesion molecule is a marker of endothelial activation in lymphocyte-rich regions of the RA synovium [24]. In addition, synovial endothelial cells situated in inflammatory infiltrates stain positively with the monoclonal antibody MECA-79 [25]. This antibody recognises sulphated carbohydrate structures on counterligands for L-selectin [26], which are expressed on HEV from lymphoid and chronically inflamed tissues [12]. P-selectin has been detected on RA endothelial cells where its distribution is endothelial selective, and there is no difference in the level of expression between RA and control samples [27].
ICAM-1 is a ligand for the β2 integrins of leukocytes and is present on most RA synovial endothelial cells, as well as on macrophages and fibroblasts [21, 23, 28]. ICAM-1 expression is upregulated on these cell types in RA compared with normal synovium. There is also increased ICAM-1 expression on cuboidal HEV-like endothelia compared with 'flat' endothelia. ICAM-1, like E-selectin, is hence considered a marker of endothelial activation. ICAM-2, another β2 integrin ligand, is mainly expressed on synovial endothelial cells and not by other cell types [23, 28]. However, this adhesion molecule is expressed by most RA endothelia and normal endothelia, suggesting that it is not an activation antigen on these cells. ICAM-3 has been detected on cells of myeloid origin in the synovium, but there is little or no expression of this molecule on the endothelium [28]. All three ICAMs are expressed by RA synovial dendritic cells that also harbour MHC class II antigens.
Vascular cell adhesion molecule (VCAM)-1 mRNA and protein is present in the RA endothelium, albeit weakly, and is mainly expressed in lining cells and macrophages [29, 30]. The CS-1 isoform of fibronectin is expressed on the luminal surface of the endothelium and by lining layer cells in RA synovia, with less expression occurring in control synovia [22, 31, 32]. Both VCAM-1 and CS-1 fibronectin are ligands for α4 integrins expressed by lymphocytes and monocytes, and may be functional in lymphocyte adhesion to synovial endothelial cells [33].
CD31 (PECAM-1) is present on most synovial endothelial cells, as well as on macrophages and lining cells, and its expression on endothelia is comparable in RA and normal synovia [23, 27]. CD146 is a cell adhesion molecule that belongs to the immunoglobulin supergene family and is potentially involved in leukocyte–endothelial interactions [34]. CD146 is expressed almost exclusively by the vascular endothelium in RA synovia and in normal synovia. The high levels of soluble CD146 found in RA synovial fluid, particularly in early disease, may reflect increased endothelial activity and angiogenesis. Vascular adhesion protein-1 was originally isolated from synovial endothelial cells, and it localises selectively to endothelial cells and not to other cell types in human RA synovia [35]. Vascular adhesion protein-1 expression is increased in joint inflammation and is a marker of activated endothelium in the pig and the dog [36]. Other adhesion molecules expressed by RA synovial endothelial cells include CD44 and cadherins [13, 23, 27].
The functional importance of adhesion molecules in human RA has been shown by the administration of antibodies in vivo. For example, anti-ICAM-1 treatment in RA patients with active disease causes a reduction in disease activity [37] and blocks trafficking of T cells [38]. In another study, anti-TNF treatment with the antibody infliximab decreases serum E-selectin and ICAM-1 levels, suggesting that this may reflect diminished activation of endothelial cells in the synovium, leading to reduced migration of leukocytes into human RA joints [39]. Furthermore, administration of anti-E-selectin in animal models of RA results in a marked decrease of polymorph and monocyte migration into arthritic rat joints, and results in inhibition of T-cell recruitment, causing reduced T-cell-mediated inflammation [40]. VCAM-1 blockade also reduces the clinical severity in collagen-induced arthritis in mice, most probably by altering B-cell trafficking [41].
Chemokines
Chemokines play several roles at inflammatory sites. They initiate firm adhesion of leukocytes to the endothelium by activating integrins on the leukocyte cell surface [42, 43]. Chemokines then direct leukocyte migration across the endothelium and through the extracellular matrix into the tissue [44].
There have been numerous reports showing the expression of chemokines in the RA synovium and the joint fluid [45], and some of these studies have detected the presence of chemokine proteins in the endothelium. The chemokines with an endothelial distribution include ENA-78 (recently designated CXCL5), IL-8 (CXCL8), SDF-1 (CXCL12), MCP-1 (CCL2), MIP-1 (CCL3), SLC (CCL21) and ELC (CCL19) [46–52]. These studies suggest that RA endothelial cells can produce numerous chemokines. However, this cannot be stated unequivocally since nearly all the studies excluded mRNA data. It is therefore not possible to determine whether the endothelial cells themselves produce the chemokines or whether they are produced elsewhere in the synovium, such as by macrophages or fibroblasts, and the endothelium is binding and internalising these mediators as part of the mechanisms of chemokine transcytosis and presentation to blood leukocytes [20, 53, 54]. In this respect, chemokine binding sites for IL-8, MCP-1, MIP-1 and RANTES, which may transcytose and present chemokines, have been shown to be expressed by the synovial endothelium [55, 56].
A few studies, however, have examined mRNA and protein expression. In the cases of IL-8, MCP-1 and SLC, their mRNAs and proteins are present in the RA synovial endothelium [50, 57] indicating that such cells can both synthesise and present these chemokines. In contrast, SDF mRNA occurs in RA synoviocytes and the protein is present in endothelial cells, where it is presented attached to heparan sulphate [58]. There may therefore be selectivity in the types of chemokines produced by synovial endothelial cells.
Chemokine receptors and other binding molecules are expressed by the synovial endothelium. CXCR3 is expressed by endothelial cells and mononuclear cells in the lymphoid aggregates of RA synovium [59]. CXCR4 is present in endothelial cells, synoviocytes and inflammatory infiltrates in RA and OA synovial tissue [58, 60]. The Duffy antigen/receptor for chemokines, a promiscuous chemokine binding protein, is present on RA and control synovial endothelial cells, where it shows a selective distribution on venules [56]. In addition, heparan sulphate proteoglycans, which bind and present a wide range of chemokines and other cytokines, are expressed by the synovial endothelial cells of the RA synovium [55].
Several studies have shown the functional importance of chemokines in animal models and human RA, and some of these have been on chemokines produced by synovial endothelial cells. For example, administration of an anti-IL-8 antibody in rabbit experimental arthritis almost completely blocks the infiltration of neutrophils into the joints and provides protection from tissue damage in the early phase of inflammation [61]. In addition, treatment with an MCP-1 antibody in rat collagen-induced arthritis decreases the number of macrophages recruited to the joints and therefore reduces ankle swelling [62]. Some of the effects of infliximab, a blocking antibody to TNF, in human RA are mediated by chemokines since patients show reduced synovial expression of IL-8 and MCP-1 and decreased leukocyte migration into joints [63].
Gene expression associated with angiogenesis
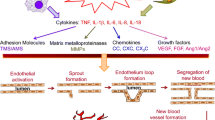
The formation of new blood vessels, termed angiogenesis, occurs in the rheumatoid synovium. It is generally accepted that angiogenesis is central to maintaining and promoting RA [7, 64–66]. The increased endothelial surface area provides the potential for enhanced leukocyte recruitment. In addition, angiogenesis is required for the formation and maintenance of the pannus since the increased volume of this invasive tissue needs a supply of oxygen and nutrients. It is proposed that angiogenesis occurs in several stages. First, at the new branch point the endothelium becomes activated by cytokines, the blood vessel permeability increases and the basement membrane is disrupted by the release of proteolytic enzymes from the endothelium. The endothelial cells then proliferate and migrate towards a chemotactic stimulus to form a new tube, and a new basement membrane is deposited. The increased permeability of the blood vessels may be mediated by proinflammatory agents, such as substance P, and results in tissue oedema. In this context, substance P receptors have been identified on endothelial cells in the RA synovium [67].
Evidence for endothelial cell proliferation in the RA synovium is the expression of cell-cycle-associated antigens (PCNA and Ki67), the αvβ3 integrin, which is associated with vascular proliferation, and vascular endothelial growth factor (VEGF) [68, 69]. Interestingly there is an increased turnover of blood vessels in the RA synovium with focal regions of angiogenesis and vascular regression. Increased cell death in the endothelium was shown by elevated labelling of DNA fragments by terminal uridyldeoxynucleotide nick-end labelling. This occurred in the same synovial tissue samples where there was a concurrent increase in the expression of proliferative markers. These changes are related to the remodelling of the synovial vasculature, leading to reduced vascular densities adjacent to the synovial lining region and to increased vascular densities in the deeper synovium.
It should be mentioned that the vascularity of the RA synovium is the subject of ongoing discussions. One study has shown the mean number of vessels per unit volume of synovium was higher in RA than in control tissue [70], whereas another reported that the vessel density was reduced in the RA synovium [71]. These differences may, in part, be explained by variation in sampling between these studies [72, 73].
Regulators of angiogenesis
Angiogenesis is regulated by the balance of angiogenic activators and inhibitors, many of which have been found in the rheumatoid joint. Several of these substances are thought to act indirectly by upregulating the expression of more potent and specific stimuli. Several growth factors that are capable of promoting angiogenesis are mitogens that act on a broad range of cell types. For example fibroblast growth factor (FGF)-1 and FGF-2 stimulate proliferation, migration and differentiation. FGF-2 mRNA and protein are expressed by RA synovial endothelial cells, as well as by fibroblasts and lining cells, and there is no expression detected in normal synovia [74, 75]. Platelet-derived growth factor (PDGF) is present in the RA synovium and is angiogenic [76]. It is also a potent mitogen for fibroblasts and is chemotactic for fibroblasts and smooth muscle cells. PDGF receptor mRNA and protein have been demonstrated in vascular endothelial and smooth muscle cells and in some stromal cells in the RA synovium [77]. The actions of the angiogenic factor hepatocyte growth factor (HGF) are dependent on its activation by HGF activator and binding to a specific HGF receptor. Both the activator and receptor mRNAs and proteins are expressed by endothelial cells, fibroblasts and macrophages in RA and OA synovia [78].
VEGF, in contrast, is a relatively endothelial-specific angiogenic factor. It has dual activities by eliciting endothelial cell division and increasing vascular permeability, which may increase oedema, and hence joint swelling, in RA. VEGF levels in the sera and synovial fluids of patients with RA are markedly higher than in OA patients or normal patients [66]. In the RA synovium, VEGF mRNA is expressed by lining cells and VEGF protein is present on lining cells, on sublining stromal cells and on the endothelium of small vessels of the pannus and other locations [79, 80]. The mRNAs and proteins for the VEGF receptors VEGFR-1, VEGFR-2 and VEGFR-3 are expressed by RA synovial endothelial cells, where they are more abundant than in controls [81, 82]. These receptors show an endothelial-selective distribution and localise in the vicinity of VEGF-producing cells. Interestingly, VEGF production is upregulated by hypoxia, and the RA joint is more hypoxic than normal. This has lead to the suggestion that the formation of new blood vessels in the pannus may be mediated by hypoxia-driven expression of VEGF [66].
Endoglin is an angiogenesis inducer. This glycoprotein is a receptor for transforming growth factor beta and also acts as an adhesion molecule, containing an arginine–glycine–aspartic acid (RGD) motif. Endoglin is expressed by RA synovial, OA synovial and normal synovial endothelial cells, with little difference in the level of expression between the three groups [83]. The endoglin ligand (transforming growth factor beta 1), however, is upregulated on RA synovial endothelial cells compared with OA and normal endothelial cells [83].
Some chemokines are angiogenic, such as IL-8, and others are angiostatic, such as IP-10 and MIG. Activation of their respective chemokine receptors results in the stimulation or inhibition of endothelial cell proliferation and chemotaxis [84]. Chemokines are mainly expressed by macrophages and fibroblasts in the RA synovium but also by endothelial cells, as shown for IL-8 [50, 51, 85]. Little is known about the expression of chemokine receptors on RA synovial endothelial cells. Some recent studies, however, have shown that CXCR3 (the receptor for IP-10 and MIG) is expressed by the RA synovial endothelium, suggesting that this receptor mediates the angiostatic response to these chemokines [59]. CXCR4, which mediates the proangiogenic activity of SDF, is present in the synovial endothelium [58, 60]. The Duffy antigen may also have an angiogenic function, and this receptor is expressed by synovial endothelial cells [56, 86].
The angiopoietins and their receptors Tie-1 and Tie-2 play a key role in the development of the vasculature. Angiopoietin-1 in adults localises to the endothelium, the lining cells and the macrophages in the RA synovium, where levels are higher than in OA or normal synovium [87]. The expression of Tie-1 and Tie-2 has been reported in the RA synovium, and there is significant upregulation of Tie-1 on endothelial cells, on lining cells and on macrophages in RA compared with normal [87–89]. Angiogenin is a 14 kDa plasma protein that has angiogenic effects, stimulating endothelial cell proliferation. It codistributes with basic FGF in the rheumatoid joint, localising to lining cells, to macrophages and to endothelial cells [75].
Thrombospondin is an inhibitor of angiogenesis and localises primarily to blood vessels, including endothelial cells, and to macrophages in the RA synovium [69, 90] Another angiogenesis regulator is angiotensin II, and angiotensin converting enzyme localises to endothelial cells and to macrophages in RA synovia [91].
The Ets 1 transcription factor has been intimately linked to the regulation of angiogenesis and it is induced in endothelial cells by VEGF. There is upregulation of Ets 1 expression in the RA synovium compared with OA synovium, with Ets 1 mRNA and Ets 1 protein localising to endothelial cells of newly formed vessels [92]. Many growth and survival factors use receptors belonging to the tyrosine kinase family. One of these tyrosine kinases, Axl, has been detected in RA synovia, localising to the capillary endothelium, to vascular smooth muscle cells of blood vessels and to other cells [93].
Cell adhesion molecules play a role in the regulation of angiogenesis. In addition to mediating leukocyte adhesion to the endothelium, VCAM and E-selectin potentiate angiogenesis, with the latter contributing to the morphogenesis of the capillary tube [94]. The invasion, migration and proliferation of endothelial cells during angiogenesis are also regulated by integrins [7]. These molecules are expressed at the cell surface of activated endothelial cells and interact with a large array of extracellular matrix proteins. Several integrins have been shown to be expressed by the RA endothelium [13]. These include β1 integrins, such as α4β1 and α5β1 that bind to fibronectin, and α6β1 that interacts with laminin. In addition, collagen-binding integrins are expressed by the vascular endothelium, including α2β1. αV can associate with several β chains and can have multiple specificities, including interactions with vitronectin and fibronectin. αVβ3 is expressed by several cell types including activated leukocytes and endothelial cells. It is minimally, if at all, expressed on resting or normal blood vessels but is highly expressed in RA synovial blood vessels, where it is viewed as a marker for endothelial activation [27, 69].
The relevance of angiogenesis in the pathophysiology in RA has been shown in animal models. For example, TNP-470 (a compound that exerts antiangiogenic, as well as other, effects) was found to suppress established disease associated with a marked inhibition of pannus formation and neovascularisation in type II collagen-induced arthritis in rats [66, 95]. In addition, a soluble form of the Flt-1 VEGF receptor significantly reduces disease severity and joint destruction in murine collagen-induced arthritis [96]. In human RA, the anti-TNF antibody infliximab reduces synovial vascularity as assessed by immunostaining for CD31, von Willebrand factor and αVβ3 integrin [66]. Observations suggest that part of the beneficial effects of anti-TNF treatment in RA may be related to decreased production of VEGF and reduced angiogenesis.
Proinflammatory cytokines, other mediators and their receptors
In the RA synovium IL-1α and IL-1β are mainly secreted by macrophages, but also localise to endothelial cells and lymphocytes [97–99]. The type 1 IL-1 receptor is expressed by the endothelium, by the lining cells and by other sublining cells in the RA synovium and the cartilage–pannus junction [97]. A similar pattern of IL-1 receptor antagonist is found, yet there is less expression. In addition, antigenic TNF-α mainly localises to macrophages in the RA lining layer and in the sublining, with some endothelial and lymphocyte staining [76, 100–102]. The TNF receptors p55 and p75 are expressed by endothelial cells and a variety of other cell types in the lining and sublining layers [99, 103]. IL-1 and TNF stimulate the production of degradative proteases and cytokines such as IL-1, IL-6, IL-8 and MCP-1 by joint tissue cells, contributing to joint destruction. Many of these cytokines are also involved in angiogenesis. In addition, IL-1 and TNF upregulate the expression of ICAM-1, VCAM-1 and E-selectin on endothelial cells, stimulating leukocyte migration into the joint [76, 104].
There have been numerous functional studies showing that blockade of cytokines have anti-inflammatory effects on RA in animal models and humans. Perhaps the most striking have been studies on TNF [105]. In humans, the use of an antibody to TNF (infliximab) or to the soluble TNF receptor (etanercept) has significantly contributed to therapy in RA. Use of infliximab results in a significant reduction in pain, stiffness, number of swollen joints, serum C-reactive protein and Paulus criteria [106, 107]. Interestingly, longer trials reveal that the antibody, in conjunction with methotrexate, reduces radiographic joint damage in 50% of patients [108].
There is some evidence that anti-inflammatory cytokines, such as IL-10 and IL-13, may inhibit leukocyte–endothelial adhesion and endothelial expression of adhesion molecules [109]. In the presence of activated leukocytes, however, IL-10 may also enhance adhesion molecule expression. IL-13 may have proangiogenic effects as it stimulates endothelial chemotaxis, inferring the expression of IL-13 receptors by these cells [76]. IL-15 protein localises to the endothelium and other cell types in the RA synovium [110]. This cytokine stimulates T-cell migration into RA synovia engrafted into the SCID mouse. IL-17 is produced by the RA synovium, induces production of metalloproteinases and could activate the endothelium enhancing leukocyte extravasation [111]. In this respect, the IL-17 receptor is expressed in RA synovia, mainly on endothelial cells, where its expression is higher than in OA synovia [112].
Elevated levels of corticotropin-releasing hormone (CRH) are produced locally in the inflamed synovium, and a role for CRH is indicated in the pathogenesis of inflammatory joint disease [113]. The CRH receptor type 1 mRNA and protein are abundantly expressed by RA synovial endothelial cells, as well as by mast cells, where this receptor may be involved in vascular permeability changes and in angiogenesis. The receptor is not expressed in normal synovia. In another study, staining for CRH receptor and the CRH ligand urocortin was demonstrated in RA endothelial cells and in a variety of other cell types [114]. Kinin levels are raised in RA and have been implicated in the pathogenesis of RA by causing release of cytokine and noncytokine mediators such as IL-1, TNF, PAF, histamine and prostaglandin E2, which contribute to joint destruction, leukocyte influx, pain, oedema and angiogenesis [115]. Kinin B2 receptors have been detected on synovial endothelial cells, on lining cells and on fibroblasts in RA and in OA samples [116].
Midkine is a retinoic acid-inducible heparin-binding cytokine that stimulates neutrophil chemotaxis. The protein is detected on the endothelial cells and synoviocytes in inflamed synovia, and less in noninflamed tissue [117]. The protein for stem cell factor, but not its receptor (c-kit), localises to synovial endothelial cells, macrophages and fibroblasts in RA and OA synovia [118]. In addition, the somatostatin receptor (sst2a) protein localises to the RA synovial endothelium and to macrophages [119].
Proteases
Proteases function in the degradation of the basement membrane and other regions of the extracellular matrix, playing a role in angiogenesis and enhancing leukocyte migration into the tissue [120]. Microvascular endothelial cells are also present in the invasive synovial pannus, such as at the cartilage–pannus junction [97, 99, 103, 121]. Proteases released by endothelial cells could therefore contribute to cartilage and bone destruction caused by the pannus. In this respect, however, it is not known how much of a contribution endothelial cells make compared with other cell types such as fibroblasts and macrophages.
Several proteases are produced by the RA synovial endothelium. In early RA the mRNAs for matrix metalloproteinase (MMP)-1 (collagenase) and the cysteine proteases cathepsin B and cathepsin L are expressed by synovial endothelial cells, as shown by in situ hybridisation [120]. There is only scant expression of these enzymes in normal synovia. MMP-3 (stromelysin) mRNA mainly occurs in the lining layer in RA synovia but is also expressed by endothelial cells [122]. The MMP-9 (gelatinase B) and MMP-13 (collagenase 3) proteins occur in endothelial cells, fibroblasts and leukocytes within the RA synovium, where they occur in elevated levels compared with the OA synovium [123, 124]. Membrane type 1-MMP mRNA is detected in endothelial cells and fibroblasts of RA synovia, and there is less expression of the protease in control synovia [125]. Regarding the regulation of MMP gene expression, the oncogene Ras (which upregulates MMP-1 and cathepsins) has been shown to colocalise with cathepsin L in the vessels of the RA synovium [126].
Proteolytic joint destruction in RA is believed to be mediated, at least in part, by the plasminogen activation system. In this context the urokinase plasminogen activator receptor is expressed by RA synovial endothelial cells, occurring in higher levels compared with normal synovial endothelial cells. The receptor is also expressed by lining cells and sublining macrophages [127]. Tissue-type plasminogen activator also localises to the synovial endothelium in RA and in OA patients but not to other cell types [128].
Other enzymes such as heparanase and plasmin are produced and secreted from endothelial cells. These enzymes may play a role in angiogenesis by releasing growth factors such as FGF, VEGF and HGF that are bound to heparan sulphate in the extracellular matrix [84, 129].
Tissue inhibitor of metalloproteinases (TIMPs) antagonise the effects of destructive MMPs. The expression of TIMP-1 and TIMP-2 has been shown in endothelial cells and lining cells in RA synovia but not in normal synovia [130]. It has been reported that the RA synovial endothelium produces decreased amounts of TIMP [131]. This may in part contribute to an imbalance between MMPs and TIMPs in the RA joint, favouring tissue destruction.
Other genes
Prostaglandins are important mediators of acute and chronic inflammation [132]. Prostaglandin E has been localised to the synovial endothelium, to lining cells and to inflammatory cells, where expression is higher in RA than in OA tissue [133]. The production of these mediators is catalysed by an enzyme cascade that includes cyclooxygenases (COXs). There are two isoforms of COX expressed in the synovium. COX-2 localises to endothelial cells, to mononuclear leukocytes and to fibroblasts, and COX-2 expression is increased in inflammatory arthritis compared with in noninflammatory arthritis [132]. COX-1, in contrast, is constitutively expressed, particularly by lining cells, and there is no difference in immunostaining between inflammatory and noninflammatory arthritis.
Another enzyme involved in prostaglandin synthesis is phospholipase A2. In this context, phospholipase A2 activating protein has been shown to be present in a variety of cells in the RA synovium, including endothelial cells, vascular smooth muscle and monocytes/macrophages [134].
Nitric oxide is synthesised by the action of a family of nitric oxide synthases, which are either constitutive or inducible. The production of nitric oxide plays a role in inflammation and physiological processes [135]. The expression of inducible nitric oxide synthase protein and mRNA has been shown in RA synovia, localising to the endothelium, to macrophage-like lining cells and, to a lesser extent, to fibroblasts [136]. There is only minimal labelling of these cells in normal synovium. Recent data suggest that nitric oxide production by inducible nitric oxide synthase has anti-inflammatory effects in experimental arthritis by mediating a reduction in leukocyte adhesion and infiltration [137].
Enhanced expression of activation markers, such as the protooncogene c-fos, has been reported in RA synovial endothelium compared with OA synovial endothelium [138]. The pentaxin PTX3 has a structure related to C-reactive protein and may play a role in inflammatory circuits in RA. PTX3 is expressed by the endothelium and synoviocytes in RA synovia [139]. Annexins are calcium-binding proteins with diverse functions including regulating inflammation and may have an intracellular role as cytoskeletal elements in exocytosis and cell differentiation. Endothelial cells in the RA synovium stain strongly for annexin IV and annexin VI, and weakly for annexin I and annexin II [140]. Lymphoid cells are also strongly positive for annexin VI in the RA synovium.
Conclusions
This review has reported 76 genes expressed by or on the synovial endothelium in the RA synovium (Table 1). Of these, 13 genes showed a preferential endothelial distribution and could be considered potential markers of these cells. In addition, 29 out of the reported 76 genes showed increased expression in the RA endothelium in comparison with non-RA control endothelial cells, and such genes may be implicated in the pathophysiology of RA. Future studies of these genes and other pathways specifically induced in RA endothelial cells may lead to the development of novel endothelium-targeted therapies for RA and to other forms of inflammatory arthritis.
Abbreviations
- COX:
-
cyclooxygenase
- CRH:
-
corticotropin-releasing hormone
- FGF:
-
fibroblast growth factor
- HEV:
-
high endothelial venules
- HGF:
-
hepatocyte growth factor
- ICAM:
-
intercellular adhesion molecule
- IL:
-
interleukin
- IFN:
-
interferon
- MCP-1:
-
monocyte chemoattractant protein-1
- MHC:
-
major histocompatibility complex
- MMP:
-
matrix metalloproteinase
- OA:
-
osteoarthritic
- RA:
-
rheumatoid arthritis
- TIMP:
-
tissue inhibitor of metalloproteinase
- TNF:
-
tumour necrosis factor
- VCAM:
-
vascular cell adhesion molecule
- VEGF:
-
vascular endothelial growth factor.
References
Silman AJ, Hochberg MC: Epidemiology of the Rheumatic Diseases. 1993, Oxford: Oxford University Press
Firestein GS: Evolving concepts of rheumatoid arthritis. Nature. 2003, 423: 356-361. 10.1038/nature01661.
Smolen JS, Steiner G: Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov. 2003, 2: 473-488. 10.1038/nrd1109.
Firestein GS: Rheumatoid synovitis and pannus. In Rheumatology. Edited by: Klippel JH, Dieppe PA. 1998, Philadelphia, PA: Mosby, 13.1-13.24. 2
Edwards JCW: The synovium. In Rheumatology. Edited by: Klippel JH, Dieppe PA. 1998, Philadelphia, PA: Mosby, 6.1-6.8. 2
Patterson AM, Schmutz C, Davis S, Gardner L, Ashton BA, Middleton J: Differential binding of chemokines to macrophages and neutrophils in the human inflamed synovium. Arthritis Res. 2002, 4: 209-214. 10.1186/ar408.
Weber AJ, De Bandt M: Angiogenesis: general mechanisms and implications for rheumatoid arthritis. Joint Bone Spine. 2000, 67: 366-383.
Kulka JP, Bocking D, Rpoes MW, Bauer W: Early joint lesions of rheumatoid arthritis. Arch Pathol. 1955, 59: 129-149.
Walsh DA: Angiogenesis and arthritis. Rheumatology. 1999, 38: 103-112. 10.1093/rheumatology/38.2.103.
Schmacher R, Kitridou RC: Synovitis of recent onset. A clinicopathologic study during the first month of disease. Arthritis Rheum. 1972, 15: 465-485.
Fitzgerald O, Soden M, Yanni G, Robinson R, Bresnihan B: Morphometric analysis of blood vessels in synovial membranes obtained from clinically affected and unaffected knee joints of patients with rheumatoid arthritis. Ann Rheum Dis. 1991, 50: 792-796.
Girard JP, Springer TA: High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today. 1995, 16: 449-457. 10.1016/0167-5699(95)80023-9.
Oppenheimer-Marks N, Lipsky PE: Adhesion molecules in rheumatoid arthritis. Springer Semin Immunopathol. 1998, 20: 95-114. 10.1007/BF00832001.
Yanni G, Whelan A, Feighery C, Fitzgerald O, Bresnihan B: Morphometric analysis of synovial membrane blood vessels in rheumatoid arthritis: associations with the immunohistologic features, synovial fluid cytokine levels and the clinical course. J Rheumatol. 1993, 20: 634-638.
Munro JM, Pober JS, Cotran RS: Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol. 1989, 135: 121-133.
Fossum S, Smith ME, Ford WL: The recirculation of T and B lymphocytes in the athymic, nude rat. Scand J Immunol. 1983, 17: 551-557.
Pallis M, Hopkinson N, Lowe J, Powell R: An electron microscopic study of muscle capillary wall thickening in systemic lupus erythematosus. Lupus. 1994, 3: 401-407.
Finol HJ, Marquez A, Rivera H, Montes de Oca I, Muller B: Ultrastructure of systemic sclerosis inflammatory myopathy. J Submicrosc Cytol Pathol. 1994, 26: 245-253.
Leinonen H, Juntunen J, Somer H, Rapola J: Capillary circulation and morphology in Duchenne muscular dystrophy. Eur Neurol. 1979, 18: 249-255.
Butcher EC, Williams M, Youngman K, Rott L, Briskin M: Lymphocyte trafficking and regional immunity. Adv Immunol. 1999, 72: 209-253.
Szekanecz Z, Koch AE: Cell–cell interactions in synovitis. Endothelial cells and immune cell migration. Arthritis Res. 2000, 2: 368-373. 10.1186/ar114.
Haskard DO: Cell adhesion molecules in rheumatoid arthritis. Curr Opin Rheumatol. 1995, 7: 229-234.
Szekanecz Z, Szegedi G, Koch AE: Cellular adhesion molecules in rheumatoid arthritis: regulation by cytokines and possible clinical importance. J Investig Med. 1996, 44: 124-135.
Kriegsmann J, Keyszer GM, Geiler T, Lagoo AS, Lagoo-Deenadayalan S, Gay RE, Gay S: Expression of E-selectin messenger RNA and protein in rheumatoid arthritis. Arthritis Rheum. 1995, 38: 750-754.
Michie SA, Streeter PR, Bolt PA, Butcher EC, Picker LJ: The human peripheral lymph node vascular addressin. An inducible endothelial antigen involved in lymphocyte homing. Am J Pathol. 1993, 143: 1688-1698.
Yeh JC, Hiraoka N, Petryniak B, Nakayama J, Ellies LG, Rabuka D, Hindsgaul O, Marth JD, Lowe JB, Fukuda M: Novel sulfated lymphocyte homing receptors and their control by a Core1 extension beta 1,3-N-acetylglucosaminyltransferase. Cell. 2001, 105: 957-969. 10.1016/S0092-8674(01)00394-4.
Johnson BA, Haines GK, Harlow LA, Koch AE: Adhesion molecule expression in human synovial tissue. Arthritis Rheum. 1993, 36: 137-146.
Szekanecz Z, Haines GK, Lin TR, Harlow LA, Goerdt S, Rayan G, Koch AE: Differential distribution of intercellular adhesion molecules (ICAM-1, ICAM-2, and ICAM-3) and the MS-1 antigen in normal and diseased human synovia. Their possible pathogenetic and clinical significance in rheumatoid arthritis. Arthritis Rheum. 1994, 37: 221-231.
Wilkinson LS, Edwards JC, Poston RN, Haskard DO: Expression of vascular cell adhesion molecule-1 in normal and inflamed synovium. Lab Invest. 1993, 68: 82-88.
Higashiyama H, Saito I, Hayashi Y, Miyasaka N: In situ hybridization study of vascular cell adhesion molecule-1 messenger RNA expression in rheumatoid synovium. J Autoimmun. 1995, 8: 947-957. 10.1016/S0896-8411(95)80028-X.
Elices MJ, Tsai V, Strahl D, Goel AS, Tollefson V, Arrhenius T, Wayner EA, Gaeta FC, Fikes JD, Firestein GS: Expression and functional significance of alternatively spliced CS1 fibronectin in rheumatoid arthritis microvasculature. J Clin Invest. 1994, 93: 405-416.
Muller-Ladner U, Elices MJ, Kriegsmann JB, Strahl D, Gay RE, Firestein GS, Gay S: Alternatively spliced CS-1 fibronectin isoform and its receptor VLA-4 in rheumatoid arthritis synovium. J Rheumatol. 1997, 24: 1873-1880.
van Dinther-Janssen AC, Horst E, Koopman G, Newmann W, Scheper RJ, Meijer CJ, Pals ST: The VLA-4/VCAM-1 pathway is involved in lymphocyte adhesion to endothelium in rheumatoid synovium. J Immunol. 1991, 147: 4207-4210.
Neidhart M, Wehrli R, Bruhlmann P, Michel BA, Gay RE, Gay S: Synovial fluid CD146 (MUC18), a marker for synovial membrane angiogenesis in rheumatoid arthritis. Arthritis Rheum. 1999, 42: 622-630. 10.1002/1529-0131(199904)42:4<622::AID-ANR4>3.0.CO;2-Y.
Salmi M, Jalkanen S: A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science. 1992, 257: 1407-1409.
Jaakkola K, Nikula T, Holopainen R, Vahasilta T, Matikainen MT, Laukkanen ML, Huupponen R, Halkola L, Nieminen L, Hiltunen J, Parviainen S, Clark MR, Knuuti J, Savunen T, Kaapa P, Voipio-Pulkki LM, Jalkanen S: In vivo detection of vascular adhesion protein-1 in experimental inflammation. Am J Pathol. 2000, 157: 463-471.
Kavanaugh AF, Davis LS, Nichols LA, Norris SH, Rothlein R, Scharschmidt LA, Lipsky PE: Treatment of refractory rheumatoid arthritis with a monoclonal antibody to intercellular adhesion molecule 1. Arthritis Rheum. 1994, 37: 992-999.
Schulze-Koops H, Lipsky PE, Kavanaugh AF, Davis LS: Elevated Th1- or Th0-like cytokine mRNA in peripheral circulation of patients with rheumatoid arthritis. Modulation by treatment with anti-ICAM-1 correlates with clinical benefit. J Immunol. 1995, 155: 5029-5037.
Paleolog EM, Hunt M, Elliott MJ, Feldmann M, Maini RN, Woody JN: Deactivation of vascular endothelium by monoclonal anti-tumor necrosis factor alpha antibody in rheumatoid arthritis. Arthritis Rheum. 1996, 39: 1082-1091.
Issekutz AC, Mu JY, Liu G, Melrose J, Berg EL: E-selectin, but not P-selectin, is required for development of adjuvant-induced arthritis in the rat. Arthritis Rheum. 2001, 44: 1428-1437. 10.1002/1529-0131(200106)44:6<1428::AID-ART238>3.0.CO;2-U.
Carter RA, Campbell IK, O'Donnel KL, Wicks IP: Vascular cell adhesion molecule-1 (VCAM-1) blockade in collagen-induced arthritis reduces joint involvement and alters B cell trafficking. Clin Exp Immunol. 2002, 128: 44-51. 10.1046/j.1365-2249.2002.01794.x.
Carveth HJ, Bohnsack JF, McIntyre TM, Baggiolini M, Prescott SM, Zimmerman GA: Neutrophil Activating Factor (NAF) induces polymorphonuclear leukocyte adherence to endothelial cells and to subendothelial matrix proteins. Biochem Biophys Res Commun. 1989, 162: 387-393. 10.1016/0006-291X(89)92009-3.
Detmers PA, Lo SK, Olsen-Egbert E, Walz A, Baggiolini M, Cohn ZA: Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J Exp Med. 1990, 171: 1155-1162. 10.1084/jem.171.4.1155.
Middleton J, Patterson A, Gardner L, Schmutz C, Ashton B: Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002, 100: 3853-3860. 10.1182/blood.V100.12.3853.
Szekanecz Z, Kim J, Koch AE: Chemokines and chemokine receptors in rheumatoid arthritis. Semin Immunol. 2003, 15: 15-21. 10.1016/S1044-5323(02)00124-0.
Harigai M, Hara M, Yoshimura T, Leonard EJ, Inoue K, Kashiwazaki S: Monocyte chemoattractant protein-1 (MCP-1) in inflammatory joint diseases and its involvement in the cytokine network of rheumatoid synovium. Clin Immunol Immunopathol. 1993, 69: 83-91. 10.1006/clin.1993.1153.
Koch AE, Kunkel SL, Harlow LA, Mazarakis DD, Haines GK, Burdick MD, Pope RM, Strieter RM: Macrophage inflammatory protein-1 alpha. A novel chemotactic cytokine for macrophages in rheumatoid arthritis. J Clin Invest. 1994, 93: 921-928.
Koch AE, Kunkel SL, Harlow LA, Mazarakis DD, Haines GK, Burdick MD, Pope RM, Walz A, Strieter RM: Epithelial neutrophil activating peptide-78: a novel chemotactic cytokine for neutrophils in arthritis. J Clin Invest. 1994, 94: 1012-1018.
Buckley CD, Amft N, Bradfield PE, Pilling D, Ross E, Arenzana-Seisdedos F, Amara A, Curnow SJ, Lord JM, Scheel-Toellner D, Salmon M: Persistent induction of the chemokine receptor CXCR4 by TGF-1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol. 2000, 165: 3423-3429.
Takahashi Y, Kasahara T, Sawai T, Rikimaru A, Mukaida N, Matsushima K, Sasaki T: The participation of IL-8 in the synovial lesions at an early stage of rheumatoid arthritis. Tohoku J Exp Med. 1999, 188: 75-87.
Koch AE, Volin MV, Woods JM, Kunkel SL, Connors MA, Harlow LA, Woodruff DC, Burdick MD, Strieter RM: Regulation of angiogenesis by the C-X-C chemokines interleukin-8 and epithelial neutrophil activating peptide 78 in the rheumatoid joint. Arthritis Rheum. 2001, 44: 31-40. 10.1002/1529-0131(200101)44:1<31::AID-ANR5>3.0.CO;2-4.
Page G, Lebecque S, Miossec P: Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J Immunol. 2002, 168: 5333-5341.
Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A: Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997, 91: 385-395. 10.1016/S0092-8674(00)80422-5.
Baekkevold ES, Yamanaka T, Palframan RT, Carlsen HS, Reinholt FP, von Andrian UH, Brandtzaeg P, Haraldsen G: The CCR7 ligand ELC (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J Exp Med. 2001, 193: 1105-1112. 10.1084/jem.193.9.1105.
Tanaka Y, Fujii K, Hubscher S, Aso M, Takazawa A, Saito K, Ota T, Eto S: Heparan sulfate proteoglycan on endothelium efficiently induces integrin-mediated T cell adhesion by immobilizing chemokines in patients with rheumatoid synovitis. Arthritis Rheum. 1998, 41: 1365-1377. 10.1002/1529-0131(199808)41:8<1365::AID-ART5>3.0.CO;2-W.
Patterson AM, Chamberlain G, Siddall H, Gardner L, Middleton J: Expression of the Duffy antigen/receptor for chemokines (DARC) by the inflamed synovial endothelium. J Pathol. 2002, 197: 108-116. 10.1002/path.1100.
Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES, Cavanagh LL, von Andrian UH: Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol. 2003, 170: 4638-4648.
Pablos JL, Santiago B, Galindo M, Torres C, Brehmer MT, Blanco FJ, Garcia-Lazaro FJ: Synoviocyte-derived CXCL12 is displayed on endothelium and induces angiogenesis in rheumatoid arthritis. J Immunol. 2003, 170: 2147-2152.
Garcia-Lopez MA, Sanchez-Madrid F, Rodriguez-Frade JM, Mellado M, Acevedo A, Garcia MI, Albar JP, Martinez C, Marazuela M: CXCR3 chemokine receptor distribution in normal and inflamed tissues: expression on activated lymphocytes, endothelial cells and dendritic cells. Lab Invest. 2001, 81: 409-418.
Blades MC, Ingegnoli F, Wheller SK, Manzo A, Wahid S, Panayi GS, Perretti M, Pitzalis C: Stromal cell-derived factor 1 (CXCL12) induces monocyte migration into human synovium transplanted onto SCID mice. Arthritis Rheum. 2002, 46: 824-836. 10.1002/art.10102.
Akahoshi T, Endo H, Kondo H, Kashiwazaki S, Kasahara T, Mukaida N, Harada A, Matsushima K: Essential involvement of interleukin-8 in neutrophil recruitment in rabbits with acute experimental arthritis induced by lipopolysaccharide and interleukin-1. Lymphokine Cytokine Res. 1994, 13: 113-116.
Ogata H, Takeya M, Yoshimura T, Takagi K, Takahashi K: The role of monocyte chemoattractant protein-1 (MCP-1) in the pathogenesis of collagen-induced arthritis in rats. J Pathol. 1997, 182: 106-114. 10.1002/(SICI)1096-9896(199705)182:1<106::AID-PATH816>3.3.CO;2-1.
Taylor PC, Peters AM, Paleolog E, Chapman PT, Elliott MJ, McCloskey R, Feldmann M, Maini RN: Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum. 2000, 43: 38-47. 10.1002/1529-0131(200001)43:1<38::AID-ANR6>3.0.CO;2-L.
Firestein GS: Starving the synovium: angiogenesis and inflammation in rheumatoid arthritis. J Clin Invest. 1999, 103: 3-4.
Walsh DA, Pearson CI: Angiogenesis in the pathogenesis of inflammatory joint and lung diseases. Arthritis Res. 2001, 3: 147-153. 10.1186/ar292.
Paleolog EM: Angiogenesis in rheumatoid arthritis. Arthritis Res. 2002, 4: S81-S90. 10.1186/ar575.
Walsh DA, Mapp PI, Wharton J, Rutherford RA, Kidd BL, Revell PA, Blake DR, Polak JM: Localisation and characterisation of substance P binding to human synovial tissue in rheumatoid arthritis. Ann Rheum Dis. 1992, 51: 313-317.
Ceponis A, Konttinen YT, Imai S, Tamulaitiene M, Li TF, Xu JW, Hietanen J, Santavirta S, Fassbender HG: Synovial lining, endothelial and inflammatory mononuclear cell proliferation in synovial membranes in psoriatic and reactive arthritis: a comparative quantitative morphometric study. Br J Rheumatol. 1998, 37: 1032-1043. 10.1093/rheumatology/37.2.170.
Walsh DA, Wade M, Mapp PI, Blake DR: Focally regulated endothelial proliferation and cell death in human synovium. Am J Pathol. 1998, 152: 691-702.
FitzGerald O, Soden M, Yanni G, Robinson R, Bresnihan B: Morphometric analysis of blood vessels in synovial membranes obtained from clinically affected and unaffected knee joints of patients with rheumatoid arthritis. Ann Rheum Dis. 1991, 50: 792-796.
Stevens CR, Blake DR, Merry P, Revell PA, Levick JR: A comparative study by morphometry of the microvasculature in normal and rheumatoid synovium. Arthritis Rheum. 1991, 34: 1508-1513.
FitzGerald O, Bresnihan B: Synovial vascularity is increased in rheumatoid arthritis: comment on the article by Stevens et al. Arthritis Rheum. 1992, 35: 1540-1541.
Stevens CR: Synovial vascularity is decreased in rheumatoid arthritis: reply [letter]. Arthritis Rheum. 1992, 35: 1541-
Nakashima M, Eguchi K, Aoyagi T, Yamashita I, Ida H, Sakai M, Shimada H, Kawabe Y, Nagataki S, Koji T, et al: Expression of basic fibroblast growth factor in synovial tissues from patients with rheumatoid arthritis: detection by immunohistological staining and in situ hybridisation. Ann Rheum Dis. 1994, 53: 45-50.
Hosaka S, Shah MR, Barquin N, Haines GK, Koch AE: Expression of basic fibroblast growth factor and angiogenin in arthritis. Pathobiology. 1995, 63: 249-256.
Szekanecz Z, Koch AE, Kunkel SL, Strieter RM: Cytokines in rheumatoid arthritis. Potential targets for pharmacological intervention. Drugs Aging. 1998, 12: 377-390.
Reuterdahl C, Tingstrom A, Terracio L, Funa K, Heldin CH, Rubin K: Characterization of platelet-derived growth factor beta-receptor expressing cells in the vasculature of human rheumatoid synovium. Lab Invest. 1991, 64: 321-329.
Nagashima M, Hasegawa J, Kato K, Yamazaki J, Nishigai K, Ishiwata T, Asano G, Yoshino S: Hepatocyte growth factor (HGF), HGF activator, and c-Met in synovial tissues in rheumatoid arthritis and osteoarthritis. J Rheumatol. 2001, 28: 1772-1778.
Ikeda M, Hosoda Y, Hirose S, Okada Y, Ikeda E: Expression of vascular endothelial growth factor isoforms and their receptors Flt-1, KDR, and neuropilin-1 in synovial tissues of rheumatoid arthritis. J Pathol. 2000, 191: 426-433. 10.1002/1096-9896(2000)9999:9999<::AID-PATH649>3.3.CO;2-5.
Wauke K, Nagashima M, Ishiwata T, Asano G, Yoshino S: Expression and localization of vascular endothelial growth factor-C in rheumatoid arthritis synovial tissue. J Rheumatol. 2002, 29: 34-38.
Fava RA, Olsen NJ, Spencer-Green G, Yeo KT, Yeo TK, Berse B, Jackman RW, Senger DR, Dvorak HF, Brown LF: Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J Exp Med. 1994, 180: 341-346. 10.1084/jem.180.1.341.
Paavonen K, Mandelin J, Partanen T, Jussila L, Li TF, Ristimaki A, Alitalo K, Konttinen YT: Vascular endothelial growth factors C and D and their VEGFR-2 and 3 receptors in blood and lymphatic vessels in healthy and arthritic synovium. J Rheumatol. 2002, 29: 39-45.
Szekanecz Z, Haines GK, Harlow LA, Shah MR, Fong TW, Fu R, Lin SJ, Rayan G, Koch AE: Increased synovial expression of transforming growth factor (TGF)-beta receptor endoglin and TGF-beta 1 in rheumatoid arthritis: possible interactions in the pathogenesis of the disease. Clin Immunol Immunopathol. 1995, 76: 187-194. 10.1006/clin.1995.1114.
Szekanecz Z, Koch AE: Chemokines and angiogenesis. Curr Opin Rheumatol. 2001, 13: 202-208. 10.1097/00002281-200105000-00009.
Szekanecz Z, Strieter RM, Kunkel SL, Koch AE: Chemokines in rheumatoid arthritis. Springer Semin Immunopathol. 1998, 20: 115-132. 10.1007/BF00832002.
Du J, Luan J, Liu H, Daniel TO, Peiper S, Chen TS, Yu Y, Horton LW, Nanney LB, Strieter RM, Richmond A: Potential role for Duffy antigen chemokine-binding protein in angiogenesis and maintenance of homeostasis in response to stress. J Leukoc Biol. 2002, 71: 141-153.
Shahrara S, Volin MV, Connors MA, Haines GK, Koch AE: Differential expression of the angiogenic Tie receptor family in arthritic and normal synovial tissue. Arthritis Res. 2002, 4: 201-208. 10.1186/ar407.
Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD: Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996, 87: 1161-1169. 10.1016/S0092-8674(00)81812-7.
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD: Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997, 277: 55-60. 10.1126/science.277.5322.55.
Koch AE, Friedman J, Burrows JC, Haines GK, Bouck NP: Localization of the angiogenesis inhibitor thrombospondin in human synovial tissues. Pathobiology. 1993, 61: 1-6.
Veale D, Yanni G, Bresnihan B, FitzGerald O: Production of angiotensin converting enzyme by rheumatoid synovial membrane. Ann Rheum Dis. 1992, 51: 476-480.
Wernert N, Justen HP, Rothe M, Behrens P, Dreschers S, Neuhaus T, Florin A, Sachinidis A, Vetter H, Ko Y: The Ets 1 transcription factor is upregulated during inflammatory angiogenesis in rheumatoid arthritis. J Mol Med. 2002, 80: 258-266. 10.1007/s00109-001-0316-0.
O'Donnell K, Harkes IC, Dougherty L, Wicks IP: Expression of receptor tyrosine kinase Axl and its ligand Gas6 in rheumatoid arthritis: evidence for a novel endothelial cell survival pathway. Am J Pathol. 1999, 154: 1171-1180.
Koch AE, Halloran MM, Haskell CJ, Shah MR, Polverini PJ: Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature. 1995, 376: 517-519. 10.1038/376517a0.
Oliver SJ, Cheng TP, Banquerigo ML, Brahn E: Suppression of collagen-induced arthritis by an angiogenesis inhibitor, AGM-1470, in combination with cyclosporin: reduction of vascular endothelial growth factor (VEGF). Cell Immunol. 1995, 166: 196-206. 10.1006/cimm.1995.9978.
Miotla J, Maciewicz R, Kendrew J, Feldmann M, Paleolog E: Treatment with soluble VEGF receptor reduces disease severity in murine collagen-induced arthritis. Lab Invest. 2000, 80: 1195-1205.
Deleuran BW, Chu CQ, Field M, Brennan FM, Katsikis P, Feldmann M, Maini RN: Localization of interleukin-1 alpha, type 1 interleukin-1 receptor and interleukin-1 receptor antagonist in the synovial membrane and cartilage/pannus junction in rheumatoid arthritis. Br J Rheumatol. 1992, 31: 801-809.
Wood NC, Dickens E, Symons JA, Duff GW: In situ hybridization of interleukin-1 in CD14-positive cells in rheumatoid arthritis. Clin Immunol Immunopathol. 1992, 62: 295-300. 10.1016/0090-1229(92)90106-X.
Miller VE, Rogers K, Muirden KD: Detection of tumour necrosis factor alpha and interleukin-1 beta in the rheumatoid osteoarthritic cartilage–pannus junction by immunohistochemical methods. Rheumatol Int. 1993, 13: 77-82. 10.1007/BF00307738.
Chu CQ, Field M, Feldmann M, Maini RN: Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage–pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991, 34: 1125-1132.
Maini RN, Brennan FM, Williams R, Chu CQ, Cope AP, Gibbons D, Elliott M, Feldmann M: TNF-alpha in rheumatoid arthritis and prospects of anti-TNF therapy. Clin Exp Rheumatol. 1993, 8: S173-S175.
Grom AA, Murray KJ, Luyrink L, Emery H, Passo MH, Glass DN, Bowlin T, Edwards C: Patterns of expression of tumor necrosis factor alpha, tumor necrosis factor beta, and their receptors in synovia of patients with juvenile rheumatoid arthritis and juvenile spondylarthropathy. Arthritis Rheum. 1996, 39: 1703-1710.
Deleuran BW, Chu CQ, Field M, Brennan FM, Mitchell T, Feldmann M, Maini RN: Localization of tumor necrosis factor receptors in the synovial tissue and cartilage–pannus junction in patients with rheumatoid arthritis. Implications for local actions of tumor necrosis factor alpha. Arthritis Rheum. 1992, 35: 1170-1178.
Abbot SE, Whish WJ, Jennison C, Blake DR, Stevens CR: Tumour necrosis factor alpha stimulated rheumatoid synovial microvascular endothelial cells exhibit increased shear rate dependent leucocyte adhesion in vitro. Ann Rheum Dis. 1999, 58: 573-581.
Feldmann M, Maini RN: Discovery of TNF-alpha as a therapeutic target in rheumatoid arthritis: preclinical and clinical studies. Joint Bone Spine. 2002, 69: 12-18. 10.1016/S1297-319X(01)00335-9.
Elliott MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, Katsikis P, Brennan FM, Walker J, Bijl H, Ghrayeb J, Woody JN: Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993, 36: 1681-1690.
Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, Leeb B, Breedveld FC, Macfarlane JD, Bijl H, Woody JN: Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994, 344: 1105-1110. 10.1016/S0140-6736(94)90628-9.
Maini RN, St Clair EW, Breedveld FC, Furst D, Kalden JR, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, Lipsky P: Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999, 354: 1932-1939. 10.1016/S0140-6736(99)05246-0.
Szekanecz Z, Koch AE: Update on synovitis. Curr Rheumatol Rep. 2001, 3: 53-63.
Oppenheimer-Marks N, Brezinschek RI, Mohamadzadeh M, Vita R, Lipsky PE: Interleukin 15 is produced by endothelial cells and increases the transendothelial migration of T cells in vitro and in the SCID mouse-human rheumatoid arthritis model in vivo. J Clin Invest. 1998, 101: 1261-1272.
Bessis N, Boissier MC: Novel pro-inflammatory interleukins: potential therapeutic targets in rheumatoid arthritis. Joint Bone Spine. 2001, 68: 477-481. 10.1016/S1297-319X(01)00310-4.
Honorati MC, Meliconi R, Pulsatelli L, Cane S, Frizziero L, Facchini A: High in vivo expression of interleukin-17 receptor in synovial endothelial cells and chondrocytes from arthritis patients. Rheumatology. 2001, 40: 522-527. 10.1093/rheumatology/40.5.522.
McEvoy AN, Bresnihan B, FitzGerald O, Murphy EP: Corticotropin-releasing hormone signaling in synovial tissue from patients with early inflammatory arthritis is mediated by the type 1 alpha corticotropin-releasing hormone receptor. Arthritis Rheum. 2001, 44: 1761-1767. 10.1002/1529-0131(200108)44:8<1761::AID-ART311>3.0.CO;2-D.
Kohno M, Kawahito Y, Tsubouchi Y, Hashiramoto A, Yamada R, Inoue KI, Kusaka Y, Kubo T, Elenkov IJ, Chrousos GP, Kondo M, Sano H: Urocortin expression in synovium of patients with rheumatoid arthritis and osteoarthritis: relation to inflammatory activity. J Clin Endocrinol Metab. 2001, 86: 4344-4352. 10.1210/jc.86.9.4344.
Sharma JN, Buchanan WW: Pathogenic responses of bradykinin system in chronic inflammatory rheumatoid disease. Exp Toxicol Pathol. 1994, 46: 421-433.
Cassim B, Naidoo S, Ramsaroop R, Bhoola KD: Immunolocalization of bradykinin receptors on human synovial tissue. Immunopharmacology. 1997, 36: 121-125. 10.1016/S0162-3109(97)00010-6.
Takada T, Toriyama K, Muramatsu H, Song XJ, Torii S, Muramatsu T: Midkine, a retinoic acid-inducible heparin-binding cytokine in inflammatory responses: chemotactic activity to neutrophils and association with inflammatory synovitis. J Biochem (Tokyo). 1997, 122: 453-458.
Ceponis A, Konttinen YT, Takagi M, Xu JW, Sorsa T, Matucci-Cerinic M, Santavirta S, Bankl HC, Valent P: Expression of stem cell factor (SCF) and SCF receptor (c-kit) in synovial membrane in arthritis: correlation with synovial mast cell hyperplasia and inflammation. J Rheumatol. 1998, 25: 2304-2314.
ten Bokum AM, Melief MJ, Schonbrunn A, van der Ham F, Lindeman J, Hofland LJ, Lamberts SW, van Hagen PM: Immunohistochemical localization of somatostatin receptor sst2A in human rheumatoid synovium. J Rheumatol. 1999, 26: 532-535.
Cunnane G, FitzGerald O, Hummel KM, Gay RE, Gay S, Bresnihan B: Collagenase, cathepsin B and cathepsin L gene expression in the synovial membrane of patients with early inflammatory arthritis. Rheumatology. 1999, 38: 34-42. 10.1093/rheumatology/38.1.34.
Kobayashi I, Ziff M: Electron microscopic studies of the cartilage–pannus junction in rheumatoid arthritis. Arthritis Rheum. 1975, 18: 475-483.
Sawai T, Uzuki M, Harris ED, Kurkinnen M, Trelstad RL, Hayashi M: In situ hybridization of stromelysin mRNA in the synovial biopsies from rheumatoid arthritis. Tohoku J Exp Med. 1996, 178: 315-330.
Ahrens D, Koch AE, Pope RM, Stein-Picarella M, Niedbala MJ: Expression of matrix metalloproteinase 9 (96-kd gelatinase B) in human rheumatoid arthritis. Arthritis Rheum. 1996, 39: 1576-1587.
Lindy O, Konttinen YT, Sorsa T, Ding Y, Santavirta S, Ceponis A, Lopez-Otin C: Matrix metalloproteinase 13 (collagenase 3) in human rheumatoid synovium. Arthritis Rheum. 1997, 40: 1391-1399.
Petrow PK, Wernicke D, Schulze Westhoff C, Hummel KM, Brauer R, Kriegsmann J, Gromnica-Ihle E, Gay RE, Gay S: Characterisation of the cell type-specificity of collagenase 3 mRNA expression in comparison with membrane type 1 matrix metalloproteinase and gelatinase A in the synovial membrane in rheumatoid arthritis. Ann Rheum Dis. 2002, 61: 391-397. 10.1136/ard.61.5.391.
Trabandt A, Aicher WK, Gay RE, Sukhatme VP, Nilson-Hamilton M, Hamilton RT, McGhee JR, Fassbender HG, Gay S: Expression of the collagenolytic and Ras-induced cysteine proteinase cathepsin L and proliferation-associated oncogenes in synovial cells of MRL/I mice and patients with rheumatoid arthritis. Matrix. 1990, 10: 349-361.
Szekanecz Z, Haines GK, Koch AE: Differential expression of the urokinase receptor (CD87) in arthritic and normal synovial tissues. J Clin Pathol. 1997, 50: 314-319.
Ronday HK, Smits HH, Van Muijen GN, Pruszczynski MS, Dolhain RJ, Van Langelaan EJ, Breedveld FC, Verheijen JH: Difference in expression of the plasminogen activation system in synovial tissue of patients with rheumatoid arthritis and osteoarthritis. Br J Rheumatol. 1996, 35: 416-423.
Brenchley PEC: Angiogenesis in inflammatory joint disease: target for therapeutic intervention. Clin Exp Immunol. 2000, 121: 426-429. 10.1046/j.1365-2249.2000.01299.x.
Nawrocki B, Polette M, Clavel C, Morrone A, Eschard JP, Etienne JC, Birembaut P: Expression of stromelysin 3 and tissue inhibitors of matrix metalloproteinases, TIMP-1 and TIMP-2, in rheumatoid arthritis. Pathol Res Pract. 1994, 190: 690-696.
Jackson CJ, Arkell J, Nguyen M: Rheumatoid synovial endothelial cells secrete decreased levels of tissue inhibitor of MMP (TIMP1). Ann Rheum Dis. 1998, 57: 158-161.
Crofford LJ: COX-2 in synovial tissues. Osteoarthritis Cartilage. 1999, 7: 406-408. 10.1053/joca.1999.0226.
Husby G, Bankhurst AD, Williams RC: Immunohistochemical localization of prostaglandin E in rheumatoid synovial tissues. Arthritis Rheum. 1977, 20: 785-791.
Bomalaski JS, Fallon M, Turner RA, Crooke ST, Meunier PC, Clark MA: Identification and isolation of a phospholipase A2 activating protein in human rheumatoid arthritis synovial fluid: induction of eicosanoid synthesis and an inflammatory response in joints injected in vivo. J Lab Clin Med. 1990, 116: 814-825.
Abramson SB, Amin AR, Clancy RM, Attur M: The role of nitric oxide in tissue destruction. Best Pract Res Clin Rheumatol. 2001, 15: 831-845. 10.1053/berh.2001.0196.
Sakurai H, Kohsaka H, Liu MF, Higashiyama H, Hirata Y, Kanno K, Saito I, Miyasaka N: Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J Clin Invest. 1995, 96: 2357-2363.
Veihelmann A, Landes J, Hofbauer A, Dorger M, Refior HJ, Messmer K, Krombach F: Exacerbation of antigen-induced arthritis in inducible nitric oxide synthase-deficient mice. Arthritis Rheum. 2001, 44: 1420-1427. 10.1002/1529-0131(200106)44:6<1420::AID-ART237>3.0.CO;2-K.
Sano H, Forough R, Maier JA, Case JP, Jackson A, Engleka K, Maciag T, Wilder RL: Detection of high levels of heparin binding growth factor-1 (acidic fibroblast growth factor) in inflammatory arthritic joints. J Cell Biol. 1990, 110: 1417-1426. 10.1083/jcb.110.4.1417.
Luchetti MM, Piccinini G, Mantovani A, Peri G, Matteucci C, Pomponio G, Fratini M, Fraticelli P, Sambo P, Di Loreto C, Doni A, Introna M, Gabrielli A: Expression and production of the long pentraxin PTX3 in rheumatoid arthritis (RA). Clin Exp Immunol. 2000, 119: 196-202. 10.1046/j.1365-2249.2000.01110.x.
Goulding NJ, Dixey J, Morand EF, Dodds RA, Wilkinson LS, Pitsillides AA, Edwards JC: Differential distribution of annexins-I, -II, -IV, and -VI in synovium. Ann Rheum Dis. 1995, 54: 841-845.
Koch AE, Burrows JC, Haines GK, Carlos TM, Harlan JM, Leibovich SJ: Immunolocalization of endothelial and leukocyte adhesion molecules in human rheumatoid and osteoarthritic synovial tissues. Lab Invest. 1991, 64: 313-320.
van Dinther-Janssen AC, Pals ST, Scheper R, Breedveld F, Meijer CJ: Dendritic cells and high endothelial venules in the rheumatoid synovial membrane. J Rheumatol. 1990, 17: 11-17.
Pufe T, Bartscher M, Petersen W, Tillmann B, Mentlein R: Expression of pleiotrophin, an embryonic growth and differentiation factor, in rheumatoid arthritis. Arthritis Rheum. 2003, 48: 660-667. 10.1002/art.10839.
Funk JL, Wei H, Downey KJ, Yocum D, Benjamin JB, Carley W: Expression of PTHrP and its cognate receptor in the rheumatoid synvoial microciculation. Biochem Biophys Res Commun. 2002, 297: 890-897. 10.1016/S0006-291X(02)02263-5.
Wharton J, Rutherford RA, Walsh DA, Mapp PI, Knock GA, Blake DR, Polak JM: Autoradiographic localization and analysis of endothelin-1 binding sites in human synovial tissue. Arthritis Rheum. 1992, 35: 894-899.
Haynes DR, Barg E, Crotti TN, Holding C, Weedon H, Atkins GJ, Zannetino A, Ahern MJ, Coleman M, Roberts-Thomson PJ, Kraan M, Tak PP, Smith MD: Osteoprotegerin expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathies and osteoarthritis and normal controls. Rheumatology. 2003, 42: 123-134. 10.1093/rheumatology/keg047.
Klareskog L, Forsum U, Malmnas Tjernlund UK, Kabelitz D, Wigren A: Appearance of anti-HLA-DR-reactive cells in normal and rheumatoid synovial tissue. Scand J Immunol. 1981, 14: 183-192.
Saalbach A, Wetzig T, Haustein UF, Anderegg U: Detection of human soluble Thy-1 in serum by ELISA. Fibroblasts and activated endothelial cells are a possible source of soluble Thy-1 in serum. Cell Tissue Res. 1999, 298: 307-315. 10.1007/s004419900079.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Rights and permissions
About this article
Cite this article
Middleton, J., Americh, L., Gayon, R. et al. Endothelial cell phenotypes in the rheumatoid synovium: activated, angiogenic, apoptotic and leaky. Arthritis Res Ther 6, 60 (2004). https://doi.org/10.1186/ar1156
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/ar1156