Abstract
Macrophages are essential immune cells and play a major role in the immune response as pro-inflammatory or anti-inflammatory agents depending on their plasticity and functions. Infiltration and activation of macrophages are usually involved in wound healing. Herein, we first described macrophage polarization and their critical functions in wound healing process. It is addressed how macrophages collaborate with other immune cells in the wound microenvironment. Targeting macrophages by manipulating or re-educating macrophages in inflammation using nanomedicines is a novel and feasible strategy for wound management. We discussed the design and physicochemical properties of nanomaterials and their functions for macrophages activation and anti-inflammatory signaling during wound therapy. The mechanism of action of the strategies and appropriate examples are also summarized to highlight the pros and cons of those approaches. Finally, the potential of nanomedicines to modulate macrophage polarization for skin regeneration is discussed.
Similar content being viewed by others
Introduction
The ultimate goal of wound management is to accelerate the wound repair and restoration process with the reduction of pain, scarring, and bacterial infections while preserving tissue function. However, it is a complicated and dynamic physiological process. To overcome the setbacks of insufficient expression of cellular markers, nutrients, and angiogenesis factors that cause wound healing delay, various types of cellular mediators, growth factors, and proteinases are required [1,2,3,4]. Different from acute wounds which take only a short time to heal through the normal process of wound healing [5, 6], chronic wounds takes several months to heal or may never heal due to disrupted healing process [7,8,9]. Chronic wounds are often characterized by persistent inflammation, presence of necrotic tissue, and poor dermal matrix integrity, which can negatively impact wound repair. They are normally associated with pathological conditions of certain diseases (e.g. diabetes), infections, or inadequate vasculature. Some common examples include pressure ulcers, diabetic ulcers, venous ulcers, and arterial ulcers. The associated complications, such as neuropathy, hyperglycemia, even gangrene or sepsis, can lead to poor quality of life of patients and increased risk of amputation [10, 11]. In addition, as the population ages, there will be a significant increase in numbers. As chronic wounds impact a large proportion of the world population, the care of chronic wound has become a major global concern for the health organizations, resulting in enormous expenses [12, 13]. In order to meet the rising demands worldwide, it is critical to find for optimum treatment options to shorten recovery time, improve patient quality of life, and lower the health-care cost [14,15,16].
Wound healing process generally divided into three continuous and overlapping phases, including inflammation, proliferation, and tissue remodeling [17, 18]. Macrophages are essential immune cells actively involved in the wound regeneration and the key regulators to ensure proper healing and tissue regeneration. The dynamic and plastic nature of macrophages allow them to change with local micro-environmental stimuli or signals [19,20,21]. They can be polarized into two distinct phenotypes, that is, pro-inflammatory macrophages (also called classically activated M1 phenotype) and anti-inflammatory macrophages (also called alternatively activated M2 phenotype). M1 macrophages (around 85%) at the initial stage infiltrates to the wound site to clean dead cells, debris, and bacteria. After about 7 days post injury, transition of macrophages from M1 phenotype to M2 phenotype (around 80–85%) is activated to promote anti-inflammatory effects, innate immunity, and tissue formation by proliferation of fibroblasts, keratinocytes, and endothelial cells in order to restore skin layers properly [22, 23]. In addition, macrophages secrete matrix metalloproteinases (MMPs) to transform temporary form of extracellular matrix (ECM) to original one at the final remodeling stage [24]. Therefore, in chronic wounds where pro-inflammatory macrophages resist converting to anti-inflammatory ones and tissue repair is impaired, it is essential to induce macrophages with favorable phenotypes for the treatment of wounds [18, 23, 25, 26].
Macrophage-based therapy has been introduced as a promising approach in chronic wound treatment [27, 28]. Currently, methods, including administration of activated macrophages, use of cytokines, dressings loaded with macrophage-modulatory therapeutic agents, and design of biomaterials or nanomedicines to manipulate macrophage infiltration or macrophage phenotypical differentiation, are utilized to engineer macrophages [29, 30]. However, more efforts are needed to overcome the limitations that lead to chronic wound treatment failure, including (1) the formation of biofilms at the wound site due to limited antibacterial activity of wound dressings, (2) lack of physical and mechanical properties of wound dressing materials such as low water vapor transmission rate, poor wound fluid absorption capacity, low porosity, and non-breathability, (3) low biocompatibility and physicochemical instability that may cause the degradation or deactivation of drugs or biological therapeutic agents such as growth factors, and (4) low level of angiogenesis, poor pharmacokinetics, and chronic inflammation that were not addressed by traditional wound cares [31,32,33,34,35]. Nanomedicines have been hailed as a huge success when it comes to treat chronic wounds by providing excellent physicochemical properties for the dressing [36,37,38], increasing the performance of antibacterial activity by prevention of biofilm formation [39,40,41], maintaining the stability of the loaded growth factors or drugs [42, 43], and improving cell functions and microenvironment for wound closure [44]. More recently, immunomodulatory bionanomatierals have been emerged as a powerful tool to enhance immune-mediated wound healing, particularly to targeted macrophage through the repolarization or depletion/repopulation approaches. Generally, nanomedicine-mediated macrophage modulation is achieved by active or passive targeting. Nanoparticles can actively target macrophages by receptor-mediated endocytosis to promote the macrophage repolarization, while physicochemical properties of nanoparticles can be tuned to regulate macrophage phenotypes by passive targeting and accumulation at the infection sites as a result of phagocytosis. Small size, large surface area, and the ability to encapsulate and deliver a wide range of therapeutic cargos are the unique advantages of nanomedicine to protect the payloads in wound healing process and infiltrate quickly to wound sites for macrophage regulation in the wound treatment [45,46,47]. Therefore, it is interesting to investigate macrophage-targeted nanotherapeutic strategies for wound treatment.
In this review, we summarize the function of macrophages in wound immune-microenvironment and healing process. Then, we focus on strategies for engineering macrophages in details to show the advantages and disadvantages of those strategies. The cargos and physicochemical properties of the biomaterials used to modulate macrophages are discussed. We also review the role of nanomedicine in restoring the function of macrophages for wound healing. Finally, the article provides a summary of challenges and future directions of the development of macrophage-targeted nanomedicines in wound healing.
The role of macrophages in wound immune-microenvironment and healing process
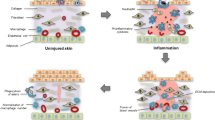
Wound immune microenvironment plays an important role in preventing pathogen invasion and tissue regeneration. Macrophages are the key immune cells for phagocytosis, regulation of inflammatory response, clearance of dead cells and pathogenic substances, and activation of tissue regeneration and remodeling. Under stimulation such as interferon gamma (IFN-γ), lipopolysaccharide (LPS), and tumor necrosis factor-α (TNF-α), M1 macrophages dominates in the early stages of wound healing. They produce pro-inflammatory mediators to foster the initial pro-inflammatory response. In addition, they involve in recruitment of other immune cells and regulate the inflammatory immune environment by interacting with T helper 1 (Th1) cells [48]. M1 macrophages are also responsible for phagocytic activity; phagocytize pathogens and foreign debris in the wound microenvironment. As wounds begin to repair, M2 macrophages are activated by interleukin-4 (IL-4) or IL-13 to generate anti-inflammatory factors, including growth factors, surface markers of scavenger receptors, IL-10, and intracellular arginase-1 (Arg-1) expression [49]. Later in the proliferation phase, M2 macrophages promote T helper 2 (Th2) responses and the adaptive immune system to provide immunomodulatory control of inflammation, which further enhance angiogenesis, fibroblast proliferation, ECM development, and re-epithelialization in the wound area [50, 51]. The M1–M2 transition prevents further damage to the wound site and guarantees the scar-free wound healing in the tissue remodeling phase. As macrophages are present throughout the entire wound recovery with dynamic changes in phenotype and function, the following sections discuss the roles of macrophages in different phases of wound healing (Fig. 1).
Inflammation phase
The immune response is innately activated by dysfunctional cellular activities and aberrant cytokine secretion, and inflammatory cells (macrophages, neutrophils, and T-lymphacytes) are recruited from the circulation during the inflammation phase. Blood-circulating monocytes, which originate from the hematopoietic stem cells located in the bone marrow of the adult mammal, are recruited and reach the dermis layer, where they differentiate to the mature macrophages to fight pathogens simultaneously with the presence of neutrophils [17, 52]. M1 macrophages release nitric oxide (NO) to kill intracellular pathogens and stabilize the host cells [53]. Macrophages are stimulated by hypoxia to produce chemoattractants, such as chemokines, to increase the number of leukocytes and leucocytic infiltration to the wound site [24, 35, 52]. M1 macrophages are also activated to express CD86 and produce pro-inflammatory cytokines such as IL-1 and IL-6, TNF-α, and reactive oxygen species (ROS) after interaction with molecular patterns associated with pathogens, damages, and peptidoglycans released from lysed cells [48, 54, 55]. The recruitment of neutrophils (the most abundant white cells) and macrophages to the wound site lead to phagocytosis, destroying and ingestion of damaged matrix, and elimination of microorganisms and dead cells [52, 56]. M1 macrophages secreted IL-12 activate Th1 cells to initiate the adaptive immune response [48].
Proliferation phase
To minimize further inflammation-induced tissue damage, macrophages polarize to M2 phenotype to facilitate wound healing, while inflammation subsides and the number of leukocytes reduces. IL-4 and IL-13 stimulate M2 macrophage polarization in order to secrete anti-inflammatory cytokine such as IL-10 to reduce the pro-inflammatory response, and growth factors such as vascular endothelial growth factors-α (VEGF-α), transforming growth factor-ꞵ (TGF-ꞵ), platelet derived growth factors (PDGF), and insulin like growth factor-1 (IGF-1) to boost cell proliferation and angiogenesis [24, 52]. These specific growth factors induce proliferation of fibroblasts and their differentiation into myofibroblasts to promote wound closure and collagen production, while prevent ECM degradation by up-regulation of tissue inhibitors of metalloproteinases [57,58,59,60]. Neutrophils can negatively influence tissue repair by destroying normal tissue using neutrophil proteases (e.g. elastase and cathepsin G) and free oxygen radicals (e.g. hydrogen peroxide) and delaying wound healing in the proliferation phase [52]. M2 macrophage-released IL-10 contributes to the apoptosis of neutrophils and increased collagen deposition to remove neutrophils, suppress inflammation, and enhance tissue repair [20, 52]. Subsequently, macrophages remove the apoptotic neutrophils by phagocytosis, which can prevent additional tissue damage and deposition of collagen in scar tissue [52].
Tissue remodeling
Tissue remodeling is the final stage of wound healing. M2 macrophages generate regulatory receptors for agonist ligands of the IL-1 family and growth factors, promoting fibroblast differentiation, ECM remodeling, and angiogenesis [61]. Macrophages, along with a variety of other cell types including fibroblasts, endothelial cells, and adipocytes, produce MMP for tissue remodeling. Zinc dependent proteases MMPs (e.g. MMP-1, MMP-3, MMP-10, and MMP-12) expressed by macrophages has enzymatic activity that enhances wound healing process and ECM remodeling via restoration of the tissue morphology and the tissue function. Macrophages are responsible for breakdown of ECM fragments by secretion of MMPs, cysteine proteinases (cathepsin B and L), and serine proteases [20]. Macrophage-derived MMP-10 is critical for collagen deposition in wound healing. Type III collagen is dominant type of collagen in proliferation phase, and then is replaced by Type I collagen which is more stable in tissue remodeling phase [35]. The degradation and depositing collagen is beneficial to organize realignment of collagen networks and increase the tensile strength of tissues, which enhance regenerative capacity of the tissue [24, 35].
Strategies for engineering macrophages
In chronic wound, persistent inflammation is normally observed due to reduced phagocytic capacity of macrophages and poor immunomodulation. Reduced M2 macrophage population results in a reduction of growth factor levels and changes the fine balance between pro-inflammatory and anti-inflammatory cytokines (Fig. 2). Therefore, efforts have been made to develop strategies to control inflammation and repolarize macrophages from an M1 to M2 phenotype in order to provide a suitable immune microenvironment for wound healing [62,63,64,65]. Recently, studies have reported that the depletion of M1 macrophages, increasing the amount of M2 macrophages or transition of M1 to M2 can benefit revascularization, re-epithelialization, and fibroblast regeneration especially for treatment of chronic wounds where normal shift from M1 macrophages to M2 macrophages is dysregulated [66,67,68].
As previously introduced, the strategies to manipulate macrophages for wound management include administration of activated macrophages, use of cytokines, dressings loaded with macrophage-modulatory therapeutic agents, and design of biomaterials or nanomedicines to manipulate macrophage infiltration or macrophage phenotypical differentiation. Although delivery of activated macrophages has been studied for decades, it is still challenging to efficiently transport activated macrophages to specific diseased sites to achieve high therapeutic benefits [69]. For example, Jetten et al. [70] reported that IL-4 or IL-10-induced M2 macrophages were found to be ineffective in the treatment of wound in wild-type mice while they delayed re-epithelialization and persistence of neutrophils in diabetic wounds. In contrast, cytokine-based strategies to balance M1–M2 macrophage phenotypes have been suggested by some studies for wound treatment [71,72,73,74]. However, disadvantages of cytokine-based therapies such as short half-life and associated systemic toxicity limited their clinical applications. Therefore, development of macrophage-modulatory biomaterials and nanomedicines has been explored as more effective strategies in wound healing [29, 69, 75]. In this review, we will mainly focus on physicochemical properties of macrophage-modulatory biomaterials and macrophage-targeted nanomedicines for wound healing.
Physiochemical properties of immunomodulatory biomaterials
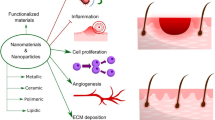
Biomaterials have attracted considerable interest as wound healing implant due to their prominent biological functions, which can provide protective environment and self-healing mechanisms after implantation of biomaterials [76,77,78]. Although they can initiate favorable response of the wound immune microenvironment for wound recovery, foreign body response to an implant surface may happen, dependent on interactions with the physical and chemical properties of the surface. This foreign body reaction, mainly involving macrophages as the immune cells, can lead to unwanted inflammatory response which hinders wound closure and recovery [17, 79, 80]. The impact of several physical and chemical properties such as pore size, topography, stiffness, and surface chemistry on macrophage behaviors has been investigated [19, 76, 81]. Here, the effects of the physicochemical properties on macrophage activation will be discussed in order to design better immunomodulatory biomaterials for wound regeneration. The most important physicochemical properties of biomaterials besides the biomaterials-based delivery of bioactive agents to promote macrophage polarization are illustrated in Fig. 3 and discussed in the following sections.
Substrate stiffness
Substrate stiffness of the biomaterials has been considered as one of the most important physical features in regulating immune response. It influences macrophage functions such as motility, morphology, and polarization [82,83,84]. Thus, understanding molecular mechanism of substrate stiffness is beneficial for the design of immunomodulatory biomaterials in order to promote wound healing. Okamoto et al. investigated the impact of substrate stiffness on in vitro activation phenotypes of the human monocyte cell line THP-1 by comparing 1% (4 kPa), 4% (15 kPa), 10% (100 kPa) agarose gel with a plastic substrate (> 10 MPa) [82]. The results showed that 1% agarose gel substrate attenuated M1-like macrophage polarization while promoted the anti-inflammatory M2-like activation phenotype of macrophages via induced peroxisome proliferator-activated receptor γ expression. Based on a few reported studies, a recent review conducted by Davenport et al. concluded that stiffness > 100 kPa activates inflammatory behavior of macrophages and their functions during wound regeneration [85]. Li and Bratlie [86] used photopolymerization to prepare methacrylated gellan gum hydrogels with compressive moduli in the range of 5 to 30 kPa. Hydrogel with the lowest crosslinking density are expected to have the least compressive modulus. The surface of the hydrogels was coated with fibronectin to modulate macrophage proliferation, adhesion, and polarization. Phenotypic shift from pro- to anti-inflammatory was investigated and their result showed that fibronectin coated gels has no impact on M1 macrophages, while fibronectin-coated gels with the highest compressive modulus (30 kPa) promote M2 macrophages polarization, in contrast to the untreated gel. Chen et al. [87] studied the effect of the substrate stiffness of polyacrylamide hydrogels on macrophage polarization. According to this study, substrates that are similar to osteoid stiffness (60 kPa) are preferable for promoting tissue repair. The ability to detect the stiffness of substrates has an impact on macrophage polarization. Collagen fiber stiffness-like substrate (5 kPa) promoted the M1 phenotype polarization, while an osteoid stiffness-like substrate resulted in M2 phenotype polarization. In a study by Sridharan et al. [88], migration mode of macrophages was affected by changing substrate stiffness. Polyacrylamide gels with stiffness of 323 kPa impact macrophages towards a M1 phenotype by decreasing production of cytokine IL-10 and IL-6. In contrast, gels with stiffness of 11 kPa and 88 kPa prime macrophages towards M2 phenotype with an increased production of IL-10. Camarero-Espinosa et al. [89] fabricated scaffolds with various mechanical properties and stiffness using copolymer of poly(lactide-co-caprolactone) with different ratio of lactide:caprolactone monomers. It was found that scaffolds with Young’s modulus more than 40 kPa led to M2 phenotype polarization and increased secretion of IL-10 and TGF-ꞵ in vitro. Their anti-inflammatory activity also resulted in remodeling and tissue regeneration in vivo. On the contrary, scaffolds with Young’s modulus less than 5 kPa resulted in M1 phenotype polarization determined by production of TNF-α. Scott et al. [90] reported that macrophages derived from cord blood CD14+ monocytes (CB CD14+) were affected by substrate modulus using poly(ethylene glycol) (PEG)-based hydrogels with shear moduli of 0.1, 3.4, and 10.3 kPa. They found that substrates with shear storage modulus of 10.3 kPa activated CB CD14+ to anti-inflammatory phenotype macrophages. Increasing the expression of VEFG, TGF-ꞵ and CD206 showed the influence of substrate with the stiffness of 10.3 kPa on M2 macrophage polarization compared to substrates with lower stiffness. Demineralized bone matrix scaffolds with varying matrix stiffness were prepared by controlling the time of decalcification by Yao et al. [91]. The high, medium, and low stiffness scaffolds were obtained by decalcification for 1 h, 12 h, and 5 days for repairing the defected rat skull. Low matrix stiffness (0.67 MPa) induced the expression of anti-inflammatory cytokines, such as Arg-1 and IL-10, as well as polarized macrophages into M2 phenotype. These findings highlighted the importance of optimizing the mechanical stiffness to modulate macrophage response during tissue regeneration.
Microstructure and geometry of biomaterials
Surface topography is a key physical parameter of biomaterials that may be used to modulate cellular response including migration, attachment, proliferation, and differentiation using macro-, micro-, and nano-patterning techniques [76, 83, 85, 92, 93]. Macrophages also respond to variations in surface roughness and are activated by topographic features of biomaterials, which can cause integration or failure of the implants [94]. Researchers have observed more macrophage accumulation and faster healing in vivo on implanted rough surface compared with smooth surface [95,96,97]. At cellular level, Barth et al. reported that RAW264.7 macrophages cultured on rough sandblasted and acid etched titanium surfaces were more likely to be activated toward anti-inflammatory M2 phenotype in healing process rather than smooth polished surface [98]. The ability of micro/nano patterned surfaces to improve macrophage adherence and polarization towards M2-like cells has been demonstrated. The effect of titanium surface roughness on human THP-1 derived macrophages was investigated by Zhang et al. [99]. The results demonstrated that low and medium rough titanium surfaces (Ra = 0.51–1.36 μm; Sa = 0.66–2.91 μm) induced the polarization of macrophages toward M2 phenotype by down-regulating the secretion and gene expression of pro-inflammatory cytokines while up-regulating the secretion and gene expression of anti-inflammatory markers compared to as-received smooth surfaces (Ra = 0.20 μm; Sa = 0.33 μm). The effect of polystyrene surface roughness on macrophage polarization was evaluated in a study by Kosoff et al. [100]. The rough micro-milled surface promoted M1 macrophage polarization, compared with the surface smoothed by acetone polishing. Therefore, modification of the surface topography is an effective approach to modulate the macrophage immune response, increase angiogenesis, and boost tissue recovery [83, 101].
In addition, the geometry of the biomaterials such as shape, size, and pore dimensions can significantly modulate macrophage behaviors. For instance, Sussman et al. examined macrophage polarization induced by subcutaneously implanted poly(2-hydroxyethyl methacrylate) hydrogels with pore size of 34 µm and 160 μm. They demonstrated that macrophages in 34 μm porous implants displayed a shift towards M1 phenotype, while macrophages immediately outside the porous structure revealed a large increase in M2 phenotypic cells [102]. In another study, the influence of pore size-induced macrophage differentiation was investigated using genipin cross-linked collagen/chitosan scaffolds with average pore size of 160 and 360 µm. The scaffolds with larger pore size induced higher degree of M1 to M2 transition and promoted macrophage secretion of anti-inflammatory and pro-angiogenic cytokines, leading to improved angiogenesis and vascularization [103]. In addition, the combinational effect of nanoporosity and chemistry on macrophage polarization into M1 and M2 phenotypes was assessed by fabricating nanoporous surfaces of controlled pore size (30, 65, and 200 nm) and lateral spacing with modified surface chemistry [104]. It is reported that macrophages on large nanoporous surfaces with acrylic acid and methyl oxazoline coatings reduced the secretion of pro-inflammatory cytokines and increased of the secretion of anti-inflammatory cytokines compared to surfaces with smaller pore sizes and methyl group rich chemistry. Camarero-Espinosa et al. demonstrated that the porosity and mechanical properties of the poly(caprolactone-co-lactide) scaffolds can influence macrophage polarization. The macrophages with a higher surface spread area polarized towards M1 phenotype, while the cells with a reduced surface spread area polarized towards M2 phenotype [89]. Moreover, the impact of nanofiber alignment on macrophage polarization was evaluated by Jia et al. [105]. Their results suggested that aligned nanofiber scaffolds significantly induced macrophage elongation and pro-healing macrophage polarization and facilitated peripheral nerve regeneration compared with random-nanofiber ones.
Surface chemistry
Function and phenotype of macrophages can be modulated through their interactions with the chemical groups on the surface of implanted biomaterials and changing of the surface wettability [83, 92, 106]. For example, Bygd et al. [107] prepared poly(N-isopropylacrylamide-co-acrylic acid) nanoparticles with the average size of 600 nm and modified with various functional groups. By measuring ex vivo TNF-α, IL-10, and Arg-1:iNOS levels, it was observed that amide, alkene, ketone, and epoxide functionalization induced more M2-like phenotypes. Amide and ketone functionalized particles also showed M2 macrophage polarization in the in vivo study. Lv et al. [108] showed that hydrophilic surfaces drove macrophages into the M2 phenotype through the existence of RGD cell-binding sites in adsorbed FN and β1 attachment most likely via PI3K/Akt signaling pathway. Macrophages on hydrophobic surfaces, on the other hand, interacted with P1/P2 segments in adsorbed FG through 2 attachments, resulting in the development of the M1 phenotype, most likely by NF-B activation. Later, Visalakshan et al. [109] studied the role of surface chemistry and wettability on macrophage response. Hydrophilic surfaces had a higher adsorption of dysopsonin albumin, leading to greater expression of anti-inflammatory cytokines by macrophages. Contrary, surface hydrophobicity caused higher opsonin IgG2 adsorption and increased production of pro-inflammatory signaling molecules. In another study, macrophage polarization on three different substrates including super-hydrophilic nanotubular Ti surfaces, air-annealed nanotubular Ti surfaces, and pure Ti substrates was evaluated. The results showed that super-hydrophilic nanotubular Ti surfaces increased expression of M2 markers (IL-10 and TGF-ꞵ) and down-regulated expression of M1 markers [110]. Kosoff et al. reported the combinational effects of surface topography and hydrophilicity on macrophage differentiation [100]. For macrophages culture on smooth surfaces, an increase in surface hydrophilicity led to increased expression of two of the three M2-associated genes evaluated, while for macrophages cultured on rough surfaces, an increase in hydrophilicity resulted in significant increases in two of the three M1-associated genes and a significant increase in only one of the M2-associated genes. Perez-Calixto et al. [111] studied how surface grafting of polypropylene and polytetrafluoroethylene with amino groups modulated macrophage polarization. They found that amino-functionalization reduces pro-inflammatory cytokine release, which could benefit wound healing. The effect of electrospun silk fibroin-silk sericin fibrous films on macrophage polarization and vascularization was assessed in a study by Wang et al. [112]. The addition of silk sericin stimulated macrophages to release more M1 and M2 cytokines. When the ratio of silk fibroin:silk sericin in electrospun films reached 7:3, the hydrophilicity enhanced, resulting in the higher ratio of M2/M1 phenotypes and the highest degree of vascularization.
In summary, the hydrophilicity or wettability of the substrate has been considered as a key feature of surface properties that influences macrophage adhesion and polarization. It has been demonstrated that increasing the hydrophilicity or wettability could up-regulate the anti-inflammatory cytokines. Design of immunomodulatory biomaterials to modulate macrophages phenotype and number are of great potential in wound tissue regeneration. To achieve successful clinical approaches, studying the behavior of macrophages in contact with biomaterials interface, optimization of physical parameters of biomaterials particularly topography, and deep understanding of the surface chemistry by employment of ions and molecules with different electrical charge density are needed in preparation of new generation of biomaterials.
Nanomedicines targeting macrophages for wound healing
Nanomaterials have been considered as a promising approach for promoting the wound regeneration due to their superior physicochemical properties, excellent drug loading capacity, and biocompatibility [113, 114]. During the last few decades, researchers have explored various types of organic or inorganic nanomaterials to be used in the development of wound dressing materials to act as antibacterial agents and stimulate wound healing process by providing sustained drug release, mimic the properties of ECM, and regulate cell behaviors [113, 115]. Particularly, nanomaterials can modulate the macrophages in the wounds via macrophage depletion or phenotype repolarization. The nanomaterials-based strategies to modulate macrophages in the wound healing application have been explained in the following subsections and summarized in Table 1.
Drug-free inorganic nanomaterials induce macrophage polarization
Inorganic nanomaterials have been used to regulate macrophage polarization because inorganic materials (e.g. metal ions) are able to improve inflammatory microenvironment and wound recovery [106]. Dong et al. studied the mechanism of bioactive glass (BG) enhancing wound healing via macrophage regulation [116]. They found that BG ionic products activated macrophages towards the M2 phenotype and stimulated macrophages to reduce inflammation and wound closure compared to control. Later, Xie et al. [117] investigated the effects of bioactive glass nanoparticles (BGNPs) on macrophage proliferation, migration, and polarization for diabetic wound healing. The M1-to-M2 macrophage phenotype switch was observed after treatment of BGNPs with the concentration 20 µg/ml, whereas higher concentration of the particles (100 µg/ml) caused prolonged M1 macrophage polarization and decelerated wound healing. The BGNPs regulated macrophage polarization, wound closure, and re-epithelialization in diabetic wounds in a dose-dependent manner. In addition, the influences of metal conjugated BG materials on wound healing were also investigated experimentally in a range of studies. Marza et al. [118] prepared ointment formulations with BG conjugated gold nanoparticles (AuNPs) mixed with Vaseline to repair the skin wound. Furthermore, Wu et al. [119] has developed a highly versatile ROS-scavenging tissue adhesive nanocomposite using ceria nanocrystals-decorated mesoporous silica nanoparticles (MSNPs). A significant decrease in the local inflammatory response was observed in ceria nanocrystals-decorated MSNPs treated rats by staining the infiltration of CD68-positive macrophages at day 5 post-wounding; suggesting this formulation efficiently accelerated the wound healing and limited scar formation.
Drug-free organic nanomaterials induce macrophage polarization
Organic nanomaterials, particularly the polymeric nanostructures, have been widely used for wound healing because of relatively simple fabrication methods, versatile surface functionalization process, biodegradability, and biocompatibility. The researchers discovered that some polymeric nanostructures can improve macrophage activation while also effectively boosting angiogenesis and re-epithelialization in the wound healing process [120, 121]. Kim et al. [122] fabricated nanofibrous electrospun hybrid scaffold using copolymer of poly(lactide-co-caprolactone) and decellularized heart tissue. By analyzing macrophage population and their immune response, the results showed that the scaffolds had anti-inflammatory effects by increasing initial M2 macrophages. In addition, the scaffolds reduced scarring by rapid replacement of collagen type III to collagen type I after 21 days post-surgery, evaluated by the immunohistochemistry studies. Liu et al. [123] synthesized electrospun thioether grafted hyaluronic acid nanofibrous hydrogels to promote macrophage modulation for diabetic wound healing. It was observed that the thioether grafted hyaluronic acid nanofibers scavenged the ROS, reduced the inflammatory response, promoted the macrophage polarization from M1 to M2 phenotype, leading to improved wound healing phase transition, compared with the control group. ECM-biomimetic coaxial nanofibrous scaffolds made of poly(lactic-co-glycolic acid) PLGA/fibrinogen as the shell and PLGA/collagen as the core were prepared for the repair of chronic wounds by Sun et al. [124]. The biomimetic coaxial scaffolds promoted adipose-derived mesenchymal stromal cells to secrete immunosuppressive factors (COX-2 and TSG-6) as well as wound healing growth factors (TGF-ꞵ and VEGF-α). The secretion of immunosuppressive factor modulated the macrophage phenotypic switch from M1 to M2. In vivo study in rat models exhibited the biomimetic coaxial scaffolds reduced inflammation and consequently accelerated diabetic wound healing. The α-gal nanoparticles with multiple α-gal epitopes as carbohydrate antigen were prepared and their effects on diabetic wound treatment were assessed. These nanoparticles were able to recruit M2 macrophages, which release pro-healing cytokines/growth factors to accelerate wound healing [125]. More recently, injectable hybrid supramolecular hydrogels were synthesized by electrostatic interactions between phosphorylated tripeptide and vinylimidazole and ketoprofen polymeric nanoparticles [126]. It was demonstrated that the hybrid hydrogels activated anti-inflammatory macrophages by causing significant reduction of NO. In a study by Peled et al. [127], amphiphilic nanoparticles were produced by the self-assembly of a copolymer of hydrolyzed galactomannan, a natural polysaccharide of galactose and mannose, grafted with poly(methyl metacrylate). By measuring M1 and M2 markers, the results suggested that these nanoparticles can polarize towards the M2-like phenotype. They also found that synthesized grafted amphiphilic nanoparticles were able to accelerate wound healing process.
Drug-free organic–inorganic hybrid nanomaterials induce macrophage polarization
As discussed, some inorganic nanoparticles possess antimicrobial, immunomodulatory, and wound healing properties by regulating cells, cytokines, and growth factors, while certain organic nanomaterials can benefit tissue regeneration by activating healing-related immune response and facilitating ECM remodeling. Therefore, researchers have incorporated inorganic nanoparticles with organic coating or matrix to form organic–inorganic hybrid nanomaterials. Recently, they have been attracted increasing attentions to be used in macrophage-modulatory wound tissue engineering [128, 129]. For instance, metallic silver nanoparticles (AgNPs) conjugated collagen/chitosan hybrid scaffold was fabricated and its therapeutic potential to improve wound healing was investigated [130]. They demonstrated that hybrid scaffold attenuated inflammatory response by regulating macrophage activation and normalized the wound healing process in rat models. The results suggested that hybrid scaffold was antibacterial, anti-inflammatory, and enhanced wound healing by modulation of macrophage activation and fibroblast migration. Adhikari et al. [131] incorporated magnesium particles in electrospun polycaprolactone nanofibers and then assessed their influence on macrophage infiltration and polarization, cytotoxicity, and collagen deposition. The animal study showed that the magnesium-loaded nanofiber mesh resulted in the presence of M2-like, reparative macrophages, well vascularization, and improved healing within 28 days compared with the mesh alone sample. Gan et al. [132], modified SiO2 nanoparticles with konjac glucomannan to enhance diabetic wound healing via reprogramming the murine bone marrow-derived macrophages. The modified SiO2 induced the formation of M2-like macrophages by clustering mannose receptor on the cells. Subsequently, the activated macrophages secreted the cytokines, leading to fibroblast proliferation and ECM secretion. These results suggested that modified SiO2 display great therapeutic potential for cutaneous wounds by effectively suppressing excessive or persistent inflammation and fibrosis. More recently, Xiang et al. [133] demonstrated that mussel-inspired zwitterionic AgNPs decorated with poly(carboxybetaine-co-dopamine methacrylamide) copolymer reduced macrophage-mediated inflammatory response during wound management. The AgNPs decorated copolymer suppressed the expression of pro-inflammatory cytokines and inhibited CD68 macrophage activation, leading to improved wound healing. In addition, Daghian et al. [134] fabricated inorganic/organic hybrid nanocomposites using silver/talc nanoparticles and their chitosan-capped derivatives. The influence of these nanocomposites on biocompatibility, anti-oxidant, antibacterial functions, and macrophage polarization in wound healing process was evaluated. In addition to controlling of bacterial infection, the hybrid nanocomposites activated M2 macrophages and increased the secretion of IL-10, CD206, Arg-1, fibroblast growth factor (bFGF), and collagen type I, which further resulted in fibroblast migration and wound closure. They demonstrated that these hybrid nanocomposites can provide a safe and effective strategy for infected wound healing. Gharehpapagh et al. [135] also reached a similar conclusion by developing AuNPs/perlite mesoporous nanocomposites with Urtica dioica extract and its chitosan-capped derivatives. It was found that the hybrid nanocomposites induced the M2 macrophages polarization, evidenced by the high expression of IL-10, bFGF, and Arg-1. They showed that the hybrid nanocomposites accelerated treatment of infected wounds through inhibiting bacterial infection and macrophage-based healing. Zhu et al. [136] prepared nanocomposite hydrogel dressings by incorporating magnesium-containing BGs into hydrogel formed by hyaluronic acid-modified phenyl boronate acid and quaternized chitosan. By analyzing the expression of pro-inflammation and anti-inflammation genes, it was reported that magnesium in the nanocomposite hydrogels suppressed inflammation. This modified hydrogel accelerated wound healing in diabetic rat models by improving granulation tissues formation, vascularization, collagen deposition, the formation of blood vessels, and decreasing inflammation in the wound sites.
Drug-loaded inorganic nanomaterials induce macrophage polarization
Inorganic nanomaterials can also be functionalized and conjugated with anti-inflammatory molecules as drug carriers for macrophage regulation in wound regeneration. Taking the advantage of antioxidant, antimicrobial, and anti-inflammatory properties of tannic acid, Orlowski et al. [137], synthesized tannic acid-modified AgNPs and evaluated their wound healing properties. Tannic acid-modified AgNPs showed an advantageous profile of pro-inflammatory cytokines produced by macrophages compared with control, which improved immune microenvironment to promote keratinocyte and fibroblast migration, proliferation, and angiogenesis. Wu et al. [138] developed bFGF-loaded dopamine-heparin-conjugated Fe3O4 magnetic nanoparticles (bFGF-HDC@Fe3O4) for enhanced wound repair. The expression of anti-inflammatory cytokines (Arg-1, IL-10, and CD206) increased, while the expression of pro-inflammatory cytokine (iNOS and TNF-α) decreased in the presence of heparin [138]. bFGF-HDC@Fe3O4 stabilized and released bFGF in a sustained manner. Both in vitro and in vivo evaluation showed bFGF-HDC@Fe3O4 induced macrophage polarization toward anti-inflammatory M2 phenotypes. The promoted cell proliferation and macrophage polarization further benefited the wound regeneration including granulation formation and collagen deposition. Snail slime from Helix aspersa can promote cell regeneration and growth, while inhibit inflammation. Thus, Gubitosa et al. [139] synthesized AuNPs with snail slime for wound healing application. Polarization of RAW264.7 macrophages into anti-inflammatory phenotypes was evaluated. They found that AuNPs conjugated with snail slime can modulate the inflammatory response induced by LPS in murine macrophages by significantly reducing the levels of IL-1β, IL-6, and iNOS.
Drug-loaded organic nanomaterials induce macrophage polarization
Organic nanomaterials can protect the therapeutic agents from degradation and exhibit sustained release of incorporated drugs, as well as overcome the limitations of conventional dressings such as bleeding, tissue damage, and preparation issues. Therefore, researchers have applied organic nanomaterials as delivery vehicles for controlled release of bioactive molecules which can regulate macrophage phenotypic switch for wound recovery. Liu et al. [140] loaded asiaticoside as an anti-inflammation and antioxidant drug in silk nanofiber hydrogels to stimulate collagen synthesis and promote angiogenesis for skin regeneration. Both the in vitro and in vivo studies demonstrated that the asiaticoside-laden hydrogel matrices regulated inflammatory reaction and vascularization towards scarless tissue regeneration. After treatment with the asiaticoside-laden hydrogel matrices, highest ratio of M2/M1 macrophages was reported during remodeling compared with other treatment groups. It was also observed that the asiaticoside-laden hydrogel caused a lower ratio of collagen type I to collagen type III and the deposited collagen microstructure, confirming scarless wound recovery. Saleh et al. [141], proposed an adhesive hydrogels containing miR-233 microRNA-loaded hyaluronic acid nanoparticles to polarize macrophages into anti-inflammatory phenotypes for wound healing. The polarization of macrophages to the M2 phenotype resulted from miR-233-loaded hydrogel treatment was observed using cellular and animal models. This immune response also induced the formation of uniform vascularized skin at the wound site and accelerated wound healing. Hu et al. [142] developed a smart pH- and ROS-responsive injectable hydrogel with self-healing and remodeling capability using phenyl boronic acid-grafted alginate to control the delivery of antibiotic amikacin and anti-inflammatory drug naproxen for wound healing. These drug-loaded micelle hydrogels inhibited the inflammatory response of macrophages by decreasing the expression of TNF-α while increased the expression of IL-10 after tropical administration, leading to promising healing outcome at the infected wound area. Ding et al. [143] reported an ibuprofen-encapsulated porphyrin-covalent organic framework-based membrane (IBU@DhaTph-membrane). They demonstrated that IBU@DhaTph-membrane dressing had excellent anti-infection and tissue remodeling activities by increasing local M2 macrophages and M2/M1 ratio, neovascularization and granulation tissue area, and collagen deposition. A study by Zhang et al. [144] explored the anti-inflammatory and pro-healing effects of apoptotic-cell-inspired deformable phosphatidylserine-containing nanoliposomes on diabetic chronic wounds. The prepared system persistently bound to macrophage membranes and efficiently induced M2-like macrophage polarization, resulting in improved anti-inflammatory and pro-healing responses of macrophages, increased vascularization, and accelerated wound closure.
Drug-loaded organic–inorganic hybrid nanomaterials induce macrophage polarization
Organic–inorganic hybrid nanocomposites are a favorable group of nanomaterials with great potentials in macrophage regulation for wound management due to their specific characteristics, including drug-loading, anti-inflammatory, antimicrobial, proangiogenic, proliferative, and remodeling properties [145, 146]. For intense, Mebert et al. [147] combined core–shell silica particles loaded with gentamicin sulfate and sodium rifamycin with concentrated collagen type I hydrogels to form a drug-loaded collagen-silica nanocomposites. These nanocomposites were able to provide prolonged release of two topical antibiotics, while caused the absence of M1 inflammatory macrophages in the wound bed and solved infection-triggered inflammation. This study illustrated the therapeutic potential of the collagen-silica nanocomposites to prevent infection and promote cutaneous wound repair. Wang et al. [148] fabricated biomimic virus-like mesoporous silica coated Ag nanocubes loaded with gentamicin in a hydrogel dressing for bacteria infected diabetic wound healing. The prepared coating allowed successful delivery of gentamicin and effective bacteria adhesion, leading to improved antibacterial activity. Wound immunohistochemical staining of CD86 was used to detect the pro-inflammatory M1 macrophage marker. The CD86 positive cells in gentamicin hydrogel treated group was higher than those of mesoporous silica coated AgNPs loaded with gentamicin hydrogel treated group. Depletion of M1 macrophages led to improve diabetic wound healing. In a similar study by Maheen et al. [149], econazole nitrate and triamcinolone acetonide was loaded in MSNPs and coated with cellulose acetate. The improved efficiency of econazole nitrate and triamcinolone acetonide loaded MSNPs was associated with controlled drug delivery at the wound site, reduced toxicity of the therapeutic agents by their encapsulation, and macrophage infiltration and polarization during 2 weeks wound healing process. Therefore, the skin tissue of these nanoparticles treated group developed faster than the free drug control groups.
Conclusion and future perspective
Regulation of macrophages is an important process during the wound development and healing. Macrophages differentiate into M1 phenotype in the early phase of wound healing with production of inflammatory factors and cytokines to phagocytize and destroy foreign bodies and necrotic tissues. Macrophages in normal wound can be effectively transitioned from the M1 phenotype in the inflammatory phase to the M2 phenotype in the proliferation and tissue remodeling phase, allowing them to dominate healing process and complete the wound repair. However, in chronic or infected wounds, it is challenging to induce the polarization of M1 macrophages to M2 phenotype in the proliferation and tissue remodeling phase, leading to impaired tissue repair.
Nanomaterials have been developed to achieve macrophage polarization as well as control of infections via controlled delivery of molecules to alter macrophage number or promote the transition of macrophage phenotypes, direct delivery of activated macrophages, delivery of cytokines, and design of biomaterials to manipulate the macrophage phenotype differentiation. Large specific area, controllable size and porosity, and loading capability are the main advantages of nanomaterials in biomedical applications, particularly wound treatment. Generally, physicochemical properties of nanomaterials, including hydrophilicity of biomaterials surfaces, surface topography, and substrate stiffness play a critical role in modulating macrophage phenotypes. Evidences suggest that smooth and hydrophobic surface, and soft substrate are associated with M1 phenotype polarization, while hydrophilic and rough surface and harder substrate can regulate macrophages toward the M2 phenotype polarization.
The interaction of biomaterials and macrophages have been investigated and showed their potentials in regulation of macrophage polarization. However, more research is needed in order to provide better understanding for preparation of engineered biomaterials as well as to overcome the remaining challenges related to immune regulation during different phases of wound healing. In addition, it is difficult to maintain the biological function of the biomaterials as degradation of biomaterials is one of the most important issue during wound treatment. There is limited information related to the degradation profile of the implant, the biocompatibility of both initial materials and degraded products, and dynamic function of materials during degradation in the complex biological environment. Therefore, comprehensive investigation of the wound dressing materials and degraded products to induce macrophage polarization is crucial for clinical success and patient safety.
References
Wang W, Lu K, Yu C, Huang Q, Du Y-Z. Nano-drug delivery systems in wound treatment and skin regeneration. J Nanobiotechnol. 2019;17:82. https://doi.org/10.1186/s12951-019-0514-y.
Naskar A, Kim K. Recent advances in nanomaterial-based wound-healing therapeutics. Pharmaceutics. 2020;12:499. https://doi.org/10.3390/pharmaceutics12060499.
Momoh FU, Boateng JS, Richardson SCW, Chowdhry BZ, Mitchell JC. Development and functional characterization of alginate dressing as potential protein delivery system for wound healing. Int J Biol Macromol. 2015;81:137–50. https://doi.org/10.1016/j.ijbiomac.2015.07.037.
Sanapalli BKR, Yele V, Singh MK, Thaggikuppe Krishnamurthy P, Karri VVSR. Preclinical models of diabetic wound healing: a critical review. Biomed Pharmacother. 2021;142:111946. https://doi.org/10.1016/j.biopha.2021.111946.
Saleh K, Sönnergren HH. 5—Control and treatment of infected wounds. In: Ågren MS, editor. Wound healing biomaterials. Duxford: Woodhead Publishing; 2016. p. 107–15. https://doi.org/10.1016/B978-1-78242-456-7.00005-2.
Rajendran S, Anand SC. 14—Woven textiles for medical applications. In: Gandhi KL, editor. Woven textiles. Sawston: Woodhead Publishing; 2012. p. 414–41. https://doi.org/10.1533/9780857095589.3.414.
Dai C, Shih S, Khachemoune A. Skin substitutes for acute and chronic wound healing: an updated review. J Dermatol Treat. 2020;31:639–48. https://doi.org/10.1080/09546634.2018.1530443.
Demidova-Rice TN, Durham JT, Herman IM. Wound healing angiogenesis: innovations and challenges in acute and chronic wound healing. Adv Wound Care. 2012;1:17–22. https://doi.org/10.1089/wound.2011.0308.
Praburaj DV, Anand SC, Dean C, Nettleton R. Conducting clinical trials in woundcare. In: Anand SC, Kennedy JF, Miraftab M, Rajendran S, editors. Medical textiles and biomaterials for healthcare. Cambridge: Woodhead Publishing; 2006. p. 310–9. https://doi.org/10.1533/9781845694104.5.310.
Noor S, Khan RU, Ahmad J. Understanding diabetic foot infection and its management. Diabetes Metab Syndr Clin Res Rev. 2017;11:149–56. https://doi.org/10.1016/j.dsx.2016.06.023.
Ahmad J. The diabetic foot. Diabetes Metab Syndr Clin Res Rev. 2016;10:48–60. https://doi.org/10.1016/j.dsx.2015.04.002.
Järbrink K, Ni G, Sönnergren H, Schmidtchen A, Pang C, Bajpai R, Car J. Prevalence and incidence of chronic wounds and related complications: a protocol for a systematic review. Syst Rev. 2016;5:152. https://doi.org/10.1186/s13643-016-0329-y.
Martinengo L, Olsson M, Bajpai R, Soljak M, Upton Z, Schmidtchen A, Car J, Järbrink K. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann Epidemiol. 2019;29:8–15. https://doi.org/10.1016/j.annepidem.2018.10.005.
Rieger KA, Birch NP, Schiffman JD. Designing electrospun nanofiber mats to promote wound healing—a review. J Mater Chem B. 2013;1:4531–41. https://doi.org/10.1039/C3TB20795A.
Gruen D. Wound healing and nutrition: going beyond dressings with a balanced care plan. J Am Coll Certif Wound Spec. 2010;2:46–9. https://doi.org/10.1016/j.jcws.2010.11.001.
Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34:599–610. https://doi.org/10.1007/s12325-017-0478-y.
Zhu Y, Ma Z, Kong L, He Y, Chan HF, Li H. Modulation of macrophages by bioactive glass/sodium alginate hydrogel is crucial in skin regeneration enhancement. Biomaterials. 2020;256:120216. https://doi.org/10.1016/j.biomaterials.2020.120216.
Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol. 2018;9:419. https://doi.org/10.3389/fphys.2018.00419.
Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33:3792–802. https://doi.org/10.1016/j.biomaterials.2012.02.034.
Minutti CM, Knipper JA, Allen JE, Zaiss DMW. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol. 2017;61:3–11. https://doi.org/10.1016/j.semcdb.2016.08.006.
Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. https://doi.org/10.1038/nri3600.
Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13.
Ley K. M1 means kill; M2 means heal. J Immunol. 2017;199:2191–3. https://doi.org/10.4049/jimmunol.1701135.
Kloc M, Ghobrial RM, Wosik J, Lewicka A, Lewicki S, Kubiak JZ. Macrophage functions in wound healing. J Tissue Eng Regen Med. 2019;13:99–109. https://doi.org/10.1002/term.2772.
Kim SY, Nair MG. Macrophages in wound healing: activation and plasticity. Immunol Cell Biol. 2019;97:258–67. https://doi.org/10.1111/imcb.12236.
Ferrante CJ, Leibovich SJ. Regulation of macrophage polarization and wound healing. Adv Wound Care. 2012;1:10–6. https://doi.org/10.1089/wound.2011.0307.
Torregrossa M, Kakpenova A, Simon JC, Franz S. Modulation of macrophage functions by ECM-inspired wound dressings—a promising therapeutic approach for chronic wounds. Biol Chem. 2021;402:1289–307. https://doi.org/10.1515/hsz-2021-0145.
Caputa G, Flachsmann LJ, Cameron AM. Macrophage metabolism: a wound-healing perspective. Immunol Cell Biol. 2019;97:268–78. https://doi.org/10.1111/imcb.12237.
Spiller KL, Koh TJ. Macrophage-based therapeutic strategies in regenerative medicine. Adv Drug Deliv Rev. 2017;122:74–83. https://doi.org/10.1016/j.addr.2017.05.010.
Alvarez MM, Liu JC, Trujillo-de Santiago G, Cha B-H, Vishwakarma A, Ghaemmaghami AM, Khademhosseini A. Delivery strategies to control inflammatory response: modulating M1–M2 polarization in tissue engineering applications. J Control Release. 2016;240:349–63. https://doi.org/10.1016/j.jconrel.2016.01.026.
Subbiahdoss G, Sharifi S, Grijpma DW, Laurent S, van der Mei HC, Mahmoudi M, Busscher HJ. Magnetic targeting of surface-modified superparamagnetic iron oxide nanoparticles yields antibacterial efficacy against biofilms of gentamicin-resistant staphylococci. Acta Biomater. 2012;8:2047–55. https://doi.org/10.1016/j.actbio.2012.03.002.
Chu C, Deng J, Liu L, Cao Y, Wei X, Li J, Man Y. Nanoparticles combined with growth factors: recent progress and applications. RSC Adv. 2016;6:90856–72. https://doi.org/10.1039/C6RA13636B.
Chigurupati S, Mughal MR, Okun E, Das S, Kumar A, McCaffery M, Seal S, Mattson MP. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials. 2013;34:2194–201. https://doi.org/10.1016/j.biomaterials.2012.11.061.
Kim JE, Lee J, Jang M, Kwak MH, Go J, Kho EK, Song SH, Sung JE, Lee J, Hwang DY. Accelerated healing of cutaneous wounds using phytochemically stabilized gold nanoparticle deposited hydrocolloid membranes. Biomater Sci. 2015;3:509–19. https://doi.org/10.1039/C4BM00390J.
Sharifi S, Hajipour MJ, Gould L, Mahmoudi M. Nanomedicine in healing chronic wounds: opportunities and challenges. Mol Pharm. 2021;18:550–75. https://doi.org/10.1021/acs.molpharmaceut.0c00346.
Mei L, Fan R, Li X, Wang Y, Han B, Gu Y, Zhou L, Zheng Y, Tong A, Guo G. Nanofibers for improving the wound repair process: the combination of a grafted chitosan and an antioxidant agent. Polym Chem. 2017;8:1664–71. https://doi.org/10.1039/C7PY00038C.
Mashinchian O, Bonakdar S, Taghinejad H, Satarifard V, Heidari M, Majidi M, Sharifi S, Peirovi A, Saffar S, Taghinejad M, Abdolahad M, Mohajerzadeh S, Shokrgozar MA, Rezayat SM, Ejtehadi MR, Dalby MJ, Mahmoudi M. Cell-imprinted substrates act as an artificial niche for skin regeneration. ACS Appl Mater Interfaces. 2014;6:13280–92. https://doi.org/10.1021/am503045b.
Kim HN, Hong Y, Kim MS, Kim SM, Suh K-Y. Effect of orientation and density of nanotopography in dermal wound healing. Biomaterials. 2012;33:8782–92. https://doi.org/10.1016/j.biomaterials.2012.08.038.
Joorabloo A, Khorasani MT, Adeli H, Mansoori-Moghadam Z, Moghaddam A. Fabrication of heparinized nano ZnO/poly(vinylalcohol)/carboxymethyl cellulose bionanocomposite hydrogels using artificial neural network for wound dressing application. J Ind Eng Chem. 2019. https://doi.org/10.1016/j.jiec.2018.10.022.
Khorasani MT, Joorabloo A, Adeli H, Milan PB, Amoupour M. Enhanced antimicrobial and full-thickness wound healing efficiency of hydrogels loaded with heparinized ZnO nanoparticles: in vitro and in vivo evaluation. Int J Biol Macromol. 2020. https://doi.org/10.1016/j.ijbiomac.2020.10.142.
Joorabloo A, Khorasani MT, Adeli H, Brouki Milan P, Amoupour M. Using artificial neural network for design and development of PVA/chitosan/starch/heparinized nZnO hydrogels for enhanced wound healing. J Ind Eng Chem. 2021. https://doi.org/10.1016/j.jiec.2021.12.027.
Rahimi HR, Nedaeinia R, SepehriShamloo A, Nikdoust S, KazemiOskuee R. Novel delivery system for natural products: nano-curcumin formulations. Avicenna J Phytomed. 2016;6:383–98.
Dizaj SM, Vazifehasl Z, Salatin S, Adibkia K, Javadzadeh Y. Nanosizing of drugs: effect on dissolution rate. Res Pharm Sci. 2015;10:95–108.
Wei Z, Zhang Y, Wang L, Wang Z, Chen S, Bao J, Xie Y, Su B, Zhao C. Photoenhanced dual-functional nanomedicine for promoting wound healing: shifting focus from bacteria eradication to host microenvironment modulation. ACS Appl Mater Interfaces. 2021;13:32316–31. https://doi.org/10.1021/acsami.1c08875.
Gaspar N, Zambito G, Löwik MWGC, Mezzanotte L. Active nano-targeting of macrophages. Curr Pharm Des. 2019;25:1951–61. https://doi.org/10.2174/1381612825666190710114108.
Zang X, Cheng M, Zhang X, Chen X. Targeting macrophages using nanoparticles: a potential therapeutic strategy for atherosclerosis. J Mater Chem B. 2021;9:3284–94. https://doi.org/10.1039/D0TB02956D.
Liu J, Wan M, Lyon CJ, Hu TY. Nanomedicine therapies modulating macrophage dysfunction: a potential strategy to attenuate cytokine storms in severe infections. Theranostics. 2020;10:9591–600. https://doi.org/10.7150/thno.47982.
Liu J, Geng X, Hou J, Wu G. New insights into M1/M2 macrophages: key modulators in cancer progression. Cancer Cell Int. 2021;21:389. https://doi.org/10.1186/s12935-021-02089-2.
Elliott MR, Koster KM, Murphy PS. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J Immunol. 2017;198:1387–94. https://doi.org/10.4049/jimmunol.1601520.
Sandoval Pacheco CM, Araujo Flores GV, Gonzalez K, de Castro Gomes CM, Passero LFD, Tomokane TY, Sosa-Ochoa W, Zúniga C, Calzada J, Saldaña A, Corbett CEP, Silveira FT, Laurenti MD. Macrophage polarization in the skin lesion caused by neotropical species of Leishmania sp. J Immunol Res. 2021;2021:5596876. https://doi.org/10.1155/2021/5596876.
Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol. 2012;32:463–88. https://doi.org/10.1615/CritRevImmunol.v32.i6.10.
Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23. https://doi.org/10.1017/S1462399411001943.
Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73:3861–85. https://doi.org/10.1007/s00018-016-2268-0.
Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514. https://doi.org/10.3389/fimmu.2014.00514.
Moghadam ZM, Henneke P, Kolter J. From flies to men: ROS and the NADPH oxidase in phagocytes. Front Cell Dev Biol. 2021;9:618. https://doi.org/10.3389/fcell.2021.628991.
Silva MT. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukoc Biol. 2010;87:93–106. https://doi.org/10.1189/jlb.0809549.
Ashouri F, Beyranvand F, BeigiBoroujeni N, Tavafi M, Sheikhian A, Varzi AM, Shahrokhi S. Macrophage polarization in wound healing: role of aloe vera/chitosan nanohydrogel. Drug Deliv Transl Res. 2019;9:1027–42. https://doi.org/10.1007/s13346-019-00643-0.
Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci. 2017. https://doi.org/10.3390/ijms18071545.
Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. https://doi.org/10.1189/jlb.0409236.
Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS−) vs. alternatively activated macrophages. Front Immunol. 2019;10:1084. https://doi.org/10.3389/fimmu.2019.01084.
Chistiakov DA, Myasoedova VA, Revin VV, Orekhov AN, Bobryshev YV. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology. 2018;223:101–11. https://doi.org/10.1016/j.imbio.2017.10.005.
Wolf SJ, Melvin WJ, Gallagher K. Macrophage-mediated inflammation in diabetic wound repair. Semin Cell Dev Biol. 2021;119:111–8. https://doi.org/10.1016/j.semcdb.2021.06.013.
Kim H, Wang SY, Kwak G, Yang Y, Kwon IC, Kim SH. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv Sci. 2019;6:1900513. https://doi.org/10.1002/advs.201900513.
Kotwal GJ, Chien S. Macrophage differentiation in normal and accelerated wound healing. Macrophages. 2017;62:353–64.
Sharifiaghdam M, Shaabani E, Sharifiaghdam Z, De Keersmaecker H, Lucas B, Lammens J, Ghanbari H, Teimoori-Toolabi L, Vervaet C, De Beer T, Faridi-Majidi R, De Smedt SC, Braeckmans K, Fraire JC. Macrophage reprogramming into a pro-healing phenotype by siRNA delivered with LBL assembled nanocomplexes for wound healing applications. Nanoscale. 2021;13:15445–63. https://doi.org/10.1039/D1NR03830C.
Miao M, Niu Y, Xie T, Yuan B, Qing C, Lu S. Diabetes-impaired wound healing and altered macrophage activation: a possible pathophysiologic correlation. Wound Repair Regen. 2012;20:203–13. https://doi.org/10.1111/j.1524-475X.2012.00772.x.
Seraphim PM, Leal EC, Moura J, Gonçalves P, Gonçalves JP, Carvalho E. Lack of lymphocytes impairs macrophage polarization and angiogenesis in diabetic wound healing. Life Sci. 2020;254: 117813. https://doi.org/10.1016/j.lfs.2020.117813.
Leal EC, Carvalho E, Tellechea A, Kafanas A, Tecilazich F, Kearney C, Kuchibhotla S, Auster ME, Kokkotou E, Mooney DJ, LoGerfo FW, Pradhan-Nabzdyk L, Veves A. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am J Pathol. 2015;185:1638–48. https://doi.org/10.1016/j.ajpath.2015.02.011.
Burke B, Sumner S, Maitland N, Lewis CE. Macrophages in gene therapy: cellular delivery vehicles and in vivo targets. J Leukoc Biol. 2002;72:417–28. https://doi.org/10.1189/jlb.72.3.417.
Jetten N, Roumans N, Gijbels MJ, Romano A, Post MJ, de Winther MPJ, van der Hulst RRWJ, Xanthoulea S. Wound administration of M2-polarized macrophages does not improve murine cutaneous healing responses. PLoS ONE. 2014;9: e102994. https://doi.org/10.1371/journal.pone.0102994.
Goren I, Müller E, Schiefelbein D, Christen U, Pfeilschifter J, Mühl H, Frank S. Systemic anti-TNFα treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. J Invest Dermatol. 2007;127:2259–67. https://doi.org/10.1038/sj.jid.5700842.
Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62:2579–87. https://doi.org/10.2337/db12-1450.
Ashcroft GS, Jeong M-J, Ashworth JJ, Hardman M, Jin W, Moutsopoulos N, Wild T, McCartney-Francis N, Sim D, McGrady G, Song X, Wahl SM. Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing. Wound Repair Regen. 2012;20:38–49. https://doi.org/10.1111/j.1524-475X.2011.00748.x.
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. PERSPECTIVE ARTICLE: growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. https://doi.org/10.1111/j.1524-475X.2008.00410.x.
Hu G, Guo M, Xu J, Wu F, Fan J, Huang Q, Yang G, Lv Z, Wang X, Jin Y. Nanoparticles targeting macrophages as potential clinical therapeutic agents against cancer and inflammation. Front Immunol. 2019;10:1998. https://doi.org/10.3389/fimmu.2019.01998.
Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O’Brien FJ. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Mater Today. 2015;18:313–25. https://doi.org/10.1016/j.mattod.2015.01.019.
Kulinets I. Biomaterials and their applications in medicine. Regul Aff Biomater Med Devices. 2015. https://doi.org/10.1533/9780857099204.1.
Abaricia JO, Shah AH, Chaubal M, Hotchkiss KM, Olivares-Navarrete R. Wnt signaling modulates macrophage polarization and is regulated by biomaterial surface properties. Biomaterials. 2020;243: 119920. https://doi.org/10.1016/j.biomaterials.2020.119920.
Sheikh Z, Brooks PJ, Barzilay O, Fine N, Glogauer M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials (Basel). 2015;8:5671–701. https://doi.org/10.3390/ma8095269.
Xia Z, Triffitt JT. A review on macrophage responses to biomaterials. Biomed Mater. 2006;1:R1–9. https://doi.org/10.1088/1748-6041/1/1/r01.
Boersema GSA, Grotenhuis N, Bayon Y, Lange JF, Bastiaansen-Jenniskens YM. The effect of biomaterials used for tissue regeneration purposes on polarization of macrophages. BioRes Open Access. 2016;5:6–14. https://doi.org/10.1089/biores.2015.0041.
Okamoto T, Takagi Y, Kawamoto E, Park EJ, Usuda H, Wada K, Shimaoka M. Reduced substrate stiffness promotes M2-like macrophage activation and enhances peroxisome proliferator-activated receptor γ expression. Exp Cell Res. 2018;367:264–73. https://doi.org/10.1016/j.yexcr.2018.04.005.
Zhou H, Xue Y, Dong L, Wang C. Biomaterial-based physical regulation of macrophage behaviour. J Mater Chem B. 2021;9:3608–21. https://doi.org/10.1039/D1TB00107H.
Ribeiro S, Carvalho AM, Fernandes EM, Gomes ME, Reis RL, Bayon Y, Zeugolis DI. Development and characterisation of cytocompatible polyester substrates with tunable mechanical properties and degradation rate. Acta Biomater. 2021;121:303–15. https://doi.org/10.1016/j.actbio.2020.11.026.
Davenport Huyer L, Pascual-Gil S, Wang Y, Mandla S, Yee B, Radisic M. Advanced strategies for modulation of the material-macrophage interface. Adv Funct Mater. 2020;30:1909331. https://doi.org/10.1002/adfm.201909331.
Li Z, Bratlie KM. Macrophage phenotypic changes on FN-coated physical gradient hydrogels. ACS Appl Bio Mater. 2021;4:6758–68. https://doi.org/10.1021/acsabm.1c00489.
Chen M, Zhang Y, Zhou P, Liu X, Zhao H, Zhou X, Gu Q, Li B, Zhu X, Shi Q. Substrate stiffness modulates bone marrow-derived macrophage polarization through NF-κB signaling pathway. Bioact Mater. 2020;5:880–90. https://doi.org/10.1016/j.bioactmat.2020.05.004.
Sridharan R, Cavanagh B, Cameron AR, Kelly DJ, O’Brien FJ. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019;89:47–59. https://doi.org/10.1016/j.actbio.2019.02.048.
Camarero-Espinosa S, Carlos-Oliveira M, Liu H, Mano JF, Bouvy N, Moroni L. 3D printed dual-porosity scaffolds: the combined effect of stiffness and porosity in the modulation of macrophage polarization. Adv Healthc Mater. 2022;11:2101415. https://doi.org/10.1002/adhm.202101415.
Scott RA, Kiick KL, Akins RE. Substrate stiffness directs the phenotype and polarization state of cord blood derived macrophages. Acta Biomater. 2021;122:220–35. https://doi.org/10.1016/j.actbio.2020.12.040.
Yao D, Qiao F, Song C, Lv Y. Matrix stiffness regulates bone repair by modulating 12-lipoxygenase-mediated early inflammation. Mater Sci Eng C. 2021;128: 112359. https://doi.org/10.1016/j.msec.2021.112359.
Rostam HM, Singh S, Vrana NE, Alexander MR, Ghaemmaghami AM. Impact of surface chemistry and topography on the function of antigen presenting cells. Biomater Sci. 2015;3:424–41. https://doi.org/10.1039/C4BM00375F.
Li M, Guo X, Qi W, Wu Z, de Bruijn JD, Xiao Y, Bao C, Yuan H. Macrophage polarization plays roles in bone formation instructed by calcium phosphate ceramics. J Mater Chem B. 2020;8:1863–77. https://doi.org/10.1039/C9TB02932J.
Sutherland J, Denyer M, Britland S. Contact guidance in human dermal fibroblasts is modulated by population pressure. J Anat. 2005;206:581–7. https://doi.org/10.1111/j.1469-7580.2005.00415.x.
Wennerberg A, Albrektsson T, Andersson B. An animal study of cp titanium screws with different surface topographies. J Mater Sci Mater Med. 1995;6:302–9. https://doi.org/10.1007/BF00120275.
Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci. 2010;107:15211–6. https://doi.org/10.1073/pnas.1006442107.
Chehroudi B, Ghrebi S, Murakami H, Waterfield JD, Owen G, Brunette DM. Bone formation on rough, but not polished, subcutaneously implanted Ti surfaces is preceded by macrophage accumulation. J Biomed Mater Res Part A. 2010;93A:724–37. https://doi.org/10.1002/jbm.a.32587.
Barth KA, Waterfield JD, Brunette DM. The effect of surface roughness on RAW 264.7 macrophage phenotype. J Biomed Mater Res Part A. 2013;101A:2679–88. https://doi.org/10.1002/jbm.a.34562.
Zhang Y, Cheng X, Jansen JA, Yang F, van den Beucken JJJP. Titanium surfaces characteristics modulate macrophage polarization. Mater Sci Eng C. 2019;95:143–51. https://doi.org/10.1016/j.msec.2018.10.065.
Kosoff D, Yu J, Suresh V, Beebe DJ, Lang JM. Surface topography and hydrophilicity regulate macrophage phenotype in milled microfluidic systems. Lab Chip. 2018;18:3011–7. https://doi.org/10.1039/C8LC00431E.
Wissing TB, Bonito V, van Haaften EE, van Doeselaar M, Brugmans MMCP, Janssen HM, Bouten CVC, Smits AIPM. Macrophage-driven biomaterial degradation depends on scaffold microarchitecture. Front Bioeng Biotechnol. 2019;7:87. https://doi.org/10.3389/fbioe.2019.00087.
Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann Biomed Eng. 2014;42:1508–16. https://doi.org/10.1007/s10439-013-0933-0.
Yin Y, He X-T, Wang J, Wu R-X, Xu X-Y, Hong Y-L, Tian B-M, Chen F-M. Pore size-mediated macrophage M1-to-M2 transition influences new vessel formation within the compartment of a scaffold. Appl Mater Today. 2020;18: 100466. https://doi.org/10.1016/j.apmt.2019.100466.
Bachhuka A, MadathiparambilVisalakshan R, Law CS, Santos A, Ebendorff-Heidepriem H, Karnati S, Vasilev K. Modulation of macrophages differentiation by nanoscale-engineered geometric and chemical features. ACS Appl Bio Mater. 2020;3:1496–505. https://doi.org/10.1021/acsabm.9b01125.
Jia Y, Yang W, Zhang K, Qiu S, Xu J, Wang C, Chai Y. Nanofiber arrangement regulates peripheral nerve regeneration through differential modulation of macrophage phenotypes. Acta Biomater. 2019;83:291–301. https://doi.org/10.1016/j.actbio.2018.10.040.
Mao J, Chen L, Cai Z, Qian S, Liu Z, Zhao B, Zhang Y, Sun X, Cui W. Advanced biomaterials for regulating polarization of macrophages in wound healing. Adv Funct Mater. 2021. https://doi.org/10.1002/adfm.202111003.
Bygd HC, Forsmark KD, Bratlie KM. Altering in vivo macrophage responses with modified polymer properties. Biomaterials. 2015;56:187–97. https://doi.org/10.1016/j.biomaterials.2015.03.042.
Lv L, Xie Y, Li K, Hu T, Lu X, Cao Y, Zheng X. Unveiling the mechanism of surface hydrophilicity-modulated macrophage polarization. Adv Healthc Mater. 2018;7:1800675. https://doi.org/10.1002/adhm.201800675.
Visalakshan RM, MacGregor MN, Sasidharan S, Ghazaryan A, Mierczynska-Vasilev AM, Morsbach S, Mailänder V, Landfester K, Hayball JD, Vasilev K. Biomaterial surface hydrophobicity-mediated serum protein adsorption and immune responses. ACS Appl Mater Interfaces. 2019;11:27615–23. https://doi.org/10.1021/acsami.9b09900.
Gao S, Lu R, Wang X, Chou J, Wang N, Huai X, Wang C, Zhao Y, Chen S. Immune response of macrophages on super-hydrophilic TiO2 nanotube arrays. J Biomater Appl. 2020;34:1239–53. https://doi.org/10.1177/0885328220903249.
Pérez-Calixto M, Diaz-Rodriguez P, Concheiro A, Alvarez-Lorenzo C, Burillo G. Amino-functionalized polymers by gamma radiation and their influence on macrophage polarization. React Funct Polym. 2020;151: 104568. https://doi.org/10.1016/j.reactfunctpolym.2020.104568.
Wang Y, Yao D, Li L, Qian Z, He W, Ding R, Liu H, Fan Y. Effect of electrospun silk fibroin-silk sericin films on macrophage polarization and vascularization. ACS Biomater Sci Eng. 2020;6:3502–12. https://doi.org/10.1021/acsbiomaterials.0c00175.
Barroso A, Mestre H, Ascenso A, Simões S, Reis C. Nanomaterials in wound healing: from material sciences to wound healing applications. Nano Select. 2020;1:443–60. https://doi.org/10.1002/nano.202000055.
Mihai MM, Dima MB, Dima B, Holban AM. Nanomaterials for wound healing and infection control. Materials. 2019. https://doi.org/10.3390/ma12132176.
Medrano-Bosch M, Moreno-Lanceta A, Melgar-Lesmes P. Nanoparticles to target and treat macrophages: the Ockham’s concept? Pharmaceutics. 2021. https://doi.org/10.3390/pharmaceutics13091340.
Dong X, Chang J, Li H. Bioglass promotes wound healing through modulating the paracrine effects between macrophages and repairing cells. J Mater Chem B. 2017;5:5240–50. https://doi.org/10.1039/C7TB01211J.
Xie W, Fu X, Tang F, Mo Y, Cheng J, Wang H, Chen X. Dose-dependent modulation effects of bioactive glass particles on macrophages and diabetic wound healing. J Mater Chem B. 2019;7:940–52. https://doi.org/10.1039/C8TB02938E.
Mârza SM, Magyari K, Bogdan S, Moldovan M, Peştean C, Nagy A, Tăbăran F, Licarete E, Suarasan S, Dreanca A, Baia L, Papuc I. Skin wound regeneration with bioactive glass-gold nanoparticles ointment. Biomed Mater. 2019;14:25011. https://doi.org/10.1088/1748-605x/aafd7d.
Wu H, Li F, Wang S, Lu J, Li J, Du Y, Sun X, Chen X, Gao J, Ling D. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials. 2018;151:66–77. https://doi.org/10.1016/j.biomaterials.2017.10.018.
Younan GJ, Heit YI, Dastouri P, Kekhia H, Xing W, Gurish MF, Orgill DP. Mast cells are required in the proliferation and remodeling phases of microdeformational wound therapy. Plast Reconstr Surg. 2011;128:649e–58e. https://doi.org/10.1097/PRS.0b013e318230c55d.
Rajendran NK, Kumar SSD, Houreld NN, Abrahamse H. A review on nanoparticle based treatment for wound healing. J Drug Deliv Sci Technol. 2018;44:421–30. https://doi.org/10.1016/j.jddst.2018.01.009.
Kim TH, Jung Y, Kim SH. Nanofibrous electrospun heart decellularized extracellular matrix-based hybrid scaffold as wound dressing for reducing scarring in wound healing. Tissue Eng Part A. 2017;24:830–48. https://doi.org/10.1089/ten.tea.2017.0318.
Liu S, Zhang Q, Yu J, Shao N, Lu H, Guo J, Qiu X, Zhou D, Huang Y. Absorbable thioether grafted hyaluronic acid nanofibrous hydrogel for synergistic modulation of inflammation microenvironment to accelerate chronic diabetic wound healing. Adv Healthc Mater. 2020;9:2000198. https://doi.org/10.1002/adhm.202000198.
Sun L, Li J, Gao W, Shi M, Tang F, Fu X, Chen X. Coaxial nanofibrous scaffolds mimicking the extracellular matrix transition in the wound healing process promoting skin regeneration through enhancing immunomodulation. J Mater Chem B. 2021;9:1395–405. https://doi.org/10.1039/D0TB01933J.
Kaymakcalan OE, Abadeer A, Goldufsky JW, Galili U, Karinja SJ, Dong X, Jin JL, Samadi A, Spector JA. Topical α-gal nanoparticles accelerate diabetic wound healing. Exp Dermatol. 2020;29:404–13. https://doi.org/10.1111/exd.14084.
Criado-Gonzalez M, Espinosa-Cano E, Rojo L, Boulmedais F, Aguilar MR, Hernández R. Injectable tripeptide/polymer nanoparticles supramolecular hydrogel: a candidate for the treatment of inflammatory pathologies. ACS Appl Mater Interfaces. 2022;14:10068–80. https://doi.org/10.1021/acsami.1c22993.
Peled E, Sosnik A. Amphiphilic galactomannan nanoparticles trigger the alternative activation of murine macrophages. J Control Release. 2021;339:473–83. https://doi.org/10.1016/j.jconrel.2021.10.017.
Liu Q, Kim Y-J, Im G-B, Zhu J, Wu Y, Liu Y, Bhang SH. Inorganic nanoparticles applied as functional therapeutics. Adv Funct Mater. 2021;31:2008171. https://doi.org/10.1002/adfm.202008171.
Cheng J, Zhang Q, Fan S, Zhang A, Liu B, Hong Y, Guo J, Cui D, Song J. The vacuolization of macrophages induced by large amounts of inorganic nanoparticle uptake to enhance the immune response. Nanoscale. 2019;11:22849–59. https://doi.org/10.1039/C9NR08261A.
You C, Li Q, Wang X, Wu P, Ho JK, Jin R, Zhang L, Shao H, Han C. Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci Rep. 2017;7:10489. https://doi.org/10.1038/s41598-017-10481-0.
Adhikari U, An X, Rijal N, Hopkins T, Khanal S, Chavez T, Tatu R, Sankar J, Little KJ, Hom DB, Bhattarai N, Pixley SK. Embedding magnesium metallic particles in polycaprolactone nanofiber mesh improves applicability for biomedical applications. Acta Biomater. 2019;98:215–34. https://doi.org/10.1016/j.actbio.2019.04.061.
Gan J, Liu C, Li H, Wang S, Wang Z, Kang Z, Huang Z, Zhang J, Wang C, Lv D, Dong L. Accelerated wound healing in diabetes by reprogramming the macrophages with particle-induced clustering of the mannose receptors. Biomaterials. 2019;219: 119340. https://doi.org/10.1016/j.biomaterials.2019.119340.
Xiang J, Zhu R, Lang S, Yan H, Liu G, Peng B. Mussel-inspired immobilization of zwitterionic silver nanoparticles toward antibacterial cotton gauze for promoting wound healing. Chem Eng J. 2021;409: 128291. https://doi.org/10.1016/j.cej.2020.128291.
Daghian SG, Farahpour MR, Jafarirad S. Biological fabrication and electrostatic attractions of new layered silver/talc nanocomposite using Lawsonia inermis L. and its chitosan-capped inorganic/organic hybrid: investigation on acceleration of Staphylococcus aureus and Pseudomonas aeruginosa infected wound healing. Mater Sci Eng C. 2021;128: 112294. https://doi.org/10.1016/j.msec.2021.112294.
ChoodariGharehpapagh A, Farahpour MR, Jafarirad S. The biological synthesis of gold/perlite nanocomposite using Urtica dioica extract and its chitosan-capped derivative for healing wounds infected with methicillin-resistant Staphylococcus aureus. Int J Biol Macromol. 2021;183:447–56. https://doi.org/10.1016/j.ijbiomac.2021.04.150.
Zhu S, Dai Q, Yao L, Wang Z, He Z, Li M, Wang H, Li Q, Gao H, Cao X. Engineered multifunctional nanocomposite hydrogel dressing to promote vascularization and anti-inflammation by sustained releasing of Mg2+ for diabetic wounds. Compos Part B Eng. 2022;231: 109569. https://doi.org/10.1016/j.compositesb.2021.109569.
Orlowski P, Zmigrodzka M, Tomaszewska E, Ranoszek-Soliwoda K, Czupryn M, Antos-Bielska M, Szemraj J, Celichowski G, Grobelny J, Krzyzowska M. Tannic acid-modified silver nanoparticles for wound healing: the importance of size. Int J Nanomed. 2018;13:991.
Wu J, Zhu J, Wu Q, An Y, Wang K, Xuan T, Zhang J, Song W, He H, Song L, Zheng J, Xiao J. Mussel-inspired surface immobilization of heparin on magnetic nanoparticles for enhanced wound repair via sustained release of a growth factor and M2 macrophage polarization. ACS Appl Mater Interfaces. 2021;13:2230–44. https://doi.org/10.1021/acsami.0c18388.
Gubitosa J, Rizzi V, Fini P, Laurenzana A, Fibbi G, Veiga-Villauriz C, Fanelli F, Fracassi F, Onzo A, Bianco G, Gaeta C, Guerrieri A, Cosma P. Biomolecules from snail mucus (Helix aspersa) conjugated gold nanoparticles, exhibiting potential wound healing and anti-inflammatory activity. Soft Matter. 2020;16:10876–88. https://doi.org/10.1039/D0SM01638A.
Liu L, Ding Z, Yang Y, Zhang Z, Lu Q, Kaplan DL. Asiaticoside-laden silk nanofiber hydrogels to regulate inflammation and angiogenesis for scarless skin regeneration. Biomater Sci. 2021;9:5227–36. https://doi.org/10.1039/D1BM00904D.
Saleh B, Dhaliwal HK, Portillo-Lara R, Shirzaei Sani E, Abdi R, Amiji MM, Annabi N. Local immunomodulation using an adhesive hydrogel loaded with miRNA-laden nanoparticles promotes wound healing. Small. 2019;15:1902232. https://doi.org/10.1002/smll.201902232.
Hu C, Zhang F, Long L, Kong Q, Luo R, Wang Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J Control Release. 2020;324:204–17. https://doi.org/10.1016/j.jconrel.2020.05.010.
Ding L-G, Wang S, Yao B-J, Li F, Li Y-A, Zhao G-Y, Dong Y-B. Synergistic antibacterial and anti-inflammatory effects of a drug-loaded self-standing porphyrin-COF membrane for efficient skin wound healing. Adv Healthc Mater. 2021;10:2001821. https://doi.org/10.1002/adhm.202001821.
Zhang G, Xue H, Sun D, Yang S, Tu M, Zeng R. Soft apoptotic-cell-inspired nanoparticles persistently bind to macrophage membranes and promote anti-inflammatory and pro-healing effects. Acta Biomater. 2021;131:452–63. https://doi.org/10.1016/j.actbio.2021.07.002.
Gobi R, Ravichandiran P, Babu RS, Yoo DJ. Biopolymer and synthetic polymer-based nanocomposites in wound dressing applications: a review. Polymers. 2021. https://doi.org/10.3390/polym13121962.
Dulińska-Litewka J, Dykas K, Felkle D, Karnas K, Khachatryan G, Karewicz A. Hyaluronic acid-silver nanocomposites and their biomedical applications: a review. Materials. 2022. https://doi.org/10.3390/ma15010234.
Mebert AM, Alvarez GS, Peroni R, Illoul C, Hélary C, Coradin T, Desimone MF. Collagen-silica nanocomposites as dermal dressings preventing infection in vivo. Mater Sci Eng C. 2018;93:170–7. https://doi.org/10.1016/j.msec.2018.07.078.
Wang P, Jiang S, Li Y, Luo Q, Lin J, Hu L, Liu X, Xue F. Virus-like mesoporous silica-coated plasmonic Ag nanocube with strong bacteria adhesion for diabetic wound ulcer healing. Nanomed Nanotechnol Biol Med. 2021;34: 102381. https://doi.org/10.1016/j.nano.2021.102381.
Maheen S, Younis H, Khan HU, Salman Shafqat S, Ali S, Rehman AU, Ilyas S, Zafar MN, Shafqat SR, Kalam A, Al-Ghamdi AA. Enhanced antifungal and wound healing efficacy of statistically optimized, physicochemically evaluated econazole-triamcinolone loaded silica nanoparticles. Front Chem. 2022. https://doi.org/10.3389/fchem.2022.836678.
Funding
Dr. Tianqing Liu is supported by the National Health and Medical Research Council (NHMRC) Early Career Fellowship (Grant No. 1112258) and WSU Vice-Chancellor’s Senior Research Fellowship.
Author information
Authors and Affiliations
Contributions
TL conceptualized, guided and edited the manuscript. AJ prepared the original manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experiments were approved by the Animal Ethics Committee of Nantong University.
Consent for publication
All authors are consent for publication.
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Joorabloo, A., Liu, T. Recent advances in nanomedicines for regulation of macrophages in wound healing. J Nanobiotechnol 20, 407 (2022). https://doi.org/10.1186/s12951-022-01616-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-022-01616-1