Abstract
Background
The assessment of clinical prognosis of pregnant COVID-19 patients at hospital presentation is challenging, due to physiological adaptations during pregnancy. Our aim was to assess the performance of the ABC2-SPH score to predict in-hospital mortality and mechanical ventilation support in pregnant patients with COVID-19, to assess the frequency of adverse pregnancy outcomes, and characteristics of pregnant women who died.
Methods
This multicenter cohort included consecutive pregnant patients with COVID-19 admitted to the participating hospitals, from April/2020 to March/2022. Primary outcomes were in-hospital mortality and the composite outcome of mechanical ventilation support and in-hospital mortality. Secondary endpoints were pregnancy outcomes. The overall discrimination of the model was presented as the area under the receiver operating characteristic curve (AUROC). Overall performance was assessed using the Brier score.
Results
From 350 pregnant patients (median age 30 [interquartile range (25.2, 35.0)] years-old]), 11.1% had hypertensive disorders, 19.7% required mechanical ventilation support and 6.0% died. The AUROC for in-hospital mortality and for the composite outcome were 0.809 (95% IC: 0.641–0.944) and 0.704 (95% IC: 0.617–0.792), respectively, with good overall performance (Brier = 0.0384 and 0.1610, respectively). Calibration was good for the prediction of in-hospital mortality, but poor for the composite outcome. Women who died had a median age 4 years-old higher, higher frequency of hypertensive disorders (38.1% vs. 9.4%, p < 0.001) and obesity (28.6% vs. 10.6%, p = 0.025) than those who were discharged alive, and their newborns had lower birth weight (2000 vs. 2813, p = 0.001) and five-minute Apgar score (3.0 vs. 8.0, p < 0.001).
Conclusions
The ABC2-SPH score had good overall performance for in-hospital mortality and the composite outcome mechanical ventilation and in-hospital mortality. Calibration was good for the prediction of in-hospital mortality, but it was poor for the composite outcome. Therefore, the score may be useful to predict in-hospital mortality in pregnant patients with COVID-19, in addition to clinical judgment. Newborns from women who died had lower birth weight and Apgar score than those who were discharged alive.
Similar content being viewed by others
Background
Coronavirus disease 2019 (COVID-19) has quickly spread worldwide with higher morbidity and lethality than other coronaviruses [1], threatening people’s lives, and more severely the most vulnerable or those under adverse social contexts [2, 3]. Pregnancy imposes physiological adaptations, including modulations of the immune system, which have important implications on the prognosis of viral conditions [4,5,6]. Most women can experience mild or asymptomatic disease [7], with fatal consequences ranging between 0 to 15.6% among the studies [6, 8,9,10,11,12,13]. Even in a largely asymptomatic population, COVID-19 has been shown to be associated with maternal inflammatory responses in the maternal-fetal junction and at the circulation [14].
Current studies have shown that pregnant women may be particularly vulnerable to COVID-19 infection [15], as well as developing critical disease and mortality [12, 16,17,18], which is cause of great concern. Direct and indirect effects of pandemics over pregnancy became a global challenge, changing many aspects of motherhood, mostly in low- and medium-income countries [19]. Large meta-analyses have shown that pregnant women with COVID-19 have higher risk of worse perinatal outcomes, higher requirement of intensive care unit (ICU) admission and invasive mechanical ventilation support, when compared to non-pregnant women with COVID-19 [7].
Furthermore, pregnant women with comorbidities, such as diabetes, hypertensive diseases, heart disease, and lung diseases seem to be more susceptible to severe/critical forms of COVID-19 and maternal mortality [8, 9, 20]. In fact, the literature indicates other risk factors for adverse outcomes, in addition to pre-existing medical conditions, such as older age, being overweight or obese, and being a member of a black or ethnic minority ethnic group [8]. As there are several physiological changes during pregnancy, the development of rapid scoring systems for prognosis applicable for this population is challenging [21].
In Brazil, a country severely hit by the pandemic, COVID-19 became the first cause of maternal death. Therefore, the assessment of clinical characteristics and outcomes in pregnant women who are hospitalized with COVID-19, as well as the factors potentially associated with adverse maternal outcomes in those patients, is of utmost importance for public health [22, 23]. However, there are specificities in clinical parameters in pregnant women, that makes it impossible to use the same scores developed for the non-pregnant without previous assessment.
Therefore, our aim was to assess the performance of a prognosis score, developed and validated for general hospitalized adults (men and women) with COVID-19 in Brazil, to predict in-hospital mortality and mechanical ventilation support in COVID-19 pregnant patients. Additionally, to assess the frequency of adverse pregnancy outcomes, and to compare characteristics of pregnant women who died to those who were discharged.
Methods
Study design and participants
The present analysis is a substudy of the Brazilian COVID-19 Registry, an ongoing retrospective multicenter cohort study of consecutive adults both sex patients with laboratory-confirmed COVID-19 patients hospitalized in public and private hospitals in Brazil. The study protocol was published elsewhere [24]. This manuscript adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline [25].
For the present analysis, pregnant patients with confirmed COVID-19 admitted to the participating hospitals from April/2020 to March/2022, at any time during the pregnancy, were consecutively enrolled. Patients transferred to hospitals not participating in the Registry without information on final patient outcomes; and those who were admitted to the hospitals due to other conditions, developed symptoms, and had COVID-19 confirmed during hospital admission were not included (Fig. 1).
Patient management was at the discretion of the treating healthcare professionals. Management protocols followed the Brazilian Ministry of Health Guidelines [26, 27].
Data collection
Study data were collected and managed by trained health professionals using Research Electronic Data Capture (REDCap), hosted at the Telehealth Center of the University Hospital, Universidade Federal de Minas Gerais [28, 29]. Clinical characteristics, laboratory data, and obstetric characteristics at admission, as well as events that occurred during the hospital stay and patient outcomes were collected from medical records. Obstetric data were gestational age, pregnancy complications at admission, whether there was delivery and, if so, mode of delivery, birth weight, five-minute Apgar score, and vital state of the newborn. The study protocol and a coding manual guiding data collection with details was agreed with the network of researchers [24]. Furthermore, over the pandemic, the management protocols were updated regularly, following the Brazilian Ministry of Health Guidelines on the management of patients with COVID-19. All patient charts were reviewed thoroughly to confirm the accuracy of the data [24].
The prognosis score ABC2-SPH
Our group previously developed and validated a prognostic scoring model for in-hospital mortality for COVID-19 patients, based on comorbidities, clinical characteristics and laboratory findings at hospital presentation, named the ABC2-SPH score [30]. In brief, it has seven variables: age, blood urea nitrogen values, comorbidities, C-reactive protein, peripheral oxygen saturation to fraction of inspired oxygen ratio (SF ratio), platelet count and heart rate, detailed in Table S1 (see Additional file 1). Score development and validation followed strict methodological criteria [31]. It is the only score validated for the Brazilian population, and it has shown high discriminatory ability (AUROC 0.844, 95% CI 0.829 to 0.859), higher than other existing scores [30].
After exclusion criteria, 350 pregnant women were identified in 24 centers, in 15 different cities from five Brazilian states (Fig. 2).
Cities of the hospital of pregnant patients included in this study. *R Core Team (R version 4.0.2). https://www.R-project.org/
Outcomes
The primary endpoints were in-hospital mortality, and the composite outcome of mechanical ventilation support and in-hospital mortality. Secondary endpoints included pregnancy complications: abortion, ectopic pregnancy, preeclampsia, eclampsia, HELLP Syndrome, abnormal bleeding in childbirth or puerperium, hysterectomy, and puerperal infection.
Statistical analysis
Descriptive analysis was performed concerning frequency, variability, and central tendency measures. Continuous variables were summarized using medians and interquartile range (IQR), whereas counts and percentages were used for categorical variables. For comparisons between pregnant women who died or were discharged alive, the Chi-squared test or Fisher test was used for the independence hypothesis, and the Mann–Whitney test compared the numerical variables between the groups. A p-value lower than 0.05 was considered statistically significant.
Overall performance of ABC2-SPH [30] score was evaluated using the Brier score [32]. Calibration was assessed graphically by plotting the predicted outcome of interest (in-hospital mortality or the composite outcome) probabilities against the observed outcome, testing intercept equals zero and slope equals one. The area under the receiver operating characteristic curve (AUROC) described the model’s discrimination. For this, the numeric variable from the score for each pregnant woman was used to predict in-hospital mortality and the composite outcome. Confidence intervals (95% CI) for AUROC were obtained through 2000 bootstrap samples.
We also calculated accuracy, sensitivity and specificity of the ABC2SPH, as well as comparison with other existing scores for the general population [33,34,35,36,37].
All statistical analyses, calibration, and plottings were performed with R software (version 4.0.2) with the tidyverse, pROC, rms packages.
Results
Clinical characteristics and laboratory findings of the 350 pregnant women are shown in Table 1, and the geographic location of the hospital they were admitted at is shown in Fig. 2. The median age was 30.0 (IQR 25.2, 35.0) years-old, and the majority of them had no previous comorbidities (76.9%). Obesity (11.7%), diabetes (9.1%), and hypertension (11.1%) were the most prevalent underlying medical conditions. Sixty-eight pregnant women needed mechanical ventilation (19.7%), and 21 (6.0%) died. Only three of those who died were not on mechanical ventilation support.
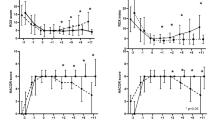
The ABC2-SPH score was able to identify high-risk pregnant women. The area under the ROC curve [AUROC] for in-hospital mortality was 0.809 (95% IC: 0.641–0.944) and for the composite outcome was 0.704 (95% IC: 0.617–0.792) (Fig. 3A and B), with good overall performance (Brier = 0.0384 and 0.1610, respectively). Calibration was also good for the prediction of in-hospital mortality, but it was poor for the composite outcome (Fig. 4A and B). Table S2 shows the comparison between ABC2-SPH and other scores (see Additional file 2).
Calibration plot of ABC2-SPH Score for in-hospital mortality (A), and composite of mechanical ventilation and in-hospital mortality (B), for each quartile of pregnant women risk. *It plots the observed and expected death, and the diagonal line represents a perfect agreement between observed and expected probability of the outcome
Women who died had a median age 4 years-old higher than those who were discharged alive, as well as a higher frequency of hypertensive disorders (38.1% vs. 9.4%, p < 0.001) and obesity (28.6% vs. 10.6%, p = 0.025). Dyspnea (55.7%), fever (47.4%), productive cough (46.9%), myalgia (33.7%), and headache (26.9%) were the most frequent symptoms, and the frequency of symptoms was similar between those who died and those who were discharged alive. With regards to clinical presentation and laboratory analysis upon hospital admission, patients who died had a significantly lower median of the SpO2/FiO2 ratio (333.0 vs 457.1, p = 0.002), higher median neutrophils-to-lymphocytes ratio (8.1 vs. 5.3, p = 0.034), and lower partial pressure of oxygen (69.0 vs 86.0, p = 0.004) than those who were discharged alive (Table 1).
Concerning obstetric characteristics (Table 2), the median gestational age was 31.0 (IQR 24.0, 36.0) weeks overall, and there was no difference between groups. However, those who died had a higher frequency of gestational complications (57.1% vs. 27.3%, p = 0.008).
Among 350 pregnant women, 139 (40.1%) gave birth during the in-hospital stay. One woman delivered twins, totaling 140 newborns. Of those, 125 (91.2%) were alive at hospital discharge. Birth weight in grams (2000 vs 2813, p = 0.001) and five-minute Apgar score (3.0 vs 8.0, p < 0.001) were lower in newborns from pregnant women who died from COVID- 19 when compared to those who were discharged alive (Table 2).
Discussion
The main contribution of the present analysis was to validate the ABC2-SPH score in 350 pregnant women from 24 Brazilian hospitals. The ABC2-SPH score has been shown to be a reliable tool in estimating in-hospital mortality risk in pregnant COVID-19 patients. In this population, the score had good overall performance for the primary outcomes and good discriminatory ability. Calibration was good for the prediction of in-hospital mortality, but it was poor for the composite outcome of in-hospital mortality and mechanical ventilation support. The score is simple, objective, uses variables easily available at hospital presentation and it may be easily calculated. Model performance comparison surpassed other existing scores, commonly used in the general population.
In fact, assessing predictors of critical outcomes in COVID-19 may advise timely treatments and better prepare facilities to overcome extra adversities during pregnancy. Our findings support the employment of the score as a tool in estimating in-hospital mortality at admission in pregnant patients. Therefore, it is of utmost importance to take into account that the score should be used in addition to the clinical judgment, to support clinical decision, for example, to help screening pregnant women who need more frequent reassessments, as well as to help to assess which one to refer to intensive care, in cases of limited resources. As a screening tool, it is of utmost importance to have a high sensitivity, to avoid missing as few cases as possible. In the present analysis, ABC2-SPH achieved 96.0% sensitivity, with a very precise confidence interval (91.8–98.4%), higher than any other score tested.
On the contrary, our findings evidence against the use of the score to predict the composite outcome of mechanical ventilation support and in-hospital mortality. In a recent analysis from our group (data not published yet), ABC2-SPH score did not have good overall performance to predict mechanical ventilation support in general (non-pregnant) patients. Therefore, the present results may reflect the fact that the score is not a good predictor for mechanical ventilation overall.
Several prediction scores have been proposed for use in the nonpregnant population with COVID-19 with varied success. The study conducted by Jones et al. (2021) [38] validated the 4C score for Canadian patients obtaining an AUC of 0.770 (95% CI 0.790–0.870). In addition, the accuracy of this prediction model (4C Mortality), beyond NEWS and CURB-65 was compared among the Romanian population with AUC of 0.818 (95% CI 0.718–0.919), 0.861 (95% CI 0784–0.939), and 0.801 (95% CI 0.681–0.922), respectively [39, 40]. In the present study, we tested these aforementioned scores, together with other scores commonly used for general COVID-19 patients, and ABC2-SPH outperformed all of them.
As aforementioned, many clinical parameters of existent scores developed for the general (non-pregnant) population are deeply modified by physiological adaptations of pregnancy. Notably, these adaptations are challenging for using the scores developed for the general population without further validation and can contribute to an understanding of the lack of prediction models for the prognosis of COVID-19 in this population, despite the fact that several prognostic scores have been developed for COVID-19 [41,42,43,44]. One multicenter retrospective cohort study including eight hospitals from four countries (n = 973) proposed two models to predict ICU admission and maternal death in pregnant women with symptomatic COVID-19 [45], however, both models are limited by methodological bias, with the absence of external (even geographic) validation.
Our study observed high in-hospital mortality in pregnant women (6%). A study based on secondary data from Brazil (975,109 cases) suggested that pregnant women with COVID-19 have approximately twice the mortality rates of men and non-pregnant women [46]. Takemoto et al. (2020) [16] reported high mortality among Brazilian pregnant women with COVID-19, approximately 20 maternal deaths out of 125,218 overall cases and 8536 deaths (as of May 7, 2020), with lethality of 15.6% in 2021 [12]. Similarly, another study found an association between COVID-19 and worse clinical outcomes for pregnant women in Brazil, with a 3.4 times higher death rate than any other acute respiratory distress syndromes (ARDS) etiologies [15]. According to a systematic review with 2670 patients from seven countries, the differences in results for maternal characteristics reflect the profile of the patient of each country of origin [9]. This study (n = 38 studies, 2670 patients, 52.6% from China), have shown a significant variation between maternal age among pregnant women with COVID-19, percentages of C-sections, maternal mortality rate and newborn outcomes [9]. In fact, is it possible that pregnant Brazilian patients have different characteristics from those from other countries, placing them in the leadership of maternal deaths due to COVID-19 worldwide [47]. One of them is the prevalence of underlying diseases, especially preeclampsia and obesity, conditions that are known inflammatory, risk factors for COVID-19 complications. Besides, an important contributor to greater mortality in the country were the barriers to access to prenatal care during the pandemic, inadequate monitoring of obstetric complications and barriers to access intensive care [4, 17, 47,48,49].
Despite having similar symptoms, our analysis showed differences between pregnant women who died and those who were discharged. Those who died had higher age, prevalence of hypertension, obesity and, as expected, in-hospital complications than the ones who were discharged alive. These findings are consistent with a large study from the Centers for Disease Control and Prevention (CDC), comparing 386,028 positive nonpregnant women in their reproductive age (15–44 years), with 23,434 SARS-CoV-2 positive pregnant women, demonstrating that death occurred more frequently among women aged 35–44 years than among those aged 15–24 years. When stratified by age, all outcomes, such as hospitalization, ICU admission, receipt of mechanical ventilation, and death were more frequently in pregnant women aged 35–44 years than among those aged 15–24 years [50]. Additionally, a brief communication conducted by Takemoto et al. (2020) [17] collected the effect of 978 pregnant women with COVID-19 in Brazil, indicating that women who died had higher maternal age (31.5 years). A living systematic review has shown that increasing age (odds ratio 1.83, 95% confidence interval 1.27 to 2.63; seven studies, 3561 women), high body mass index (2.37, 1.83 to 3.07; five studies, 3367 women), any pre-existing maternal comorbidity (1.81, 1.49 to 2.20; 3 studies; 2634 women), chronic hypertension (2.0, 1.14 to 3.48; two studies, 858 women), pre-eclampsia (4.21, 1.27 to 14.0; 4 studies; 274 women), and pre-existing diabetes (2.12, 1.62 to 2.78; 3 studies, 3333 women) are maternal risk factors associated with severe COVID-19 [8]. Non-white ethnicity (1.61, 1.05 to 2.47; 3 studies, 31,469 women; 2.23, 1.25 to 3.97; 1 study, 669 women; respectively) and high body mass index (2.27, 1.20 to 4.31; 3 studies, 31,085 women; 6.61, 1.98 to 22.02; 2 studies, 485 women; respectively; Table 2) were associated with maternal death and the need for invasive ventilation [8].
In the present analysis, the most common laboratory findings among patients who died from COVID-19 were lower median SpO2/FiO2 ratio (333.0 vs 457.1, p = 0.002), higher median neutrophils-to-lymphocytes ratio (8.1 vs 5.3, p = 0.034), and lower partial pressure of oxygen (69.0 vs 86.0, p = 0.004). During pregnancy, vital signals had proper values, including a slight drop in SpO2 [51]. It is important to mention that in this period the circulatory system undergoes physiological changes, starting early in its course, driven by peripheral vasodilatation, increased heart rate and stroke volume, reduced pulmonary vascular resistance, and reduced pulmonary residual capacity. These changes may affect the course of viral infections [52, 53]. Regarding inflammatory markers, the existing evidence is conflicting on whether the pregnancy is an immunological contributor to the severe progression of COVID-19 [54]. Successful pregnancy depends on a responsive immune system, which explains reports of universal COVID-19 testing during pregnancy, that the vast majority is asymptomatic or has mild COVID-19 [54, 55]. The unit maternal and the fetoplacental immune system is responsive, protecting both the mother and the fetus against threats from the environment [56].
Nevertheless, we observed that childbirth had an impact of COVID-19. C-section was performed in 71.0% of childbirths, with lower birth weight in babies of pregnant women who died (low birth weight, 2000 vs 2813, p = 0.001), and 13 babies died. An aforementioned systematic review [9] analyzed cesarean delivery rates geographically and found rates to be considerably higher in China (83.9%), followed by the United Kingdom (71.9%), with Spain with the lowest rate of C-sections (35.9%). The reasons for these practices are unclear, but it may be attributable to the habitual medical practice of each country, in addition to the lack of guidelines and recommendations at the beginning of the pandemic. Regarding low birth weight, the placenta is a selective barrier able to protect the developing fetus against infections, including SARS-CoV-2 virus infection [57], and it acts as an immunity-modulating organ, regulating immune responses of cells present both at the implantation site and systemically [58]. However, evidence of fetal vascular malperfusion or thrombosis has been observed in COVID-19, which may be related to an exacerbated maternal systemic inflammatory response and hypercoagulable state [59, 60]. In the meantime, our study observed lower median Apgar score in newborns of pregnant women who died from COVID-19, compared to those who were discharged alive (3.0 vs 8.0, p < 0.001). In cases of fetal distress, prematurity, and severe/critical maternal disease the Apgar scores are lower [61, 62].
Based on our results, we warn against the use of non-pregnant COVID-19 prognosis scores in pregnant patients to predict adverse outcomes without proper validation. While the control of COVID-19 pandemic is still challenging in many places, fast and efficient assessment of the prognosis of the COVID-19 is of utmost importance. We can expect the downstream effects of COVID-19 to be apparent for a number of years. Further studies with large sample sizes are required for the development and validation of a more accurate model to predict other adverse outcomes, such as mechanical ventilation and pregnancy outcomes, concerning the specificities of pregnant patients affected by COVID-19. Scores for pregnant women would be useful for early identification of cases at higher risk of worse outcomes in this highly vulnerable group of women. Further studies are also necessary to identify risks in pregnancy-related critical illness [21] due to COVID-19 or other causes. Evidence-based modeling could provide a proper prognosis score assessment tool that will help guide decision-making, develop patient care plans, and better allocate resources.
Even with its multiple strengths, the present study has some limitations. Recalibration of the ABC2-SPH score may improve its prediction of the effects of COVID-19 on pregnant women. However, our sample size is not large enough for this analysis (at least 100 events for recalibration) [31]. This is a topic for future studies with larger sample sizes. Additionally, details about diagnosis of hypertensive syndromes of pregnancy, subtypes of diabetes during pregnancy may differ among the perinatal centers involved in this cohort. Besides, the study missed details to specify gestational complications. The newborn data was used to infer possible complications related to pregnancy and delivery, the analysis of newborn outcomes secondary to COVID-19 requires a different study design and was not the purpose of the present analysis. In fact, this is a topic for an ongoing study from our group. Additionally, we have not investigated the impact of each individual SARS-CoV-2 variant on pregnant women. Different variants had different rates of adverse obstetric outcomes and different prognosis [63]. Lastly, due to the exclusion of pregnant/lactating women from the preliminary vaccine trials [64], the Brazilian vaccination campaign for pregnant women started in July 2021, and our sample size did not allow a stratified analysis. Further studies are required on both topics.
Conclusions
This study has shown that the ABC2-SPH score, developed in Brazilian general patients, was not able to sufficiently identify adverse clinical outcomes in pregnant patients with COVID-19.
We warn against the use of prediction models for general inpatients COVID-19 prognosis in pregnant women. Further studies with large sample sizes are required for the development and validation of a more accurate model to predict poor outcomes, concerning the specificities of pregnant patients affected by COVID-19.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Change history
30 January 2023
In order for author names to appear in the Pubmed indexing, only last names were captured as FamilyName.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- AUROC:
-
Receiver Operating Characteristic Curve
- BMI:
-
Body mass index
- CDC:
-
Centers for Disease Control and Prevention
- COVID-19:
-
Coronavirus Disease 2019
- HCO3 − :
-
Bicarbonate
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile Ranges
- NL ratio:
-
Neutrophils-to-lymphocytes ratio
- pCO2 :
-
Carbon dioxide partial pressure
- pH:
-
Hydrogen potential
- pO2 :
-
Oxygen partial pressure
- REDCap:
-
Research Electronic Data Capture
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome Coronavirus 2
- SF ratio:
-
Peripheral oxygen saturation to fraction of inspired oxygen ratio
- SpO2 :
-
Peripheral Oxygen Saturation
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
References
Carta MG, Scano A, Lindert J, Bonanno S, Rinaldi L, Fais S, et al. Association between the spread of COVID-19 and weather-climatic parameters. Eur Rev Med Pharmacol Sci. 2020;24(15):8226–31. https://doi.org/10.26355/eurrev_202008_22512.
Breitling LP. Global epidemiology and socio-economic development correlates of the reproductive ratio of COVID-19. Int Health. 2021;13(6):514–9. https://doi.org/10.1093/inthealth/ihab006.
Ribeiro KB, Ribeiro AF, Veras MASM, de Castro MC. Social inequalities and COVID-19 mortality in the city of São Paulo, Brazil. Int J Epidemiol. 2021;50(3):732–42. https://doi.org/10.1093/ije/dyab022.
Wastnedge EAN, Reynolds RM, van Boeckel SR, Stock SJ, Denison FC, Maybin JA, et al. Pregnancy and COVID-19. Physiol Rev. 2021;101(1):303–18. https://doi.org/10.1152/physrev.00024.2020.
Kotlar B, Gerson E, Petrillo S, Langer A, Tiemeier H. The impact of the COVID-19 pandemic on maternal and perinatal health: a scoping review. Reprod Health. 2021;18(1):1–39. https://doi.org/10.1186/s12978-021-01070-6.
Smith V, Seo D, Warty R, Payne O, Salih M, Chin KL, et al. Maternal and neonatal outcomes associated with COVID-19 infection: a systematic review. PLoS One. 2020;15(6):e0234187. https://doi.org/10.1371/journal.pone.0234187.
Jafari M, Pormohammad A, Sheikh Neshin SA, Ghorbani S, Bose D, Alimohammadi S, et al. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: a systematic review and meta-analysis. Rev Med Virol. 2021;31(5):1–16. https://doi.org/10.1002/rmv.2208.
Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. https://doi.org/10.1136/bmj.m3320.
Cuñarro-López Y, Pintado-Recarte P, Cueto-Hernández I, Hernández-Martín C, Payá-Martínez MP, Muñóz-Chápuli MDM, et al. The profile of the obstetric patients with SARS-CoV-2 infection according to country of origin of the publication: a systematic review of the literature. J Clin Med. 2021;10(2):360. https://doi.org/10.3390/jcm10020360.
Young EM, Green O, Stewart J, King Y, O'Donoghue K, Walker KF, et al. COVID-19 and pregnancy: a comparison of case reports, case series and registry studies. Eur J Obstet Gynecol Reprod Biol. 2022;268:135–42. https://doi.org/10.1016/j.ejogrb.2021.12.002.
Mascio D, Khalil A, Saccone G, Rizzo G, Buca D, Liberati M, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2(2):25. https://doi.org/10.1016/j.ajogmf.2020.100107.
Takemoto MLS, Nakamura-Pereira M, Menezes MO, Katz L, Knobel R, Amorim MMR, et al. Higher case fatality rate among obstetric patients with COVID-19 in the second year of pandemic in Brazil: do new genetic variants play a role? medRxiv. 2021; 2021.05.06.21256651. https://doi.org/10.1101/2021.05.06.21256651.
Ayed A, Embaireeg A, Benawadh A, Al-Fouzan W, Hammoud M, Al-Hathal M, et al. Maternal and perinatal characteristics and outcomes of pregnancies complicated with COVID-19 in Kuwait. BMC Pregnancy Childbirth. 2020;20(1):754. https://doi.org/10.1186/s12884-020-03461-2.
Garcia-Flores V, Romero R, Xu Y, Theis K, Arenas-Hernandez M, Miller D, et al. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat Commun. 2022;13(320):1–20. https://doi.org/10.1038/s41467-021-27745-z.
Scheler CA, Discacciati MG, Vale DB, Lajos GJ, Surita F, Teixeira JC. Mortality in pregnancy and the postpartum period in women with severe acute respiratory distress syndrome related to COVID-19 in Brazil, 2020. Int J Gynaecol Obstet. 2021;155(3):475–82. https://doi.org/10.1002/ijgo.13804.
Takemoto MLS, Menezes MO, Andreucci CB, Knobel R, Sousa LAR, Katz L, et al. Maternal mortality and COVID-19. J Matern Fetal Neonatal Med. 2020a:1–7. https://doi.org/10.1080/14767058.2020.1786056.
Takemoto MLS, Menezes MO, Andreucci CB, Nakamura-Pereira M, Amorim MMR, Katz L, et al. The tragedy of COVID-19 in Brazil: 124 maternal deaths and counting. Int J Gynaecol Obstet. 2020b;151(1):154–6. https://doi.org/10.1002/ijgo.13300.
Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641–7. https://doi.org/10.15585/mmwr.mm6944e3.
Kingsley JP, Vijay PK, Kumaresan J, Sathiakumar N. The changing aspects of motherhood in face of the COVID-19 pandemic in low- and middle-income countries. Matern Child Health J. 2021;25(1):15–21. https://doi.org/10.1007/s10995-020-03044-9.
Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222(5):415–26. https://doi.org/10.1016/j.ajog.2020.02.017.
Aoyama K, D'Souza R, Pinto R, Ray JG, Hill A, Scales DC, et al. Risk prediction models for maternal mortality: a systematic review and meta-analysis. PLoS One. 2018;13(12):e0208563. https://doi.org/10.1371/journal.pone.0208563.
Musa SS, Bello UM, Zhao S, Abdullahi ZU, Lawan MA, He D. Vertical transmission of SARS-CoV-2: a systematic review of systematic reviews. Viruses. 2021;13(9):1877. https://doi.org/10.3390/v13091877.
Savasi VM, Parisi F, Patanè L, Ferrazzi E, Frigerio L, Pellegrino A, et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2020;136(2):252–8. https://doi.org/10.1097/AOG.0000000000003979.
Marcolino MS, Ziegelmann PK, Souza-Silva MVR, do Nascimento IJB, Oliveira LM, Monteiro LS, et al. Clinical characteristics and outcomes of patients hospitalized with COVID-19 in Brazil: results from the Brazilian COVID-19 registry. Int J Infect Dis. 2021a;107:300–10. https://doi.org/10.1016/j.ijid.2021.01.019.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8. https://doi.org/10.1136/bmj.39335.541782.AD.
BRASIL. Ministério da Saúde. Protocolo De Manejo Clínico Do Coronavírus (Covid-19) Na Atenção Primária À Saúde. Brasília: Ministério da Saúde; 2020a. https://saude.rs.gov.br/upload/arquivos/202004/14140606-4-ms-protocolomanejo-aps-ver07abril.pdf.
BRASIL. Ministério da Saúde. Portaria nº 467, de 20 de março de 2020. Brasília: Ministério da Saúde; 2020b. https://www.planalto.gov.br/ccivil_03/portaria/prt/portaria%20n%C2%BA%20467-20-ms.htm.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95(103208):9. https://doi.org/10.1016/j.jbi.2019.103208.
Marcolino MS, Pires MC, Ramos LEF, Silva RT, Oliveira LM, Carvalho RLR, et al. ABC2-SPH risk score for in-hospital mortality in COVID-19 patients: development, external validation and comparison with other available scores. Int J Infect Dis. 2021b;110:281–308. https://doi.org/10.1016/j.ijid.2021.07.049.
Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–73. https://doi.org/10.7326/M14-0698.
Rufibach K. Use of brier score to assess binary predictions. J Clin Epidemiol. 2010;63(8):938–9. https://doi.org/10.1016/j.jclinepi.2009.11.009.
Knight SR, Ho A, Pius R, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: devel- opment and validation of the 4C mortality score. BMJ. 2020;370:m3339.
Liu JL, Xu F, Zhou H, et al. Expanded CURB-65: a new score system predicts severity of community-acquired pneumonia with superior efficiency. Sci Rep. 2016;6:22911.
Miyashita N, Matsushima T, Oka M, Japanese Respiratory Society. The JRS guidelines for the management of community-acquired pneumonia in adults: an update and new recommendations. Intern Med. 2006;45(7):419–28.
National Health Service (NHS) England. Resources to support the safe adoption of the revised National Early Warning Score (NEWS2). www.england.nhs.uk/wp-content/uploads/2019/12/Patient_Safety_Alert_-_adoption_of_NEWS2.pdf Date last updated: 25 April 2018; date last Accessed: 2 Apr 2020.
Medeiros GAA, Nóbrega RV, Beltrammi D, Gottardo PC, Correia LGCB. Protocolo clínico-centro estadual de disseminação de evidências em saúde da Covid-19: National Early Warning Score (NEWS) para COVID-19 modificado/simplificado (NEWS-FAST-COVID) para ambiente Pré-Hospitalar / USF. Brasil: Secretaria de Estado de Saúde; 2020.
Jones A, Pitre T, Junek M, Kapralik J, Patel R, Feng E, et al. External validation of the 4C mortality score among COVID-19 patients admitted to hospital in Ontario, Canada: a retrospective study. Sci Rep. 2021;11(1):18638. https://doi.org/10.1038/s41598-021-97332-1.
Citu C, Gorun F, Motoc A, Ratiu A, Gorun OM, Burlea B, et al. Evaluation and comparison of the predictive value of 4C mortality score, NEWS, and CURB-65 in poor outcomes in COVID-19 patients: a retrospective study from a single Center in Romania. Diagnostics. 2022;12(3):1–10. https://doi.org/10.3390/diagnostics12030703.
Wibisono E, Hadi U, Bramantono AMV, Rusli M, Rahman BE, et al. National early warning score (NEWS) 2 predicts hospital mortality from COVID-19 patients. Ann Med Surg (Lond). 2022;76:103462. https://doi.org/10.1016/j.amsu.2022.103462.
Bouwmeester W, Zuithoff NP, Mallett S, Geerlings MI, Vergouwe Y, Steyerberg EW, et al. Reporting and methods in clinical prediction research: a systematic review. PLoS Med. 2012;9(5):1–12. https://doi.org/10.1371/journal.pmed.1001221.
Christodoulou E, Ma J, Collins GS, Steyerberg EW, Verbakel JY, Van Calster B. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J Clin Epidemiol. 2019;110:12–22. https://doi.org/10.1016/j.jclinepi.2019.02.004.
Collins GS, de Groot JA, Dutton S, Omar O, Shanyinde M, Tajar A, et al. External validation of multivariable prediction models: a systematic review of methodological conduct and reporting. BMC Med Res Methodol. 2014;14(40):1–11. https://doi.org/10.1186/1471-2288-14-40.
Paiva BBM d, Delfino-Pereira P, Andrade CMV d, Gomes VMR, Lima MCPB, Souza-Silva MVR, et al. Effectiveness, explainability and reliability of machine meta-learning methods for predicting mortality in patients with COVID-19: results of the Brazilian COVID-19 registry. medRxiv. 2021. https://doi.org/10.1101/2021.11.01.21265527.
Kalafat E, Prasad S, Birol P, Tekin AB, Kunt A, Di Fabrizio C, et al. An internally validated prediction model for critical COVID-19 infection and intensive care unit admission in symptomatic pregnant women. Am J Obstet Gynecol. 2022;226(3):403.e1–403.e13. https://doi.org/10.1016/j.ajog.2021.09.024.
Gonçalves BMM, Franco RPV, Rodrigues AS. Maternal mortality associated with COVID-19 in Brazil in 2020 and 2021: comparison with non-pregnant women and men. PLoS One. 2021;16(12):e0261492. https://doi.org/10.1371/journal.pone.0261492.
Nakamura-Pereira M, Amorim MMR, Pacagnella RC, Takemoto MLS, Penso FCC, Rezende-Filho J, et al. COVID-19 and maternal death in Brazil: an invisible tragedy. Rev Bras Ginecol Obstet. 2020;42:445–7.
Wali AA, Abd-El-Fatah SM. Prognosis and outcomes of COVID-19 infection during pregnancy. Covid-19 Infect Pregnancy. 2021:145–65. https://doi.org/10.1016/B978-0-323-90595-4.00003-0.
Maza-Arnedo F, Paternina-Caicedo A, Sosa CG, Mucio B, Rojas-Suarez J, Say L, et al. Maternal mortality linked to COVID-19 in Latin America: results from a multicountry collaborative database of 447 deaths. Preprints with Lancet Specialties. 2022. https://doi.org/10.2139/ssrn.4035411.
Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):769–75. https://doi.org/10.15585/mmwr.mm6925a1.
Green LJ, Mackillop LH, Salvi D, Pullon R, Loerup L, Tarassenko L, et al. Gestation-specific vital sign reference ranges in pregnancy. Obstet Gynecol. 2020;135(3):653–64. https://doi.org/10.1097/AOG.0000000000003721.
Soma-Pillay P, Nelson-Piercy C, Tolppanen H, Mebazaa A. Physiological changes in pregnancy. Cardiovasc J Afr. 2016;27(2):89–94. https://doi.org/10.5830/CVJA-2016-021.
Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370(23):2211–8. https://doi.org/10.1056/NEJMra1213566.
Hanna N, Hanna M, Sharma S. Is pregnancy an immunological contributor to severe or controlled COVID-19 disease? Am J Reprod Immunol. 2020;84(5):e13317. https://doi.org/10.1111/aji.13317.
Crovetto F, Crispi F, Llurba E, Pascal R, Larroya M, Trilla C, et al. Impact of SARS-CoV-2 infection on pregnancy outcomes: a population-based study. Clin Infect Dis. 2021;73(10):1768–75. https://doi.org/10.1093/cid/ciab104.
Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17(8):469–82. https://doi.org/10.1038/nri.2017.64.
Komine-Aizawa S, Takada K, Hayakawa S. Placental barrier against COVID-19. Placenta. 2020;99:45–9. https://doi.org/10.1016/j.placenta.2020.07.022.
Vianna FSL, Fraga LR, Abeche AM, Silva AAD, Sanseverino MTV, Schuler-Faccini L. COVID-19 during pregnancy and adverse outcomes: concerns and recommendations from the Brazilian teratology information service. Gen Molec Biol. 2021;44(1):e20200224. https://doi.org/10.1590/1678-4685-GMB-2020-0224.
Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154(1):23–32. https://doi.org/10.1093/ajcp/aqaa089.
Baergen RN, Heller DS. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatr Dev Pathol. 2020;23(3):177–80. https://doi.org/10.1177/1093526620925569.
Pettirosso E, Giles M, Cole S, Rees M. COVID-19 and pregnancy: a review of clinical characteristics, obstetric outcomes and vertical transmission. Aust N Z J Obstet Gynaecol. 2020;60(5):640–59. https://doi.org/10.1111/ajo.13204.
Turan O, Hakim A, Dashraath P, Jeslyn WJL, Wright A, Abdul-Kadir R. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS-CoV-2 infection among hospitalized pregnant women: a systematic review. Int J Gynaecol Obstet. 2020;151(1):7–16. https://doi.org/10.1002/ijgo.
Sahin D, Tanacan A, Anuk AT, et al. Comparison of clinical features and perinatal outcomes between pre-variant and post-variant periods in pregnant women with SARS-CoV-2: analysis of 1935 cases [published online ahead of print, 2022 Mar 7]. Arch Gynecol Obstet. 2022:1–10. https://doi.org/10.1007/s00404-022-06493-5.
Şahin D, Tanaçan A, Webster SN, Moraloğlu TÖ. Pregnancy and COVID-19: prevention, vaccination, therapy, and beyond. Turk. J Med Sci. 2021;51(SI-1):3312–26. https://doi.org/10.3906/sag-2106-134.
Acknowledgments
We would like to thank the hospitals which are part of this collaboration, for supporting this project: Hospitais da Rede Mater Dei, Hospital das Clínicas da UFMG, Hospital Tacchini, Hospital Márcio Cunha, Hospital Risoleta Tolentino Neves, Hospital Santa Rosália, Hospital São João de Deus, Hospital Universitário Canoas, Hospital Moinhos de Vento, Hospital Nossa Senhora da Conceição, Hospital Júlia Kubitschek, Hospital Regional Antônio Dias, Hospital Mãe de Deus, Hospital das Clínicas da Faculdade de Medicina de Botucatu, Hospital das Clínicas da Universidade Federal de Pernambuco, Hospital Bruno Born, Hospital SOS Cárdio, Hospital Metropolitano Odilon Behrens, Hospital Santa Cruz, Hospital Universitário de Santa Maria, Santa Casa de Misericórdia de Belo Horizonte. We also thank all the clinical staff at those hospitals, who cared for the patients, and all undergraduate students who helped with data collection.
Funding
This study was supported in part by Minas Gerais State Agency for Research and Development (Fundação de Amparo à Pesquisa do Estado de Minas Gerais - FAPEMIG) [grant number APQ-00208-20], National Institute of Science and Technology for Health Technology Assessment (Instituto de Avaliação de Tecnologias em Saúde – IATS)/ National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq) [grant numbers 465518/2014-1 and 147122/2021-0], and CAPES Foundation (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) [grant number 88887.507149/2020-00]. ZSNR was partially funded by CNPq Foundation [grant number 305837/2021-4].
Author information
Authors and Affiliations
Contributions
MSM, MCP, ZSNR had substantial contributions to the conception or design of the work. ZSNR, MCP, LEFR, TLSS, PDP, KPMPM, AFG, AGRG, BPP, CCM, CCRC, CR, DP, FFMGA, FA, GPC, GMSG, GANB, GMSG, LSMM, MC, MFT, MAMC, MMA, MHGJ, PAAD, PMSR, PJLM, RSCA, RCM, SPA, EB, RALPA, MSM had substantial contributions to the acquisition, analysis, or interpretation of data for the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Brazilian National Commission for Research Ethics (CAAE 30350820.5.1001.0008). Individual informed consent was waived due to the severity of the situation and the use of unidentified data, based on medical chart review only. Additionally, administrative permissions to access and use the medical records were obtained from each institution.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
ABC2-SPH score for in-hospital mortality in patients with COVID-19*.
Additional file 2: Table S2.
Discrimination of risk scores within validation cohort (complete cases).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Reis, Z.S.N., Pires, M.C., Ramos, L.E.F. et al. Mechanical ventilation and death in pregnant patients admitted for COVID-19: a prognostic analysis from the Brazilian COVID-19 registry score. BMC Pregnancy Childbirth 23, 18 (2023). https://doi.org/10.1186/s12884-022-05310-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-022-05310-w