Abstract
Glycoprotein IIb/IIIa inhibitors represent a new promising class of antiplatelet medications. Their use in acute coronary syndromes and for patients undergoing percutaneous coronary intervention has been the subject of a number of large controlled trials using both the intravenous and the oral forms. In this review, we present a systematic overview of these trials.
Similar content being viewed by others
Introduction
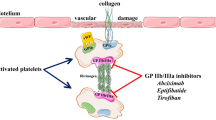
Platelets play a pivotal role in acute coronary syndromes [1,2]. Plaque rupture exposes highly thrombogenic components that induce platelet activation and initiate coagulation cascade. Platelet activation involves a conformational change in the membrane glycoproteins (GP) that are receptors for adhesive proteins [3]. The recent development of a new class of drugs that allow direct inhibition of platelet GP IIb/IIIa receptors, the 'final common pathway' of platelet activation, has raised the possibility that these potent agents may reduce thrombotic complications after percutaneous coronary interventions or in acute coronary syndromes [4]. In the following review, we summarize the trials conducted to evaluate the use of GP IIb/IIIa inhibitors in these clinical settings, and discuss issues of efficacy and safety.
Intravenous GP IIb/IIIa antagonists
Four intravenous GP IIb/IIIa antagonists have been investigated: abciximab (c7E3 Fab), eptifibatide (Integrilin), tirofiban (Aggrastat), and lamifiban (Ro 44-9883) (Table 1).
Percutaneous coronary interventions
The role of periprocedural intravenous GP IIb/IIIa inhibition in percutaneous coronary revascularization was established in nine placebo-controlled randomized trials and one comparative trial enrolling, in total, over 24,000 patients [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. All trials were blinded throughout the follow-up period. Inclusion criteria varied (Table 2).
The EPIC trial
The Evaluation of c7E3 for Prevention of Ischemic Complications (EPIC) trial included only patients at higher risk than normal for ischemic complications [5]. The administration of an intravenous bolus and 12-hour infusion of abciximab resulted in a 35% reduction of relative risk of the primary endpoint and reduced the risk of procedure-related myocardial infarction (MI). This benefit was more pronounced in patients with unstable angina and those undergoing angioplasty for MI. The major limitation of the EPIC trial, however, was the existence of a substantially increased risk of bleeding subsequently attributed to the high heparin doses.
The EPILOG trial
The Evaluation in PTCA to Improve Long-term Outcome with Abciximab GP IIb/IIIa Blockade (EPILOG) trial extended the application of abciximab to all patients undergoing coronary angioplasty, using the same abciximab regimen as the EPIC trial, but with a lower heparin dose [6]. There was a 56% reduction in the relative risk of the 30-day endpoint, with a substantial reduction in the risk of bleeding – from 14% in EPIC to just 2% in EPILOG.
The CAPTURE trial
The c7E3 Antiplatelet Therapy in Unstable Refractory Angina (CAPTURE) trial enrolled patients with unstable angina requiring coronary angioplasty [7]. Patients were treated with an abciximab infusion for 18–24 hours before angioplasty but for only 1 hour afterwards. The trial reported a 23% reduction in the relative risk of primary endpoints after 30 days.
The EPISTENT trial
The Evaluation of Platelet Inhibition in Stenting (EPISTENT) trial assessed the efficacy of abciximab with coronary stenting in patients undergoing elective or urgent coronary intervention [8,9,10]. Patients were randomized to receive either stenting with placebo, stenting with abciximab, or balloon angioplasty with abciximab. The primary endpoint at 30 days confirmed the efficacy of abciximab with a 51% decrease in the relativerisk of death, MI or urgent revascularization. This benefit was maintained throughout the 6-month follow-up period. Furthermore, the rate of repeat intervention on target vessels following stent implantation in diabetics was significantly reduced (by 51%) with treatment with abciximab. Moreover, after 1 year, the combination of stenting and abciximab resulted in a significant reduction (60%) in mortality compared with either therapy administered alone.
The RAPPORT
The ReoPro and Primary PTCA Organization and Randomized Trial (RAPPORT) focused on angioplasty as a primary reperfusion strategy for MI with acute ST elevation [11]. A total of 483 patients were assigned, within 12 hours of the onset of acute MI, to receive either an abciximab bolus followed by infusion of abciximab for 12 hours or placebo. Abciximab significantly reduced the incidence of death, reinfarction, or urgent target vessel revascularization at all time points assessed (at 7 days, 9.9% versus 3.3%, P = 0.003; at 30 days, 11.2% versus 5.8%, P = 0.03; and at 6 months, 17.8% versus 11.6%, P = 0.05). A higher incidence of major bleeding was noted in the abciximab group (16.6% versus 9.5%, P = 0.02), similar to the EPIC trial in which a higher dose of heparin was also used.
The IMPACT II and ESPRIT trials
Eptifibatide was evaluated in patients undergoing coronary intervention in the Integrilin to Minimize Platelet Aggregation and Coronary Thrombosis – II (IMPACT II) [12] trial and the Enhanced Suppression of the Platelet IIb/IIIa Receptor with Integrilin Therapy (ESPRIT) trial [13,14].
Patients undergoing balloon angioplasty or directional atherectomy in the IMPACT II trial were randomly assigned to receive either a bolus and 24-h low-dose infusion (0.5 g/kg/min) of eptifibatide, or a bolus and high-dose infusion (0.75 g/kg/min) of eptifibatide, or placebo. Although there was no significant reduction in the primary composite endpoint after 30 days with eptifibatide, there was a 10.5% reduction in ischemic events when data from the two eptifibatide groups were combined.
The ESPRIT trial, in contrast, enrolled patients undergoing routine stent implantation [13]. The patients were randomized to receive either eptifibatide in two 180 μg/kg boluses 10 min apart with a continuous infusion of 2.0 μg/g/min for 18–24 hours, or placebo. The results showed a significant reduction in the primary endpoints from 10.5 to 6.6% (P = 0.0017). There was a 38% reduction in the relative risk of death or MI at 30 days (6.3% versus 10.2%, P = 0.002), which was maintained throughout the 6-month follow-up period (7.5% versus 11.5%, P = 0.002, 95% confidence interval = 0.47–0.84) [14]. The higher dose of eptifibatide used in the ESPRIT trial resulted in more platelet inhibition (90–95%) than in the IMPACT II trial (50–60%) and may have contributed to a better outcome.
The GOLD study
The GOLD study was a prospective multicenter study to determine the optimal level of platelet inhibition with GPIIb/IIIa inhibitors in patients undergoing coronary intervention [15]. This study of GP IIb/IIIa inhibition in conjunction with percutaneous coronary intervention found that patients who achieved greater than 70% inhibition had much lower rates of major cardiac events than patients with lower levels of inhibition (12% versus 32%, P = 0.02).
The RESTORE trial
Tirofiban was evaluated in patients with unstable angina undergoing coronary intervention in the Randomized Efficacy Study of Tirofiban for Outcomes and Restenosis (RESTORE) trial [16]. A trend towards improvement in outcome at 30 days was observed in the tirofiban-treated patients when compared with placebo (10.3% versus 12.2%, P = 0.16). Furthermore, the bleeding rates were low and not significantly different from placebo.
The ADMIRAL trial
The Abciximab before Direct Angioplasty and Stenting in Myocardial Infarction Regarding Acute and Long-term follow-up (ADMIRAL) trial randomized patients suffering acute MI with ST elevation to either abciximab (0.25 mg/kg bolus, 0.125 μg/kg/min [10 μg/kg/min maximum] for 12 hours) plus stenting or placebo plus stenting [17]. The composite endpoint of death, reinfarction or urgent revascularization at 30 days was significantly lower in the abciximab group (6.0% versus 14.6%, P = 0.01) and remained significant throughout 6 months of follow-up (7.4% versus 15.9%, P = 0.02). The better clinical outcomes in the abciximab group were related to the greater frequency of thrombolysis in MI (TIMI) grade 3 when compared with placebo (before the procedure, 16.8% versus 5.4%, P = 0.01; immediately after the procedure, 95.1% versus 86.7%, P = 0.04; and at 6 months after the procedure, 94.3% versus 82.8%, P = 0.04). One major bleeding event occurred in the abciximab group and none occurred in the placebo group.
The TACTICS-TIMI 18 trial
The Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy – Thrombolysis in Myocardial Infarction 18 (TACTICS-TIMI 18) trial studied patients with unstable angina and MI without ST elevation [18]. Patients were treated with heparin and tirofiban in a loading dose of 0.4 μg/kg, followed by 0.1 μg/kg/min for 48 hours or until revascularization; tirofiban was administered for at least 12 hours after percutaneous revascularization. Patients were randomized to receive either early invasive strategy with routine catheterization (within 4–48 hours) or conservative treatment with catheterization reserved for recurrent pain or provocable ischemia. When compared with conservative therapy, the combination of tirofiban and early invasive strategy resulted in significant reduction in the primary endpoints of death, myocardial infarction or rehospitalization at 6 months (15.9% versus 19.4%, P = 0.025). The rate of death or non-fatal MI at 6 months was similarly reduced (7.3% versus 9.5%, P < 0.05). The benefit of the early invasive strategy was greatest in high-risk and intermediate-risk patients with elevated troponin T levels, whereas the outcome was similar with the use of either strategy in patients at low risk and in those without elevated troponin T levels.
TARGET
More recently, the Do Tirofiban and Reopro Give Similar Efficacy Trial (TARGET) randomized patients undergoing stenting for coronary artery disease, with or without an acute coronary syndrome, to receive either abciximab or tirofiban [19]. The combined primary endpoint of death, MI, or urgent revascularization occurred in 7.6% of the tirofiban group and 6.0% of the abciximab group (P = 0.037). Abciximab was found to be superior particularly in patients with acute coronary syndrome.
The difference in efficacy may, in part, be due to the pharmacodynamics of receptor binding. Abciximab dissociates slowly in contrast to the rapid reversibility of tirofiban. Furthermore, the non-specific blockade by abciximab of both the GP IIb/IIIa receptor and the αvβ3 receptor may theoretically provide an advantage over tirofiban. Ex vivo studies have suggested that dual receptor blockade more completely suppresses platelet-mediated thrombin generation than does inhibition of either receptor alone [20].
Summary
In conclusion, as an adjunct to percutaneous coronary interventions, GP IIb/IIIa inhibition results in a significant reduction in early ischemic events that is sustained throughout the 1-year follow-up period. Furthermore, this benefit is independent of the interventional devices used and of lesion complexities, and has been reported across all the aforementioned interventional trials. Hemorrhagic risk was reduced when the heparin dose was limited and the vascular sheath was removed early. Rates of intracranial hemorrhage were not increased by GP IIb/IIIa blockade. It unclear whether the superiority of abciximab over tirofiban is related to the differences in the mechanisms of antagonism of GP IIb/IIIa, the specific effects of abciximab in blocking interactions between platelets and endothelial cells and leukocytes, differences in doses, differences in patients, or statistical variation [21].
Acute coronary syndromes
The role of intravenous GP IIb/IIIa antagonists in the treatment of unstable ischemic syndromes, independent of the use of coronary revascularization, was tested in more than 31,000 patients in six placebo-controlled trials (Table 3) [22,23,24,25,26].
The PRISM trial
The Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) trial randomized patients with unstable angina or non-Q-wave MI to receive either standard heparin or a 48-hour infusion of tirofiban [22]. At 48 hours, tirofiban was superior to heparin in reducing the composite endpoint of death, MI and refractory ischemia (3.8% versus 5.6%, P = 0.01), with a 36% reduction in relative risk.
The PRISM-PLUS trial
Patients in the Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs (PRISM-PLUS) trial were randomized to receive either tirofiban alone, tirofiban with heparin, or heparin alone [23]. The tirofiban-only arm was subsequently discontinued because of a higher mortality rate than heparin alone. The combination of tirofiban and heparin was, however, superior to heparin in reducing the combined endpoint of death, MI and recurrent ischemia after 7 days (12.9% versus 17.9%, P = 0.004). This significant difference persisted throughout 6 months of follow-up (18.5% versus 22.3%, P = 0.03).
The PURSUIT trial
In the Platelet IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) trial, an epti-fibatide infusion for 72 hours reduced the combined incidence of death or MI by 10% after 30 days (14.2% versus 15.7%, P = 0.04) [24].
The PARAGON trials
The Platelet IIb/IIIa Antagonism for the Reduction of Acute Coronary Syndrome events in the Global Organization Network (PARAGON A [25] and PARAGON B [26]) trials, in contrast to PRISM, PRISM-PLUS and PURSUIT, failed to demonstrate an advantage of lamifiban over placebo. This failure did not include results in patients with elevated troponin-T levels (30-day composite endpoint, 11% versus 19.4%, P = 0.01).
The GUSTO IV-ACS trial
Similarly disappointing results have recently been demonstrated in the Global Use of Strategies To Open Occluded Arteries – IV – Acute Coronary Syndrome (GUSTO IV-ACS) trial [27]. A total of 7800 patients with non-ST-elevation acute coronary syndromes, for whom percutaneous coronary interventions were not planned, were randomized to receive either 24 hours of abciximab, 48 hours of abciximab, or placebo. Abciximab use in this trial was not associated with a reduced risk of death or MI after 30 days (8% for placebo, 8.2% for 24-hour abcix-imab and 9.2% for 48-hour abciximab; not significant).
Summary
In conclusion, high-risk patients (patients with ST depression or elevated troponin levels) should receive GP IIb/IIIa inhibitors (either tirofiban or eptifibatide) in addition to the usual antithrombotic regimen.
Adjuncts to reperfusion therapy
Several completed and ongoing studies are evaluating the role of GP IIb/IIIa inhibitors as an adjunct to thrombolytic therapy.
The TAMI 8 trial
The Thrombolysis and Angioplasty in MI (TAMI 8) trial was a preliminary dose-ranging trial, testing abciximab combined with thrombolytic therapy [28]. Sixty patients with acute MI receiving recombinant tissue-type plasminogen activator (rt-PA) also received a bolus injection of abcix-imab. The infarct-related artery was patent in 56% of control patients and 92% of the GP IIb/IIIa inhibitor patients. The incidence of major bleeding was increased in the control group (abciximab, 25% versus control, 50%).
The PARADIGM trial
The Platelet Aggregation Receptor Antagonist Dose Investigation and Reperfusion Gain in MI (PARADIGM) trial studied patients with acute MI who received treatment with either alteplase or streptokinase [29]. The patients were randomly assigned treatment with either adjunctive lamifiban or placebo. Despite the higher patency of the infarct-related artery in the lamifiban group when compared with placebo (75% versus 62.5%), the clinical outcomes in the lamifiban and placebo groups were not significantly different (death, 2.1% versus 2.6%; reinfarction, 8.9% versus 6.0%; refractor ischemia, 6.4% versus 8.5%; and revascularization, 11.4% versus 12.0%). Furthermore, there was more bleeding associated with lami-fiban (transfusions, 16% versus 10.3%; major bleeding, 3.0% versus 1.7%).
The IMPACT-AMI trial
The Integrilin to Minimize Platelet Aggregation and Prevent Coronary Thrombosis AMI (IMPACT-AMI) trial was a randomized, placebo-controlled, dose-ranging trial in which 132 patients who received accelerated alteplase (rt-PA) were randomized to eptifibatide (Integrilin) or placebo [30]. Patients treated with the highest eptifibatide dose achieved 90-min TIMI 3 flow in 66% of patients, compared with 39% of patients receiving placebo. Composite clinical end-points were similar in both groups (43% versus 42%). The incidence of excessive bleeding was not increased in the active treatment group compared with the placebo group (4% versus 5%).
The TIMI 14 trial
In the Thrombolysis in MI (TIMI 14) trial, 888 patients who suffered a MI with ST elevation were randomized to receive either 100 mg accelerated-dose alteplase (control), abciximab alone, or abciximab in combination with either reduced doses of alteplase (20, 35, or 65 mg) or streptokinase (500,000 U–1.5 MU) [31]. Excessive bleeding was noted with the highest dose of streptokinase plus abciximab, and this arm was discontinued after five patients were enrolled. The most promising regimen was the combination of abciximab and a 50 mg dose of alteplase, which resulted in 77% TIMI grade 3 flow at 90 min compared with 62% for alteplase alone (P = 0.02). This improvement in reperfusion with alteplase occurred without an increase in the risk of major bleeding.
The SPEED trial
The Strategies for Patency Enhancement in the Emergency Department (SPEED) trial was a dose escalation trial testing the combination of abciximab and reteplase in patients with acute MI [32,33]. All patients received a full-dose of abciximab (0.25 mg/kg bolus, 0.125 μg/kg/min [10 μg/kg/min maximum] for 12 hours) and were randomly assigned in a 4:1 ratio to receive either reteplase (5, 7.5, 10, or 5 + 5 U) with abciximab, or abciximab alone. TIMI 3 flow in the various groups at 60 min was 19% (abciximab alone), 52% (5 U reteplase plus abciximab), 48% (7.5 U reteplase plus abciximab), 51% (10 U reteplase plus abciximab) and 62% (5 + 5 U reteplase plus abciximab).
The GUSTO V trial
In the Global Use of Strategies To Open Occluded Arteries – V (GUSTO V) trial, a total of 16,588 patients who suffered an acute MI with ST elevation were randomized to receive two bolus doses of reteplase (10 U) or two half-boluses of reteplase (5 U) with a full dose of abciximab (0.25 mg/kg bolus, 0.125 μg/kg/min [10 μg/kg/min maximum] for 12 hours) [34]. The medication was administered on an open label basis. The combination of half-dose reteplase and abciximab has failed to show a significant reduction in mortality at 30 days compared with full-dose reteplase alone (5.6% versus 6.9%, P = 0.43). The trial did, however, show that the combination was 'non-inferior' to the fibrinolytic alone. There were, however, fewer deaths or reinfarctions with the combination (7.4% versus 8.8%, P = 0.001) and less need for urgent revascularization, but more non-cranial bleeds (severe bleeding, 1.1% versus 0.5%, P < 0.0001; moderate bleeding, 3.5% versus 1.8%, P < 0.0001; transfusion, 5.7% versus 4.0%, P < 0.0001).
Summary
In conclusion, the combination of intravenous GP IIb/IIIa antagonists and fibrinolytic therapy results in more rapid reperfusion than conventional therapy. The incidence of 30-day mortality was not reduced, however, and its use was associated with more non-cranial bleeds. There are other ongoing trials; both angiographic (i.e. looking at effects on TIMI flow) with TNKase and each of the three GP IIb/IIIa inhibitors (such as the Integrilin and Tenecteplase for Acute Myocardial Infarction [INTEGRITI] trial), and larger mortality trials (such as Assessment of the Safety and Efficacy of a New Thrombolytic – III [ASSENT III]) with TNKase and abciximab. These ongoing trials are exploring further the potential role of GP IIb/IIIa inhibitors combined with reduced doses of thrombolytic therapy and may further clarify its role as an adjunct to reperfusion therapy.
Oral platelet GP IIb/IIIa antagonists
Prodrugs with RGD specificity (Arg-Gly-Asp sequence) now include oral GP IIb/IIIa receptor blockers, such as xemilofiban, orofiban, sibrafiban and lotrafiban, which have longer half-lives and are excreted renally. Five trials evaluated the use of these agents in patients with acute coronary syndrome or undergoing percutaneous coronary intervention. Four of these trials enrolled patients presenting with acute coronary syndromes and one trial enrolled patients undergoing percutaneous coronary interventions (Table 4).
The EXCITE trial
The Evaluation of Xemilofiban in Controlling Thrombotic Events (EXCITE) trial compared xemilofiban (10–20 mg, administered 3 times a day for 2 weeks) with placebo in 7232 patients undergoing percutaneous coronary interventions [35]. The primary endpoint was death, recurrent MI, and urgent revascularization at 30 and 182 days. The incidence of death, MI, and urgent revascularization within 182 days was comparable among the three groups (13.5% in placebo, 13.9% in those receiving 10 mg xemilofiban, and 12.7% in those receiving 20 mg xemilofiban).
The OPUS-TIMI 16 trial
The Orofiban in Patients with Unstable Coronary Syndromes Thrombolysis in MI (OPUS-TIMI 16) trial randomized 10,302 patients presenting with acute coronary syndromes [36]. They received either 50 mg orofiban twice daily for 6 months, 50 mg orofiban for 30 days followed by 30 mg twice daily for 6 months, 50 mg orofiban twice daily for 5 months, or placebo. The primary endpoint was death, recurrent MI, recurrent ischemia requiring rehospitalization or revascularization, and stroke, at 30 days and at 10 months. This trial was stopped prematurely because of a statistically significant increase in mortality observed after 30 days with orofiban therapy (2.0% versus 1.4%, P = 0.02).
The SYMPHONY trials
The Sibrafiban Versus Aspirin to Yield Maximum Protection From Ischemic Heart Events Post Acute Coronary Syndromes (SYMPHONY [37] and SECONDSYMPHONY [38]) trials studied sibrafiban, with doses adjusted to weight and serum creatinine, in acute coronary syndrome patients. The SYMPHONY trial randomized 9233 patients to low-dose sibrafiban, high-dose sibrafiban, or aspirin therapy for 90 days. The SECONDSYMPHONY trial compared the same low-dose regimen of sibrafiban with aspirin or high-dose sibrafiban without aspirin with aspirin therapy alone for 90 days. The primary endpoint for both studies was death, MI, and severe recurrent ischemia requiring revascularization at 90 days. The SYMPHONY trial demonstrated a lack of benefit with sibrafiban compared with aspirin alone (9.8% aspirin, 10.1% low-dose sibrafiban and 10.1% high-dose sibrafiban). This SECOND SYMPHONY trial was terminated prematurely, after 6637 patients were enrolled, when the results of the SYMPHONY trial were known.
The BRAVO trial
The Blockade of the GP IIb/IIIa Receptor to Avoid Vascular Occlusion (BRAVO) trial studied the role of lotrafiban in patients who had suffered a recent MI, unstable angina, transient ischemic attack or a stroke, or patients who presented at any time after a diagnosis of peripheral vascular disease [39]. The trial was stopped when it was found at an interim analysis that lotrafiban had a higher mortality than placebo (2.7% versus 2.0%, P = 0.022), more major bleeding (4.2% versus 1.3%, P < 0.022) and a greater incidence of serious thrombocytopenia (2.2% versus 0.5%) [26].
Summary
Overall, each trial reported an increased risk of mortality during the follow-up period, with an overall 31% increase in mortality. Furthermore, high-dose GP IIb/IIIa inhibition is associated with an even greater fatality risk. No trial demonstrated a statistically significant effect on MI. Conversely, the need for urgent revascularization was reduced in each study except in the EXCITE trial. Moreover, a statistically significant increase in bleeding was observed in each trial. In a meta-analysis of the first four published trials, Chew et al demonstrated that there was a consistent and statistically significant increase in mortality with oral GP IIb/IIIa therapy (odds ratio = 1.37, 95% confidence interval = 1.13–1.66, P = 0.001) [40].
Conclusion
Intravenous GP IIb/IIIa inhibition used as an adjunct to percutaneous coronary interventions results in significant reduction in early ischemic events that may be sustained for 1 year. This benefit is independent of the interventional devices used and independent of lesion complexities. Abciximab seems to provide better results than eptifibatide. Preliminary data suggest that intravenous GP IIb/IIIa inhibition may be a useful adjunct to conventional thrombolytic therapy by accelerating the process of fibri-nolysis. However, this awaits confirmatory evidence on the efficacy and safety of this combination regimen from ongoing megatrials.
In contrast to the beneficial effects of intravenous GP IIb/IIIa inhibitors, oral GP IIb/IIIa inhibitors were associated with a significant increase in mortality. Further investigation to elucidate the cause of this increased fatality risk is warranted.
Abbreviations
- GP:
-
= glycoproteins
- MI:
-
= myocardial infarction
- rt-PA:
-
= recombinant tissue-type plasminogen activator
- TIMI:
-
= thrombolysis in MI. ADMIRAL = Abciximab before Direct Angioplasty and Stenting in Myocardial Infarction Regarding Acute and Long-term follow-up
- BRAVO:
-
= Blockade of the GP IIb/IIIa Receptor to Avoid Vascular Occlusion
- CAPTURE:
-
= c7E3 Antiplatelet Therapy in Unstable Refractory Angina
- EPIC:
-
= Evaluation of c7E3 for Prevention of Ischemic Complications
- EPILOG:
-
= Evaluation in PTCA to Improve Long-term Outcome with Abciximab GP IIb/IIIa Blockade
- EPISTENT:
-
= Evaluation of Platelet Inhibition in Stenting
- ESPRIT:
-
= Enhanced Suppression of the Platelet IIb/IIIa Receptor with Integrilin Therapy
- EXCITE:
-
= Evaluation of Xemilofiban in Controlling Thrombotic Events
- GUSTO IV-ACS:
-
= Global Use of Strategies To Open Occluded Arteries – IV – Acute Coronary Syndrome
- IMPACT II:
-
= Integrilin to Minimize Platelet Aggregation and Coronary Thrombosis – II
- OPUS-TIMI 16:
-
= Orofiban in Patients with Unstable Coronary Syndromes Thrombolysis in MI
- PARAGON:
-
= Platelet IIb/IIIa Antagonism for the Reduction of Acute Coronary Syndrome events in the Global Organization Network
- PRISM:
-
= Platelet Receptor Inhibition in Ischemic Syndrome Management
- PRISM-PLUS:
-
= Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs
- PURSUIT:
-
= Platelet IIb/IIIa in Unstable Angina Receptor Suppression Using Integrilin Therapy
- RAPPORT:
-
= ReoPro and Primary PTCA Organization and Randomized Trial
- RESTORE:
-
= Randomized Efficacy Study of Tirofiban for Outcomes and Restenosis
- SYMPHONY:
-
= Sibrafiban Versus Aspirin to Yield Maximum Protection From Ischemic Heart Events Post Acute Coronary Syndromes
- TACTICS-TIMI 18:
-
= Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy – Thrombolysis in Myocardial Infarction 18
- TARGET:
-
= Do Tirofiban and Reopro Give Similar Efficacy Trial.
References
Fuster V, Badimon L, Badimon JJ, Chesebro JH: The pathogenesis of coronary artery disease and the acute coronary syndromes (part I). N Engl J Med. 1992, 326: 242-250.
Fuster V, Badimon L, Badimon JJ, Chesebro JH: The pathogenesis of coronary artery disease and the acute coronary syndromes (part II). N Engl J Med. 1992, 326: 310-318.
Steele PM, Chesebro JH, Stanson AW, Holmes DR, Dewanjee MK, Badimon L, Fuster V: Balloon angioplasty. Natural history of the pathophysiological response to injury in a pig model. Circulation Res. 1985, 57: 105-112.
Lincoff AM, Califf RM, Topol EJ: Platelet glycoprotein IIb/IIIa receptor blockade in coronary artery disease. J Am Coll Cardiol. 2000, 35: 1103-1115. 10.1016/S0735-1097(00)00554-4.
EPIC Investigators: Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med. 1994, 330: 956-961. 10.1056/NEJM199404073301402.
EPILOG Investigators: Platelet glycoprotein IIb/IIIa blockade with abciximab with low-dose heparin during percutaneous coronary revascularization. N Engl J Med. 1997, 336: 1689-1696. 10.1056/NEJM199706123362401.
CAPTURE Investigators: Randomized placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina: the CAPTURE study. Lancet. 1997, 349: 1429-1435. 10.1016/S0140-6736(96)10452-9.
EPISTENT Investigators: Randomized placebo-controlled and balloon-angioplasty controlled trial to assess safety of coronary stenting with use of platelet glycoprotein IIb/IIIa blockade. Lancet. 1998, 352: 87-92.
Lincoff AM, Califf RM, Moliterno DJ, Ellis SG, Ducas J, Kramer JH, Kleiman NS, Cohen EA, Booth JE, Sapp SK, Cabot CF, Topol EJ: Complementary clinical benefits of coronary artery stenting and blockade of platelet glycoprotein IIb/IIIa receptors. N Engl J Med. 1999, 341: 319-327. 10.1056/NEJM199907293410503.
Topol EJ, Mark DB, Lincoff AM, Cohen E, Burton J, Kleiman N, Talley D, Sapp S, Booth J, Cabot CF, Anderson KM, Califf RM: Enhanced survival with platelet glycoprotein IIb/IIIa blockade in patients undergoing coronary stenting: 1 year outcomes and health care economic implications from a multicenter, randomized trial. Lancet. 1999, 354: 2019-2024. 10.1016/S0140-6736(99)10018-7.
Brener SJ, Barr LA, Burchenal JEB, Katz S, George BS, Jones AA, Cohen ED, Gainey PC, White HJ, Cheek HB, Moses JW, Moliterno DJ, Effron MB, Topol EJ: A randomized, placebo-controlled trial of platelet glycoprotein IIb/IIIa blockade with primary angioplasty for acute myocardial infarction. Circulation. 1998, 98: 734-741.
IMPACT II Investigators: Randomized placebo-controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention: IMPACT II. Lancet. 1997, 349: 1422-1428. 10.1016/S0140-6736(96)10172-0.
The ESPRIT Investigators: Novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomized, placebo-controlled trial. Lancet. 2000, 356: 2037-2044. 10.1016/S0140-6736(00)03400-0.
O'Shea JC, Hafley GE, Greenberg S, Hasselblad V, Lorenz TJ, Kitt MM, Strony J, Tcheng JE: Platelets glycoprotein IIb/IIIa integrin blockade with eptifibatide in coronary stent intervention. JAMA. 2001, 285: 2468-2473. 10.1001/jama.285.19.2468.
Steinhubl S, Talley D, Braden G, Tcheng JE, Casterella PJ, Moliterno DJ, Navetta FI, Berger PB, Popma JJ, Dangas G, Gallo R, Sane DC, Saucedo JF, Jia G, Lincoff AM, Theroux P, Holmes DR, Teirstein PS, Kereiakes DJ: Point-of-Care Measured Platelet Inhibition Correlates With a Reduced Risk of an Adverse Cardiac Event After Percutaneous Coronary Intervention: Results of the GOLD (AU-Assessing Ultegra) Multicenter Study. Circulation. 2001, 103: 2572-2578.
RESTORE Investigators: Effects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplasty. Circulation. 1997, 96: 1445-1453.
Montalescot G, Barragan P, Wittenberg O, Ecollan P, Elhadad S, Villain P, Boulenc JM, Morice MC, Maillard L, Pansieri M, Choussat R, Pinton P: Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med. 2001, 334: 1895-1903. 10.1056/NEJM200106213442503.
Cannon CP, Weintraub WS, Demopoulos LA, Cannon CP, Wein-traub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, Neumann FJ, Robertson DH, DeLucca PT, DiBattiste PM, Gibson CM, Braunwald E: Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001, 344: 1879-1887. 10.1056/NEJM200106213442501.
The TARGET Investigators: Comparison of Two Platelet Glyco-protein IIb/IIIa Inhibitors, Tirofiban and Abciximab, for the Prevention of Ischemic Events with Percutaneous Coronary Revascularization. N Engl J Med. 2001, 344: 1888-1894. 10.1056/NEJM200106213442502.
Reverter JC, Beguin S, Kessel H, Kumar R, Hemker HC, Coller BS: Inhibition of platelet-mediated, tissue factor-induced thrombin generation by the mouse/human chimeric 7E3 antibody. J Clin Invest. 1996, 98: 863-874.
Boden WE, McKay RG: Optimal treatment of acute coronary syndromes – an evolving strategy. N Engl J Med. 2001, 334: 1939-1942. 10.1056/NEJM200106213442510.
PRISM Study Investigators: A comparison of aspirin plus tirofiban with aspirin plus heparin for unstable angina. N Engl J Med. 1998, 338: 1498-1505. 10.1056/NEJM199805213382103.
PRISM-PLUS Study Investigators: Inhibition of the platelet gly-coprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q wave myocardial infarction. N Engl J Med. 1998, 338: 1488-1497. 10.1056/NEJM199805213382102.
PURSUIT Trial Investigators: Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. N Engl J Med. 1998, 339: 436-443. 10.1056/NEJM199808133390704.
PARAGON Investigators: International, randomized, controlled trial of lamifiban (a platelet glycoprotein IIb/IIIa inhibitor), heparin or both in unstable angina. Circulation. 1998, 97: 2386-2395.
Cardiosource. [http://www.cardiosource.com/trials]
The GUSTO IV-ACS Investigators: Effect of glycoprotein IIb/IIIa receptor blocker abciximab on outcome in patients with acute coronary syndromes without early revascularisation: the GUSTO IV-ACS randomized trial. Lancet. 2001, 357: 1915-1924. 10.1016/S0140-6736(00)05060-1.
Kleiman NS, Ohman M, Califf RM, George BS, Kereiakes D, Aguirre FV, Weisman H, Schaible T, Topol EJ: Profound inhibition of platelet aggregation with a monoclonal antibody 7E3 Fab after thrombolytic therapy: results of the Thrombolysis and Angioplasty in Myocardial Infarction (TAMI 8) Pilot Study. J Am Coll Cardiol. 1993, 22: 381-389.
The PARADIGM Investigators: Combining thrombolysis with the platlet glycoprotein IIb/IIIa inhibitor lamifiban: results of the platelet aggregation receptor antagonist dose investigation and reperfusion gain in myocardial infarction trial. J Am Coll Cardiol. 1998, 32: 2003-2010. 10.1016/S0735-1097(98)00474-4.
Ohman EM, Kleiman NS, Gocioch G, Worley SJ, Navetta FI, Talley JD, Anderson HV, Ellis SG, Cohen MD, Spriggs D, Miller M, Kereiakes D, Yakubov S, Kitt MM, Sigmon KN, Califf RM, Krucoff MW, Topol EJ, for the IMPACT-AMI Investigators: Combined accelerated tissue-plasminogen activator and platelet glyco-protein IIb/IIIa integrin receptor blockade with Integrilin in acute myocardial infarction. Circulation. 1997, 95: 846-854.
Antaman EM, Giugliano RP, Gibson CM, McCabe CH, Coussement P, Kleiman NS, Vahanian A, Adgey AA, Menown I, Rupprecht HJ, Van der Wieken R, Ducas J, Scherer J, Anderson K, Van de Werf F, Braunwald E, for the TIMI 14 Investigators: Abcix-imab facilitates the rate and extent of thrombolysis results of the Thrombolysis in Myocardial Infarction (TIMI) 14 trial. Circulation. 1999, 99: 2720-2732.
The Strategies for Patency Enhancement in the Emergency Department (SPEED) Group: Randomized trial of abciximab with and without low-dose reteplase for acute myocardial infarction. Circulation. 2000, 101: 2788-2794.
Herrman HC, Moliterno DJ, Ohman EM, Stebbins AL, Bode C, Betriu A, Forycki F, Miklin JS, Bachinsky WB, Lincoff AM, Califf RM, Topol EJ: Facilitation of early percutaneous coronary intervention after reteplase with or without abciximab in acute myocardial infarction. Results of the SPEED (Gusto-4Pilot) trial. J Am Coll Cardiol. 2000, 36: 1489-1496. 10.1016/S0735-1097(00)00923-2.
The GUSTO V Investigators: Reperfusion therapy for acute myocardial infarction with fibrinolytic therapy or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: the GUSTO V randomized trial. Lancet. 2001, 357: 1905-1914. 10.1016/S0140-6736(00)05059-5.
O'Neil WW, Serruys P, Knudtson M, van Es GA, Timmis GC, van der Zwaan C, Kleiman J, Gong J, Roecker EB, Dreiling R, Alexander J, Anders R: Long-term treatment with a platelet glycopro-tein-receptor antagonist after percutaneous coronary revascularization: EXCITE Trial Investigators: Evaluation of Oral Xemilofiban in Controlling Thrombotic Events. N Engl J Med. 2000, 342: 1316-1324. 10.1056/NEJM200005043421803.
Cannon CP, McCabe CH, Wilcox RG, Langer A, Caspi A, Berink P, Lopez-Sendon J, Toman J, Charlesworth A, Anders RJ, Alexander JC, Skene A, Braunwald E: Oral glycoprotein IIb/IIIa inhibition with orofiban in patients with unstable coronary syndromes (OPUS-TIMI 16) trial. Circulation. 2000, 102: 149-156.
SYMPHONY Investigators: Comparison of sibrafiban with aspirin for prevention of cardiovascular events after acute coronary syndromes: a randomized trial: Sibrafiban Versus Aspirin to Yield Maximum Protection From Ischemic Heart Events Post-Acute Coronary Syndromes. Lancet. 2000, 355: 337-345. 10.1016/S0140-6736(99)11179-6.
Second SYMPHONY Investigators: Randomized trial of aspirin, sibrafiban, or both for secondary prevention after acute coronary syndromes. Circulation. 2001, 103: 1727-1733.
Topol EJ, Easton JD, Amarenco P, Califf R, Harrington R, Graffagnino C, Davis S, Diener HC, Ferguson J, Fitzgerald D, Shuaib A, Koudstaal PJ, Theroux P, Van de Werf F, Willerson JT, Chan R, Samuels R, Ilson B, Granett J: Design of the blockade of the glycoprotein IIb/IIIa receptor to avoid vascular occlusion (BRAVO) trial. Am Heart J. 2000, 139(6): 927-933. 10.1067/mhj.2000.105107.
Chew DP, Bhatt DL, Sapp S, Topol EJ: Increased mortality with oral platelet glycoprotein IIb/IIIa antagonists. A meta-analysis of phase III multicenter randomized trials. Circulation. 2001, 103: 201-206.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Rights and permissions
About this article
Cite this article
Suwaidi, J.A., Salam, A.M. Platelet glycoprotein IIb/IIIa receptor blockade in coronary artery disease. Trials 2, 171 (2001). https://doi.org/10.1186/cvm-2-4-171
Published:
DOI: https://doi.org/10.1186/cvm-2-4-171