Abstract
Zirconium thin films were deposited on a glass substrate using direct current magnetron sputtering technique and then post-annealed at different temperatures (100°C to 500°C in steps of 100°C) in an oxygen constant flow. The dependence of crystallographic structure, surface morphology, chemical composition, and electrical and decorative properties of the films on the annealing temperature was investigated. X-ray diffraction showed different phases of zirconium oxide at different annealing temperatures. It is observed that crystallite size and nanostrain increase with annealing temperature. Atomic force microscopy results showed granular structure in all samples, while both grain size and film surface roughness increased with the annealing temperature. Energy dispersive X-ray analysis data showed that the ratio of O/Zr was approximately 1.6, 1.7, 1.9, 2.1, and 2.2 at annealing temperatures of 100°C, 200°C, 300°C, 400°C, and 500°C, respectively. The annealed films at higher temperatures (400°C and 500°C) were transparent, while annealed films at lower temperatures (100°C to 300°C) were grey and brown, respectively. The variation of electrical resistance of samples with applied voltage was approximately constant, while it increased with annealing temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The study of metal oxides, especially in the form of thin films, has both scientific and technological significance. Zirconia (ZrO2) is one of these materials that exhibit remarkable properties. Because of its desirable physical and chemical properties, such as high melting point (2,700°C), low thermal conductivity (4.2 W m−1 K−1 at room temperature) [1], high hardness (18 GPa for monoclinic phase and 14 to 11 GPa for amorphous [2, 3]), excellent chemical and corrosion resistance [2, 4], high refractive index (2.2), large band gap (5 eV), high transparency in the visible and near-IR region [5], high dielectric constant (approximately 25 [6]), and biocompatibility [7], it is widely used in coating materials against corrosion, wear and oxidation, electronic devices, high refractive mirrors, broadband interference filters, decorative devices, active electro-optical devices, and anti-reflection coatings in optical industries [6, 8–12]. Furthermore, because of its color (similar to tooth color), biocompatibility, and adequate mechanical properties, zirconia is widely used in dentistry [13].
Among various physical and chemical methods for preparation of zirconium oxide thin films [14–22], post-annealing of zirconium layers is a simple, low-cost, suitable, and flexible method. In this technique, variation of annealing conditions including annealing temperature, time, and environment results in different nanostructures with interesting properties. Some researchers have also used post-annealing of Zr or ZrO2 layers for preparation of zirconia thin films with new and different properties [23–25].
In this paper, in order to more deeply understand the post-annealing effect on the Zr thin films, we have performed the post-annealing procedure on sputter-coated Zr/glass thin films with constant flow of oxygen at different temperatures and investigated the effect of this process on the crystallographic structure, surface morphology, chemical composition, and decorative and electrical properties of the produced zirconium oxide thin films.
Experimental details
Zirconium thin films of 90-nm thickness were deposited by means of a direct current (DC) magnetron sputtering system using a circular sputtering target (99.998% purity) of 76-mm diameter and 1-mm thickness. The target-to-substrate distance was 10 cm. A continuously variable DC power supply of 600 V and 150 mA was used as a power source for sputtering. The thickness and deposition rate of Zr films were checked in situ using a quartz crystal monitor (6 MHz gold, INFICON Company, East Syracuse, NY, USA) located near the substrate during the sputtering process. The zirconium thin films were deposited with a deposition rate of 9 Å/s at room temperature. The base pressure was 4×10−5 mbar which changed to 2.8×10−3 mbar during deposition of Zr. The purity of argon gas in this work was 99.998% and controlled by a mass flow controller. The substrates for the deposition were 20×20 mm2 glass (cut from a microscope slide) and were cleaned with acetone and ethanol in an ultrasound cleaner for a few minutes. The surface roughness of the substrates was measured by a Talysurf profilometer (Taylor-Hobson, Leicester, UK). The root mean square (rms) substrate roughness was 0.3 nm. Post-annealing of Zr/glass films was performed using a tube furnace at five different temperatures (100°C to 500°C in steps of 100°C) with a 200 sccm (standard cubic centimeter per minute) flow of oxygen (purity of 99.98%). The samples reached the selected temperature with a thermal gradient of 5°/min and were kept at the annealing temperature for 60 min, then gradually cooled down to room temperature. The flow rate of oxygen gas was controlled by a mass flow controller. Nanostructure and crystallographic orientation of the samples were obtained using a Philips XRD X'pert MPD diffractometer (Cu Kα radiation, 40 kV and 30 mA; FEI Co., Hillsboro, OR, USA) with a step size of 0.02° and count time of 1 s per step. A field emission scanning electron microscope (model: MV2300, CamScan, Czech and England) and an atomic force microscope (AFM; Auto Probe PC, Park Scientific Instrument, Santa Clara, CA, USA) were also employed for investigation of chemical composition and surface morphology of the samples, respectively. The electrical resistance of the films was also measured by a two-probe instrument with an excitation wavelength at 320 nm. The excitation wavelength in UV range was needed due to the high dielectric constant (approximately 25 [6]) of zirconium oxide, so that the electrical resistance measurement of samples without the UV excitation wavelength was impossible.
Results and discussion
Crystallographic structure
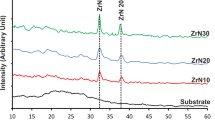
Zirconium oxide exists in different crystalline phases, namely amorphous [26, 27], cubic [28, 29], monoclinic [28, 30], tetragonal [31, 32], orthorhombic [33, 34], and combination of different phases [24]. All these structures are strongly affected by the preparation method and growth parameters. X-ray diffraction patterns of Zr/glass thin films annealed at different temperatures for 60 min are shown in Figure 1, while the numerical data of this analysis are given in columns 3 and 4 of Table 1. The X-ray diffraction (XRD) pattern of an annealed sample at 100°C shows two peaks at 31.89° and 36.53° that can be related to the ZrO2(012) diffraction line of orthorhombic phase (with reference to JCPDS card no. 33-1483, 2θ: 31.889°) and Zr(101) diffraction line (with reference to JCPDS card no. 05-0665, 2θ: 36.510°). At 200°C annealing temperature, the intensity of the zirconium peak is decreased, while the ZrO2(012) peak intensity is increased. By increasing the annealing temperature to 300°C, the abovementioned peaks disappeared and three new peaks appeared at 28.05°, 29.81°, and 34.21° diffraction angles. These peaks can be attributed to the ZrO2(111) crystallographic orientation of monoclinic phase (with reference to JCPDS card no. 05-0543, 2θ: 28.036°), ZrO2(101) crystallographic orientation of tetragonal phase (with reference to JCPDS card no. 24-1164, 2θ: 29.807°), and ZrO2(002) crystallographic orientation of monoclinic phase (with reference to JCPDS card no. 05-0543, 2θ: 34.195°), respectively. When the annealing temperature increased to the higher temperatures (i.e., 400°C and 500°C), only the intensities of these peaks are increased and no new peaks are observed. On the other hand, it may be suggested that by increasing the annealing temperature between 300°C and 500°C, little change may occur in the structure of ZrO2 films.
In summary, the results of XRD analysis of the samples produced in this work show that one may distinguish two groups of nanostructures. Group I (samples I and II) includes the films annealed at lower temperatures (100°C and 200°C) with a complex structure of ZrO2 (orthorhombic) and Zr (hexagonal). Group II (samples III to V) includes the films annealed at higher temperatures (300°C to 500°C) that were completely oxidized and showed a mixed crystallographic structure of monoclinic and tetragonal.
It can be seen in column 3 of Table 1 that the position of all diffraction lines is shifted to higher diffraction angles relative to that of the powder sample. Furthermore, the sample annealed at the highest annealing temperature shows a larger shift than those annealed at lower temperatures. The observed shifts provide information to calculate the nanostrain (ϵ) in the body of the film [35, 36] using
where d is the plane spacing of the sample (column 5 of Table 1) and d0 is the plane spacing of the standard powder sample. Variations of nanostrain for all diffraction lines are shown in column 6 of Table 1. The results for all samples show compressive strain. Considering the ZrO2(101) (i.e., T(101), tetragonal) diffraction line in the samples annealed at higher temperatures of 300°C to 500°C, it can be deduced that this compressive strain is increased to its highest value at 500°C. In general, the volume energy of the thin film decreases with increasing of the substrate (annealing) temperature due to increased diffusion effect, which in turn reduces the nanostrain in the structure of the thin film [37]; at the same time by increasing the temperature, the contribution of the thermal strain on the total strain is increased, causing the strain to increase.
The competition between the diffusion process and thermal strain at different substrate (annealing) temperatures may have been the cause of the above observations in the nanostrain in zirconium oxide films reported in Table 1.
In order to obtain the crystallite size (coherently diffracting domains) of the samples, we used the Scherrer relation [38]
where λ is the wavelength of X-ray, θ is the Bragg angle, and k is a dimensionless constant which is related to the shape and distribution of crystallites [39] (usually taken as unity). For obtaining the value of B, we used the usual procedure of full width at half maximum (FWHM) measurement technique [40]; therefore,
where W0 is the FWHM of the sample and W i is the FWHM of the stress-free sample (standard SiO2 single-crystal sample). The calculated crystallite size D (coherently diffracting domains) obtained from the above procedure is given in column 7 of Table 1. This result shows that the crystallite size in each group of films (i.e., groups I and II) increases with the annealing temperature. This behavior may be due to the increased diffusion effect with annealing temperature. A similar effect is reported by Ciosek et al. [23].
Surface morphology
3-D AFM images of selected zirconium oxide samples prepared in this work are shown in Figure 2, while grain size (obtained from 2-D AFM images by JMicrovision code) and surface roughness parameters of all samples are given in columns 8 to 10 of Table 1. The results show a granular structure for all samples, while grain size in each group of the samples (consistent with XRD results) and surface roughness increase with annealing temperature. This behavior is due to the high annealing temperature that provides energy of film atoms to enhance mobility. Increasing of mobility also increases the coalescence and produces larger grains with deeper valleys between them; hence, higher surface roughness is obtained. A similar behavior with annealing temperature is also obtained and reported in [41].
The grain sizes obtained from XRD data (27 to 74 nm) are smaller than the results obtained from AFM images (32 to 102 nm). These differences can be attributed to the fact that the AFM images show only surface of the grains.
Chemical composition and color of the produced samples
In addition to other factors such as hardness, color of zirconia coatings is important in dentistry and decorative devices. For this reason, we have studied the dependence of color and chemical composition of samples on annealing temperature. The chemical composition deduced from energy dispersive X-ray (EDAX) measurements and colors of the films produced in this work are given in Figure 3. These results indicate that the O/Zr ratio in the structure of the samples (i.e., x in ZrO x ) increases with the annealing temperature. The stoichiometry of the zirconium oxide thin films was found to be approximately 1.6, 1.7, 1.9, 2.1, and 2.2 at annealing temperatures of 100°C, 200°C, 300°C, 400°C, and 500°C, respectively. On the other hand, the result shows that the degree of film oxidation increases with annealing temperature. Koski et al. [42] have reported that zirconium oxide is colorless and transparent when it is fully oxidized. This color is due to the profusion of oxygen atoms in the lattice structure. The color is grey or brown when the O/Zr ratios are 1.6 and 1.8, respectively. A color of grey happens when the thin films are rich in metal. These results are consistent with the color of our samples in this work. Figure 4 shows the images of our samples annealed at 100°C, 300°C, and 500°C with grey, brown, and transparent (colorless) colors, respectively.
The results of EDAX analysis and color of samples are consistent with XRD patterns. The films annealed at lower temperatures (100°C and 200°C) due to the low influence of oxygen had a mixed structure of ZrO2 and Zr and were substoichiometric and grey, whereas the films annealed at higher temperatures (400°C and 500°C) due to the deeper/more influence of oxygen were completely oxidized, colorless, and overstoichiometric.
Electrical resistance
The I-V curve was measured to study the electrical behavior of the films. This work was done with a two-probe system equipped with a nano-ammeter with an excitation wavelength at 320 nm. Figures 5 and 6 show the variation of current as a function of applied voltage and the voltage dependence of electrical resistance of ZrO x thin films obtained from Figure 5 data, respectively.
It can be seen that variation of electrical resistance with applied voltage is approximately constant within the experimental error of 10%, for each ZrO x sample, while it increases with the annealing temperature. This behavior can be related to the increased oxidation rate as discussed above and is consistent with the EDAX results. When the number of oxygen atoms that should bond to the zirconium atoms is not enough, the remainder Zr atoms act as donors and can provide free electrons. With these free electrons, the films have a high carrier density and low resistivity. However, as the number of oxygen atoms integrated within the zirconium atoms increases, the number of these free electrons decreases and the film resistance increases.
Conclusion
A DC magnetron sputtering system was used to deposit Zr thin films of 90-nm thickness on glass substrates. The influence of post-annealing temperature in the presence of constant oxygen flow on crystallographic structure, surface morphology, and chemical composition of the produced samples was studied by XRD, AFM, and EDAX techniques, respectively. A two-probe instrument with an excitation wavelength of 320 nm was employed for electrical resistance investigation. The annealed films at lower temperatures of 100°C and 200°C (group I) had a combined structure of ZrO2 (orthorhombic) and Zr (hexagonal), while the annealed films at higher temperatures >300°C (group II) were completely oxidized and showed a mixed crystallographic structure of monoclinic and tetragonal. The crystallite size, nanostrain, and surface roughness were increased with the annealing temperature in each group of the samples. The stoichiometry and color of the zirconium oxide thin films were found to be approximately 1.6 (grey), 1.7 (grey), 1.9 (brown), 2.1 (transparent), and 2.2 (transparent) at annealing temperatures of 100°C, 200°C, 300°C, 400°C, and 500°C, respectively. The variation of electrical resistance of the films was almost constant with applied voltage for each sample. The electrical resistance of ZrO x thin films was increased with the annealing temperature.
References
Askeland DR, Phulé PP: The Science and Engineering of Materials. 4th edition. Pacific Grove: Thomson Brooks/Cole; 2003.
Gan Z, Yu GQ, Zhao ZW, Tan CM, Tay BK: Mechanical properties of zirconia thin films deposited by filtered cathodic vacuum arc. J. Am. Ceram. Soc 2005, 88: 2227–2229.
Martin PJ, Bendavid A: Properties of zirconium oxide films prepared by filtered cathodic vacuum arc deposition and pulsed DC substrate bias. Thin Solid Films 2010, 518: 5078–5082.
Sui JH, Cai W: Formation of ZrO 2 coating on the NiTi alloys for improving their surface properties. Nucl. Instrum. Methods Phys. Res. B 2006, 251: 402–406.
Zhao ZW, Tay BK, Yu GQ, Lau SP: Optical properties of filtered cathodic vacuum arc-deposited zirconium oxide thin films. J. Phys. Condens. Matter 2003, 15: 7707–7716.
Yu GQ, Tay BK, Zhao ZW: Structure and properties of zirconium oxide thin films prepared by filtered cathodic vacuum arc. Appl. Phys. A Mater. Sci. Proc 2005, 81: 405–411.
Liu X, Huang A, Ding C, Chu PK: Enhanced bioactivity of self-organized ZrO 2 nanotube layer by annealing and UV irradiation. Biomaterials 2006, 27: 3904–3911.
Ohtsu Y, Hino Y, Misawa T, Fujita H, Yukimura K, Akiyama M: Influence of ion-bombardment-energy on thin zirconium oxide films prepared by dual frequency oxygen plasma sputtering. Surf. Coat. Technol 2007, 201: 6627–6630.
Xu KW, Zhao S, Ma F, Song ZX: Thickness-dependent structural and optical properties of sputter deposited ZrO 2 films. Opt. Mater 2008, 30: 910–915.
Lai LJ, Lu HC, Chen HK, Cheng BM, Lin MI, Chu TC: Photoluminescence of zirconia films with VUV excitation. J. Electron. Spectrosc 2005, 144: 865–868.
Cyviene E, Laurikaitis M, Dudonis J: Deposition of nanocomposite Zr-ZrO 2 films by reactive cathodic vacuum arc evaporation. Mat. Sci. Eng. B-Solid 2005, 118: 238–241.
Korkmaz S, Pat S, Ekem N, Zafer Balbag M, Temel S: Thermal treatment effect on the optical properties of ZrO 2 thin films deposited by thermionic vacuum arc. Vac 2012, 6: 1930–1933.
Manicone PF, Iommetti PR, Raffaelli L: An overview of zirconia ceramics: basic properties and clinical applications. J. Dent 2007, 35: 819–826.
Cueto LF, Sanchez E, Torres-Martinez LM, Hirata GA: On the optical, structural, and morphological properties of ZrO 2 and TiO 2 dip-coated thin films supported on glass substrates. Mater. Charact 2005, 55: 263–267.
Lee BJ, Sohn HL, Cho YT: The effect of the RF-sputtering condition on the ZrO 2 thin film's characteristics. J. Korean. Phys. Soc 2007, 51: 1038–1041.
Shen YM, Shao SY, Yu H, Fan ZX, He HB, Shao JD: Influences of oxygen partial pressure on structure and related properties of ZrO 2 thin films prepared by electron beam evaporation deposition. Appl. Surf. Sci 2007, 254: 552–556.
Huang AP, Chu PK: Microstructural improvement of sputtered ZrO 2 thin films by substrate biasing. Mat. Sci. Eng. B-Solid 2005, 121: 244–247.
Huy LD, Laffez P, Daniel P, Jouanneaux A, Khoi NT, Simeone D: Structure and phase component of ZrO 2 thin films studied by Raman spectroscopy and X-ray diffraction. Mat. Sci. Eng. B-Solid 2003, 104: 163–168.
Ehrhart G, Capoen B, Robbe O, Boy P, Turrell S, Bouazaoui M: Structural and optical properties of n-propoxide sol–gel derived ZrO 2 thin films. Thin Solid Films 2006, 496: 227–233.
Vrejoiu I, Matei DG, Morar M, Epurescu G, Ferrari A, Balucani M, Lamedica G, Dinescu G, Grigoriu C, Dinescu M: Properties of ZrO 2 thin films prepared by laser ablation. Mat. Sci. Semicon. Proc 2002, 5: 253–257.
Liu WC, Wu D, Li AD, Ling HQ, Tang YF, Ming NB: Annealing and doping effects on structure and optical properties of sol–gel derived ZrO 2 thin films. Appl. Surf. Sci 2002, 191: 181–187.
Zhu JF, Gao YG Y, Zhang LA, Pan YH, Wang GD, Xu Y, Zhang WH, Z.H.U.J.F: Epitaxial growth of ultrathin ZrO 2 (111) films on Pt(111). Chinese Sci. Bull 2011, 56: 502–507.
Ciosek J, Paszkowicz W, Pankowski P, Firak J, Stanislawek U, Patron Z: Modification of zirconium oxide film microstructure during post-deposition annealing. Vac 2004, 72: 135–141.
Kuei PY, Chou JD, Huang CT, Ko HH, Su SC: Growth and characterization of zirconium oxide thin films on silicon substrate. J. Cryst. Growth 2011, 314: 81–84.
Venkataraj S, Kappertz O, Liesch C, Detemple R, Jayavel R, Wutti M: Thermal stability of sputtered zirconium oxide films. Vac 2004, 75: 7–16.
Chua DHC, Milne WI, Zhao ZW, Tay BK, Lau SP, Carney T, White RG: Properties of amorphous ZrO x thin films deposited by filtered cathodic vacuum arc. J. Non-Cryst. Solids 2003, 332: 185–189.
Zhao ZW, Tay BK, Huang L, Yu GQ: Study of the structure and optical properties of nanocrystalline zirconium oxide thin films deposited at low temperatures. J. Phys. D Appl. Phys 2004, 37: 1701–1705.
Martin PJ, Netterfield RP, Sainty WG: Modification of the optical and structural properties of dielectric ZrO 2 films by ion-assisted deposition. J. Appl. Phys 1984, 55: 235–241.
Mehner A, Kluemper-Westkamp H, Hoffmann F, Mayr P: Crystallization and residual stress formation of sol–gel-derived zirconia films. Thin Solid Films 1997, 308–309: 363–368.
Martin PJ, Netterfiend RP, Kinder TJ: Ion-beam-deposited films produced by filtered arcevaporation. Thin Solid Films 1990, 193–194: 77–83.
Pawkewicz WT, Hays DD: Microstructure control for sputter-deposited ZrO 2 , ZrO 2 -CaO and ZrO 2 -Y 2 O 3 . Thin Solid Films 1982, 94: 31–45.
Ochando IM, Vila M, Prieto C: Optical and structural study of EB-PVD ZrO 2 thin films. Vac 2007, 81: 1484–1488.
Haung AP, Di ZF, Fu RKY, Chu PK: Improvement of interfacial and microstructure properties of high- k ZrO 2 thin films fabricated by filtered cathodic arc deposition using nitrogen incorporation. Surf. Coat. Technol 2007, 201: 8282–8285.
Huang AP, Fu RKY, Chu PK, Wang L, Cheung WY, Xu JB, Wong SP: Plasma nitridation and microstructure of high-k ZrO 2 thin films fabricated by cathodic arc deposition. J. Cryst. Growth 2005, 277: 422–427.
Savaloni H, Gholipour-Shahraki M, Player MA: A comparison of different methods for x-ray diffraction line broadening analysis of Ti and Ag UHV deposited thin films: nanostructural dependence on substrate temperature and film thickness. J. Phys. D Appl. Phys 2006, 39: 2231–2247.
Chung FH, Smith DK: Industrial Applications of X-ray Diffraction. New York: Marcel Dekker; 1999.
Shi Z, Player MA: Preferred orientation of evaporated Ni films on Mo and SiO 2 substrates. Vac 1998, 49: 257–263.
Warren BE: X-Ray Diffraction. London: Addison Wesley; 1969.
Langford JI, Wilson AJ: Scherrer after sixty years: a survey and some new results in the determination of crystallite size: J. Appl. Cryst 1978, 11: 102–113.
Huang TC, Lim G, Parmigiani F, Kay E: Effect of ion bombardment during deposition on the x-ray microstructure of thin silver films. J. Vac. Sci. Technol 1985, A3: 2161–2166.
Khojier K, Savaloni H, Kangarloo H, Ghoranneviss M, Yari M: Influence of annealing temperature on the nanostructure and corrosivity of Ti/stainless steel substrates. Appl. Sur. Sci 2008, 254: 2528–2533.
Koski V, HÖlsä J, Juliet P: Properties of zirconium oxide thin films deposited by pulsed reactive magnetron sputtering. Sur. Coat. Technol 1999, 120–121: 303–312.
Acknowledgements
This work was carried out with the support of the Islamic Azad University, Chalous Branch and Central Tehran Branch. HS acknowledges the University of Tehran and is grateful to the Centre of Excellence for Physics of Structure and Microscopic Properties of Matter, Department of Physics, University of Tehran for partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors provided the same contributions in this article. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Khojier, K., Savaloni, H. & Jafari, F. Structural, electrical, and decorative properties of sputtered zirconium thin films during post-annealing process. J Theor Appl Phys 7, 55 (2013). https://doi.org/10.1186/2251-7235-7-55
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-7235-7-55