Abstract
In recent years, there has been an increasing interest in finding innovative solutions for the efficient removal of contaminants from water, soil, and air. The present study reviews the adsorptive removal of catechol (C), resorcinol (R), hydroquinone (HQ), and their derivatives from various adsorbents. As an effective, efficient, and economic approach for water purification, adsorbents and adsorption processes have been widely studied and applied in different aspects for a long time. The role of various adsorbent materials like activated carbon, activated carbon cloth, carbon nanotubes, polymeric resins, organic clays, Fe(OH)2, and TiO2 was discussed together with that of other experimental parameters. In all the synthetic resins, particularly, aminated hypercrosslinked polymers have good adsorption capability for phenols. These polymeric adsorbents are suitable for industrial effluents containing C, R, HQ, and their derivatives. The adsorption capacities of the adsorbents reviewed here vary significantly depending on the characteristics of the individual adsorbent, the extent of chemical modifications, and the concentrations of solutes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Review
Introduction
Phenol (P) and substituted P are important organic intermediates for the products of industry and agriculture[1]. For example, hydroxy aromatic compounds, such as catechol (C), resorcinol (R), and hydroquinone (HQ), were used widely as industrial solvents. C (1,2-dihydroxybenzene) is also widely used to produce food additive agents, hair dyes, and antioxidants[2]. R (1,3-dihydroxybenzene) is usually employed to produce dyes, plastics, and synthetic fibers[3–5]. Phenolic compounds, such as C, R, and HQ, were found in the effluents of industries such as textile, paper and pulp, steel, petrochemical, petroleum refinery, rubber, dye, plastic, pharmaceutical, cosmetic, etc. and in the wastewater of synthetic coal fuel conversion processes[6, 7]. The effluents from synthetic coal fuel conversion processes may contain C and R concentrations ranging from 1 to 1,000 mg/l[8].
-
H.
Reinsch first isolated C in 1839 by distilling catechin (from catechu, the juice of Mimosa catechu (Acacia catechu L.f)). The pyrocatechol is formed by the heating of catechin above its decomposition point. C is produced industrially by the hydroxylation of P using hydrogen peroxide [9]. C has also been produced by the hydrolysis of 2-substituted phenols, especially 2-chlorphenol, with hot aqueous solutions containing alkali metal hydroxides. Its methyl ether derivative, guaiacol, converts to C via hydrolysis of the CH3-O bond as promoted by hydriodic acid. R is obtained by fusing many resins (galbanum, asafoetida, etc.) with potassium hydroxide or by the distillation of brazilwood extract. It may be prepared synthetically by fusing 3-iodophenol, phenol-3-sulfonic acid, or benzene-1,3-disulfonic acid with potassium carbonate; by the action of nitrous acid on 3-aminophenol; or by the action of 10% HCl on 1,3-diaminobenzene [10]. Many ortho- and para-compounds of the aromatic series (for example, the bromophenols, benzene-para-disulfonic acid) also yield R by fusion with potassium hydroxide. HQ is obtained from few methods: oxidation of aniline with manganese dioxide and sulfuric acid, followed by reduction with iron dust and water [11]. The second method consists of the alkylation of benzene with propylene to produce a mixture of diisopropylbenzene isomers from which, in a first step, the para-isomer is isolated. This is oxidized with oxygen to produce the corresponding dihydroperoxide, which is treated with an acid to produce HQ and acetone [12]. Finally, the oxidation of P with hydrogen peroxide can be used to produce a mixture of products from which both HQ and C (1,2-dihydroxybenzene) can be isolated [12].
C occurs freely in kino and in beechwood tar; its sulfonic acid has been detected in the urine of horses and humans[13]. HQ exists in a free state in pear leaves[14]. Arbutin, a glucoside of HQ, occurs widely in the leaves, barks, buds, and fruits of many plants[11], especially the Ericaceae[15]. C (o-benzenediol, 1,2-benzenediol, or 1,2-dihydroxybenzene) is a natural polyphenolic compound that widely exists in higher plants such as teas, vegetables, fruits, tobaccos, and some traditional Chinese medicines[16]. The smoke from 100 cigarettes contains about 10 mg of P and 50 mg of C besides other phenols[17]. HQ has been detected in cigarette smoke[18, 19]. This compound occurs in the effluents resulting from different industrial activities such as photoprocessing[20], coal-tar production[21], and the paper industry[22].
The toxicity of C for microorganisms has been demonstrated in the past years[23–25] and has been suggested to be the reason for the difficulties in cultivating microorganisms on benzene, toluene, or chlorobenzene[23]. Several studies additionally indicated the toxicity of C for water flea, zebra fish, trout, rabbit, cat, rat, and mouse and for human cell lines[26]. C is strongly irritating to the eyes, skin, and respiratory tract, and it has been proven to cause DNA damage, vascular collapse, coma, and death. Between R and C, C is considered more toxic[11, 27]. However, these compounds are considered as the primary pollutants in wastewater due to their high toxicity, high oxygen demand, and low biodegradability[8, 28]. Consequently, their removal from wastewater has attracted significant environmental concerns. C, like other phenols, is of particular interest from a sanitary point of view due to its toxicity and deleterious effect on the quality of water supplies. In human medicine, HQ is used as a topical application in skin whitening to lighten the color of the skin as it does not have the same predisposition to cause dermatitis as metal does. There is inadequate evidence in humans for the carcinogenicity of HQ; however, it causes toxicity in several organs, notably the kidney and forestomach[12].
Additional file1: Table S1 shows the hazard ranking of C, R, and HQ. The compound is readily absorbed from the gastrointestinal tract, causes hemolysis, degenerates the renal tubes, diminishes liver function, and accumulates in the bone marrow[29]. Its metabolites may initiate many cancers and neurodegenerative diseases[30]. C is even more toxic than P since it provokes changes in the function of erythrocytes at doses as low as 50 μg/l compared to 250 μg/l of P[31].
The United States Environmental Protection Agency (USEPA) has designated granular activated carbon (GAC) adsorption as the ‘best available technology’ for removing organic pollutants[32]. P and associated compounds have been listed as priority pollutants by the Ministry of Environment and Forests (MoEF), Government of India, and USEPA. MoEF has prescribed that the concentration of phenols should not exceed 1.0 mg/l for their discharge into surface waters and 5.0 mg/l for their discharge into public sewers, on land for irrigation, and on marine coastal areas. These limits have generally been on the basis of the total phenols present in the effluent.
Conventional methods used in the remediation/degradation of C, R, and HQ are biodegradation[33, 34], anaerobic biodegradation[35–37], anodic oxidation[38, 39], photocatalysis[40], oxidative catalysis[41], etc. Adsorption techniques have gained favor in recent years because they are considered efficient for the removal of trace organic pollutants from water that cannot be removed using other treatment processes.
In addition, adsorption and desorption kinetics are technologically important because the diffusion within solid particles is a phenomenon of great importance in catalysis, metallurgy, microelectronics, materials science, and other numerous scientific and technological applications. Chemical kinetics explains how fast the rate of chemical reaction occurs and also on the factors affecting the reaction rate. The nature of the sorption process will depend on physical or chemical characteristics of the adsorbent systems and also on the system conditions.
Adsorption using GAC has been found to be an attractive process for the removal of phenols[6, 42–50]. However, the cost of GAC and the loss of adsorption efficiency after regeneration of the exhausted GAC have limited its use in effluent treatment. Therefore, alternative low-cost, non-conventional adsorbents such as activated carbon cloth[51, 52], waste Fe(III)/Cr(III) hydroxide[53], hypercrosslinked resin[53–55], TiO2 surface[56, 57], organoclays[58], bagasse fly ash[59], activated cashew nut shell[60], etc. have been investigated for the treatment of effluents. So, in this context, the present review is made for the adsorption of C, R, and HQ, and their derivatives onto different adsorbents.
Properties of C, R, and HQ
C, R, and HQ are available in the form of colorless crystalline and white crystals. C and R have phenolic odor and unpleasant sweet taste and become brown on exposure to air and light and pink on contact with air and light[61]. Small amounts of C occur naturally in fruits and vegetables, along with the enzyme polyphenol oxidase. R crystallizes from benzene as colorless needles which are readily soluble in water, alcohol, and ether, but insoluble in chloroform and carbon disulfide. With concentrated nitric acid, in the presence of cold concentrated sulfuric acid, it yields trinitro-resorcin (styphnic acid), which forms yellow crystals, exploding violently on rapid heating. It reduces Fehling's solution and ammoniacal silver solutions. It does not form a precipitate with lead acetate solution, as the isomeric pyrocatechol does. Iron(III) chloride colors its aqueous solution a dark violet, and bromine water precipitates tribromoresorcin. Some of the physicochemical properties of adsorbents affect the adsorption process like water solubility, acid dissociation constant (pKa) value, and octanol/water partition coefficient. R has more solubility (1,100 g/l) than C and HQ. The octanol/water partition value of R is 0.93, which is more than those of C and HQ. The pKa values of C, R, and HQ were 9.25, 13; 9.4, 12.3; and 9.9, 11.6, respectively. Some more properties are given in Table1. C can form stable complexes with various di- and trivalent metal ions, the complexes with trivalent ions being the most stable. C can also undergo redox reactions (Scheme 1 of[7]), cycling between C, semiquinone radicals, and ortho-benzoquinone.
Physicochemical characteristics of various adsorbents for removal of C, R, and HQ
Physicochemical characteristics of various adsorbents for the removal of C, R, HQ, and their derivatives from water were listed in Tables2 and3.
Usually, there are different processes for the preparation and pretreatment of adsorbents, namely either thermal procedures (physical) or chemical routes. In comparison with physical activation, there are two important advantages of chemical activation: One is the lower temperature in which the process is accomplished, and the other is that the global yield of the chemical activation tends to be greater since burn-off char is not required. For impregnation, the precursor with dehydrating agents was widely used as a chemical agent in the preparation of adsorbents. Knowledge of different variables during the activation process is very important in developing the porosity of materials which is sought for a given application. For example, chemical activation done by using H2SO4, HCl or HNO3, ZnCl2, AgNO3, H3PO4, H2O2, etc. can improve the pore distribution and increase the surface area of adsorbents in the structure due to the use of different chemicals[42, 44, 51–53, 58, 70] and then heat treatment at moderate temperatures in a one-step process.
The raw sawdust was carbonized at 873 K in a nitrogen atmosphere, and concentrated H2SO4 was impregnated with carbonized materials in different concentrated H2SO4/sawdust ratios from 3:1 to 6:1 (w/w) at 575 K for 4 h. The product was cooled to room temperature, washed with 5% H2SO4 and then with bi-distilled water until free from sulfate ions, and dried at 393 K for 6 h. GACs are placed in the reducing system with hydrogen under vacuum condition and then warmed at 573 K for 6 days. Finally, the AC is placed with HNO3 solution in Soxhlet equipment for 9 h. The sample is then washed with distilled water until a constant pH value is obtained, and the AC is then dried at 383 K for 24 h. These GACs are used for the removal of C[42], HQ, and other phenols[44]. The chloromethylated PS beads were dried at 323 K in vacuum for 8 h and then swollen with nitrobenzene at 298 K overnight with anhydrous ZnCl2 added into the reaction flask with the temperature at 323 K, and the beads were obtained after 12 h. The polymeric beads are washed with 1% HCl (w/w) aqueous solution and ethanol until the solution becomes transparent. Finally, they were extracted with ethanol for 8 h[55]. Shakir et al.[58] prepared the organobentonite by treating natural bentonites which are crushed and oven-heated at 85°C for 3 h and dried for 1 h at 105°C. The treated clay was washed with AgNO3. Finally, the organobentonite was at last separated from water by vacuum filtration and dried for 1 h at 105°C, and a similar procedure was reported for the removal of HQ onto AC by drying under vacuum at 120°C[51, 52].
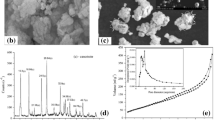
Figures1a,b,c and2a,b,c show the yearly progress of publications and citations, respectively, in the late twentieth century and during the present decade. These figures clearly indicate the increasing interest in the removal of C, R, and HQ by adsorption.
Number of research papers appearing with ‘adsorption’ and (a) ‘catechol,’ (b) ‘resorcinol,’ and (c) ‘hydroquinone. In the topic as listed in[71] for ‘All Years’ (1983 to 2010; out of a total of 267 and 14; 282 and 49; and 239 and 18, respectively, articles appearing). Note that early adsorption articles appeared in the late 1990 and 1983. The small number of papers in pre-1990 and 1982 means that they cannot be properly shown in the figure.
Number of citations appearing with ‘adsorption’ and (a) ‘catechol,’ (b) ‘resorcinol,’ and (c) ‘ hydroquinone.’ In the topic as listed in[71] for ‘All Years’ (1983 to 2010; out of a total of 4,734 and 326; 3,812 and 686; and 3,358 and 394, respectively, articles appearing). Note that early adsorption articles appeared in the late 1991 and 1992. The small number of papers in pre-1991 and 1968 means that they cannot be properly shown in the figure.
Removal by adsorption onto various adsorbents
Adsorption of organic solutes from the aqueous phase is a very important application of ACs, and it has been cited by the USEPA as one of the best available environmental control technologies[72]. The removal by adsorption onto various adsorbents of C, R, HQ, and their derivatives can be found in the literature[6, 43–45, 52–57, 68, 70, 73–75].
Activated carbon
Adsorption onto AC is widely used for wastewater treatment. Various adsorbents prepared from AC and low-cost adsorbents used in wastewater treatment were reported by Lin and Juang[76]. Thus, AC is used in the control of color and odors and in the removal of organic compounds or trihalomethane precursors, chlorine, and toxic compounds in general. A large amount of work has been devoted to the study of phenolic compound adsorption onto AC for water treatment purposes. The development of the investigation on the subject has been recently reviewed by Dabrowski[77]. Dobbs and Cohen[78] reported an extensive list of isotherm data of several toxic compounds. Ozkaya[79] compared different isotherm models used to describe the adsorption of phenols on AC. Garcia-Araya et al.[80] studied the adsorption of some phenolic acid on AC.
ACs present an outstanding adsorption capacity that stems from their high surface area, pore structure, and surface chemical properties. These materials are effective adsorbents for priority pollutants, therefore being suitable for the decontamination of water and wastewater. The AC surface is usually divided in three zones: basal planes, heterogeneous superficial groups (mainly oxygen-containing surface groups), and inorganic ash. For aromatic compounds, most of the adsorption sites are found on the basal planes[81]. However, heterogeneous groups have a higher activity and define the chemical characteristics of the carbon surface. The nature of the surface groups can be modified through physical, chemical, and electrochemical treatments. The most common are liquid phase treatments using HNO3 and H2O2, gas-phase oxidation with O2 or N2O, and heat treatment under inert gas to selectively remove some of the functional groups[42, 81, 82].
ACs possess excellent adsorption ability for relatively low molecular weight organic compounds such as phenols[82]. From a general point of view, an AC to be used in such processes must have adequate adsorptive capacity, mechanical strength, and chemical purity. Furthermore, all of these specifications should coexist with a low production cost. The most common materials used for the preparation of ACs are wood, coal, lignin, petroleum coke, and polymers[83]. Recently, many investigators have studied the feasibility of using many agricultural by-products and wastes, which are available at very little or no cost to prepare AC[84]. Both the texture and surface chemistry of ACs determine performance, and the final application of the carbon material will depend on its characteristics. The porous structure of ACs is a function of the precursor used in the preparation, the activation method followed, and the extent of activation. AC types produced by applying chemical activation processes contain mainly mesopores, while carbons obtained via gas activation are of the microporous type[85], although, depending on the nature of the parent material and by adjustment of the process conditions, different pore sizes that cover the micro-, meso-, and macropore ranges can be obtained[86]. Therefore, in recent years, it has prompted a growing research interest in the production of AC from renewable and cheaper precursors which are mainly agricultural by-products, such as corncob[87], rattan sawdust[88], rice straw[59, 89], apricot shell[90], jute fiber[91], rubber wood sawdust[33, 92], bamboo[93], oil palm fiber[94], bagasse fly ash, coconut shell[59], and activated cashew shell nut[60].
Table4 compares various parameters like adsorbent dosage (m), time (t), pH, etc. for the adsorption of C, R, HQ, and their derivatives onto GAC. Kumar et al.[6] investigated in a batch mode the adsorption behavior of R and C on GAC from a basic salt medium at optimum pH ≈ 7.1, optimum temperature ≈ 30°C, and optimum m ≈ 10 g/l for both R and C with 48 h as equilibrium time. However, Mohamed et al.[42] found 5 g/l as the optimum dosage of modified AC with 48 h as equilibrium time for the removal of C and R. Mondal and Balomajumder[68] observed adsorption and simultaneous adsorption biodegradation (SAB) for the removal of R and P from two separate synthetic wastewater over a 2- to 4-mm particle size at 28°C with 10 h as equilibrium time. They conducted the experiments over various adsorbent doses (2 to 12 g/l). They found that the removal of R reaches its constant value at the dose of around 8 g/l. Richard et al.[43] have reported that the adsorption of polyfunctional phenols encountered in olive oil mill wastewater was carried out onto AC at 20°C. They studied the effect of m in the range of 2 to 15 g/l and the effect of pH in the range of 5 to 9. They found that 16 g/l and 6.25 are the optimum dosage and pH, respectively, for the removal of C from olive oil mill wastewater. Huang et al.[55] reported the adsorption of C and HQ on modified GAC at pH 7, 9, and 11 with 48 h as equilibrium time. They observed that as the pH of the solution increases from 7 to 11, the amount of the phenols adsorbed diminishes. This indicates that adsorption increases when more protonated species are present in the solution. The adsorption isotherms show a linear behavior at Co< 200 mg/l, and later, the retained quantity increases slightly at pH 9. The adsorbed species are the protonated and monobasic anions, and due to the positive charge of the carbon surface, attractive and dispersive electrostatic forces begin to interact between the surface and the anion, favoring their accumulation. At pH 11, the surface is loaded negatively, the pH > pHPZC and the species present are the anionic monohydroxylated phenols. Therefore, the anionic species present in the solution repulse the surface AC. The adsorption occurs when the repulsive electrostatic forces predominate, and the adsorption at low concentrations is less than that at high concentrations[55].
Activated carbon cloth
Generally, utilization of AC can be in the form of powder, granule, and fiber or cloth. Activated carbon cloth (ACC), having very high specific surface area and adsorption capacity, uniform pore size, and mechanical strength, has attracted attention in recent years. Not only the adsorption but also the electrosorption characteristics of ACC when acting as a working electrode material have been investigated on various adsorbates[94–97]. Table5 compares various parameters like m, t, pH, etc. for the adsorption of C, R, HQ, and their derivatives onto ACC.
Bayram et al.[69] investigated the removal of C and R from aqueous solutions by adsorption and electrosorption onto high-area ACC with a dosage of 0.9 g/l, with 25°C and 24 h as operating temperature and contact time, respectively. They found that the extent of electrosorption of C was higher than R. It was attributed partly to a higher dipole moment of the former. It was demonstrated that ACC can partly be regenerated by an electrodesorption process. It was predicted that adsorption and electrosorption of C and R resulted mainly from dispersion interactions between surface charges and π electrons on adsorbate molecules. Ayranci and Duman[52] reported the adsorption of P, HQ, m-cresol, p-cresol, and p-nitrophenol from aqueous solutions onto ACC. The effect of ionization on the adsorption of these ionizable phenolic compounds was examined by studying the adsorption from acidic, basic, and natural pH (7.6) solutions at an operating temperature of 30°C.
Carbon nanotubes
Carbon nanotubes (CNTs), because of their chemical and physical properties, attract wide applications such as in the field of polymer composites[98], field emissions[93], energy storage[99], and sensors[100]. The large specific surface area of CNTs makes them suitable for the adsorption of gas[101], metal ions[102, 103], and organic compounds[104]. Recent studies suggest that CNTs can serve as good adsorbent for a variety of gas and liquid molecules, like hydrogen, butane, dioxins, dichlorobenzene, and heavy metal ions[105–110]. The considerable attentions about CNTs depend on their unique structural and mechanical properties, high thermal stability, and large specific surface area[62, 90, 90, 111].
Carbon nanomaterials include fullerenes, single-walled carbon nanotubes (SWCNTs), and multi-walled carbon nanotubes (MWCNTs). The monomer structure of fullerenes is a closed graphite ball, while those of CNTs are rolled-up graphite sheets forming a coaxial tube. A single rolled-up graphite sheet forms the SWCNT structure, while several rolled-up graphite sheets form the MWCNT structure. They consist of sheets of carbon atoms covalently bonded in hexagonal arrays that are seamlessly rolled into a hollow cylindrical shape[112]. The cylindrical surface geometry of CNTs has been well defined with outer diameters in the range of approximately 1 to 100 nm and length up to several tens of micrometers. Knowledge of environmental risk assessment of both toxic chemicals and CNTs once they are released to the environment[113–116] is also important. Table5 compares various parameters like m, t, pH, etc. for the adsorption of C, R, HQ, and their derivatives onto GAC by CNTs.
Liao et al.[70] studied the adsorption of P, C, R, HQ, and pyrogallol onto untreated MWCNTs and HNO3-treated MWCNTs. The uptake of R was 19.7 mg/ml (60% of total amounts adsorbed) within 1 min, when the total contact time was 660 min. Liao et al.[70] have suggested that the adsorption mechanism of MWCNTs is not totally the same as that of AC. The adsorption onto AC is dependent on the porous structure, so it takes time for adsorbates to diffuse through pores[117]. It is confirmed by the adsorption of N2 to CNTs that most available spaces of CNTs for adsorption are the cylindrical external surfaces, neither the inner cavities nor the inner-wall spacings[116].
Liao et al.[70] observed a pH < 6. There is a slight increase in the uptake of R with the decrease of pH because the solubility of R is dependent on pH; due to the weak acidity of R, the solubility of R decreases with the decrease of pH. The adsorption onto AC suggested that the uptake of phenolic derivatives was inversely proportional to solubility[118]; thus, the uptake of R on MWCNTs increased with the decrease of pH. Lin and Xing[75] have conducted experiments for the adsorption of P, C, and pyrogallol onto CNTs over a range of pH. It was found that the final solution pH (4.0 to 6.5) was lower than the pKa of the phenolics and close to the point of zero charge (pHPZC) of CNTs, which suggested that phenolics used for adsorption should be primarily in neutral form. Therefore, electrostatic interaction might not greatly influence the sorption of phenolics to CNTs in the weakly acidic environment. Sorption was found to increase sharply from P to C and then to pyrogallol, while their hydrophobicity decreased. Thus, the hydrophobic interaction can be insignificant as a major influencing factor regulating the sorption of these phenolics to CNTs.
Liao et al.[70] found that the acid-treated MWCNTs showed decreased adsorption capacity for R compared to untreated MWCNTs because of the increased electrostatic repulsion between carboxylic groups weakening the π-π interaction and water adsorption. The uptake of the adsorbates increased with the increase in the number of hydroxyl groups and their location in meta-position on the aromatic ring. Four possible solute-sorbent interactions are possible for the adsorption of phenolics onto CNTs as follows: (a) hydrophobic interaction, (b) electrostatic attraction or repulsion, (c) hydrogen bonding between the -OH and the tube surface -OH or -COOH groups, and (d) -OH substitution-enhanced π-π interactions between the phenolics and the CNTs. The -OH substitution on the phenolics and the hydroxy/carboxylic groups on the CNT surface may form hydrogen bonds; the hydrogen bonds may also form between the surface-adsorbed and dissolved phenolics. However, very low hydrogen and oxygen contents were detected on the CNTs, indicating that hydrogen bonding (if any) might not be significant between the phenolics and the functional groups on the CNTs[70, 119, 120]. An insignificant effect of hydrogen bonding on the sorption of nitroaromatics to CNTs was also recently reported[121], and hydrogen bond formation may have favorable adsorption at high concentration.
Polymeric resins
Adsorption onto polymeric resins has been widely studied for the treatment of effluents containing phenolic compounds[54, 122]. In comparison to classical adsorbents such as silica gel, alumina, and AC, polymeric adsorbents have high chemical stability, easy regeneration ability, excellent selectivity, and longevity. Among them, the commercial resin Amberlite XAD-4 has been considered as one of the best for the removal of phenolic compounds from wastewater[54, 123]. Many researchers have made more efforts on the chemical modification of polymeric adsorbents with functional groups such as phenolic hydroxyl, acetyl, benzoyl, and hypercrosslinked polymers[124, 125] to improve their adsorption properties by increasing the interactions between adsorbates and adsorbents[54, 55, 126, 127]. Styrene-divinylbenzene matrix has also been used for the removal of hydrophobic organic pollutants[128–130]. Table4 compares various parameters like m, t, pH, etc. for the adsorption of C, R, HQ, and their derivatives onto various polymeric resins.
Adsorption of C and R from an aqueous solution onto AH-1, AH-2, AH-3, NDA-100, and HJ-1 resins was reported by Sun et al.[54] and Huang et al.[55]. Sun et al.[54] found that the qm of C and R onto aminated hypercrosslinked resins AH-1, AH-2, and AH-3 are higher than that onto hypercrosslinked resin NDA-100, and more time was required to reach the equilibrium for higher Co and the required time for the adsorption of R is shorter than that of C[54, 55]. Thus, effects of the solubility and position of the hydroxyl group at the ortho- position may probably account for higher adsorbability of C than R onto HJ-1. Specific surface area, micropore structure, and content of tertiary amino groups play a combined role during the adsorption of both compounds onto the aminated hypercrosslinked polymers[54, 55]. Generally, the removal of these compounds mainly results from van der Waals interaction between the solute and the sorbent phase[131]. However, many hydrophilic or water-soluble organic compounds cannot be readily removed by those sorbents partly due to the strong solute-water interaction[130].
Organoclays
Over the last few decades, organoclays have gained much importance in the removal of organic pollutants from aqueous solutions. Among the various types of clays, montmorillonite, which is the main constituent of the low-cost and naturally abundant mineral bentonite, possesses several properties that make it very appropriate for organoclay preparation. Montmorillonite has a 2:1 type of layer consisting of one octahedral sheet of alumina inserted in between two silica tetrahedral sheets. In the tetrahedral sheets, Al3+ can substitute for Si4+, and in the octahedral sheets, Mg2+ or Zn2+ can replace Al3+. These isomorphous substitutions in the clay lattice result into a net negative charge on the clay surface[132]. Montmorillonite, with its lower surface charge density, has substitutions mostly in the octahedral sheet[133].
The adsorption properties of the organoclay surfaces may be significantly altered by exchange reactions, thus making the clay more organophilic in nature, and this increases its capability to remove organic pollutants from aqueous solutions[134–136]. Hydrophilic clays can be changed to organophilic clays through organic chemicals[137]. The modified organoclays have acted as a partition medium in the sorption of organic pollutants[138–141]. Table4 compares various parameters like m, t, pH, etc. for the adsorption of C, R, HQ, and their derivatives onto organoclays.
The removal of C from aqueous solutions onto cetyltrimethylammonium bromide-modified bentonite (CTAB-B) surfaces was reported by Shakir et al.[58]. They carried out the experiment with a wide range of pH (5 to 12), contact time (1 to 250 min), and concentration (0.8 to 15.3 mmol/l) at 30 ± 1°C. The percentage adsorption is ≤ 11% at pH ≤ 7.5, whereas it increases sharply with pH, attaining about 100% at pH ≥ 9.9. However, the sorption capacities increase with decreasing pH. Yildiz et al.[51] reported the removal of HQ and benzoic acid on synthesized organobentonites (ODTMA-B, HDTMA-B). Zhu et al.[134] and Smith and Grum[135] indicated that the magnitude and mechanism of sorption are functions of the cation-exchange capacity of the clay, the molecular structure of the exchanged organic cation, the extent of cation exchange, and the molecular structure of the solute. Sorption may take place either by partition or by adsorption depending mainly on the characteristics of the exchanged organic cation and the molecular structure of the solute. Adsorption of the large cationic surfactant molecule, CTAB, greatly modifies the nature of the clay surface which may exhibit both hydrophilic and hydrophobic as well as electrostatic properties and van der Waals interaction between the -R group of the surfactant and adsorbate[136–142], and it is also observed that the percentage adsorption increases from 81.5% to approximately 100% as the initial adsorbate concentration is reduced from the initial concentration of 88 mg/l. This observed increase in the percentage adsorption is due to the availability of larger sorbent surface sites for a relatively smaller amount of C at lower Co[58].
Metal surface
TiO 2
The adsorption of organic compounds onto oxides, hydroxides, and other minerals is known to retard their migration in soils and aquifers and to alter their susceptibility toward chemical and biological transformations[143]. Few literatures on the adsorption of organic compounds with hydroxyl or amino groups as the sole ligand donor groups are available[144]. It has been indicated that certain adsorbates can interact with active centers such as hydroxyl groups or bridging oxygen on TiO2 surface, resulting in a different catalytic activity[145]. Also, some of them can act as poisons[146]. Table4 compares various parameters like m, t, pH, etc. for the adsorption of C, R, HQ, and their derivatives onto TiO2 surface.
The adsorption removal of phenols was 90% at 1 g/l onto TiO2 surface. 4-Nitrocatechol adsorbs to a significantly greater extent than 4-nitro-2-aminophenol over the entire pH range at 1 g/l TiO2 dosage[56]. When pH < pHPZC of the surface, positive surface charge increases with decreasing pH, and when pH > pHPZC, negative surface charge increases with increasing pH[56]. Generally, the adsorption behavior of a number of organic compounds possessing carboxylic acid groups and the effects of pH have been successfully modeled by choosing appropriate stoichiometries and equilibrium constants for adsorption[147, 148].
Waste Fe(III)/Cr(III) OH. Adsorption has gained wide acceptance and popularity for the removal of phenolic compounds including industrial solid wastes[149]. Chromium(VI) compounds are used as corrosion inhibitors in cooling water systems in industries. Fe(II), which is generated electrolytically, reduces chromium(VI) in wastewater to Cr(III) under acidic conditions[150]. The Fe(III)/Cr(III) ions, produced in solution, are precipitated as Fe(III)/Cr(III) hydroxide by the use of lime. The resultant sludge is discarded as waste by the industries. Waste Fe(III)/Cr(III) hydroxide has been investigated in the laboratory for the removal of heavy metals[151], dyes, and pesticides[152]. The percentage C removal at equilibrium decreased from 80% to 65% as the Co increased from 10 to 40 mg/l onto ‘waste’ Fe(III)/Cr(III) hydroxide[53].
Which (C, R, HQ, and their derivatives) gets more adsorbed by different adsorbents?
Kumar et al.[6] found that C was adsorbed more than R, suggesting that a lesser quantity of AC would be required to remove the same amount of C as compared to R in the individual compounds. The results may differ when both R and C are simultaneously present in solution[6]. Mohamed et al.[42] found that for various carbons, the amount of adsorbate adsorbed follows the order P > HQ > R > C. Mondal and Balomajumder[68] concluded that the efficiency of SAB is more than that of adsorption and the percentage removal of P is more than that of R for both adsorption and SAB because of the increase of specific surface area as well as micropore volume in the SAB system. Kumar et al.[6] concluded that C is adsorbed to a greater extent than R because of the difference in adsorbability which can be explained in terms of the compound's solubility, pH, density, and the presence and position of the hydroxyl group on the aromatic benzene ring[63]. Liao et al.[70] have suggested that the uptake of R and other phenols increased with the increasing number of hydroxyl and its location in meta-position on the aromatic ring, which resulted in the highest adsorptive capacity. The solubility of R in water is higher than that of C[153]; thus, it has more affinity towards water, i.e., hydrophilic. A similar result was shown by Sun et al.[54] on different polymer resins as adsorbents. C adsorption is much higher than those of the other phenolics, and its interaction occurs preferentially through the formation of a catecholate monodentate. R and the cresols interact by means of hydrogen bonds through the hydroxyl group, and their adsorption is much lower than that of C[57]. According to the Lewis acid–base theory, the benzene ring of the aromatic-based resin and the tertiary amino groups on it can be viewed as Lewis bases, while phenolic compounds can be viewed as Lewis acids[134]. Therefore, the Lewis acid–base interaction may occur between phenolic compounds and the benzene ring as well as the tertiary amino group of the resins, which leads to the formation of a hydrogen-bonded complex. In addition, the matching of polarity between adsorbent and adsorbate is also an important factor affecting adsorption of phenolic compounds. The tertiary amino nitrogen on the resins has a large dipole moment, and the dipole moment of C is larger than that of R (2.620 and 2.071 D, respectively[64, 65, 154]); therefore, the interaction between the resins and C is expected to be stronger than that between the resins and R. Furthermore, some researches revealed that the same functional group but at the ortho-position greatly enhances the adsorption energy of C[6]. These may be possible reasons for its lower adsorbability. Kumar et al.[6] have also observed that the same functional group but at the ortho-position greatly enhances the adsorption energies of these compounds, such as C. However, R was adsorbed more compared to C which may refer to the geometry and molecular size of the adsorbate molecule[42]. The changes induced in the texture of the prepared ACs as a result of H2SO4 activation do not seem to have a significant role in the adsorption of phenols (P, C, R, and HQ), although activation with sulfuric acid leads to a continuous increase in both micropore volume and surface area[42].
Kinetics study
The study of the adsorption equilibrium and kinetics is essential to supply the basic information required for the design and operation of adsorption equipment[59]. A mass transfer occurs during the adsorption process; the first step is the solute transfer through the adsorbent external surface film, and the others are the solute fluid diffusion into the pore holes and the adsorbed molecules' migration along the pore surfaces, if it takes place. The former is characterized by the external mass transfer coefficient, and the last ones, by the internal pore and surface diffusivities. Available bulk adsorbate concentration in the liquid phase and adsorbed solute concentration in the solid phase are considered time-dependent.
Various kinetic models, namely pseudo-first-order, pseudo-second-order, and intraparticle diffusion models, have been used to test their validity with the experimental adsorption data for C, R, and HQ onto GAC and other adsorbents. The most commonly used kinetic expressions to explain the solid/liquid adsorption processes are the pseudo-first-order and pseudo-second-order kinetic models[59, 155]. The pseudo-second-order expression as proposed by Ho[156] and Srivastava et al.[59] was found to explain the kinetics of most of sorption systems very well for the entire range of sorption period. Weber and Morris had presented an intraparticle diffusion model in 1962[47, 59, 157]. Recently, the intraparticle diffusion model was reviewed by Wu et al.[158]. Various models are reported for the removal of C, R, and HQ onto various adsorbents[6, 43–45, 52–57, 68, 70, 73–75]. Table5 shows a list of the first- and second-order kinetic constants for the adsorption of C, R, HQ, and their derivatives on various adsorbents.
The first-order kinetic model was fitted for the adsorption of C and R onto different polymers and ACC over a period of 80 and 90 min, respectively, at natural pH[52, 54], and it was found by Sun et al. that the k1 value of R was higher than that of C[54]. Similar results were found by Ayranci and Duman[52] in the rate constants which decreased in the order p-nitrophenol > m-cresol > p-cresol > HQ > P onto ACC. Various researchers have proposed and fitted the first-order kinetic model for the removal of C, R, and other phenols onto different adsorbents[54, 58, 94, 95]. The adsorption and electrosorption of C and R onto ACC follows the pseudo-second-order model for adsorption periods of 1,042 and 1,508 min, and the electrosorption of C was higher than that of R which was attributed partly to the higher dipole moment of the former[69]. The pseudo-second-order model rather than the pseudo-first-order model was fitted by Bayram et al.[69] because of smaller error and the R2 value was close to 1. Namasivayam and Sumithra[53] and Huang et al.[55] have reported an adsorption dynamic curve which followed the second-order rate kinetics onto metal surface and HJ-1 resin.
Isotherm and thermodynamic study
Adsorption equilibrium measurements are used to determine the maximum or ultimate adsorbed capacity. Adsorption equilibrium is established when the amount of solute being adsorbed onto the adsorbent surface is equal to that being desorbed from the surface to the bulk fluid[59, 159]. At this point, the equilibrium solution concentration remains constant. Adsorption equilibrium data are formulated into an isotherm model. Six types of adsorption isotherms exist including types I to VI[59, 160].
The various adsorption isotherms were reviewed[161]. Equilibrium isotherms are measured to determine the capacity of the adsorbent for the adsorbate. The equilibrium of adsorption processes is usually dependent on the amounts adsorbed on the fluid phase composition for a constant temperature[162]. To design and optimize separations using adsorption isotherms, knowledge on the powerful concept adsorption isotherms is mandatory[163–167].
An increase in temperature increases the chemical potential of the organic molecules to penetrate through the surface pores of GAC. Also, the mobility of the adsorbate increases with an increase in temperature. Together, these result in the enhancement in the adsorptive capacity of the GAC at higher temperature. An increase in the phenol adsorption capacity of the carbonaceous adsorbents with an increase in temperature has also been reported by other investigators[59, 168, 169]. The investigators have ascribed different reasons for the endothermic nature of the adsorption of phenolics onto ACs. A rise in adsorption temperature weakens hydrogen bonds formed among water molecules and between water molecules and the solute or the adsorbent[170] and enhances pore diffusion[59, 80, 171]. Therefore, an increase in temperature favors the dehydration of adsorbate molecules, which makes them more planar and gives them a larger dipolar moment. An increase in planarity provides solute molecules a greater access to the pores of the GAC, while an increase in dipolar moment results in enhanced adsorbent-adsorbate interactions. As a result, the adsorption is found to be endothermic because of the endothermicity of dehydration adsorbate molecules. Therefore, the adsorptive uptake increases with an increase in temperature.
Adsorption isotherm modeling
Various isotherm equations like those of Freundlich, Langmuir, Temkin, Dubinin-Radushkevich, and Redlich-Peterson have been used in the literature to describe the equilibrium characteristics of adsorption for the removal of C, R, HQ, and their derivatives onto different adsorbents. Some researchers already explained the theory, assumption, and equation or models for the removal of phenolic compounds[43, 59].
The R2 values alone are not sufficient in determining the best isotherm model to represent the experimental data because they are generally found to be >0.91 for all the four models. Table5 lists the Freundlich, Langmuir, and other constants for the adsorption of C, R, HQ, and their derivatives on various adsorbents.
The most important parameter to compare in Table5 is the qmax value of the Langmuir model because this measures the adsorption capacity of AC and others for the adsorbates. By comparing the results for the adsorption capacity of AC for C, R, or HQ, it is seen that the qmax value is clearly greater for C at higher temperature (45°C), whereas it is greater for R at lower temperatures (15°C and 30°C).
The adsorption capacity as measured by KF of the Freundlich model was best fitted for R and C onto GAC as compared to other models[6, 69]. The Freundlich model is more suitable for characterizing adsorption than the Langmuir model[55], indicating that the adsorption may be a multilayer process and the adsorbent possesses a heterogeneous nature[119]. However, the adsorption was not a pure monolayer type, and the mixed model was expected for the isotherms of R and HQ as reported by Liao et al.[70].
There were higher adsorption constant (KL) and average adsorption energy for the adsorption of C than those of R and other phenolic compounds onto Degussa P-25 TiO2. The Langmuir model was best fitted for the adsorption of C and other phenolic compounds having KL which was much larger than those of P and R. However, the Langmuir equation is valid up to a concentration limit, which is a characteristic of each phenolic compound[53, 57, 172].
The adsorption isotherms of C, R, HQ, and pyrogallol have been well fitted by both the Freundlich and Langmuir models[58, 70], which means that physical adsorption occurred[173] and the adsorbed amount of phenolic derivatives follows the order R >> C onto MWCNTs[70]. Lu et al.[102] indicated that the adsorption capacity of AC was closely related with the molecular weight and boiling point of the compound. The difference in boiling point of the three phenolic derivatives (HQ, 285.2°C > R, 281°C > C, 245°C) might be the reason for the lowest adsorbability of C. However, the uptake of R is the highest, which might be due to other complex factors and need further exploration. The adsorption capacity of MWCNTs was more than that of the AC treated by Mohamed et al.[42], even though the specific surface area of AC (SSA = 210 m2/g) was much larger than that of MWCNTs (SSA = 72 m2/g) in our experiments.
Adsorption isotherms of pyrogallol, C, and P show that the effect of -OH substitution on sorption was not proportional to the number of -OH groups. The higher KD ratios of pyrogallol/C than C/P at high equilibrium concentrations were due to the possible greater hydrogen-bonding interaction between the surface-adsorbed pyrogallol and the ones dissolved in water; the hydrogen bonding interaction may not be significant between the surface-adsorbed and dissolved C or P molecules due to fewer -OH groups on the molecules. Multilayer sorption of pyrogallol, but not C and P, on the CNTs could be the evidence for the sorption mechanism difference between these phenolics at high concentrations[75].
Adsorption capacity of C was higher than that of R[52, 55, 69] due to its smaller molecular size. The value of ΔH for C was more negative than that for R which can be explained in terms of the solubility and the polarity of the two adsorbates[55]. However, the adsorption capacity decreases with increase in temperature[54]. The Langmuir isotherm was best fitted for C, and the Freundlich isotherm, for the other compounds[43]. The adsorption behavior of phenolic compounds on the same kind of AC was also studied[42], and it was also concluded that the adsorption capacities are of the same order of magnitude, but the other parameters appear to be strongly dependent upon the chemical groups around the aromatic ring. However, acidic phenols exhibit a more progressive isotherm than the non-acidic species[42, 43].
Adsorption thermodynamics
The Gibbs free energy change (ΔG0) of the adsorption process is related to the equilibrium constant (KD) by the classical Van't Hoff equation and also related to the entropy change (ΔS0) and heat of adsorption (ΔH0) at constant temperature. The equilibrium adsorption constant (KD) can be related to as follows:
where T is the absolute temperature (K), R is the universal gas constant (8.314 × 10−3 kJ/mol K), and KD (=qe/Ce) is the single point or linear sorption distribution coefficient. Thus, ΔH0, which is the enthalpy change (kJ/mol), can be determined from the slope of the linear Van't Hoff plot, i.e., ln KD versus (1/T). This ΔH0 corresponds to the isosteric heat of adsorption (ΔHst,0) with zero surface coverage (i.e., qe = 0)[174]. KD at qe = 0 can be obtained from the intercept of the ln qe/Ce versus qe plot[175]. The adsorption ΔH0, ΔS0, and ΔG0 corresponding to different percentages of the adsorbent surface, adsorption free energies, and adsorption entropies are reviewed in Table6.
Generally, the ΔG0 value is in the range of −20 to 0 kJ/mol for physisorption and in the range of −400 to −80 kJ/mol for chemisorption[176]. ΔG0 values were negative, indicating that the adsorption process led to a decrease in ΔG0 and that the adsorption process is feasible and spontaneous[59, 177]. The ΔG0 values achieved at four different temperatures are negative, indicating a spontaneous physisorption process[58]. The ΔH0 of the adsorption of organic molecules from an aqueous solution onto AC is usually within the range of 8 to 65 kJ/mol[178]. The positive values of ΔH0 indicate the endothermic nature of the adsorption process. The ΔH0 value decreases with increase in the percentage of the adsorbent surface which resulted from the energetic heterogeneity of the adsorbent surface[119]. In physisorption, the bond between adsorbent and adsorbate is the van der Waals interaction, and ΔH0 is typically in the range of 5 to 10 kJ/mol for liquid-phase adsorption. In the case of chemisorption, a chemical bond is formed between adsorbate molecules and the surface, and the chemisorption energy is, generally, in the range of 30 to 70 kJ/mol[179]. The value of ΔH0 of C was little more negative than that of R at the same percentage of the adsorbent surface[55]. The initial adsorption enthalpy of C is greater than that of R, displaying that the interaction of the adsorbent with C is a little stronger[54]. The negative adsorption free energies imply a favorable and spontaneous process, and the adsorption free energy is independent on the occupancy percentage of the adsorbent surface. The ΔH0 indicates exothermic adsorption, and the positive value of ΔS0, indicates increased randomness at the solid/solution interface with some structural changes in the adsorbates and the adsorbents[57]. The positive ΔS0 value also corresponds to an increase in the degree of freedom of the adsorbed species[180]. The negative value of ΔS0 reveals that a more ordered arrangement of the adsorbates is shaped on the adsorbent surface and also a weaker activity of adsorbate molecules on the adsorbents than on the aqueous solution[54].
Conclusions
Chemical contamination of water from a wide range of toxic compounds, in particular aromatic molecules, is a serious environmental problem owing to their potential human toxicity especially C, R, HQ, and their derivatives which appear to be the major organic pollutants globally. They, derived from industrial effluent discharges, present an ongoing and serious threat to human health and to natural water. Most of the researchers concluded that there is higher adsorbability of C than R and HQ because of the effects of solubility and the position of the (−OH) functional group at the ortho-position.
The role of AC and other adsorbents in the removal of C, R, HQ, and their derivatives from water and wastewater was discussed. However, it is only able to remove few milligrams of phenols per gram of AC, and there are still some problems encountered in the regeneration process. This makes AC an expensive adsorbent for this purpose. The solid waste can be converted into low-cost adsorbents for the treatment of discharged wastewater; the cost of removal might decrease. Although the organoclays revealed good adsorption capability, they were still non-economic. The ability and efficiency of the adsorption technologies in water treatment depend on the characteristics and functions of adsorbents. Therefore, to transfer the pollutants in promoting the adsorption rate, we can design and prepare some special composite adsorbents with good adsorptive functions.
Author’s information
Dr. SS obtained his Ph.D. in 2010 from the Indian Institute of Technology Roorkee. Currently, he is an assistant professor in the Department of Chemical Engineering, Maulana Azad National Institute of Technology Bhopal. He has more than 20 papers in peer-reviewed journals. His research interests include reactor design, biochemical engineering, and energy and environmental engineering. He received a Young Scientist Award from the Uttarakhand State Council for Science and Technology, Government of Uttarakhand, India, and a Visiting Scientist fellowship from the Jawaharlal Nehru Centre for Advanced Scientific Research, International Centre for Materials Science, Bangalore, India, and was associated with Prof. CNR Rao. Dr. VCS obtained his Ph.D. in 2006 from the Indian Institute of Technology Roorkee. Currently, he is an assistant professor in the Department of Chemical Engineering, Indian Institute of Technology Roorkee. He has published more than 45 papers in peer-reviewed journals and more than 40 papers in conferences/seminars. His major research interests are separation processes, physicochemical treatment of industrial wastes, multicomponent adsorption, electrochemical treatment, etc. He was awarded with the ProSPER.Net-Scopus Young Researcher Award 2010 - First Runner-up Prize for research work on ‘Treatment of industrial wastes’ and the ‘INSA Young Scientist Medal 2012’ by the Indian National Science Academy in the Engineering Science category. He has more than 900 citations of research papers with an h-index of 15. Prof. IMM obtained his Ph.D. (Chemical Engineering) in 1977 from BHU, India, and finished his postdoctoral research at the University of Hannover, Germany, from 1980 to 1982. Currently, he is the Dean of the Indian Institute of Technology Roorkee, Saharanpur Campus. He served as the Head of the Department of Chemical Engineering for two terms (1991 to 1994 and 1999 to 2002). He has published more than 110 papers in peer-reviewed journals and more than 150 papers in conferences/seminars. His research interests include transport phenomenon, biochemical engineering, environmental engineering, and biomass energy engineering. He has received several awards to his credit and more than 1,150 citations to his research papers with an h-index of 17.
References
Korbahti BK, Tanyolac A: Continuous electrochemical treatment of phenolic wastewater in a tubular reactor. Water Res. 2003, 37: 1505-1514. 10.1016/S0043-1354(02)00523-7
Dellinger B, Pryor WA, Cueto R, Squadrito GL, Hegde V, Deutsch WA: Role of free radicals in the toxicity of airborne fine particulate matter. Chem. Res. Toxicol. 2001, 14: 1371-1377. 10.1021/tx010050x
Merck: The Merck Index. 11th edition. Merk, Rahway; 1989.
Milligan PW, Haggblom MM: Biodegradation of resorcinol and catechol by denitrifying enrichment cultures. Environ. Toxicol. Chem. 1998, 17: 1456-1461. 10.1002/etc.5620170804
Hays MD, Fine PM, Geron CD, Kleeman MJ, Gullett BK: Open burning of agricultural biomass: physical and chemical properties of particle-phase emissions. Atmos. Environ. 2005, 39: 6747-6764. 10.1016/j.atmosenv.2005.07.072
Kumar A, Kumar S, Kumar S: Adsorption of resorcinol and catechol on activated carbon: equilibrium and kinetics. Carbon 2003, 41: 3015-3025. 10.1016/S0008-6223(03)00431-7
Schweigert N, Zehnder AJB, Eggen RIL: Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ. Microbiol. 2001, 3: 81-91. 10.1046/j.1462-2920.2001.00176.x
Phutdhawong W, Chowwanapoonpohn S, Buddhasukh D: Electro coagulation and subsequent recovery of phenolic compounds. Anal. Sci. 2000, 16: 1083-1084. 10.2116/analsci.16.1083
Fiegel H, Voges HW, Hamamoto T, Umemura S, Iwata T, Miki H, Fujita Y, Buysch HJ, Garbe D, Paulus W: Phenol Derivatives in Ullmann’s Encyclopedia of Industrial Chemistry. Wiley, New York; 2002.
Meyer J: Article for resorcinol: 10% hydrochloric acid on 1,3-diaminobenzene. Ber. 1897, 30: 2569.
Raff R, Ettling BV: Hydroquinone, resorcinol and pyrocatechol. In Encyclopedia of Chemical Technology. 2nd edition. Edited by: Kirk RE, Othmer DF. Wiley, New York; 1966:462-492.
IARC: Some fumigants, the herbicides 2,4-D and 2,4,5-T, chlorinated dibenzodioxins and miscellaneous industrial chemicals. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. IARC, Lyon; 1977:155-l75.
Zheng LT, Ryu GM, Kwon BM, Lee WH, Suk K: Anti-inflammatory effects of catechols in lipopolysaccharide-stimulated microglia cells: inhibition of microglial neurotoxicity. Eur. J. Pharmacol. 2008, 588: 106-113. 10.1016/j.ejphar.2008.04.035
Smale BC, Keil HL: A biochemical study of the intervarietal resistance of Pyrus communis to fire blight. Phytochem. 1966, 5: 1113-1120. 10.1016/S0031-9422(00)86104-X
Ahtardjieff C: Uber das Vorkommen von Arbutin und Gerbstoffen in einheimischen Vertretem der Familie der Ericaceen. Pharmazie. 1966, 21: 59-60.
Sun YG, Cui H, Li YH, Lin X: Determination of some catechol derivatives by a flow injection electrochemiluminescent inhibition method. Talanta 2000, 53: 661-666. 10.1016/S0039-9140(00)00550-6
Wynder EL, Hoffmann D: Certain constituents of tobacco products: phenolic compounds and carboxylic acids. In Tobacco and Tobacco Smoke. Academic, New York; 1967:381-410.
Izard C, Lacharpagne J, Testa P: Sur l’activite biologique de divers condensats de fumee de cigarettes, lee par les tests auxiniques. C.R. Acad. Sci. Paris Ser D 1966, 262: 1859-1861.
Lazar P, Izard C, Morse-testa P, Chouroulinkov I: Interaction entre l’hydroquinone et le condensat de fumee de cigarette dans les tests cutanes i court terme du pouvoir carcinogene. C.R. Acad. Sci. Paris Ser D 1972, 274: 496-499.
Dagon TJ: Biological treatment of photo processing effluents. J. Water Poll. Control Fed. 1973, 45: 2123-2135.
Umpelev VL, Kogan LA, Gagarinova LM: Thin-layer chromatographic separation of polyhydric phenols in effluents. Zh. Anal. Khim. 1974, 29: 179-180.
Jacquemain R, Remy F, Guinchard C: Etudes et comparaisons des determinations des phenols dans les eaux; application a l’examen dun rejet de papeterie. J. Fr. Hydrol. 1975, 16: 25-31.
Fritz H, Reineke W, Schmidt E: Toxicity of chlorobenzene on Pseudomonas sp. strain RHO1, a chlorobenzene-degrading strain. Biodegradation 1992, 2: 165-170. 10.1007/BF00124490
Capasso R, Evidente A, Schivo L, Orru G, Marcialis MA, Cristinzio G: Antibacterial polyphenols from olive oil mill waste waters. J. Appl. Bacteriol. 1995, 79: 393-398. 10.1111/j.1365-2672.1995.tb03153.x
Boyd GE, Adamson AW, Meyers LS: The exchange adsorption of ions from aqueous solution by organic zeolites. II Kinetics. J. Am. Chem. Soc. 1947, 69: 2836-2848.
Rahouti M, Steiman R, Seigle-Murandi F, Chritov LP: Growth of 1044 strains and species of fungi on 7 phenolic lignin model compounds. Chemosphere 1999, 38: 2549-2559. 10.1016/S0045-6535(98)00462-7
Prager JC: Environmental Contaminant Reference Data Book. Van Nostrand Reinhold, New York; 1997.
Van Duursen MBM, Sanderson JT, de Jong PC, Kraaij M, van den Berg M: Phytochemicals inhibit catechol-o-methyltransferase activity in cytosolic fractions from healthy human mammary tissues: implications for catechol estrogen-induced DNA damage. Toxicol. Sci. 2004, 81: 316-324. 10.1093/toxsci/kfh216
Irons RD, Sawahata R: Phenols, catechols, and quinones. In Bioactivation of Foreign Compounds. Edited by: Anders MW. Academic, San Diego; 1985:259-279.
Cavalieri EL, Li K-M, Balu N, Saeed M, Devanesan P, Higginbotham S, Zhao J, Gross ML, Rogan EG: Catechol ortho-quinones: the electrophilic compounds that form depurinating DNA adducts and could initiate cancer and other diseases. Carcinogenesis 2002, 23: 1071-1077. 10.1093/carcin/23.6.1071
Bukowska B, Kowalaska S: Phenol and catechol induce prehemolytic and hemolytic changes in human erythrocytes. Toxicol. Lett. 2004, 152: 73-84. 10.1016/j.toxlet.2004.03.025
Moreno-Castilla C: Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon 2004, 42: 83-94. 10.1016/j.carbon.2003.09.022
Kumar A, Kumar S, Kumar S: Biodegradation kinetics of phenol and resorcinol using Pseudomonas putida MTCC 1194. Biochem. Eng. J. 2005, 22: 151-159. 10.1016/j.bej.2004.09.006
Stoilova I, Krastanov A, Stanchev V, Daniel D, Gerginova M, Alexieva Z: Biodegradation of high amounts of phenol, resorcinol, 2,4-dichlorophenol and 2,6-dimethoxyphenol by Aspergillus awamori cells. Enzyme Microb. Tech. 2006, 39: 1036-1041. 10.1016/j.enzmictec.2006.02.006
Latkar M, Swaminathan K, Chakrabarti T: Kinetics of anaerobic biodegradation of resorcinol and hydroquinone in upflow fixed film–fixed bed reactors. Bioresour. Technol. 2003, 88: 69-74. 10.1016/S0960-8524(02)00261-4
Subramanyam R, Mishra IM: Biodegradation of catechol (2-hydroxy phenol) bearing wastewater in an UASB reactor. Chemosphere 2007, 69: 816-824. 10.1016/j.chemosphere.2007.04.064
Subramanyam R, Mishra IM: Co-degradation of resorcinol and catechol in an UASB reactor. Bioresour. Technol. 2008, 99: 4147-4157. 10.1016/j.biortech.2007.08.060
Nasr B, Abdellatif G, Canizares P, Saez C, Lobato J, Rodrigo MA: Electrochemical oxidation of hydroquinone, resorcinol, and catechol on boron doped diamond anodes. Environ. Sci. Technol. 2005, 39: 7234-7239. 10.1021/es0500660
Chien SWC, Chen HL, Wang MC, Seshaiah K: Oxidative degradation and associated mineralization of R, hydroquinone and resorcinol catalyzed by birnessite. Chemosphere 2009, 74: 1125-1133. 10.1016/j.chemosphere.2008.10.007
Arana J, Rodriguez CF, Diaz OG, Melian JAH, Pena JP: Role of Cu in the Cu–TiO2 photocatalytic degradation of dihydroxybenzenes. Catal. Today 2005, 101: 261-266. 10.1016/j.cattod.2005.03.006
Ahn MY, Martinez CE, Archibald DD, Zimmerman AR, Bollag J-M, Dec J: Transformation of resorcinol in the presence of a laccase and birnessite. Soil Biol. Biochem. 2006, 38: 1015-1020. 10.1016/j.soilbio.2005.08.016
Mohamed FS, Khater WA, Mostafa MR: Characterization and phenols sorptive properties of carbons activated by sulphuric acid. Chem. Eng. J. 2006, 116: 47-52.
Richard D, Delgado ML, Schweich D: Adsorption of complex phenolic compounds on activated charcoal: adsorption capacity and isotherms. Chem. Eng. J. 2009, 148: 1-7. 10.1016/j.cej.2008.07.023
Blanco-Martinez DA, Giraldo L, Moreno-Pirajan JC: Effect of the pH in the adsorption and in the immersion enthalpy of monohydroxylated phenols from aqueous solutions on activated carbons. J. Hazard. Mater. 2009, 169: 291-296. 10.1016/j.jhazmat.2009.03.099
Richard D, Delgado ML, Schweich D: Adsorption of complex phenolic compounds on active charcoal: breakthrough curves. Chem. Eng. J. 2010, 158: 213-219. 10.1016/j.cej.2009.12.044
Suresh S, Srivastava VC, Mishra IM: Isotherm, thermodynamics, desorption and disposal study for the adsorption of catechol and resorcinol onto granular activated carbon. J. Chem. Eng. Data 2011, 56: 811-818. 10.1021/je100303x
Suresh S, Srivastava VC, Mishra IM: Study of catechol and resorcinol adsorption mechanism through granular activated carbon characterization, pH and kinetic study. Sep. Sci. Technol. 2011, 46: 1750-1766. 10.1080/01496395.2011.570284
Suresh S, Srivastava VC, Mishra IM: Adsorption of hydroquinone in aqueous solution by granular activated carbon. J. Environ. Eng. 2011, 137: 1145-1157. 10.1061/(ASCE)EE.1943-7870.0000443
Suresh S, Srivastava VC, Mishra IM: Adsorptive removal of aniline-phenol and 4-nitrophenol-phenol binaries from aqueous solution by granular activated carbon. Chem. Eng. J. 2011, 171: 997-1003. 10.1016/j.cej.2011.04.050
Suresh S, Srivastava VC, Mishra IM: Adsorptive removal of aniline by granular activated carbon from binary solutions with catechol and resorcinol. Environ. Technol. 2012, 33: 773-781. 10.1080/09593330.2011.592228
Yildiz N, Gonulsen R, Koyuncu H, Calimli A: Adsorption of benzoic acid and hydroquinone by organically modified bentonites. Colloid. Surf. A: Physicochem. Eng. Aspects 2005, 260: 87-94. 10.1016/j.colsurfa.2005.03.006
Ayranci E, Duman O: Adsorption behaviors of some phenolic compounds onto high specific area AC cloth. J. Hazard. Mater. B 2005, 124: 125-132. 10.1016/j.jhazmat.2005.04.020
Namasivayam C, Sumithra S: Adsorptive removal of catechol on waste Fe(III)/Cr(III) hydroxide: equilibrium and kinetics study. Ind. Eng. Chem. Res. 2004, 43: 7581-7587. 10.1021/ie0496636
Sun Y, Chen J, Li A, Liu F, Zhang Q: Adsorption of resorcinol and catechol from aqueous solution by aminated hypercrosslinked polymers. React. Funct. Polym. 2005, 64: 63-73. 10.1016/j.reactfunctpolym.2005.03.004
Huang J, Huang K, Yan C: Application of an easily water-compatible hyper crosslinked polymeric adsorbent for efficient removal of catechol and resorcinol in aqueous solution. J. Hazard. Mater. 2009, 167: 69-74. 10.1016/j.jhazmat.2008.12.120
Vasudevan D, Stone TA: Adsorption of catechols, 2-aminophenols, and 1,2-phenylenediamines at the metal (hydr) oxide/water interface: effect of ring substituents on the adsorption onto TiO2. Environ. Sci. Technol. 1996, 30: 1604-1613. 10.1021/es950615+
Arana J, Melian EP, Lopez VMR, Alonso AP, Rodriguez JMD, Diaz OG, Pena JP: Photocatalytic degradation of phenol and phenolic compounds Part I. Adsorption and FTIR study. J. Hazard. Mater. 2007, 146: 520-528. 10.1016/j.jhazmat.2007.04.066
Shakir K, Ghoneimy HF, Elkafrawy AF, Beheir SG, Refaat M: Removal of catechol from aqueous solutions by adsorption onto organophilic-bentonite. J. Hazard. Mater. 2008, 150: 765-773. 10.1016/j.jhazmat.2007.05.037
Srivastava VC, Swamy MM, Mall ID, Prasad B, Mishra IM: Adsorptive removal of phenol by bagasse fly ash and activated carbon: equilibrium, kinetics and thermodynamics. Colloid. Surf. A: Physicochem. Eng. Aspects 2006, 272: 89-104. 10.1016/j.colsurfa.2005.07.016
Suresh S, Vijayalakshmi G, Rajmohan B, Subbaramaiah V: Adsorption of benzene vapor onto activated biomass from cashew nut shell: batch and column study. Recent Patents Chem. Eng. 2012,5(2):116-133. 10.2174/2211334711205020116
Lewis RJ Sr: Sax’s Dangerous Properties of Industrial Materials (11th edn). 728th edition. Wiley, Hoboken; 2004.
Yang K, Wu W, Jing Q, Zhu L: Aqueous adsorption of aniline, phenol, and their substitutes by multi-walled carbon nanotubes. Environ. Sci. Technol. 2008, 42: 7931-7936. 10.1021/es801463v
International Programme on Chemical Safety (IPCS): International Chemical Safety Cards. (1994). Accessed 04 July 1997 http://www.cdc.gov/niosh/ipcsneng/neng0411.html
Yogesh R, Gupta S, Javiya S, Paul P, Basu S, Singh K, Ganguly B, Bhattacharya A: Studies of performances by the interchanging of the sequence of the photomodified layer in the thin film composite (TFC) membrane. J. Appl. Polymer Sci. 2008, 108: 2611-2616. 10.1002/app.27924
Kamlet MJ, Doherty RM, Abraham MH, Marcus Y, Taft RW: Linear solvation energy relationships. 46. An improved equation for correlation and prediction of octanol-water partition coefficients of organic nonelectrolytes (including strong hydrogen bond donor solutes). J. Phys. Chem 1988, 92: 5244-5255. 10.1021/j100329a035
Rajkumar D, Palanivelu K, Balasubramanian N: Combined electrochemical degradation and activated carbon adsorption treatments for wastewater containing mixed phenolic compounds. J. Environ. Eng. Sci. 2005, 4: 1-9.
Rodriguez E, Encinas A, Masa FJ, Beltran FJ: Influence of resorcinol chemical oxidation on the removal of resulting organic carbon by activated carbon adsorption. Chemosphere 2008, 70: 1366-1374. 10.1016/j.chemosphere.2007.09.035
Mondal P, Balomajumder C: Treatment of resorcinol and phenol bearing wastewater by simultaneous adsorption biodegradation (SAB): optimization of process parameters. Int. J. Chem. React. Eng. 2007, 5: S1.
Bayram E, Hoda N, Ayranci E: Adsorption/electrosorption of catechol and resorcinol onto high area activated carbon cloth. J. Hazard. Mater. 2009, 168: 1459-1466. 10.1016/j.jhazmat.2009.03.039
Liao Q, Sun J, Gao L: Adsorption of chlorophenols by multiwalled carbon nanotubes treated with HNO3 and NH3. Carbon 2008, 46: 553-555. 10.1016/j.carbon.2007.12.009
ISI Web of Science Database
Derbyshire F, Jagtoyen M, Andrews R, Rao A, Martin-Gullon I, Grulke EA: Carbon materials in environmental applications. In Chemistry and Physics of Carbon. Edited by: Radovic LK. Dekker, New York; 2001.
Figueiredo JL, Pereira MFR, Freitas MMA, Orfao JJM: Modification of the surface chemistry of activated carbons. Carbon 1999, 37: 1379-1389. 10.1016/S0008-6223(98)00333-9
Suresh S, Srivastava VC, Mishra IM: Studies of adsorption kinetics and regeneration of Aniline, Phenol, 4-Chlorophenol and 4-Nitrophenol by Activated Carbon. Chem Ind. Chem. Eng. Q. 2012. 10.2298/CICEQ111225054S
Lin D, Xing B: Adsorption of phenolic compounds by carbon nanotubes: role of aromaticity and substitution of hydroxyl groups. Environ. Sci. Technol. 2008, 42: 7254-7259. 10.1021/es801297u
Lin S-H, Juang R-S: Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: a review. J. Environ. Manage. 2009, 90: 1336-1349. 10.1016/j.jenvman.2008.09.003
Dabrowski A: Adsorption—from theory to practice. Adv. Colloid Interface Sci. 2001, 93: 135-224. 10.1016/S0001-8686(00)00082-8
Dobbs RA, Cohen JM: Carbon Adsorption Isotherm for Toxic Organics, Tech. Re EPA-600/8-80-023. Environment Protection Agency, Cincinnati; 1980.
Ozkaya B: Adsorption and desorption of phenol on activated carbon and a comparison of isotherm models. J. Hazard. Mater. B 2006, 129: 158-163. 10.1016/j.jhazmat.2005.08.025
Garcia-Araya J, Beltrn De Heredia J, Alvarez P, Masa FJ: Activated carbon adsorption of some phenolic compounds present in agroindustrial wastewater. Adsorption 2003, 9: 107-115. 10.1023/A:1024228708675
Franz M, Arafat HA, Pinto NG (2000) Effect of chemical surface heterogeneity on the adsorption mechanism of dissolved aromatics on activated carbon. Carbon 38, 1807–1819.
Haghseresht F, Nouri S, Lu GQ: Effects of the solute ionization on the adsorption of aromatic compounds from dilute aqueous solutions by activated carbon. Langmuir 2002, 18: 1574-1579. 10.1021/la010903l
Streat M, Patrick JW, Camporro Perez MJ: Sorption of phenol and parachlorophenol from water using conventional and novel ACs. Water Res 1995, 29: 467-472. 10.1016/0043-1354(94)00187-C
Zhang T, Walawender WP, Fan LT, Fan M, Daugaard D, Brown RC: Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem. Eng. J. 2004, 105: 53-59. 10.1016/j.cej.2004.06.011
Rodriguez-Reinoso F: Controlled Gasification of Carbon and Pore Structure Development. Kluwer, Dordrecht; 1991.
Rodriguez-Reinoso F, Molina-Sabio M, Gonzalez MT: The use of steam and CO2 as activating agents in the preparation of activated carbons. Carbon 1995, 33: 15-23. 10.1016/0008-6223(94)00100-E
Tseng RL, Tseng SK, Wu FC: Preparation of high surface area carbons from corncob with KOH etching plus CO2 gasification for the adsorption of dyes and phenols from water. Colloid. Surf. A: Physicochem. Eng. Aspects 2006, 279: 69-78. 10.1016/j.colsurfa.2005.12.042
Hameed BH, Ahmad AL, Latiff KNA: Adsorption of basic dye (methylene blue) onto AC prepared from rattan sawdust. Dyes Pigments 2007, 75: 143-149. 10.1016/j.dyepig.2006.05.039
Johns MM, Toles CA, Marshall WE: ACs from low-density agricultural waste. US Patent March 2003, 834051: 24.
Karagozoglu B, Tasdemir M, Demirbas E, Kobya M: The adsorption of basic dye (Astrazon Blue FGRL) from aqueous solutions onto sepiolite, fly ash and apricot shell AC: kinetic and equilibrium studies. J. Hazard. Mater. 2007, 147: 297-306. 10.1016/j.jhazmat.2007.01.003
Kumar S, Varadarajan PR, Porkodi K, Subbhuraam CV: Adsorption of methylene blue onto jute fiber carbon: kinetics and equilibrium studies. J. Colloid Interface Sci. 2005, 284: 78-82. 10.1016/j.jcis.2004.09.027
Kalavathy MH, Karthikeyan T, Rajgopal S, Miranda LR: Kinetic and isotherm studies of Cu(II) adsorption onto H3PO4-activated rubber wood sawdust. J. Colloid Interface Sci. 2005, 292: 354-362. 10.1016/j.jcis.2005.05.087
Tans IAW, Hameed BH, Ahmad AL: Equilibrium and kinetic studies on basic dye adsorption by oil palm fibre AC. Chem. Eng. J. 2007, 127: 111-119. 10.1016/j.cej.2006.09.010
Ayranci E, Conway BE: Adsorption and electrosorption at high-area carbon felt electrodes for waste-water purification: systems evaluation with inorganic S-containing anion. J. Appl. Electrochem. 2001, 31: 257-266. 10.1023/A:1017528002713
Niu J, Conway BE: Development of techniques for purification of wastewaters: removal of pyridine from aqueous solution by adsorption at high area AC-cloth electrodes using in situ optical spectrometry. J. Electroanal. Chem. 2002, 521: 16-28. 10.1016/S0022-0728(02)00660-5
Han Y, Quan X, Chen S, Zhao H, Cui C, Zhao Y: Electrochemically enhanced adsorption of aniline on activated carbon fibers. Sep. Purif. Technol. 2006, 50: 365-372. 10.1016/j.seppur.2005.12.011
Han Y, Quan X, Ruan X, Zhang W: Integrated electrochemically enhanced adsorption with electrochemical regeneration for removal of acid orange 7 using activated carbon fibers. Sep. Purif. Technol. 2008, 59: 43-49. 10.1016/j.seppur.2007.05.026
Baughman RH, de Zakhidov AA, Heer WA: Carbon nanotubes-the route toward applications. Science 2002, 297: 787. 10.1126/science.1060928
Dillon AC, Jones KM, Bekkedahl TA, Kiang CH, Bethune DS, Heben MJ: Storage of hydrogen in single-walled carbon nanotubes. Nature 1997, 386: 377-379. 10.1038/386377a0
Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, Cho K, Dai H: Nanotube molecular wires as chemical sensors. Science 2000, 287: 622-625. 10.1126/science.287.5453.622
Cinke M, Li J, Bauschlicher CW Jr, Ricca A, Meyyappan M: CO2 adsorption in single-walled carbon nanotubes. Chem. Phys. Lett. 2003, 376: 761-766. 10.1016/S0009-2614(03)01124-2
Lu C, Chiu H, Liu C: Removal of zinc(II) from aqueous solution by purified carbon nanotubes: kinetics and equilibrium studies. Ind. Eng. Chem. Res. 2006, 45: 2850-2855. 10.1021/ie051206h
Wang HJ, Zhou AL, Peng F, Yu H, Chen LF: Adsorption characteristic of acidified carbon nanotubes for heavy metal Pb(II) in aqueous solution. Mater. Sci. Eng. A 2007, 466: 201-206. 10.1016/j.msea.2007.02.097
Lu C, Chung Y-L, Chang K-F: Adsorption of trihalomethanes from water with carbon nanotubes. Water Res. 2005, 39: 1183-1189. 10.1016/j.watres.2004.12.033
Long RQ, Yang RT: Carbon nanotubes as superior sorbent for dioxin removal. J. Am. Chem. Soc. 2001, 123: 2058-2059. 10.1021/ja003830l
Cai YQ, Jiang GB, Liu JF, Zhou QX: Multiwalled carbon nanotubes as a solid-phase extraction adsorbent for the determination of bisphenol A, 4-n-nonylphenol, and 4-tert-octylphenol. Anal. Chem. 2003, 75: 2517-2521. 10.1021/ac0263566
Fagan SB, Souza Filho AG, Lima JOG, Mendes Filho J, Ferreira OP, Mazail IO, Alves OL, Dresselhaus MS: 1,2-Dichlorobenzene interacting with carbon nanotubes. Nano. Lett 2004, 4: 1285. 10.1021/nl0493895
Efremenko I, Sheintuch M: Enthalpy and entropy effects in hydrogen adsorption on carbon nanotubes. Langmuir 2005, 21: 6282-6288. 10.1021/la046757b
Hilding JM, Grulke EA: Heat of adsorption of butane on multiwalled carbon nanotubes. J. Phys. Chem. B 2004, 108: 13688-13695. 10.1021/jp036387k
Peng XJ, Luan ZK, Di ZC, Zhang ZG, Zhu CL: Carbon nanotubes iron oxides magnetic composites as adsorbent for removal of Pb(II) and Cu(II) from water. Carbon 2005, 43: 880-883. 10.1016/j.carbon.2004.11.009
Coleman JN, Khan U, Gunko YK: Mechanical reinforcement of polymers using carbon nanotubes. Adv. Mater. 2006, 18: 689-706. 10.1002/adma.200501851
Masciangioli T, Zhang WX: Environmental technologies at the nanoscale. Environ. Sci. Technol. 2003, 37: 102A-108A. 10.1021/es0323998
Helland A, Wick P, Koehler A, Schmid K, Som C: Reviewing the environmental and human health knowledge base of carbon nanotubes. Environ. Health Perspect. 2007, 115: 1125-1131. 10.1289/ehp.9652
Nowack B, Bucheli TD: Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150: 5-22. 10.1016/j.envpol.2007.06.006
Yang K, Zhu LZ, Xing BS: Adsorption of polycyclic aromatic hydrocarbons by carbon nanomaterials. Environ. Sci. Technol. 2006, 40: 1855-1861. 10.1021/es052208w
Yang K, Xing B: Desorption of polycyclic aromatic hydrocarbons from carbon nanomaterials in water. Environ. Pollut. 2007, 145: 529-537. 10.1016/j.envpol.2006.04.020
Peng X, Li Y, Luan Z, Di Z, Wang H, Tian B, Jia Z: Adsorption of 1,2-dichlorobenzene from water to carbon nanotubes. Chem. Phys. Lett. 2003, 376: 154-158. 10.1016/S0009-2614(03)00960-6
Daifullah AAM, Girgis BS: Removal of some substituted phenols by activated carbon obtained from agricultural waste. Water Res. 1998, 32: 1169-1177. 10.1016/S0043-1354(97)00310-2
Li HT, Xu MC, Shi ZQ, He BL: Isotherm analysis of phenol adsorption on polymeric adsorbents from nonaqueous solutions. J. Colloid Interface Sci. 2004, 271: 47-54. 10.1016/j.jcis.2003.10.026
Zhu DQ, Pignatello JJ: Characterization of aromatic compound sorptive interactions with black carbon (charcoal) assisted by graphite as a model. Environ. Sci. Technol. 2005, 39: 2033-2041. 10.1021/es0491376
Chen W, Duan L, Zhu DQ: Adsorption of polar and non-polar organic chemicals to carbon nanotubes. Environ. Sci. Technol. 2007, 41: 8295-8300. 10.1021/es071230h
He BL, Huang WQ: Ion Exchange and Adsorption Resins. The Science and Education, Shanghai; 1992.
Davankov VA, Tsyurupa MP: Structure and properties of porous hypercrosslinked polystyrene sorbents Styrosorb. Pure Appl. Chem. 1989, 61: 1881. 10.1351/pac198961111881
Davankov VA, Tsyurupa MP: Structure and properties of hypercrosslinked polystyrene—the first representative of a new class of polymer networks. React. Polym. 1990, 13: 27-42. 10.1016/0923-1137(90)90038-6
Leon-Gonzalez ME, Perez-Arribas LV: Chemically modified polymeric sorbents for sample preconcentration. J. Chromatogr. A. 2003, 902: 3-16.
Li AM, Zhang QX, Chen JL: Adsorption of phenolic compounds on Amberlite XAD-4 and its acetylated derivative MX-4. React. Funct. Polym. 2001, 49: 225-233. 10.1016/S1381-5148(01)00080-3
Pan BC, Xiong Y, Su Q, Li AM, Chen JL, Zhang QX: Role of amination of a polymeric adsorbent on phenol adsorption from aqueous solution. Chemosphere 2003, 51: 953. 10.1016/S0045-6535(03)00038-9
Cai JG, Li AM, Shi HY: Equilibrium and kinetic studies on the adsorption of aniline compounds from aqueous phase onto bifunctional polymeric adsorbent with sulfuric groups. Chemosphere 2005, 61: 502-509. 10.1016/j.chemosphere.2005.03.001
Xu ZY, Zhang QX, Fang HP: Applications of porous resin sorbents in industrial wastewater treatment and resource recovery. Crit. Rev. Environ. Sci. Technol. 2003, 33: 363. 10.1080/10643380390249512
Pan BC, Zhang WM, Zhang QX, Xu ZY, Chen JL: Application of resin adsorbents in pollution control of toxic compounds in water systems. In International Symposium on Nanotechnology in Environmental Protection and Pollution (ISNEPP). Hongkong; June 2006:18-21.
He BL, Ma JB: Polymeric adsorbents for blood purification. Chem. J. Chin. U. 1997, 18: 1212.
Stumm W, Morgan JM: Aquatic Chemistry. Wiley, New York; 1981.
Giese RF, Van Oss CJ: Organophilicity and hydrophobicity of organo-clays. In Organo-Clay Complexes and Interactions. Edited by: Yariv S, Cross H. Marcel Dekker, New York; 2002:175-192.
Zhu L, Ren X, Yu S: Use of cetyltrimethylammonium bromide-bentonite to remove organic contaminants of varying polar character from water. Environ. Sci. Technol. 1998, 32: 3374-3378. 10.1021/es980353m
Smith JA, Grum A: Sorption of nonionic organic contaminants to single and dual organication bentonite from water. Environ. Sci. Technol. 1995, 29: 685-692. 10.1021/es00003a016
Boyd SA, Mortland MM, Chiou CT: Sorption characteristics of organic compounds on hexadecyltrimethylammonium-smectite. Soil Sci. Soc. Am. J. 1988, 52: 652-657. 10.2136/sssaj1988.03615995005200030010x
Dentel SK, Jamrah AI, Sparks DL: Sorption and co-sorption of 1,2,4-trichlorobenzene and tannic acid by organoclays. Water Res. 1998, 32: 3689-3697. 10.1016/S0043-1354(98)00148-1
Mortland MM: Clay-organic complexes and interactions. Adv. Agron. 1970, 22: 75-117.
Janes WF, Boyd SA: Clay mineral type and organic compound sorption by hexadecyltrimethyl ammonium-exchanged clays. Soil Sci. Soc. Am. J. 1991, 55: 43-48. 10.2136/sssaj1991.03615995005500010007x
Chern JM, Chien YW: Adsorption of nitrophenol onto activated carbon beds: isotherms and breakthrough curves. Water Res. 2002, 36: 647-655. 10.1016/S0043-1354(01)00258-5
Yariv S: Organo-clay complexes and interactions. In Introduction to Organo-Clay Complexes and Interactions. Edited by: Yariv S, Cross H. Marcel Dekker, New York; 2002:39-112.
Kim YS, Song DI, Jecn YW, Choi SJ: Adsorption of organic phenols onto hexadecyltrimethylammonium-treated montmorillonite. Sep. Sci. Technol. 1996, 31: 2815-2830. 10.1080/01496399608000829
McCarthy JF, Zachara JM: Subsurface transport of contaminants. Environ. Sci. Technol. 1989, 23: 496-502.
Zachara JM, Resch CT, Smith SC: Influence of humic substances on CO2+ sorption by a subsurface mineral separate and its mineralogic components. Geochim. Cosmochim. Acta. 1994, 58: 553-566. 10.1016/0016-7037(94)90488-X
Cao Y, Yang W, Chen Y, Du H, Yue P: Effect of chemisorbed surface species on the photocatalytic activity of TiO2 nanoparticulate films. Appl. Surf. Sci. 2004, 236: 223-230. 10.1016/j.apsusc.2004.04.020
Piera E, Ayllon JA, Domenech X, Peral J: TiO2 deactivation during gas-phase photocatalytic oxidation of ethanol. Catal. Today 2002, 76: 259-270. 10.1016/S0920-5861(02)00224-9
Regazzoni AE, Blesa MA, Marotao AJG: Electrophoretic behavior of the hematite/complexing monovalent anion solution interface. J. Colloid Interface Sci. 1988, 122: 315-325. 10.1016/0021-9797(88)90367-0
Rubio J, Matijevic E: Interactions of metal hydrous oxides with chelating agents: I -FeOOH-EDTA. J. Colloid Interface Sci. 1979, 68: 408-421. 10.1016/0021-9797(79)90299-6
Jain AK, Gupta VK, Jain S, Suhas A: Removal of chlorophenols using industrial wastes. Environ. Sci. Technol. 2004, 38: 1195-1200. 10.1021/es034412u
Ramakrishnan SR, Subramanian G: A continuous process for the purification of cooling tower blow down-water or the effluent containing hexavalent chromium. 1986.
Namasivayam C, Senthilkumar S: Removal of arsenic (V) from aqueous solution using industrial solid waste: adsorption rates and equilibrium studies. Ind. Eng. Chem. Res. 1998, 37: 4816-4822. 10.1021/ie970774x
Namasivayam C, Jayakumar R, Yamuna RT: Dye removal from wastewater by adsorption on waste Fe(III)/Cr(III) hydroxide. Waste Mangt. 1994, 14: 643-648. 10.1016/0956-053X(94)90036-1
Perry RH, Chilton CH: Chemical Engineers’ Handbook. 5th edition. McGraw-Hill, Kogakusha; 1973.
Chen GR: In: Chemical Industry Encyclopedia. Chemical Industry, Beijing; 1990.
Ho YS, McKay G: Pseudo-second order model for sorption processes. Process Biochem. 1999, 34: 451-465. 10.1016/S0032-9592(98)00112-5
Ho YS: Adsorption of Heavy Metals from Waste Streams by Peat. PhD Thesis, University of Birmingham; 1995.
Weber WJ, Morris JC: Kinetics of adsorption on carbon from solution. J. Sanitary Engg. Div. ASCE 1963, 89: 31-60.
Wu F-C, Tseng R-L, Juang R-S: Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153: 1-8. 10.1016/j.cej.2009.04.042
Shapiro A, Stenby EH: Multicomponent adsorption approaches to modeling adsorption equilibria. In Encyclopedia of Surface and Colloid Science. Taylor & Francis, New York; 2006:4180-4189.
Giles CH, Mac Ewan TH, Nakhwa SN, Smith D: Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface area of solids. J. Am. Chem. Soc 1960, 11: 3973-3993.
Nicoud RM, Seidel-Morgenstern A: Adsorption isotherms: experimental determination and application to preparative chromatography. Isolat. Purif. 1996, 2: 165-200.
Ruthven DM: Principles of Adsorption and Adsorption Processes. Wiley, New York; 1984.
Brougton DB, Gerhold CG: Continuous sorption process employing fixed beds of sorbent moving inlets and outlets. 23 May 1961.
Mazzotti M, Storti G, Morbidelli M: Operation of simulated moving bed units for nonlinear chromatographic separations. J. Chromatogr. A. 1997, 769: 3-24. 10.1016/S0021-9673(97)00048-4
Migliorini C, Mazzotti M, Morbidelli M: Continuous chromatographic separation through simulated moving beds under linear and nonlinear conditions. J. Chromatogr. A. 1998, 827: 161-173. 10.1016/S0021-9673(98)00643-8
Kniep H, Mann G, Vogel C, Seidel-Morgenstern A: Separation of enantiomers through simulated moving-bed chromatography. Chem. Eng. Technol. 2000, 23: 853-857. 10.1002/1521-4125(200010)23:10<853::AID-CEAT853>3.0.CO;2-2
Kaspereit M, Jandera P, Skavrada M, Seidel-Morgenstern A: Impact of adsorption isotherm parameters on the performance of enantioseparation using simulated moving bed chromatography. J. Chromatogr. A. 2002, 944: 249. 10.1016/S0021-9673(01)01341-3
Vijayalakshmi PR, Raksh VJ, Thirumaleswara SGB: Adsorption of phenol, cresol isomers and benzyl alcohol from aqueous solution on activated carbon at 278, 298 and 323 K. J. Chem. Technol. Biotechnol. 1998, 71: 173-179. 10.1002/(SICI)1097-4660(199802)71:2<173::AID-JCTB818>3.0.CO;2-N
Banat F, Sameer AA, Leema AM: Utilization of raw and activated date pits for the removal of phenol from aqueous solutions. Chem. Eng. Technol 2004, 27: 80-86. 10.1002/ceat.200401868
Costa E, Calleja G, Marijuan L: Comparative adsorption of phenol, p-nitrophenol and p-hydroxybenzoic acid on activated carbon. Adsorpt. Sci. Technol. 1988, 5: 213-228.
Terzyk AP: Further insights into the role of carbon surface functionalities in the mechanism of phenol adsorption. J. Colloid Interface Sci. 2003, 268: 301-329. 10.1016/S0021-9797(03)00690-8
Arana J, Rodriguez JMD, Diaz OG, Melian JAH, Rodriguez CF, Pena JP: The effect of acetic acid on the photocatalytic degradation of catechol and resorcinol. Appl. Catal. A: Gen. 2006, 299: 274-284.
Bansal RC, Goyalo M: Activated Carbon Adsorption. CRC, Boca Raton; 2005.
Suzuki M, Fujii T: Concentration dependence of surface diffusion coefficient of propionic acid in activated carbon particles. AICHE J 1982, 28: 380-385. 10.1002/aic.690280303
Srivastava VC, Mall ID, Mishra IM: Adsorption thermodynamics and isosteric heat of adsorption of toxic metal ions onto bagasse fly ash (BFA) and rice husk ash (RHA). Chem. Eng. J. 2007, 132: 267-278. 10.1016/j.cej.2007.01.007
Faust SD, Aly OM: Adsorption Processes for Water. Treatment Butterwort, Boston; 1987.
Sabah E, Cinar M, Celik MS: Decolorization of vegetable oils: adsorption mechanism of β-carotene on acid-activated sepiolite. Food Chem. 2007, 100: 1661-1668. 10.1016/j.foodchem.2005.12.052
Mattson J, Mark H: Activated Carbon: Surface Chemistry and Adsorption from Solution. Marcel Dekker, New York; 1971.
Murzin D, Salami T: Chemical Kinetics. Elsevier, Amsterdam; 2005.
Raymon C: Chemistry: Thermodynamic. McGraw-Hill, Boston; 1998.
Scorecard: Pollution Information Site. (2011). Accessed 16 May 2012 http://www.scorecard.org/chemical-profiles
Acknowledgements
This study was financially supported by MHRD, Government of India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SS, VCS, and IMM conceived the concept and procedures of the analysis and drafted the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
40095_2012_40_MOESM1_ESM.doc
Additional file 1: Table S1: Hazard ranking of C, R, and HQ[181]. (DOC 89 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Suresh, S., Srivastava, V.C. & Mishra, I.M. Adsorption of catechol, resorcinol, hydroquinone, and their derivatives: a review. Int J Energy Environ Eng 3, 32 (2012). https://doi.org/10.1186/2251-6832-3-32
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6832-3-32