Abstract
Background
The aim of this study was to evaluate whether global levels of DNA methylation status were associated with albuminuria and progression of diabetic nephropathy in a case-control study of 123 patients with type 2 diabetes- 53 patients with albuminuria and 70 patients without albuminuria.
Methods
The 5-methyl cytosine content was assessed by reverse phase high pressure liquid chromatography (RP-HPLC) of peripheral blood mononuclear cells to determine individual global DNA methylation status in two groups.
Results
Global DNA methylation levels were significantly higher in patients with albuminuria compared with those in normal range of albuminuria (p = 0.01). There were significant differences in global levels of DNA methylation in relation to albuminuria (p = 0.028) and an interesting pattern of increasing global levels of DNA methylation in terms of albuminuria severity.
In patients with micro- and macro albuminuria, we found no significant correlations between global DNA methylation levels and duration of diabetes (p > 0.05). In both sub groups, there were not significant differences between global DNA methylation levels with good and poor glycaemic control (p > 0.05). In addition, in patients with albuminuria, no differences in DNA methylation levels were observed between patients with and without other risk factors including age, gender, hypertension, dyslipidaemia and obesity.
Conclusions
These data may be helpful in further studies to develop novel biomarkers and new strategies for clinical care of patients at risk of diabetic nephropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Diabetic nephropathy (DN) is one of the most serious micro vascular complications and remains a leading cause of end-stage renal disease (ESRD) and kidney replacement therapy [1]. Consequenctly, DN is associated with reduced life expectancy and increased morbidity, thereby imposing a significant burden on national healthcare system in diabetic care [2, 3].
The persistent albuminuria has been considered to be a central hallmark of DN and also a clinically informative predictor of kidney damage in diabetes patients [4, 5]. Albuminuria was categorized into two types: to micro albuminuria and macro albuminuria, and clinically, micro albuminuria is used extensively as a helpful predictor of first stage of kidney damage. However, despite its capacity to herald kidney damages for clinicians, micro albuminuria is not a specific biomarker for prediction and prevention of DN prior to the onset of this devastating complication. In principle, there are numerous cellular and molecular defects prior to appearing a clinical symptom and researchers still represent a major medical challenge in the hope to unravel an easy and accurate way to detect DN before its beginning. Moreover, better understanding of the disease pathogenesis might be helpful to identify new biomarkers and also novel mechanisms underlying DN.
Although DN is a common disorder with a heritable manner affected by genetic factors [6–8], this heritability could not be fully explained by strict genetic inheritance patterns [9]. It is now accepted that the initiation and progression of DN are a result of complex interactions between genetic and environmental factors that could be explained by epigenetic modifications [10, 11]. Environmental signals could alter the intracellular pathways by chromatin modifiers and regulate gene expression patterns leading to diabetes and its complications. A hyperglycaemic environment in diabetic patients causes an imbalance in metabolic homeostasis that may alter gene expression [12]. Recent epigenetic studies have focused on how gene transcription controls cell behaviour in response to environmental changes [13]. It will help to recognize novel approaches to inhibit, attenuate, or reverse the persistent deleterious consequences of diabetes in the kidney.
DNA methylation of cytosine residues is a heritable epigenetic modification of chromatin structure [14]. Alterations in genomic methylation patterns have been described in several complex disorders such as cancers that making it interesting for researchers as a plausible biomarker and a target of treatment. Emerging evidence regarding the relationship between a hyperglycaemic environment and alterations in DNA methylation, suggests that evaluation of global DNA methylation could be used as a possible biomarker to identify diabetic complications [13]. Thus far, there are few studies investigating DNA methylation alterations in DN and chronic kidney disease (CKD) patients [15–17].
They have demonstrated the role of DNA methylation patterns in DN and also methylation alterations in key genes to be responsible for DN development. However, the contribution of global DNA methylation associated with albuminuria and DN remains to be explored. Here, we evaluated whether global levels of DNA methylation status are associated with albuminuria and progression of DN.
Material and methods
Study population
This clinical-based case–control study of diabetes patients was conducted from July 2102 to September 2013 in a referral diabetes clinic that is affiliated with the Tehran University of Medical Sciences. The study was approved by the Ethics Committee of the Endocrinology and Metabolism Research Institute and written informed consent was obtained from all participants.
The case subjects were type 2 diabetic patients with persistence micro- and macro albuminuria (n = 53). The control subjects (n = 70) were diabetic patients with urinary albumin excretion values within the normal range, without retinopathy and history of hypertension and not on antihypertensive medications.
The primary exclusion criteria for case and control groups were a history of cancer, chronic disorders such rheumatoid arthritis, liver disease, clinical sings of acute infection, current smoker, pregnancy at the time of study and type 1 diabetes.
The diagnosis of type 2 diabetes was carried out and/or confirmed following American Diabetes Association criteria; which include a fasting blood glucose ≥ 126 mg/dL on two separate occasions, random (non-fasting) blood glucose ≥ 200 mg/dL on two separate occasions, or a blood glucose > 200 mg/dL at 2 hours during a standard oral glucose tolerance test.
To investigate micro- and macro albuminuria, a urine albumin-to-creatinine ratio was determined from a random urine collection of all patients and classified as normal (urine microalbumin: creatinine ratio ≤ 30 μg/mg), micro albuminuria (urine microalbumin: creatinine ratio >30 μg/mg and ≤ 299 μg/mg) and macro albuminuria (urine microalbumin: creatinine ratio ≥ 300 μg/mg) at least on two separate occasions.
Data collection and measurements
At first visit, demographic characteristics and clinical features including sex, age, age at diabetes diagnosis, diabetes duration, cigarette smoking status, and current use of medications were evaluated with a questionnaire. Standard anthropometric techniques were used to measure height, and weight. Body mass index was calculated as body weight (kg)/(height (m)2. Blood pressure was measured twice after participants were seated for at least for 10 minutes. Blood samples were taken after an overnight fast of 10–14 hours. The separated sera was kept at -80°C until analysis. Serum levels of glucose, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), blood urea nitrogen (BUN), uric acid, and creatinine were measured by enzymatic colorimetric assay [Pars-Asmun kits, Iran] using an auto analyzer [Hitachi 902, Japan]. The fasting serum insulin levels were assessed by an immunoenzymometric assay [Monobind Inc., USA]. The intra- and inter-assay coefficients of variation (CVs) for insulin were 5.9% and 9.2%, respectively. Glycated hemoglobin (HbA1c) levels were measured using ion exchange chromatography with a DS5 set [DREW, United Kingdom].
A random urine sample was collected the same day. Urine microalbumin and creatinine levels were measured by enzymatic colorimetric assay [Pars-Asmun kits, Iran] using an auto analyzer [Hitachi 902, Japan]. In case group urine microalbumin: creatinine ratio was >30 μg/mg (without any likely cause for error). For confirmation, a second urine sample was collected by the investigator at the next outpatient visit. The second urinalysis was handled the same way as the urinalysis for the first sample. If the second urine microalbumin: creatinine ratio was >30 μg/mg without cause for error, then the patients were eligible for the study as a case group.
Definition of diabetes risk factors
Diabetes predisposing factors were defined based on ADA criteria [18]. Hypertension was defined in subjects with a BP ≥ 140/90 mm Hg or current use of high blood pressure medications. Dyslipidemia was defined as TG > 250 mg/dL and/or HDL < 35 mg/dL or using lipid-lowering medications. Glycemic control was categorized into poor and good glycemic control based on HbA1c ≥ 7% or HbA1c < 7%, respectively [18, 19]. Obesity was classified based on BMI > 30 kg/m2.
Genomic DNA preparation and DNA hydrolysis
Genomic DNA was extracted from peripheral blood mononuclear cells (PBMCs) using a phenol chloroform method. Genomic DNA hydrolysis was performed as described elsewhere [20]. Briefly, RNA was first removed by treating 50 μg DNA in 300 μL 1X Tris-EDTA buffer with RNase A and RNase T1 [both from Fermentas Life Science, Lithuania] at final concentrations of 100 μg/mL and 2000 units/mL, respectively, for 2 h at 37°C, which was followed by ethanol precipitation. The dissolved DNA was then digested with 50 μg/mL DNase I [Fermentas Life Science, Lithuania] for 14 h at 37°C, denatured by heating at 100°C for 3 minutes, and rapidly cooled on ice. Then, 2 volumes 30 mM sodium acetate pH 5.2 [Carlo Erba Reagenti SpA, Rodano, Italy] with ZnSO4 and nuclease P1 [Sigma-Aldrich, St. Louis, MO, USA] at final concentrations of 1 mM and 50 μg/ml, respectively, were added and the mixture incubated for a further 16 h at 37°C. Hydrolyzed DNA from all subjects was maintained at -80°C until analysis.
Reverse phase high pressure liquid chromatography (RP-HPLC) analysis
Chemicals
Deionized water and HPLC-grade methanol [Merck, Darmstadt, Germany] were used in all experiments and all chemicals were of analytical reagent grade. The deoxynucleoside standards, 2-deoxycytidine 5-monophosphate sodium salt hydrate (dC), 2-deoxyguanosine 5-monophosphate sodium salt hydrate (dG), 2-deoxyadenosine 5-monophosphate (dA), thymidine 5-monophosphate disodium salt hydrate (dT), 5-methylcytidine (metC), were obtained from Sigma [St. Louis, MO, USA].
Instrumentation
A Waters 2487 dual absorbance detector chromatograph equipped with a Waters 1500 series HPLC pump was used in this study. The chromatographic column was a C8, 4.6 × 150 mm, 5 μm [Waters Spherisorb, Ireland].
Analytical procedure
Isocratic RP-HPLC analysis was performed at 10°C with a flow rate of 0.6 ml/min. The separation was achieved with 50 mM ammonium phosphate dibasic (titrated to pH 4.0 with phosphoric acid) [Merck KGaA, Darmstadt, Germany] as the mobile phase. A detection wavelength of 280 nm was used to identify specific spectra peaks originating from nucleosides. The injection volume of all samples was 100 μL. The area under the peaks was used for quantitative analysis. For linear calibration curve was obtained of ten concentration solutions range of 0–10 μM/ml for dA, dG, dC and dT and range 0–0.34 μM/ml for metC. The correlation coefficient of the curve was 0.999 for every compound. All samples were measured in duplicate. Coefficient of variation of deoxinoclotides between experiments ranged from 1.25% to 2.3%. The 5methyl-cytosine percentage was calculated using the equation: 5Methyl - cyto sin e(%) = 5Methyl - cyto sin e/(5Methyl - cyto sin e + Cyto sin e) ∗ 100.
Data analysis
For the analysis, we used the first urine collection from patients with normal range of albuminuria (control group) and the second confirmatory urine collection with >30 μg/mg for patients with persistent microalbuminuria (case group).
Statistical analysis was conducted using SPSS software (version 16). As certain data, including fasting serum LDL, TG, and insulin levels did not have normal distributions; log transformation was applied to correct their normality distribution. Student’s t-test was used to compare the differences of global DNA methylation levels in diabetic patients with and without albuminuria. One-way ONOVA test was used to compare the clinical and laboratory features in subgroups; normal, micro- and macro albuminuria. Kruskal Wallis test was used for variables not normally distributed. A Pearson correlation was applied to examine correlation of clinical, laboratory findings and diabetes risk factors with global DNA methylation levels. Numerical variables are reported as the mean ± standard error and categorical variables are presented as percentages. Two-tail p-values less than 0.05 were considered as statistically significant.
Results
Clinical characteristic and laboratory features
The clinical characteristics of the study population in each subgroup are shown in Table 1. Patients with micro and macro albuminuria were older, and had longer duration of diabetes, higher HbA1c, TG, BUN and uric acid and lower HDL serum levels.
A family history of diabetes in primary relatives was reported for 77.4% in patients with albuminuria (combined patients with micro- and macro albuminuria) and 60% of patients without albuminuria (with normal range of albuminuria), respectively (p = 0.04). The prevalence of obesity in patients with albuminuria (51%) was higher than those with normal albuminuria (30%) (p = 0.019). Poor control of glycemia was observed on 71.7% of patients with albuminuria compared with 54.3% of patients with normal albuminuria (p = 0.049). There was no significant difference in the prevalence of dyslipidaemia in patients with and without albuminuria, 79.2% and 84.3%; respectively (p = 0.47). The prevalence of coronary artery disease was observed in 17% of patients with albuminuria and 10% in patients without albuminuria.
Global DNA methylation and albuminuria
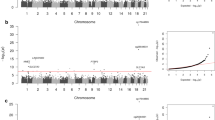
Global DNA methylation levels were significantly higher in patients with albuminuria compared with those without albuminuria (4.86 ± 0.15 vs. 4.25 ± 0.16, respectively, p = 0.01). There was significant increasing trend in global DNA methylation levels in relation to albuminuria (normal range of albuminuria: 4.25 ± 0.16, micro albuminuria: 4.76 ± 0.18, macro albuminuria: 5.05 ± 0.28 (F = 3.75, p = 0.028) (Figure 1).
Quantification of global DNA related to age and gender
No significance differences were observed in global DNA methylation levels between men and women in three groups (p > 0.05). There was also no significant correlation between age and DNA methylation levels in three groups (p >0.05). Patients with albuminuria (combined patients with micro- and macro albuminuria) had higher levels of DNA methylation independent of age and sex (p = 0.028).
Global DNA methylation and glycemic control and duration of diabetes
In diabetic patients without albuminuria, there was significant correlation between global DNA methylation levels and duration of diabetes (p = 0.002). In contrast, there was no significant correlation between global levels of DNA methylation and duration of diabetes in patients with albuminuria (p = 0.84).
In subgroups patients with micro- and macro albuminuria, we found no significant correlation between duration of diabetes and global DNA methylation levels (Table 2).
Global DNA methylation and diabetic risk factors in patients with albuminuria
In case group; global DNA methylation levels were higher in patients with family history of diabetes in first-degree relatives than those without family history of diabetes but it was not significant (4.94 ± 0.17 vs. 4.61 ± 0.34, p = 0.37). There were no significant differences in global DNA methylation levels in patients with and without hypertension (p = 0.53), dyslipidaemia (p = 0.37), and obesity (p = 0.41).
Conclusions
The main focus of current study was to examine global DNA methylation levels in relation to albuminuria in type 2 diabetic patients. We found higher levels of global DNA methylation in patients with micro- and macro albuminuria compared those with normal ranges of albuminuria even after adjusting age and sex. Interestingly, we did observe an interesting pattern of increasing global levels of DNA methylation in terms of albuminuria severity.
Although the role of DNA methylation in diabetic kidney gene expression remains poorly characterized, few studies have shown that altered DNA methylation at CpG sites to be associated with diabetic nephropathy [16, 21]. Moreover, DNA methylation changes in key genes were correlated with the development of diabetic nephropathy [16, 21]. Experimental studies and animal models have confirmed that long-lasting hyperglycaemia exposure causes DNA methylation changes in the promoter region of genes that are key components in diabetes and its complications [22–26].
This increasing body of evidence implicates that aberrant DNA methylation in response to hyperglycaemia may account for diabetic complications. It was revealed that the duration of diabetes prior to the onset of nephropathy was correlated with CpG methylation at those key genes [21]. Interestingly, our results showed glycaemic control and duration of diabetes were not associated with global DNA methylation in patients with albuminuria. It is accepted that chronic hyperglycaemia is the hallmark of development and progression of diabetic complications. This inconsistency may be due to the role of early hyperglycaemia exposure and may prime future transcriptional and metabolic profiles related to diabetes clinical endpoints [27–30]. There is also an increasing body of evidence to suggest that memory of early hyperglycaemic exposure can be retained in target cells that could subsequently alter gene expression patters even under euglycaemic conditions [27, 31–35]. As broadly shown in epidemiological studies and trials, good glycaemic control could not prevent diabetes progression and associated vascular complications in most diabetic patients and early prevention of hyperglycaemia is more important in prevention of diabetes complications [36–39]. Our results, which are consistent with these studies, indicate that aberrant DNA methylation alterations might occur in response to a chronic hyperglycaemic environment rather than glucose fluctuations that arisen by glycaemic control. Moreover, the main known risk factors that attend to diabetic nephropathy such as dyslipidaemia, hypertension, inflammation and oxidative stress could alter DNA methylation patterns [40–43].
In the current study, we observed no association between global DNA methylation levels and obesity, dyslipidemia and hypertension in patients with albuminuria. In contrast, higher methylation levels in peripheral blood cell genomes were reported in essential hypertensive patients compared with subjects without hypertension [41]. Also, the importance of DNA methylation modification to the development of cardiovascular disease is evidenced in some studies [44, 45]. Additionally, in another study in chronic kidney disease patients, global levels of DNA hypermethylation were correlated with systemic inflammation [40].
In a study in non-diabetic patients, a positive correlation was observed between Long Interspersed Nucleotide Element 1 (LINE-1) DNA methylation (an index of global DNA methylation) and total cholesterol, triglycerides and LDL-cholesterol concentrations. A negative association was also observed between LINE-1 methylation and HDL cholesterol concentration [46]. While, in a study in obese patients, DNA methylation changes in peripheral blood leukocytes were assessed and showed no methylation differences in CpG sites between the obese and lean groups [47].
Although we detected no significant association between global DNA methylation levels and obesity, hypertension, and dyslipidemia in patients with albuminuria, a possible relationship between these factors and DNA methylation cannot be ignored. How these characters modulate intracellular pathways inducing epigenetic alterations and cause long-lasting effects is not completely understood.
Our study takes a global approach to determine DNA methylation alterations in diabetic patients related to albuminuria. The capacity to analyse DNA methylation of the entire genome could provide an overall overview of cellular DNA methylation levels and be also crucial for clear the mechanisms by which gene-environment interplay leads to altered DNA methylation [48]. It also will enable us to understand the role of DNA methylation in normal development as well as disease state. Since the design of present study is a clinical case-control, it could not show causality between global DNA methylation and severity of albuminuria. Therefore, future studies with longitudinal design will be required to confirm this possibility. In this study, we evaluated global DNA methylation using HPLC assay which would be expected to be a standard method with higher sensitivity and more reproducibility than other methods used to determine global DNA methylation levels [49]. HPLC is a quantitative method and the single nucleotides are separated according to size and both cytosine and methylated cytosine are quantified [50].
Our study measured global DNA methylation levels in PBMCs. It is expected that DNA methylation is a specific- tissue manner and global DNA methylation tissue patterns have been found to significantly differ between tissue types [51]. However, human tissues availability set limitations and are not always accessible to evaluate global DNA methylation changes. In addition, PBMCs have been used for exploring gene expression in various diseases and predicting clinical outcome [52–54]. PBMCs can also reflect the effect of metabolic and environmental factors on chromatin structure and DNA methylation [55] and can useful in understanding etiology of complex disorders modulated by gene– environment interplay such as diabetes.
In summary, we have shown that global DNA methylation alterations associated with albuminuria and there was a significant increasing trend in global DNA methylation levels in relation to severity of albuminuria. Advances in understanding of epigenetic modifications in the progression of diabetic nephropathy could be helpful to clear pathogenic pathways and subsequent translation of this information into development of novel biomarkers and new strategies for clinical care of patients at risk of diabetic nephropathy.
Abbreviations
- DN:
-
Diabetic nephropathy
- PBMC:
-
peripheral blood mononuclear cells.
References
Gilbertson DT, Liu J, Xue JL, Louis TA, Solid CA, Ebben JP, Collins AJ: Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J Am Soc Nephrol 2005, 16: 3736–3741.
Thomas MC, Weekes AJ, Broadley OJ, Cooper ME, Mathew TH: The burden of chronic kidney disease in Australian patients with type 2 diabetes (the NEFRON study). Med J Aust 2006, 185: 140–144.
Ayodele OE, Alebiosu CO, Salako BL: Diabetic nephropathy–a review of the natural history, burden, risk factors and treatment. J Natl Med Assoc 2004, 96: 1445–1454.
Mogensen CE: Microalbuminuria as a predictor of clinical diabetic nephropathy. Kidney Int 1987, 31: 673–689.
Caramori ML, Fioretto P, Mauer M: The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes 2000, 49: 1399–1408.
Seaquist ER, Goetz FC, Rich S, Barbosa J: Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med 1989, 320: 1161–1165.
Harjutsalo V, Katoh S, Sarti C, Tajima N, Tuomilehto J: Population-based assessment of familial clustering of diabetic nephropathy in type 1 diabetes. Diabetes 2004, 53: 2449–2454.
Maeda S, Araki S, Babazono T, Toyoda M, Umezono T, Kawai K, Imanishi M, Uzu T, Watada H, Suzuki D, Kashiwagi A, Iwamoto Y, Kaku K, Kawamori R, Nakamura Y: Replication study for the association between four Loci identified by a genome-wide association study on European American subjects with type 1 diabetes and susceptibility to diabetic nephropathy in Japanese subjects with type 2 diabetes. Diabetes 2010, 59: 2075–2079.
Thomas MC, Groop PH, Tryggvason K: Towards understanding the inherited susceptibility for nephropathy in diabetes. Curr Opin Nephrol Hypertens 2012, 21: 195–202.
Freedman BI, Bostrom M, Daeihagh P, Bowden DW: Genetic factors in diabetic nephropathy. Clin J Am Soc Nephrol: CJASN 2007, 2: 1306–1316.
Keating ST, El-Osta A: Glycemic memories and the epigenetic component of diabetic nephropathy. Curr Diab Rep 2013, 13: 574–581.
Forbes JM, Fukami K, Cooper ME: Diabetic nephropathy: where hemodynamics meets metabolism. Exp Clin Endocrinol Diabetes 2007, 115: 69–84.
Cooper ME, El-Osta A: Epigenetics: mechanisms and implications for diabetic complications. Circ Res 2010, 107: 1403–1413.
Jin B, Li Y, Robertson KD: DNA methylation: superior or subordinate in the epigenetic hierarchy? Genes Cancer 2011, 2: 607–617.
Geisel J, Schorr H, Heine GH, Bodis M, Hubner U, Knapp JP, Herrmann W: Decreased p66Shc promoter methylation in patients with end-stage renal disease. Clin Chem Lab Med: CCLM/FESCC 2007, 45: 1764–1770.
Sapienza C, Lee J, Powell J, Erinle O, Yafai F, Reichert J, Siraj ES, Madaio M: DNA methylation profiling identifies epigenetic differences between diabetes patients with ESRD and diabetes patients without nephropathy. Epigenetics 2011, 6: 20–28.
Zawada AM, Rogacev KS, Heine GH: Clinical relevance of epigenetic dysregulation in chronic kidney disease-associated cardiovascular disease. Nephrol Dial Transplant 2013, 28: 1663–1671.
American Diabetes Association: Standards of medical care for patients with diabetes mellitus. Diabetes Care 2002, 25: 213–229.
American Diabetes Association: Standards of medical care for patients with diabetes mellitus. Diabetes care. Conn Med 1991, 55: 630–633.
Ramsahoye BH: Measurement of genome-wide DNA cytosine-5 methylation by reversed-phase high-pressure liquid chromatography. Methods Mol Biol 2002, 200: 17–27.
Bell CG, Teschendorff AE, Rakyan VK, Maxwell AP, Beck S, Savage DA: Genome-wide DNA methylation analysis for diabetic nephropathy in type 1 diabetes mellitus. BMC Med Genom 2010, 3: 33.
Ling C, Del Guerra S, Lupi R, Ronn T, Granhall C, Luthman H, Masiello P, Marchetti P, Groop L, Del Prato S: Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia 2008, 51: 615–622.
Tewari S, Zhong Q, Santos JM, Kowluru RA: Mitochondria DNA replication and DNA methylation in the metabolic memory associated with continued progression of diabetic retinopathy. Invest Ophthalmol Vis Sci 2012, 53: 4881–4888.
Toperoff G, Aran D, Kark JD, Rosenberg M, Dubnikov T, Nissan B, Wainstein J, Friedlander Y, Levy-Lahad E, Glaser B, Hellman A: Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Hum Mol Genet 2012, 21: 371–383.
Pirola L, Balcerczyk A, Tothill RW, Haviv I, Kaspi A, Lunke S, Ziemann M, Karagiannis T, Tonna S, Kowalczyk A, Beresford-Smith B, Macintyre G, Kelong M, Hongyu Z, Zhu J, EI-Osta A: Genome-wide analysis distinguishes hyperglycemia regulated epigenetic signatures of primary vascular cells. Genome Res 2011, 21: 1601–1615.
Yokomori N, Tawata M, Onaya T: DNA demethylation during the differentiation of 3T3-L1 cells affects the expression of the mouse GLUT4 gene. Diabetes 1999, 48: 685–690.
Roy S, Sala R, Cagliero E, Lorenzi M: Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci U S A 1990, 87: 404–408.
Gotzsche O, Gundersen HJ, Osterby R: Irreversibility of glomerular basement membrane accumulation despite reversibility of renal hypertrophy with islet transplantation in early experimental diabetes. Diabetes 1981, 30: 481–485.
Okabe J, Orlowski C, Balcerczyk A, Tikellis C, Thomas MC, Cooper ME, El-Osta A: Distinguishing hyperglycemic changes by Set7 in vascular endothelial cells. Circ Res 2012, 110: 1067–1076.
El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M: Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 2008, 205: 2409–2417.
Zhao J, Goldberg J, Bremner JD, Vaccarino V: Global DNA methylation is associated with insulin resistance: a monozygotic twin study. Diabetes 2012, 61: 542–546.
Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005, 353: 2643–2653.
Engerman RL, Kern TS: Progression of incipient diabetic retinopathy during good glycemic control. Diabetes 1987, 36: 808–812.
Kowluru RA: Effect of reinstitution of good glycemic control on retinal oxidative stress and nitrative stress in diabetic rats. Diabetes 2003, 52: 818–823.
Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC: Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes 1988, 37: 622–628.
The Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993, 329: 977–986.
UK Prospective Diabetes Study (UKPDS) Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352: 837–853.
Ceriello A, Ihnat MA, Thorpe JE: Clinical review 2: The "metabolic memory": is more than just tight glucose control necessary to prevent diabetic complications? J Clin Endocrinol Metab 2009, 94: 410–415.
Keenan HA, Costacou T, Sun JK, Doria A, Cavellerano J, Coney J, Orchard TJ, Aiello LP, King GL: Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50-year medalist study. Diabetes Care 2007, 30: 1995–1997.
Stenvinkel P, Karimi M, Johansson S, Axelsson J, Suliman M, Lindholm B, Heimburger O, Barany P, Alvestrand A, Nordfors L, Qureshi AR, Ekström TJ, Schalling M: Impact of inflammation on epigenetic DNA methylation - a novel risk factor for cardiovascular disease? J Intern Med 2007, 261: 488–499.
Smolarek I, Wyszko E, Barciszewska AM, Nowak S, Gawronska I, Jablecka A, Barciszewska MZ: Global DNA methylation changes in blood of patients with essential hypertension. Med Sci Mon Int Med J Exp Clin Res 2010, 16: CR149-CR155.
Zaina S, Dossing KB, Lindholm MW, Lund G: Chromatin modification by lipids and lipoprotein components: an initiating event in atherogenesis? Curr Opin Lipidol 2005, 16: 549–553.
Zaina S, Lindholm MW, Lund G: Nutrition and aberrant DNA methylation patterns in atherosclerosis: more than just hyperhomocysteinemia? J Nutr 2005, 135: 5–8.
Sharma P, Kumar J, Garg G, Kumar A, Patowary A, Karthikeyan G, Ramakrishnan L, Brahmachari V, Sengupta S: Detection of altered global DNA methylation in coronary artery disease patients. DNA Cell Biol 2008, 27: 357–365.
Kim M, Long TI, Arakawa K, Wang R, Yu MC, Laird PW: DNA methylation as a biomarker for cardiovascular disease risk. PLoS One 2010, 5: e9692.
Pearce MS, McConnell JC, Potter C, Barrett LM, Parker L, Mathers JC, Relton CL: Global LINE-1 DNA methylation is associated with blood glycaemic and lipid profiles. Int J Epidemiol 2012, 41: 210–217.
Wang X, Zhu H, Snieder H, Su S, Munn D, Harshfield G, Maria BL, Dong Y, Treiber F, Gutin B, Shi H: Obesity related methylation changes in DNA of peripheral blood leukocytes. BMC Med 2010, 8: 87.
Romerio AS, Fiorillo G, Terruzzi I, Senesi P, Testolin G, Battezzati A: Measurement of DNA methylation using stable isotope dilution and gas chromatography-mass spectrometry. Anal Biochem 2005, 336: 158–163.
Lisanti S, Omar WA, Tomaszewski B, De Prins S, Jacobs G, Koppen G, Mathers JC, Langie SA: Comparison of methods for quantification of global DNA methylation in human cells and tissues. PLoS One 2013, 8: e79044.
Kuo KC, McCune RA, Gehrke CW, Midgett R, Ehrlich M: Quantitative reversed-phase high performance liquid chromatographic determination of major and modified deoxyribonucleosides in DNA. Nucleic Acids Res 1980, 8: 4763–4776.
Lokk K, Modhukur V, Rajashekar B, Martens K, Magi R, Kolde R, Kolt Ina M, Nilsson TK, Vilo J, Salumets A, Tonisson N: DNA methylome profiling of human tissues identifies global and tissue-specific methylation patterns. Genome Biol 2014, 15: R54.
Ma J, Dempsey AA, Stamatiou D, Marshall KW, Liew CC: Identifying leukocyte gene expression patterns associated with plasma lipid levels in human subjects. Atherosclerosis 2007, 191: 63–72.
Wettinger SB, Doggen CJ, Spek CA, Rosendaal FR, Reitsma PH: High throughput mRNA profiling highlights associations between myocardial infarction and aberrant expression of inflammatory molecules in blood cells. Blood 2005, 105: 2000–2006.
Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S: Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab 2001, 86: 3257–3265.
Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M: Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A 2005, 102: 10604–10609.
Acknowledgments
We appreciate the help from S. Safari and S. Zorpaykar for subject recruitment and supervisors of the diabetes clinic of Tehran University of Medical Sciences for their helpful cooperation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors designed the study. ZM and MA gathered the clinical data. ZM and SE performed experiments. ZM, BL and AK analyzed the data. ZM, BL, SE, MA, and AK wrote the main paper. All authors discussed the results and commented on the manuscript at all stages. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Maghbooli, Z., Larijani, B., Emamgholipour, S. et al. Aberrant DNA methylation patterns in diabetic nephropathy. J Diabetes Metab Disord 13, 69 (2014). https://doi.org/10.1186/2251-6581-13-69
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6581-13-69