Abstract
Background
The healthy properties of conjugated linoleic acid (CLA) such as weight loss, reducing cardiovascular risk factors and inflammation have been reported. The trans-10, cis-12 CLA isomer is related to increasing insulin resistance, but the effects of cis-9, trans-11 isomer is not clear. The aim of this study was to investigate the effects of CLA with and without Vitamin E on body weight, body composition, glycemic index, inflammatory and coagulation factors, lipid profile, serum leptin and adiponectin, malondialdehyde (MDA), and blood pressure in type2 diabetes.
Methods
56 patients with type2 diabetes were included in 8 week double-blind control trial that used metformin. They randomly divided into three groups: CLA + VitE, CLA + VitE placebo, CLA placebo + VitE placebo. All variables, anthropometric measurements, and body composition were evaluated at the beginning and the end of study. Statistical analysis and analysis of dietary data were performed using SPSS and nutritionist IV software, respectively.
Results
There were not any significant differences in variable changes among three groups. However, there was a trend to increase in MDA and decrease in apoB100 among CLA consumers.
Conclusion
The results of this study showed that administration of CLA supplementation for 8 weeks does not affect any indicators of metabolic control in overweight type2 diabetic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Conjugated linoleic acid (CLA) is referred to a group of 18 carbon unsaturated fatty acids with two double bond that is isomers of octadecanoic acid [1, 2]. Normally, CLA is produced in the rumen of ruminants through partial biohydration or bacterial fermentation as the first intermediate of linoleic to stearic acid transformation or from trans-11 octadecanoic acid (vaccenic acid) by delta 9-desaturase (oxidation) [3]. Therefore, the major food sources of this unusual fatty acid are ruminant products (meat and milk). Some studies have reported useful properties for CLA such as anti-obesity [4–6], anti-Atherogenic [7, 8], anti-diabetic [9–11] and anti-inflammatory properties [12].
Two active isomers of CLA that have been investigated so far are cis-9, trans-11 and trans-10, cis-12 that first one is more dominant in dairy products [3, 13]. These isomers have several effects in different biological systems and their effects are similar or different sometimes [2]. Results of some studies have shown that trans-10, cis-12 isomers are the active form of CLA reduces body weight and fat accumulation [5]. However, in some cases this isomer lead to worsening peripheral insulin sensitivity and increased concentrations of blood glucose, free fatty acids, and lipid profiles [14–17] and cis-9, trans-11 isomer reduces serum triglyceride levels and plasma free fatty acids [2]. However, the results of 50:50 isomer blend of CLA on weight, body fat, insulin resistance index and inflammation are controversial [10, 18].
Animal studies have shown that isomer trans-10, cis-12 isomers of CLA increase insulin resistance, but the results of studies on cis-9, trans-11 isomer and mixtures (50:50) is contradictory. In some animal studies, CLA had no effect on the blood glucose and insulin resistance [12, 19]. Also, various researches have shown that CLA, even in different species of animals have different effects. For example, in rats, CLA increases PPAR-γ expression as a ligand for this receptor and in this way plays its role in controlling insulin resistance. Also, CLA decreases insulin resistance in adipose tissue of rats. But some studies have shown that CLA increases insulin resistance on mice [19].
Also, the results of CLA supplementation on type II diabetic patients are inconsistent. One of the possible reasons may be the types of consuming drugs, heterogeneity of population in the cases of age, BMI and etc. [7, 20].
In some studies supplementation of CLA led to increment in oxidative stress and inflammation [9, 21]. Since these conditions are related to diabetes, we suppose that supplementation with vitamin E may compensate the possible effects of CLA on oxidative stress and diabetes control. Furthermore, regarding the decrement of Antioxidant capacity in diabetic patients, it raises the possibility that this issue in previous studies may mask some effects of CLA or lead to a slight increase in insulin resistance. Regarding the possible intake of CLA supplements among type 2 diabetic patients to lose weight and tendency of some food industries such as dairy products and oil to enrich their products with CLA,
So, the aim of current study is to investigate the effects of CLA alone and combined with VitE on glycemic response, cardiovascular risk factors, inflammatory indicators, malondialdehyde (MDA) and some adipocytikines associated to diabetes and obesity. Important notable excellence of our study is that to date no well designed controlled study with such inclusion criteria on type 2 diabetic patients has been done.
Methods
This study was approved by the Ethic Committee of Institute of Food Technology and Nutrition research, Shahid Beheshti University of Medical Sciences and was conducted in 2008 at Research Institute for Endocrine Sciences (RIES), Tehran, Iran.
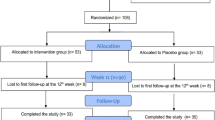
Sixty-three participants with type2 diabetes whose diabetes was controlled by metformin were recruited and 56 completed the trial. At the beginning, the protocol and the aim of study were fully explained to the participants and written informed consent was obtained from each volunteers. Inclusion criteria, including diagnosed type2 diabetes mellitus for more than 5Y, diagnosis after 30 years old, age of 35 to 50Y, body mass index (BMI; in kg/m2) >25 and <30 and fasting blood glucose of 126–180 mg/dl. Exclusion criteria were the history of myocardial infarction, angina pectina and stroke, diagnosis of cardiovascular disease, liver or renal disease or chronic inflammatory and thyroid disease, being vegetarians or vegans, smoking, consuming of alcohol and any supplements (e.g. vitamins such as C and E, fish oils, CLA, etc.) 2 months before intervention, pregnancy and menopause.
The study was an eight weeks randomized double blind, placebo-controlled (RCT) parallel intervention. The participants were stratified according to their sex, age and BMI into one of three groups (A, B, and C) receiving 3.0 g CLA/d (3×1 g capsules; a 50:50 isomer blend of c-9, t-11 and t-10, c-12 CLA) with 100 IU/d VitE, 3.0 g CLA/d with VitE placebo, or CLA placebo (soy bean oil) and VitE placebo respectively. Ineffectiveness of soybean oil as a placebo in the quantities being used in this study on reviewed variables has shown in other studies [20]. The CLA used in this study was Tonalin SG1000T FFA and soft gelatin capsules with clear, transparent shell and pale amber fill. They contain Tonalin FFA 80 i.e., free fatty acids containing about 80% conjugated Linoleic. All supplements were supplied by Cognis, Norway. Each volunteers received the capsules in 2 batches, at the beginning of the study. All participants were asked to maintain their usual physical activity and dietary and lifestyle habits and these were checked by food record and physical activity questionnaire. There was no change in prescribed medication throughout the trial.
Acceptance of the supplements was investigated via weekly phone calling and meetings through asking and counting the remaining capsules in the package delivered. During these calls possible problems such as supplement intolerance and medication use, possibly changes in food consumption, getting a new disease or a change in physical activity was followed and if this situation occurred, the patient desired were excluded. In the fourth and eighth weeks of study through counting the remaining capsules, compliance rates of patients was evaluated and patients were not consumed more than ten percent of received capsules, were excluded from the study.
To evaluate the mean dietary intake two 1-d 24-h food recall and 2-d food records (one day a week holiday) were used on the baseline and the end of the fourth and eighth week of the study. In the first taking the 24 days recall, how to diet properly recording, including how to weigh and measure food was trained by an expert. This dietary information was analyzed with N4 software (Nutritionist: version 4.0; Tinuviel Software, Warrington, United Kingdom).
Body weight was measured with light clothing but no shoes on a digital balance (with 0.1 kg sensitivity). Height was assessed by using a stadiometer that measured to the nearest 0.1 cm. Body mass index (BMI) was estimated as the ratio of body weight to height squared and expressed as kg/m2. Waist circumference (with 0.1 cm sensitivity) was measured at the minimum circumference between the iliac crest and the last rib cage at the end of exhalation. The hip circumference was measured using tape as the maximal circumference over the hip and Waist-to-hip ratio (WHR) was calculated [22]. Bioelectrical impedance analysis was used to measurement of body fat and lean percentage (Body Stat 1500, Douglas Isle of man, British islets, England). For this purpose, first two glasses of water consumed by patients and after one hour and urination without any metal on the body, body composition was measured. Measuring body composition was done in lying state and installing the electrodes to the right hand and foot. Patients were taught avoiding intense activity the day before testing [23]. These measurements were done at the beginning and end of the study.
Blood pressure measuring was done to the nearest 2 mmHg, after resting for at least 15min and sitting on the seat handle. Right arm blood pressure was measured, twice, at least five minutes interval, by Korotkoff’s auscultatory method at the beginning and end of the study.
Seven ml 12-hour fasting state and 3 ml postprandial brachial vein blood samples were taken at the baseline and end of eighth week and blood collected into EDAT containing tubes. A standard breakfast in diabetic patients is contained about 360kcal, including: 56.6 g carbohydrate (50-55% Cal), 19.5g protein (15-20% Cal) and 11.5 g fat (30% Cal) [24, 25]. Localized standard breakfast for Iranians that has been used in previous studies included two servings bread; one serving cheese, 2x dates, and four small cubes that total provided about 390kcal and 14g protein [26]. To maintain comparability of Iranian studies with the international studies in which the breakfast induced energy is 360 kcal, and also given that patients participating in this study did not normally use refined s such as in the diet, of breakfast was removed and replaced as a number of dates were added to the breakfast.
Serum glucose concentration was measured by using enzymatic colorimetric method according glucose oxidase principle (Glucose determination kit, Parsazmun, Tehran, Iran) through auto-analyzer instrument (Sellectra II, Dieren, Netherland). Serum insulin, proinsulin and C-peptide levels were measured by Enzyme Linked Immuno assay (ELISA) kit (Mercodia AB, Uppsala, Sweden). Glycated hemoglobin was determined on whole blood sample by ion exchange chromatography method (HbA1c Kit, Inter Medical, Villaricca, Italy).
The intra assay coefficient of variation (CV%) for glucose, insulin, proinsulin, C- peptide and HbA1c were 4.7%, 5.5%, 4.5%, 4.7% and 5.6% and the inter assay coefficient of variation were 4.9%, 5.8%, 4.9%, 5%, and 5.8% respectively. The assays sensitivity was 1mg/dl, 1mU/L, 0.5 pmol/l, 5 pmol/l and 1% respectively.
Insulin resistance was calculated according the homeostasis model of assessment ratio (HOMA-IR) formula, as an index of insulin resistance:
[Insulin (μU/ml)×glucose (mmol/L)]/22.5 [27]. That 5<HOMA IR is defined as insulin resistance and 3> HOMA IR as not-insulin resistance [28].
Insulin sensitivity was calculated from following formula [29]:
And beta cell function determined through proinsulin/ insulin ratio [30]. Beta cell function index as HOMA-B% and C-peptide to insulin molar ratio as an index of liver insulin clearance was calculated [31].
Fasting b-cell responsiveness (M0) represents the ability of fasting glucose to stimulate b-cell secretion and postprandial b-cell responsiveness (M1) represents the ability of postprandial glucose to step up b-cell secretion and they were calculated using the formula of Hovorka et al. [32].
Serum concentration of triglyceride and HDL cholesterol were measured using kits and enzymatic colorimetric method (Parsazmun, Tehran, Iran). Total cholesterol Concentration was measured by enzymatic photometric method kits (Parsazmun, Tehran, Iran). LDL cholesterol concentration calculated using the Friedewald formula. Also, the ratio of LDL to HDL cholesterol concentrations was calculated. The intra assay coefficient of variation (CV%) for triglycerides, total cholesterol and HDL cholesterol were 2.7%, 2.3% and 5.1% and the inter assay coefficient of variation were 2.9%, 2.5T and 5.5% respectively. Also, the related assays sensitivity was 1 mg/dL, 3mg/dL and 1mg/dl respectively.
Serum leptin levels were measured by Enzyme Linked Immuno assay (ELISA) kit (Diagnostics Biochem Canada Inc., ontario, Canada). Serum adiponectin levels were measured by Enzyme Linked Immuno assay (ELISA) kit (Mercodia AB, Uppsala, Sweden). The intra assay coefficient of variation (CV%) for leptin and adiponectin were 2% and 1.5% respectively and the inter assay coefficient of variation were 4% and 2%. The sensitivity of the assays was 0.50 ng/ml and 1.25 ng/ml respectively.
Serum concentrations of interleukin-1 beta, interleukin-6 and TNF-α were measured using ELISA kits (Diaclone, France) and CRP concentration using ELISA kit (Diagnostics Biochem Canada Inc., Ontario, Canada). Intra assay coefficient of variation of inflammatory IL-1 beta, interleukin 6, CRP and TNF-α, were 7.1%, 4.6%, 1.8%, 3% and the related inter assay coefficient of variation were 7%, 4.7%, 2% and 3.2% respectively. Sensitivity of assays were also 7 pg/ml, 2 pg/ml, 10 ng/ml and 8 pg/ml. Measuring fibrinogen concentration and PAI-1was done using ELISA kits (Hyphen BioMed, Neuville-Sur-Oise, France) and MDA concentration by using the colorimetric method kit (Cayman Chemical Company, Ann Arbor, USA). The intra and inter assay coefficient of variation for fibrinogen and PAI-1, were 2.6%, 3.2% and 3%, 3.5% respectively and assays sensitivity were 1 mg/ml and 0.5 ng/ml respectively. These values for apoB-100 and MDA were 1.4%, 3% and 2%, 3.5% and 0.1 μg/dl, 1 μmol/L respectively.
Statistical analysis
All statistical tests were performed with the use of SPSS (version 13.0; SPSS Inc, Chicago) and a p-value<0.05 showed statistical significance. Normality and homogeneity of variance was tested with Kolmogrov-Smirinov. ANOVA test were used to compare mean differences before and after intervention for anthropometric data and body composition. Other changes were compared using ANCOVA test adjusted to body composition and waist circumference. To compare food consumption at the baseline and end of the fourth and eighth weeks among groups, and comparison of food consumption in each group among baseline and end of the fourth and eighth weeks of study the ANOVA test and repeated measure ANOVA test were used.
Results
Among 63 individuals participated in this research, one patient was excluded due to reporting heartburn and digestive discomfort, one due to herbal drug use and five patients due to not taking the supplements regularly. At the end, 56 (30females and 26males) completed the study. Baseline characteristics of patients of A, B, and C groups listed in Table 1.
Patients’food intakes at the baseline, the end of the fourth and eighth weeks has been shown in Table 2. During the study, participants’ food intake did not change significantly. It is notable that we analyzed dietary SFA, MUFA and PUFA and also, physical activity level and no changes were shown. Data was not shown in article.
Table 3, 4, and 5 summarize the results of supplementation on the studied variables.
Discussion
In this study, consumption of CLA alone and in combination with VitE did not result in significant changes in any of the measured variables.
So far, few studies regarding the effect of CLA has been done in diabetic patients. In a study, usual dose of commercial CLA supplement improved the insulin sensitivity index in young diabetic adults, but some differences were observed in their responsiveness [10]. In another study, supplemental CLA significantly increased fasting glucose concentrations and reduced insulin sensitivity (homeostasis model), oral glucose insulin sensitivity and insulin sensitivity index (ISI) [7], while in our study any of related parameters were not affected. Inconsistency in the results of studies on diabetic patients may be due to differences in patients ‘responsiveness. As shown in Eyjolfson et al. study, despite average increment in insulin sensitivity with 4g supplementation, two participants demonstrated essential no, and two had a decrease in insulin sensitivity. In that study, among six patients, insulin sensitivity found to increase and therefore caused to increase in the average insulin sensitivity in the intervention group [23]. Elusive changes in blood biochemistry presented in our study may also be due to differences in the patients’ responsiveness. This could be caused by differences in duration of diabetes, patients ‘weight, the severity of insulin resistance and other parameters measured at baseline and different amounts of CLA intake from the usual diet. But what is clear, in patients with normal BMI or overweight, CLA has no or a little effect on improvement of blood glucose, insulin and insulin sensitivity [10, 33]. So, it is likely that in obese individuals, CLA causes a small increase in insulin resistance [9, 34]. However in our study, CLA supplementation did not change weight and glycemic indicators.
In most studies, serum glucose, insulin, and sometimes, fasting pro insulin and C-peptide have been measured and only indicators of insulin resistance and insulin sensitivity has been reported. While, several studies have shown that serum pro insulin, pro insulin/insulin are better predictors of the risk of heart disease compared to insulin concentration [35]. In T2D pro insulin, insulin ratio increases, that can be the result of insulin resistance or impaired conversion of pro insulin to insulin [36]. In the present study, serum concentrations of C peptide and pro insulin were also measured at the beginning and end of the study and no changes were detected.
Recently, using pure isomers of CLA has shown that the effects of CLA on body composition that is related to trans-10, cis-12 isomer [6, 37]. This fatty acid decreases appetite and energy intake in laboratory animals, increases energy expenditure in the body by increasing expression of Mitochondrial uncoupling protein 2 (UCP2). CLA decreases lypogenesis through inhibition of lipoprotein lipase (LPL2), fatty acid synthase, acylcoenzyme A carboxylase and PPAR-γ. Also, CLA increases fat oxidation. Thus, CLA reduces fatty cell size with no effect on cell numbers [5]. Changes in weight and body composition observed in human studies are not as changes observed in experimental animals. In some human studies, CLA supplementation was associated to decrease in body weight and fat mass [21, 38], but in others there was not any changes in body composition [39, 40]. It seems that factors such as current state of health, degree of obesity, and activity levels might influence the impact that CLA supplementation has on altering body composition. Also, given the fact that the molecular mechanism of CLA is through changing gene expression [12, 39], the differences in gene expression in several species can cause contradictory results. Regarding human studies, Belury and colleagues showed that plasma levels of CLA has been inversely associated with body weight and the trans-10, cis-12 isomer is responsible for this negative correlation and biological active responsible for weight change in people with type2 diabetes. Short duration of intervention in these studies may be one reason of elusive changes in anthropometric measurements, body fat and lean body mass. In the present study the daily intake of 3g of CLA capsules for eight weeks, did not occur significant changes in weight, BMI, waist circumference and body fat mass percentage. However, the results of this study is not comparable to the results of separated isomers, because the commercial CLA supplements contain the two active isomer in equal ratio, so each isomers differentially act on expression of related enzymes [12]. In long-term studies of 6 and 12 months using supplemental CLA isomers in equal ratio, weight loss and a reduction in body fat mass have been reported [41, 42]. It seems that appearing of CLA effects on weight and body composition, more time is needed. As in our study which lasted eight weeks, no changes in physical parameters were seen. However, in some three months and even six-week studies changes in body weight and fat mass have been seen [42, 43]. The differences in individuals’ response in expression of enzymes associated with energy expenditure and body composition, type of drugs and CLA dietary intake can justify the inconsistency in the results of studies. As this study was on glucose-lowering drug consumers that may interfere with the mechanisms of CLA in some metabolic pathways. Also, calculation of dietary CLA intake was not possible.
In this study, daily supplementation with 3 g of CLA capsules alone and combined with 100 IU of VitE for eight weeks compared to placebo, did not result in significant changes in serum triglycerides, total cholesterol, LDL, HDL cholesterol and apoB100. Some animal studies using pure CLA isomers have shown that cis-9, trans-11 isomer lead to lowering triglyceride levels and plasma free fatty acids and trans-10, cis-12 isomer lead to increasing plasma free fatty acids and LDL cholesterol [2] that is confirmed by human studies [21]. However, the results of equal ratio of isomers are contradictory. In a study on patients with type2 diabetes, taking 3g of CLA supplementation for 8 weeks, resulting in increased HDL2 cholesterol and decreased LDL to HDL cholesterol ratio [7], although in another study, reduced serum HDL cholesterol following 12 weeks of supplementation has been reported [44]. In other studies with 6 weeks and 6 months duration, no significant changes were shown in serum lipids and Apo proteins [33, 43]. Overall, the effects of commercial CLA capsules on lipids profiles are unknown. Further studies should be done in this regard to identify the mechanisms of CLA in regulating gene expression involved in lipid metabolism.
In our study, it was not shown any change in systolic and diastolic blood pressure. In two animal studies, CLA has reduced blood pressure [45, 46]. In another study, trans-10, cis-12 isomer lowered blood pressure and cis-9, trans-11 did not affect [47]. CLA effects on blood pressure could be related to the pathway of eicosanoids production. Inhibition of Arashidonic acid released from cell membranes and cyclooxygenase by CLA has been reported [11].
In our study, 3 g daily CLA capsules alone and combined with 100 IU of VitE for eight weeks did not affect any inflammatory parameters IL-1 beta, interleukin-6, TNF-α, CRP, Serum leptin and adiponectin. Animal studies show that trans-10, cis-12 isomer increases indicators of inflammation and cis-9; trans-11 is associated with reduction of inflammatory indicators [12]. In this way the CLA supplementation in rats leads to decreased TNF-α and in mice increased it [19]. In some human studies, CLA did not affect serum inflammatory parameters [7], but in the some others led to increase in these mediators [45], in the present study also no significant changes developed.
In one study, CLA supplementation reduced leptin and adiponectin [8] and in two other studies any changes are not mentioned [9, 28]. In the present study changes in serum leptin and adiponectin showed no statistically differences among the three groups.
In our study there was not seen any significant differences in serum fibrinogen and PAI-1 changes among three groups. Also, no information is regarding the effects of CLA supplementation on these variables.
In the present study 3 g of CLA capsules alone and combined with 100IU VitE did not make a difference in the oxidative stress index, MDA compared with placebo. In some studies, taking an active CLA isomer has increased lipid peroxidation indexes, including urinary excretion of isoprostaglandins [9, 21].
One limitation of this study is that estimation of dietary and serum CLA was not possible. Differences in dietary intake and thus serum CLA levels can affect patients’ responsiveness to supplementation. Also, the use of 2-d diet records may not accurately reflect changes in calories during intervention. Duration of this study was 8 weeks. It may be required longer time to appearance CLA effects.
Because of insufficient information regarding the molecular mechanisms of CLA in humans, human cell culture studies are suggested.
Conclusion
Overall, our results show that commercial CLA supplementation alone or combined with VitE for 8weeks, does not significantly affect glycemic indicators, body composition, lipid concentrations, indicators of inflammation, coagulation, leptin, adiponectin, MDA and blood pressure in type2 diabetes, but there was a significant trend to increase in MDA and decrease in apoB100 among CLA consumers.
Abbreviations
- ANCOVA:
-
Analysis of covariance
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- Cal:
-
Calorie
- CLA:
-
Conjugated linoleic acid
- CRP:
-
C - reactive protein
- CV:
-
Coefficient of variation
- EDTA:
-
Ethylenediaminetetraacetic Acid
- ELISA:
-
Enzyme Linked Immuno assay
- FFA:
-
Free fatty acid
- HbA1c:
-
Hemoglobin A1C
- HDL:
-
High density lipoprotein
- HOMA-IR:
-
Homeostasis model of assessment ratio- insulin resistance
- ISI:
-
Insulin sensitivity index
- LDL:
-
Low density lipoprotein
- LPL:
-
Lipoprotein lipase
- MDA:
-
Malondialdehyde
- PAI-1:
-
Plasminogen activator inhibitor-1
- PPAR:
-
Peroxisome proliferator-activated receptor
- QUICKI:
-
Quantitative insulin sensitivity check index
- RCT:
-
Randomized double blind, placebo-controlled trial
- RIES:
-
Research Institute for Endocrine Sciences body mass index
- T2D:
-
Type 2 diabetes
- TNF-α:
-
Tumor necrosis factor-alpha
- UCP2:
-
Mitochondrial uncoupling protein 2
- WHR:
-
Waist-to-hip ratio.
References
Aminot-Gilchrist DV, Anderson HD: Insulin resistance-associated cardiovascular disease: potential benefits of conjugated linoleic acid. Am J Clin Nutr 2004,79(6 Suppl):1159S-1163S. Jun
Bhattacharya A, Banu J, Rahman M, Causey J, Fernandes G: Biological effects of conjugated linoleic acids in health and disease. J Nutr Biochem 2006,17(12):789–810. 10.1016/j.jnutbio.2006.02.009
Jiang J, Wolk A, Vessby B: Relation between the intake of milk fat and the occurrence of conjugated linoleic acid in human adipose tissue. Am J Clin Nutr 1999,70(1):21–27.
Larsen TM, Toubro S, Gudmundsen O, Astrup A: Conjugated linoleic acid supplementation for 1 y does not prevent weight or body fat regain. Am J Clin Nutr 2006,83(3):606–612.
Wang YW, Jones PJ: Conjugated linoleic acid and obesity control: efficacy and mechanisms. Int J Obes Relat Metab Disord 2004,28(8):941–955. 10.1038/sj.ijo.0802641
Poirier H, Shapiro JS, Kim RJ, Lazar MA: Nutritional supplementation with trans-10, cis-12-conjugated linoleic acid induces inflammation of white adipose tissue. Diabetes 2006,55(6):1634–1641. Jun 10.2337/db06-0036
Moloney F, Yeow TP, Mullen A, Nolan JJ, Roche HM: Conjugated linoleic acid supplementation, insulin sensitivity, and lipoprotein metabolism in patients with type 2 diabetes mellitus. Am J Clin Nutr 2004,80(4):887–895.
Valeille K, Férézou J, Parquet M, Amsler G, Gripois D, Quignard-Boulangé A, Martin JC: The natural concentration of the conjugated linoleic acid, cis-9, trans-11, in milk fat has antiatherogenic effects in hyperlipidemic hamsters. J Nutr 2006,136(5):1305–1310.
Riserus U, Vessby B, Arnlov J, Basu S: Effects of cis-9, trans-11 conjugated linoleic acid supplementation on insulin sensitivity, lipid peroxidation, and proinflammatory markers in obese men. Am J Clin Nutr 2004,80(2):279–283.
Eyjolfson V, Spriet LL, Dyck DJ: Conjugated linoleic acid improves insulin sensitivity in young, sedentary humans. Med Sci Sports Exerc 2004,36(5):814–820.
Noto A, Zahradka P, Ryz NR, Yurkova N, Xie X, Taylor CG: Dietary conjugated linoleic acid preserves pancreatic function and reduces inflammatory markers in obese, insulin-resistant rats. Metabolism 2007, 56: 142–151. 10.1016/j.metabol.2006.09.009
de Roos B, Rucklidge G, Reid M, Ross K, Duncan G, Navarro MA, Arbones-Mainar JM, Guzman-Garcia MA, Osada J, Browne J, Loscher CE, Roche HM: Divergent mechanisms of cis9, trans11-and trans10, cis12-conjugated linoleic acid affecting insulin resistance and inflammation in apolipoprotein E knockout mice: a proteomics approach. FASEB J 2005,19(12):1746–1748.
Lin H, Boylston TD, Chang MJ, Luedecke LO, Shultz TD: Survey of the conjugated linoleic acid contents of dairy products. J Dairy Sci 1995,78(11):2358–2365. 10.3168/jds.S0022-0302(95)76863-1
Riserus U, Smedman A, Basu S, Vessby B: CLA and body weight regulation in humans. Lipids 2003,38(2):133–137. 10.1007/s11745-003-1043-7
Riserus U, Smedman A, Basu S, Vessby B: Metabolic effects of conjugated linoleic acid in humans: the Swedish experience. Am J Clin Nutr 2004,79(6 Suppl):1146S-1148S.
Riserus U, Vessby B, Arner P, Zethelius B: Supplementation with trans10cis12-conjugated linoleic acid induces hyperproinsulinaemia in obese men: close association with impaired insulin sensitivity. Diabetologia 2004,47(6):1016–1019.
Brown JM, McIntosh MK: Conjugated linoleic acid in humans: regulation of adiposity and insulin sensitivity. J Nutr 2003,133(10):3041–3046.
Raff M, Tholstrup T, Sejrsen K, Straarup EM, Wiinberg N: Diets rich in conjugated linoleic acid and vaccenic acid have no effect on blood pressure and isobaric arterial elasticity in healthy young men. J Nutr 2006,136(4):992–997.
Taylor CG, Zahradka P: Dietary conjugated linoleic acid and insulin sensitivity and resistance in rodent models. Am J Clin Nutr 2004,79(6 Suppl):1164S-1168S.
Belury MA, Mahon A, Banni S: The conjugated linoleic acid (CLA) isomer, t10c12-CLA, is inversely associated with changes in body weight and serum leptin in subjects with type 2 diabetes mellitus. J Nutr 2003,133(1):257S-260S.
Riserus U, Arner P, Brismar K, Vessby B: Treatment with dietary trans10cis12 conjugated linoleic acid causes isomer-specific insulin resistance in obese men with the metabolic syndrome. Diabetes Care 2002,25(9):1516–1521. 10.2337/diacare.25.9.1516
Gerber LM, Madhavan S, Alderman MH: Waist-to-hip ratio as an index of risk for hyperglycemia among hypertensive patients. Am J Prev Med 1987,3(2):64–68.
Rajeswari K, Lakshmi VV: Body composition of preadolescents by skinfold measurements and Body Stat 1500 Analyzer. Indian Pediatr 2006,43(12):1092–1093.
Montazeri A, Goshtasebi A, Vahdaninia M, Gandek B: The Short Form Health Survey (SF-36): translation and validation study of the Iranian version. Qual Life Res 2005,14(3):875–882. 10.1007/s11136-004-1014-5
Montazeri A, Harirchi AM, Shariati M, Garmaroudi G, Ebadi M, Fateh A: The 12-item General Health Questionnaire (GHQ-12): translation and validation study of the Iranian version. Health Qual Life Outcomes 2003, 1: 66. 10.1186/1477-7525-1-66
Baghdadchi J, Amani R, Khajeh Mugahi N: Assessment of the effects of breakfast on concentration span shortterm memory of school children. Journal of Iran University of medicine 2002,8(27):535–540.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985,28(7):412–419. 10.1007/BF00280883
Miyazaki Y, Pipek R, Mandarino LJ, DeFronzo RA: Tumor necrosis factor alpha and insulin resistance in obese type 2 diabetic patients. Int J Obes Relat Metab Disord 2003,27(1):88–94. 10.1038/sj.ijo.0802187
Vanhala P, Vanhala M, Kumpusalo E, Keinanen-Kiukaanniemi S: The quantitative insulin sensitivity check index QUICKI predicts the onset of type 2 diabetes better than fasting plasma insulin in obese subjects: a 5-year follow-up study. J Clin Endocrinol Metab 2002,87(12):5834–5837. 10.1210/jc.2002-020591
Shim WS, Kim SK, Kim HJ, Kang ES, Ahn CW, Lim SK, Lee HC, Cha BS: Decrement of postprandial insulin secretion determines the progressive nature of type-2 diabetes. Eur J Endocrinol 2006,155(4):615–622. 10.1530/eje.1.02249
Uwaifo GI, Fallon EM, Chin J, Elberg J, Parikh SJ, Yanovski JA: Indices of insulin action, disposal, and secretion derived from fasting samples and clamps in normal glucose-tolerant black and white children. Diabetes Care 2002,25(11):2081–2087. 10.2337/diacare.25.11.2081
Hovorka R, Chassin L, Luzio SD, Playle R, Owens DR: Pancreatic beta-cell responsiveness during meal tolerance test: model assessment in normal subjects and subjects with newly diagnosed noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1998,83(3):744–750. 10.1210/jc.83.3.744
Gaullier JM, Halse J, Høivik HO, Høye K, Syvertsen C, Nurminiemi M, Hassfeld C, Einerhand A, O’Shea M, Gudmundsen O: Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. Br J Nutr 2007,97(3):550–560. Mar 10.1017/S0007114507381324
Riserus U, Basu S, Jovinge S, Fredrikson GN, Arnlov J, Vessby B: Supplementation with conjugated linoleic acid causes isomer-dependent oxidative stress and elevated C-reactive protein: a potential link to fatty acid-induced insulin resistance. Circulation 2002,8;106(15):1925–1929.
Zethelius B, Byberg L, Hales CN, Lithell H, Berne C: Proinsulin is an independent predictor of coronary heart disease: Report from a 27-year follow-up study. Circulation 2002,7;105(18):2153–2158.
Pivatto I, Bustos P, Amigo H, Acosta AM, Arteaga A: Association between proinsulin, insulin, proinsulin/insulin ratio, and insulin resistance status with the metabolic syndrome. Arq Bras Endocrinol Metabol 2007,51(7):1128–1133. 10.1590/S0004-27302007000700016
Simon E, Macarulla MT, Churruca I, Fernandez A, Portillo MP: Trans – 10, cis -12 conjugated linoleic acid prevents adiposity but not insulin resistance induced by an atherogenic diet in hamsters. J Nutr Biochem 2006, 17: 126–131. 10.1016/j.jnutbio.2005.06.001
Gaullier J, Halse J, Hoye K, Kristiansen K, Fagertun H, Vik H: Supplementation with Conjugated Linoleic Acid for 24 Months Is Well Tolerated by and Reduces Body Fat Mass in Healthy, Overweight Humans. J Nutr 2005, 135: 778–784.
Nazare JA, de la Perrière AB, Bonnet F, Desage M, Peyrat J, Maitrepierre C, Louche-Pelissier C, Bruzeau J, Goudable J, Lassel T, Vidal H, Laville M: Daily intake of conjugated linoleic acid-enriched yoghurts: effects on energy metabolism and adipose tissue gene expression in healthy subjects. Br J Nutr 2007,97(2):273–280. 10.1017/S0007114507191911
Taylor JS, Williams SR, Rhys R, James P, Frenneaux MP: Conjugated linoleic acid impairs endothelial function. Arterioscler Thromb Vasc Biol 2006,26(2):307–312.
Watras AC, Buchholz AC, Close RN, Zhang Z, Schoeller DA: The role of conjugated linoleic acid in reducing body fat and preventing holiday weight gain. Int J Obes (Lond) 2007,31(3):481–487. 10.1038/sj.ijo.0803437
Laso N, Brugué E, Vidal J, Ros E, Arnaiz JA, Carné X, Vidal S, Mas S, Deulofeu R, Lafuente A: Effects of milk supplementation with conjugated linoleic acid (isomers cis-9, trans-11 and trans-10, cis-12) on body composition and metabolic syndrome components. Br J Nutr 2007,98(4):860–867.
Colakoglu S, Colakoglu M, Taneli F, Cetinoz F, Turkmen M: Cumulative effects of conjugated linoleic acid and exercise on endurance development, body composition, serum leptin and insulin levels. J Sports Med Phys Fitness 2006,46(4):570–577.
Steck SE, Chalecki AM, Miller P, Conway J, Austin GL, Hardin JW, Albright CD, Thuillier P: Conjugated linoleic acid supplementation for twelve weeks increases lean body mass in obese humans. J Nutr 2007,137(5):1188–1193.
Nagao K, Inoue N, Wang YM, Yanagita T: Conjugated linoleic acid enhances plasma adiponectin level and alleviates hyperinsulinemia and hypertension in Zucker diabetic fatty (fa/fa) rats. Biochem Biophys Res Commun 2003,17;310(2):562–566.
Inoue N, Nagao K, Hirata J, Wang YM, Yanagita T: Conjugated linoleic acid prevents the development of essential hypertension in spontaneously hypertensive rats. Biochem Biophys Res Commun 2004,15;323(2):679–684.
Nagao K, Inoue N, Wang YM, Hirata J, Shimada Y, Nagao T, Matsui T, Yanagita T: The 10trans,12cis isomer of conjugated linoleic acid suppresses the development of hypertension in Otsuka Long-Evans Tokushima fatty rats. Biochem Biophys Res Commun 2003,20;306(1):134–138.
Acknowledgement
This study was supported by a research grant from Endocrine Research Center, Shahid Beheshti University of Medical Sciences. We are indebted to kind collaboration of several endocrinology specialists. We express our gratitude to the staffs of Laboratory at Endocrine Research Center, for their skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
There are not any financial or non-financial competing interests (political, personal, religious, ideological, academic, intellectual, commercial, or any other) to declare in relation to this manuscript.
Authors’ contributions
ZS conceived of the study, carried out its designing and implementation, drafted the manuscript, and performed the statistical analysis. FAT participated in the design of the study and revised the manuscript. NS participated in the design of the study and revised the manuscript. MH conceived of the study, coordinated implementation, and participated in analysis of data. All authors read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Shadman, Z., Taleban, F.A., Saadat, N. et al. Effect of conjugated linoleic acid and vitamin E on glycemic control, body composition, and inflammatory markers in overweight type2 diabetics. J Diabetes Metab Disord 12, 42 (2013). https://doi.org/10.1186/2251-6581-12-42
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6581-12-42