Abstract
Background
Since both dietary carbohydrate and fatty acids separately affect carbohydrate metabolism, how dietary macronutrients distribution may have different effects on carbohydrate metabolism pathways and regulation of blood glucose especially in diabetic patients.
Methods
In this cross-sectional study 750 type 2 diabetic patients (261 men and 489 women, aged 35–65 years),who at least two years were followed in Diabetes and Metabolic disease Clinic of Tehran University of Medical Sciences, were recruited according to inclusion and exclusion criteria by simple sampling. Dietary data were collected by a validated food frequency questionnaire. Other variables were anthropometric measurements, Stress, physical activity level, Biochemical analyses including fasting and postprandial plasma glucose, Glycated hemoglobin, total cholesterol, low and high density lipoproteins, triglycerides and 25-hydoxy D3. Linear regression models were used to assess the association of covariates with the mean concentrations of HbA1C in quintiles and multivariate linear regression model was used to distinguish the impacts of dietary macronutrient composition of the diet.
Results
Carbohydrate and dietary fiber intakes were inversely (P: < 0.0001 and 0.003 respectively) and dietary amount and proportion of saturated, mono-unsaturated and poly-unsaturated fatty were positively (P: < 0.0001, 0.03, 0.01 and 0.01 respectively) associated with HbA1C concentrations.
Multivariate linear regression macronutrient density model that controlled for age, sex, diabetes duration and calorie intake showed that carbohydrate was inversely associated with HbA1C (P < 0.0001, R2 = 15%). Results were also the same in the other three models adjusted for stress and exercise levels in model 2, waist circumference and sum of meals in model 3 and serum triglyceride and 25-hydroxy vitamin D in model 4(P < .0001, <.0001 and 0.0003 respectively). Calorie intake of 25 Kcal/body weight was identified as a cut of point of the negative effect of dietary carbohydrate and 30 for the positive effect of fat on HbA1c respectively (P = 0.04 and 0.03). Moreover, carbohydrate intake was positively (β = 0.08, P = 0.01) and protein (β = −0.04, P < 0.0001), SAFA (β = −0.04, P < 0.0001) and MUFA (β = −0.02, 0.07) proportion were negatively associated with increment in calorie intake.
Conclusion
This study showed that the substitution of fat for carbohydrate is associated with low concentrations of HbA1c in high calorie consuming type 2 diabetic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Medical nutrition therapy is an integral component of diabetes management [1]. In the process of designing an individualized diet, after estimating energy requirement, determining the distribution of dietary macronutrients (percent of carbohydrate, fat and protein of total calorie) is the next step. The current American Diabetes Association (ADA) recommendations suggest a range of carbohydrate intake of between 45% and 65% of total calories, protein 10–20%, total fat ≤ 30%, saturated fatty acids (SFAs) <7%, mono-unsaturated fatty acids (MUFAs) up to 20% and poly unsaturated fatty acids (PUFAs) up to 10% of total calorie [2].
In diabetes management, carbohydrate modification is the first step recommendation and the more emphasis is on it [3], but each macronutrient may involve in carbohydrate metabolism by different biochemical pathways. Since dietary fatty acids (FAs) play a key role in the cell membrane and insulin sensitivity, some fatty acids may induce development of insulin resistance and consequently affect diabetes metabolic control. Observational studies assessing serum or tissues fatty acid composition suggest that insulin resistance is associated with relatively high intakes of saturated fat (e.g. palmitic acid) and low intakes of polyunsaturated fat (e.g. linoleic acid), findings that are supported by recent clinical data [4].
It has been emphasized the ability of low carbohydrate diets to improve glycemic control, hemoglobin A1C (HbA1c) and to reduce medication [5]. In a 2-year follow-up study, HbA1c levels were significantly improved in the carbohydrate restricted diet [6]. A number of short duration trials have demonstrated improvements in insulin resistance with a high total and mono unsaturated fat diet [7–10]; whereas, some others have shown high carbohydrate diets are associated with a better glycemic control [11–13]. In a study, the magnitude of blood glucose decrements was similar after consuming two low-caloric diets (high-glycemic index and the high-fat/low-carbohydrate diets) [14]. Also, several studies have examined the effects of dietary macronutrients on postprandial glucose [15–19] not glycated hemoglobin as a diabetes control indicator.
In several studies so far have been done, the association of dietary macronutrients and calorie intake with the risk of diabetes has been explored. For example, regarding to the type 2 diabetes, high caloric diets were associated to increase [5], high carbohydrate, in some cases, diet was associated to increase [6–8] and in others, associated to decrease [9–12], and diets high in glycemic index and glycemic load were associated to increase [6, 13–16] the risk.
So the question is whether only carbohydrate containing food groups should be taken into diabetes management programs or the amount and type of dietary fat and oil also should be considered. Furthermore, the extent of recommended ranges of macronutrients such as carbohydrate may cause extreme values show opposite effects. Moreover, considering differences in genetic variations, dietary patterns, eating habits and etc. among populations, the proportion of macronutrients in calorie intake may have several effects on glucose metabolism [20, 21].
Based on our best knowledge, no study has evaluated the role of dietary macronutrients on glycemic control in Iranian diabetic patients. The aim of this study was to examine the association of dietary composition of macronutrients with HbA1c and blood glucose in type 2 diabetic patients.
Methods
This cross-sectional study was approved by the Endocrinology and Metabolism Research Center ethic committee (EC-00146).
Subjects
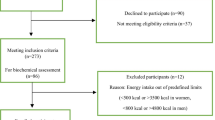
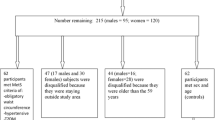
In this study 750 type 2 diabetic patients (261 men and 489 women, aged 35–65 years),who at least two years have been followed in Diabetes and Metabolic disease Clinic of Tehran University of Medical Sciences, were recruited according to inclusion and exclusion criteria by simple sampling. Inclusion criteria were 35–65 years old, diagnosis of diabetes after 30 years old, and diabetes mellitus for more than 5 years. Exclusion criteria were insulin therapy, myocardial infarction, angina pectina, stroke, acute liver or renal disease during the past year, chronic inflammation, thyroid disease, vegetarianism, alcohol consumption and pregnancy). At the beginning, the protocol and the aim of the study were fully explained to the subjects and written informed consent was obtained each volunteers.
Dietary data
A validated 168-item food frequency questionnaire (FFQ) [22] was completed by trained dietitians by face-to-face interviews to assess the usual dietary intakes of participants. To estimate portion sizes, a set of 2-dimensional shapes and in some cases 3-dimensional food models was used. Amounts were documented in household units, eg, teaspoons, cups, and ounces. Data were analyzed for total calorie intake, carbohydrate, protein, saturated and unsaturated fatty acids using adjusted N4 software (Nutritionist: version 4.0; Tinuviel Software, Warrington, United Kingdom).
Anthropometric measurements
Height was measured with a wall-mounted stadiometer to the nearest 0.1 cm. Weight was determined to the nearest 0.1 kg on the same properly calibrated electronic digital scale, without shoes, with minimal clothing, and after voiding. Two measurements were obtained and averaged; with a third measurement taken if the first two differed by 0.1. Body mass index (BMI) was estimated as the ratio of body weight to height squared and expressed as kg/m2. Waist circumference was determined by placing a measuring tape in a horizontal plane around the abdomen just above the right iliac crest. Three measurements were made to the nearest 0.1 cm and averaged.
Physical activity and stress
Physical activity level was assessed by a validated questionnaire in which nine different metabolic equivalent (MET) levels were ranged on a scale from sleep/rest (0.9 METs) to high-intensity physical activities (> 6 METs) [23]. Over 24 hours, for each activity level, the MET value was multiplied by the time spent at that particular level. The sum of MET-time at each level and, finally, its average was calculated dividing by 24. Measurement of the three related negative emotional states of depression, anxiety and tension/stress was done by the self report validated Depression Anxiety Stress Scales (DASS-42) [24].
Biochemical tests
Three ml 12-hour fasting state and three ml postprandial brachial vein blood samples were taken and collected into EDTA containing tubes. Samples were centrifuged at 3000 g for 10 minutes and 4°C, and promptly plasma aliquoted into separate tubes that were stored at −75°C until analyzed. One ml was stored as whole blood to A1C measurements. Plasma glucose concentration was measured by fluorometric method according glucose oxidase principle (Glucose determination kit, Parsazmun, Tehran, Iran) through auto-analyzer instrument (Hitachi 902, Roche, Basel, Switzerland). Glycated hemoglobin was determined on whole blood sample by HbA1c Pink Kit and DS5 analyzer. The intra assay coefficient of variation (CV%) for glucose and HbA1c were 1.4% and 3.7%, and the inter assay coefficient of variation were 1.9% and 3.5% respectively. Serum triglyceride (TG), total cholesterol (TC), LDL (low density lipoprotein) and HDL (high density lipoprotein) cholesterol were measured by the related biochemical kits (Parsazmun, Tehran, Iran) by the auto-analyzer (Hitachi 902, Roche, Basel, Switzerland). The intra assay CV% were 4.1, 1.3, 2.0 and 1.8 and the inter assay CV% were 4.5, 2.0, 2.3, and 2.0 respectively. Serum 25-hydroxy vitamin D was measured by Enzyme-linked immunosorbent assay (ELAISA) (IDS, Boldon, UK). The intra assay CV% was 5.4% and inter assay CV% 5.5%.
Statistical analysis
All statistical analyses were performed by SPSS software (version 16.0; SPSS Inc, Chicago) and a p-value < 0.05 showed statistical significance. One sample T test was used to compare participants’ dietary intake and quintiles with common nutritional recommendations. In each case that recommendation was as a range, the mean value was used to comparison with each quintile value. First, linear regression models adjusted for age, sex and diabetes duration were used to assess the association of covariates with the mean concentrations of HbA1C in quintiles. In the next step, multivariate linear regression model including the percentage of energy intake from carbohydrate and protein was used to distinguish the impacts of macronutrient composition of the diet and energy intake per body weight and some other variables that may affect diabetes control. Because protein intake is often stable, these models can show the effect of submitting dietary carbohydrate for fat while calorie intake and other covariates are constant.
To determine foods which are responsible for changing in HbA1C; fats, oils and other fatty acid containing foods that have correlation with HbA1C (based on Pearson correlation coefficient) were entered into the linear regression model. Pearson correlation was used for assessing the association between dietary macronutrients and fiber. Then to find the true effect of carbohydrate on HbA1c, fiber was added to the model. Since total calorie intake is one of the most effective factors on glucose level control [2, 25], we compared the relation (regression coefficient) of macronutrients and HbA1c between 2 groups of daily calorie intake.
To identify that by increasing of calorie intake in studied population the proportion of which macronutrient is increased, analysis of regression was used for each macronutrient.
Results
Table 1 shows the basic characteristic of patients. This data show that the mean BMI and glucose control are higher than normal.
Participants’ dietary intakes in quintiles and its comparison with common nutritional recommendations have been shown in Table 2. In each case that recommendation was as a range the mean recommended range was used to comparison with each quintile value and in the cases that the recommendation was not as a range, the specified value was used. These data show high consumption of calorie in 80%, total and saturated fat in 60% and 80%, and low consumption of fiber in 60% of the participants (Table 2).
Age, sex and duration of diabetes adjusted estimates of the mean concentration of HbA1C within the quintiles of stress, physical activity level and dietary variables showed that carbohydrate and dietary fiber intakes were inversely (P < 0.0001 and 0.003 respectively) and dietary amount and type of fat were positively (P:<0.0001, 0.03, 0.01 and 0.01 for the percentages of total fat, SAFA, MUFA and PUFA from calorie respectively) associated with HbA1C concentrations (Table 3).
Multivariate linear regression macronutrient density model that controlled for age, sex, DD and calorie intake showed that carbohydrate was inversely associated with HbA1C (P < 0.0001, R2 = 15%). Results were also the same in the other three models adjusted for stress and exercise levels in model 2, waist circumference and sum of meals in model 3 and serum triglyceride and 25-hydroxy vitamin D in model 4(P < .0001, <.0001 and 0.0003 respectively) (Table 4). Analysis of regression adjusted for age, sex, and DD showed no association between the source of carbohydrate (e.g. whole and refined grains, legumes, beans, and fruits) and HbA1c.
Pearson correlation showed that dietary carbohydrate was positively (r = 0.78, p < 0.0001) and protein (r = −0.07, p = 0.13) and fat were negatively associated (r = −0.23, p < 0.0001) to dietary fiber. Controlling for fiber in macronutrients density regression model showed a reduction of carbohydrate regression coefficient (P = 0.001, β = −0.087).
Among all of fat containing food items, animal fat, Hydrogenated oils, High fat dairy products, Butter, cream, and Ground meat were positively associated with HbA1C variations (data not shown). In the next step that this determined food items were entered in a regression model showed that consumption of hydrogenated vegetable oils and ground meat were significantly associated with HbA1C (P < 0.0001 and P = 0.007 respectively)(Table 5).
Then we compared the regression coefficients of macronutrients with HbA1c between 2 groups based on calorie intake classification because it was assumed that the effect of macronutrients on blood glucose may be affected by a cut of point of calorie intake.
Table 6 shows that the inverse effect of carbohydrate on HbA1c at the levels of calorie intake lower than 25 kcal/body weight significantly is stronger than higher levels of calorie intake (P = 0.04). Also, calorie intake of 30 Kcal/body weight was identified as a cut of point of the positive effect of dietary total fat on HbA1c (P = 0.03). In contrast, association of dietary SAFA with HbA1c was stronger at the levels higher than the cut of point of 27 Kcal/Kg (P = 0.04). In regard to dietary MUFA, PUFA and fiber no significant differences were identified at any levels of calorie intake (Table 6).
Multivariate regression model showed that carbohydrate proportion was positively (β = 0.08, P = 0.01) and protein (β = −0.04, P < 0.0001), SAFA (β = −0.04, P < 0.0001) and MUFA (β = −0.02, 0.07) proportion were negatively associated with increment in calorie intake (Table 7).
Discussion
This study showed that in type 2 diabetic patients on oral hypoglycemic agents, the substitution of fat for carbohydrate (ie, diets high in carbohydrate versus low in fat and saturated fat) is associated with low concentrations of HbA1c independent of age, sex, diabetes duration, stress and physical activity level, waist circumference, calorie intake, sum of daily meals, serum triglyceride and 25(OH) calciferol. By inserting the dietary fiber intake in the regression model, the regression coefficient was decreased but still significant.
Noticeable, in comparison to dietary macronutrients distribution recommendation, the intakes of total and saturated fat were high, dietary fiber was low and carbohydrate was in the recommended range. This composition of the diet has been observed in another studies on Iranian diabetics [26], the population based study of Tehran Lipid and Glucose Study [27] and in some other studies [12, 28–31]. Also, results in Table 7 showed that in this studied Iranian patients along with increment in calorie intake, among all dietary macronutrients, proportion of dietary carbohydrate and PUFA increased. In the other words, increment in calorie intake was associated to the intake of foods high in carbohydrate especially grains (e.g. bread and rice) and greasy foods prepared with high PUFA vegetable oils. Furthermore, the negative association between calorie intake and dietary protein and SAFA showed that our patients on high calorie diets, because of personal preferences or limitation in financial ability, did not increase the consumption of high protein containing foods (e.g. meat and dairy products) and foods high in SAFA.
In a population-based study on non-diabetic persons, total dietary fat and saturated fat were positively associated with HbA1c; but the association of PUFA and MUFA was not statistically significant [32]. Several studies have indicated beneficial effect of high MUFA diets, for example Mediterranean diet in prevention and managing diabetes [9, 33–35]. One meta-analysis including long-term trials with duration of at least 6 months comparing high-MUFA (>12% of total energy content) versus low-MUFA (≤12% of total energy content) diets on glycemic control in participants with abnormal glucose metabolism found that high MUFA diets appear to be effective in reducing HbA1c [36]. Energy restriction was applied in seven of nine included trials.
In a study on overweight subjects with relatively high serum insulin, low carbohydrate and low fat hypocaloric diets both made a reduction in serum glucose but the reduction was not statistically significant. However, the low carbohydrate diet led to an improvement in insulin sensitivity [37]. These results were constant on diabetics; so there was a trend toward a greater decrease in mean fasting glucose level and glycosylated hemoglobin values and an improvement in insulin sensitivity of diabetic subjects on the hypocaloric low-carbohydrate diet, as compared with those on the low-fat diet [38]. It should be noted that the participants’ diet in these two studies was associated with reduction in calorie intake. However, the energy intake in our study was changeless during the past year and was higher than recommended values. It seems that the beneficial effects of low carbohydrate and low fat diets in these two studies are attributable to the calorie restriction. Such an effect was not involved in our study.
In contrast to the commonly held view, this study showed that type 2 diabetic patients on high carbohydrate and low saturated fat diet have a better blood glucose control. Our results is according to the conclusion of two meta-analysis of the evidence that has shown high carbohydrate, high fiber diets compared to moderate carbohydrate, low fiber diets are associated with lower values for fasting, postprandial and average plasma glucose; hemoglobin A1c[39, 40]. This effect may be partly explained by carbohydrate and lipid metabolism pathways. Carbohydrate as the easiest to break down is the body proffered energy source. Carbohydrate effect in stimulating insulin secretion leads to increase in carbohydrate, but a decrease in fat oxidation [41]. So, it can be expressed that fat oxidation is determined primarily by the gap between total energy expenditure and the amount of energy ingested in the form of carbohydrate and protein, rather than by the amount of fat consumed [42]. Indeed, it seems that the effect of dietary macronutrient composition on several aspects of metabolic control may be the most important in a high calorie diet compared to low calorie or iso-caloric diet; because in low calorie or iso-caloric diet all of ingested and absorbed macronutrients should be oxidized to supply body needs. But, if the calorie intake is more than energy expenditure, more dietary fat may remain and induce weight gain, change cell membrane fatty acid composition and increase insulin resistance [33]. Also, it has been determined that saturated fatty acid oxidation rate is slower than unsaturated [43]. In the other word, dietary saturated fat has more opportunities to enter cell membrane, affect membrane fluidity, and promote insulin resistance.
In our study, the reason of no significant relationship between energy intake and HbA1c might be due to the increment of carbohydrate proportion of the diet following to increment in caloric intake, that high carbohydrate may attenuate the effects of high calorie intake on blood glucose control.
In addition, analysis of data showed that calorie intakes of 25 and 30 kcal/kg body weight were respectively the cut off points of the effects of carbohydrate and total fat on HbA1c; so, the association coefficients of dietary carbohydrate or fat with HbA1c were significantly higher in the lower values. In respect to dietary saturated fat, this association is more pronounced at higher calorie intake levels with cut off point of 27 kcal/kg body weight.
When caloric intake exceeds 27 kcal/kg body weight, dietary saturated fatty acids would probably replace in cells membrane, altering insulin receptors and insulin secretion, so promoting insulin resistance.
Other beneficial effects of high carbohydrate diet in our study may be related to high contents of dietary fiber, Fructo-oligosaccharides, resistant starch and indigestible carbohydrates that may increase peripheral insulin sensitivity and insulin secretion and decrease glucose release of the liver [44–47].
Conclusions
In this study both BMI and calorie intakes were more than appropriate levels regard to Participants’ characteristics. Furthermore, more total and saturated fat consumption may be responsible for failure to control blood glucose. Also, it appears that diabetic’s diet, which consuming high calorie diets should be high in carbohydrate to fasilate improvement in glycemic control.
Abbreviations
- ADA:
-
American diabetes association
- BMI:
-
Body mass index
- CV:
-
Coefficient of variation
- DASS:
-
Depression anxiety stress scales
- EDTA:
-
Ethylenediaminetetraacetic acid
- ELAISA:
-
Enzyme-linked immunosorbent assay
- FFQ:
-
Food frequency questionnaire
- FAs:
-
Fatty acids
- HbA1c:
-
Hemoglobin A1C
- HDL:
-
High density lipoprotein
- LDL:
-
Low density lipoprotein
- MET:
-
Metabolic equivalent
- MUFAs:
-
Mono-unsaturated fatty acids
- PUFAs:
-
Poly unsaturated fatty acids
- SFAs:
-
Saturated fatty acids
- SPSS:
-
Statistical package for the social sciences
- TG:
-
Triglyceride
- TC:
-
Total cholesterol.
References
Lindstrom J, Eriksson JG, Valle TT, Aunola S, Cepaitis Z, Hakumaki M, et al.: Prevention of diabetes mellitus in subjects with impaired glucose tolerance in the Finnish Diabetes Prevention Study: results from a randomized clinical trial. J Am Soc Nephrol 2003,14(Suppl 2):108–113.
Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, et al.: Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008,31(Suppl 1):S61-S78.
Franz MJ: Medical nutrition therapy for diabetes mellitus and hypoglycemia of nondiabetic origin. 12th edition. Edited by: Mahan LK, Escott-Stump S. Canada: Saunders; 2008.
Riserus U: Fatty acids and insulin sensitivity. Curr Opin Clin Nutr Metab Care 2008, 11: 100–105. 10.1097/MCO.0b013e3282f52708
Feinman RD, Volek JS: Carbohydrate restriction as the default treatment for type 2 diabetes and metabolic syndrome. Scand Cardiovasc J 2008, 42: 256–263. 10.1080/14017430802014838
Haimoto H, Iwata M, Wakai K, Umegaki H: Long-term effects of a diet loosely restricting carbohydrates on HbA1c levels, BMI and tapering of sulfonylureas in type 2 diabetes: a 2-year follow-up study. Diabetes Res Clin Pract 2008, 79: 350–356. 10.1016/j.diabres.2007.09.009
Rallidis LS, Lekakis J, Kolomvotsou A, Zampelas A, Vamvakou G, Efstathiou S, et al.: Close adherence to a Mediterranean diet improves endothelial function in subjects with abdominal obesity. Am J Clin Nutr 2009, 90: 263–268. 10.3945/ajcn.2008.27290
Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, et al.: Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med 2006, 145: 1–11. 10.7326/0003-4819-145-1-200607040-00004
Brehm BJ, Lattin BL, Summer SS, Boback JA, Gilchrist GM, Jandacek RJ, et al.: One-year comparison of a high-monounsaturated fat diet with a high-carbohydrate diet in type 2 diabetes. Diabetes Care 2009, 32: 215–220.
Schwingshackl L, Strasser B: High-MUFA diets reduce fasting glucose in patients with type 2 diabetes. Ann Nutr Metab 2012, 60: 33–34. 10.1159/000335162
Komiyama N, Kaneko T, Sato A, Sato W, Asami K, Onaya T, et al.: The effect of high carbohydrate diet on glucose tolerance in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2002, 57: 163–170. 10.1016/S0168-8227(02)00053-0
Delahanty LM, Nathan DM, Lachin JM, Hu FB, Cleary PA, Ziegler GK, et al.: Association of diet with glycated hemoglobin during intensive treatment of type 1 diabetes in the Diabetes Control and Complications Trial. Am J Clin Nutr 2009, 89: 518–524. 10.3945/ajcn.2008.26498
Allick G, Bisschop PH, Ackermans MT, Endert E, Meijer AJ, Kuipers F, et al.: A low-carbohydrate/high-fat diet improves glucoregulation in type 2 diabetes mellitus by reducing postabsorptive glycogenolysis. J Clin Endocrinol Metab 2004, 89: 6193–6197. 10.1210/jc.2004-1041
Ferland A, Brassard P, Lemieux S, Bergeron J, Bogaty P, Bertrand F, et al.: Impact of high-fat/low-carbohydrate, high-, low-glycaemic index or low-caloric meals on glucose regulation during aerobic exercise in Type 2 diabetes. Diabet Med 2009, 26: 589–595. 10.1111/j.1464-5491.2009.02734.x
Alssema M, Schindhelm RK, Dekker JM, Diamant M, Nijpels G, Teerlink T, et al.: Determinants of postprandial triglyceride and glucose responses after two consecutive fat-rich or carbohydrate-rich meals in normoglycemic women and in women with type 2 diabetes mellitus: the Hoorn Prandial Study. Metabolism 2008, 57: 1262–1269. 10.1016/j.metabol.2008.04.022
Alssema M, Schindhelm RK, Rijkelijkhuizen JM, Kostense PJ, Teerlink T, Nijpels G, et al.: Meal composition affects insulin secretion in women with type 2 diabetes: a comparison with healthy controls. The Hoorn prandial study. Eur J Clin Nutr 2009, 63: 398–404. 10.1038/sj.ejcn.1602953
De Natale C, Annuzzi G, Bozzetto L, Mazzarella R, Costabile G, Ciano O, et al.: Effects of a plant-based high-carbohydrate/high-fiber diet versus high-monounsaturated fat/low-carbohydrate diet on postprandial lipids in type 2 diabetic patients. Diabetes Care 2009, 32: 2168–2173. 10.2337/dc09-0266
Pearce KL, Noakes M, Keogh J, Clifton PM: Effect of carbohydrate distribution on postprandial glucose peaks with the use of continuous glucose monitoring in type 2 diabetes. Am J Clin Nutr 2008, 87: 638–644.
Powers MA, Cuddihy RM, Wesley D, Morgan B: Continuous glucose monitoring reveals different glycemic responses of moderate- vs high-carbohydrate lunch meals in people with type 2 diabetes. J Am Diet Assoc 2010, 110: 1912–1915. 10.1016/j.jada.2010.09.010
Hudgins LC, Baday A, Hellerstein MK, Parker TS, Levine DM, Seidman CE, et al.: The effect of dietary carbohydrate on genes for fatty acid synthase and inflammatory cytokines in adipose tissues from lean and obese subjects. J Nutr Biochem 2008, 19: 237–245. 10.1016/j.jnutbio.2007.02.013
Venema K: Role of gut microbiota in the control of energy and carbohydrate metabolism. Curr Opin Clin Nutr Metab Care 2010, 13: 432–438. 10.1097/MCO.0b013e32833a8b60
Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi F: Dairy consumption is inversely associated with the prevalence of the metabolic syndrome in Tehranian adults. Am J Clin Nutr 2005, 82: 523–530.
Kelishadi R, Rabiee K, Khosravi A, Famori F, Sadeghi M, Roohafza H: Assessment of physical activity in adolescents of Isfahan. J Shahrekord Univ Med Sci 2004, 3: 55–65. (in Persian)
Lovibond PF, Lovibond SH: The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther 1995, 33: 335–343. 10.1016/0005-7967(94)00075-U
UK Prospective Diabetes Study 7: Response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism 1990, 39: 905–912.
Shirinzadeh M, Shakerhosseini R, Hoshiyar rad A: Nutritional Value Assessment and Adequacy of Dietary Intake in Type 2 Diabetic Patients. J Diabetes Metab Disord 2009,11(1):25–32. (in Persian)
Mirmiran P, Esmaillzadeh A, Azizi F: Diet composition and body mass index in Tehranian adults. Asia Pac J Clin Nutr 2006, 15: 224–230.
Helmer C, Bricout H, Gin H, Barberger-Gateau P: Macronutrient intake and discrepancy with nutritional recommendations in a group of elderly diabetic subjects. Br J Nutr 2008, 99: 632–638.
Toeller M, Klischan A, Heitkamp G, Schumacher W, Milne R, Buyken A, et al.: Nutritional intake of 2868 IDDM patients from 30 centres in Europe. EURODIAB IDDM Complications Study Group. Diabetologia 1996, 39: 929–939. 10.1007/BF00403912
Gauthier-Chelle K, Mennen L, Arnault N, Rigalleau V, Hercberg S, Gin H: Comparison of the diet of self-declared diabetics with non-diabetic patients in the SU.VI.MAX study: did the diabetics modify their nutritional behavior? Diabetes Metab 2004, 30: 535–542. 10.1016/S1262-3636(07)70152-0
Shimakawa T, Herrera-Acena MG, Colditz GA, Manson JE, Stampfer MJ, Willett WC, et al.: Comparison of diets of diabetic and nondiabetic women. Diabetes Care 1993, 16: 1356–1362. 10.2337/diacare.16.10.1356
Harding AH, Sargeant LA, Welch A, Oakes S, Luben RN, Bingham S, et al.: Fat consumption and HbA(1c) levels: the EPIC-Norfolk study. Diabetes Care 2001, 24: 1911–1916. 10.2337/diacare.24.11.1911
Galgani JE, Uauy RD, Aguirre CA, Diaz EO: Effect of the dietary fat quality on insulin sensitivity. Br J Nutr 2008, 100: 471–479. 10.1017/S0007114508894408
Mediterranean diet and type 2 diabetes risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study: The InterAct project. Diabetes Care 2011, 34: 1913–1918.
Hodge AM, English DR, Itsiopoulos C, O’Dea K, Giles GG: Does a Mediterranean diet reduce the mortality risk associated with diabetes: evidence from the Melbourne Collaborative Cohort Study. Nutr Metab Cardiovasc Dis 2011, 21: 733–739. 10.1016/j.numecd.2010.10.014
Schwingshackl L, Strasser B, Hoffmann G: Effects of monounsaturated fatty acids on glycaemic control in patients with abnormal glucose metabolism: a systematic review and meta-analysis. Ann Nutr Metab 2011, 58: 290–296. 10.1159/000331214
Meckling KA, O’Sullivan C, Saari D: Comparison of a low-fat diet to a low-carbohydrate diet on weight loss, body composition, and risk factors for diabetes and cardiovascular disease in free-living, overweight men and women. J Clin Endocrinol Metab 2004, 89: 2717–2723. 10.1210/jc.2003-031606
Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al.: A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 2003, 348: 2074–2081. 10.1056/NEJMoa022637
Anderson JW, Randles KM, Kendall CW, Jenkins DJ: Carbohydrate and fiber recommendations for individuals with diabetes: a quantitative assessment and meta-analysis of the evidence. J Am Coll Nutr 2004, 23: 5–17. 10.1080/07315724.2004.10719338
Post RE, Mainous AG 3rd, King DE, Simpson KN: Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Board Fam Med 2012, 25: 16–23. 10.3122/jabfm.2012.01.110148
Flatt JP, Ravussin E, Acheson KJ, Jequier E: Effects of dietary fat on postprandial substrate oxidation and on carbohydrate and fat balances. J Clin Invest 1985, 76: 1019–1024. 10.1172/JCI112054
Flatt JP: Opposite effect of variations in food intake on carbohydrate and fat oxidation in ad libitum fed mice. J Nutr Biochem 1991, 2: 186–192. 10.1016/0955-2863(91)90015-W
Leyton J, Drury PJ, Crawford MA: Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. Br J Nutr 1987, 57: 383–393. 10.1079/BJN19870046
Fukagawa NK, Anderson JW, Hageman G, Young VR, Minaker KL: High-carbohydrate, high-fiber diets increase peripheral insulin sensitivity in healthy young and old adults. Am J Clin Nutr 1990, 52: 524–528.
Kiehm TG, Anderson JW, Ward K: Beneficial effects of a high carbohydrate, high fiber diet on hyperglycemic diabetic men. Am J Clin Nutr 1976, 29: 895–899.
Brunzell JD, Lerner RL, Hazzard WR, Porte D Jr, Bierman EL: Improved glucose tolerance with high carbohydrate feeding in mild diabetes. N Engl J Med 1971, 284: 521–524. 10.1056/NEJM197103112841004
Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al.: Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58: 1509–1517. 10.2337/db08-1637
Acknowledgments
We thank Dr. Mohammad Abbasi and Afsaneh Vosoogh who provided and coordinated biochemical analysis on behalf of laboratory of endocrine and metabolism research center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Any of this article authors do not have received reimbursements, fees, funding, or salary from an organization that may in any way gain or lose financially from the publication of this manuscript.
Any of this article authors do not hold any stocks or shares in an organization that may in any way gain or lose financially from the publication of this manuscript.
Any of authors are not applying for any patents relating to the content of this manuscript.
Any of this article authors do not have received reimbursements, fees, funding, or salary from an organization that holds or has applied for patents relating to the content of the manuscript.
Do you have any other financial competing interests?
There are not any non-financial competing interests (political, personal, religious, ideological, academic, intellectual, commercial or any other) to declare in relation to this manuscript.
Authors’ contributions
ZS conceived of the study, carried out its designing, coordinated the implementation, drafted the manuscript, and performed the statistical analysis. MK participated in the design of the study and revised the manuscript. NP participated in acquisition of data and revised the manuscript. MA participated in acquisition of data and revised the manuscript. MO participated in analysis and interpretation of data and revised the manuscript. BL participated in the design of the study and revised the manuscript. SH participated in the design of the study and revised the manuscript. All authors read and approved the final manuscript.
Zhaleh Shadman, Mohsen Khoshniat, Nooshin Poorsoltan, Mahdieh Akhoundan, Maryam Omidvar, Bagher Larijani and Saeed Hoseini contributed equally to this work.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Shadman, Z., Khoshniat, M., Poorsoltan, N. et al. Association of high carbohydrate versus high fat diet with glycated hemoglobin in high calorie consuming type 2 diabetics. J Diabetes Metab Disord 12, 27 (2013). https://doi.org/10.1186/2251-6581-12-27
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6581-12-27