Abstract
Corrosion control of metals is an important activity of technical, economical, environmental, and aesthetical importance. The use of inhibitors is one of the best options of protecting metals and alloys against corrosion. The toxicity of organic and inorganic corrosion inhibitors to the environment has prompted the search for safer corrosion inhibitors such as green corrosion inhibitors as other more environmental friendly corrosion inhibitors, most of which are biodegradable and do not contain heavy metals or other toxic compounds. Investigations of corrosion-inhibiting abilities of polymeric substances, e.g., plant gums, in addition to being environmentally friendly and ecologically acceptable, have shown that plant products are inexpensive, readily available, and renewable sources of materials. Need for more effective inhibitors has propelled companies such as the Montazhkhimzashchita Trust to develop a pool method of welding sheet vinyl, a method widely employed in gluing on roller sticky bands from thermoplast on a pipe. The article enumerates several kinds of polymeric materials which are suitable for use in combating corrosion, and several which have suitable strength characteristics as to serve in place of scarce expensive metals and alloys.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Review
Introduction
Several researches have indicated that some polymers can be used as corrosion inhibitors because, through their functional groups, they form complexes with metal ions and on metal surfaces. These complexes occupy a large surface area, thereby blanketing the surface and protecting the metals from corrosive agents present in the solution. The corrosion inhibition by various cationic polymers such as polyethyleneimine derivative, polyacrylamide derivative, polydicyanodiamide derivative, and anionic polymers such as polymaleic acid derivative, polyacrylic acid derivative, and polyacrylic acid, have been investigated by Sekine et al. [1]. They found that polymers having the COOH group are effective polymer-based systems that can function as corrosion inhibitors. Muller et al. [2] studied the corrosion inhibition of polymethacrylic acid and styrene maleic acid co-polymer on zinc pigments in aqueous alkaline medium.
Abdel Rehim et al. [3] found that the amino polycarboxylic acids, such as diethylenetriaminepentaacetic acid, polyacrylic acid, and polymethacrylic acid, are good inhibitors for metal corrosion. Sedahmed et al. [4] investigated the use of a formulation containing polyethylene oxide, polyacrylamide, and carboxymethyl cellulose (CMC) as corrosion inhibitors for iron in acidic and neutral media using electrochemical methods and found that this formulation is an excellent inhibitor. Khairou and Sayed [5] studied the inhibiting action of polyacrylamide, Polyvinyl alcohol (PVA), sodium polyacrylate, poly(ethylene glycol), pectin, and CMC on the corrosion of Cd in 0.5 M HCl solution. Meena et al. [6] investigated the synergistic effect between CMC and Zn2+on the corrosion inhibition of carbon steel in NaCl solutions and found that the combination produces strong inhibition potential.
Studies on the use of some macromolecules (i.e., natural polymers) have also been carried out. For example, Umoren et al. [7, 8] studied the potential of gum arabic as a corrosion inhibitor for aluminum in alkaline medium. The inhibition of aluminum corrosion by gum arabic was attributed to the presence of arabinogalactan, oligosaccharides, polysaccharides, and glucoproteins since these compounds contain oxygen and nitrogen atoms which are the centers of adsorption. The inhibitive effect of exudate gum from Dacroydes edulis on the corrosion of aluminum in HCl solutions was studied using weight loss and thermometric methods at 30°C to 60°C by Umoren et al. [9, 10], and the results revealed that the exudate gum acted as an inhibitor for the corrosion of aluminum in HCl solution. The inhibition efficiency increases with an increase in the concentration of the exudate gum but decreases with increase in temperature.
The effect of naturally occurring exudate gum from Raphia hookeri on the corrosion of mild steel in H2SO4 between 30°C and 60°C has also been investigated by Umoren et al. [10] using weight loss and hydrogen evolution techniques. Results obtained revealed that the exudate gum is a good inhibitor for the corrosion of mild steel in acidic media. The inhibition efficiency increased with an increase in exudate gum content and decreases with increase in temperature. The adsorption of exudate gum from R. hookeri on the mild steel follows Langmuir adsorption isotherm. Guar gum has been shown to be an effective corrosion inhibitor for some metals in aggressive acid environment according to the study by Abdallah [11]. Results obtained show that natural substances act as effective corrosion inhibitors in the different test media. Inhibition efficiency was found to increase with increase in the concentration of the tested material.
It should be pointed out that the potentials of polymers as corrosion inhibitors are dependent on the chemical composition of the polymers, and most of the studies carried out on polymers are done without reference to their chemical properties.
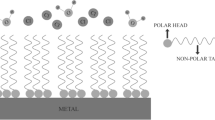
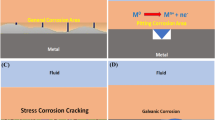
It is well known in surface chemistry that surface reactions are strongly affected by the presence of foreign molecules. Corrosion processes, being surface reactions, can be controlled by compounds known as inhibitors which adsorb on the reacting metal surface.
The term adsorption refers to molecules attached directly to the surface, normally only one molecular layer thick, and not penetrating into the bulk of the metal itself. The technique of adding inhibitors to the environment of a metal is a well known method of controlling corrosion in many branches of technology. A corrosion inhibitor may act in a number of ways: it may restrict the rate of the anodic process or the cathodic process by simply blocking active sites on the metal surface. Alternatively, it may act by increasing the potential of the metal surface so that the metal enters the passivation region where a natural oxide film forms. A further mode of action of some inhibitors is that the inhibiting compound contributes to the formation of a thin layer on the surface which stifles the corrosion process, for example, in order to mitigate aluminum corrosion. The main strategy is to effectively isolate the metal from corrosive agents by the use of corrosion inhibitors. Inorganic substances such as phosphates, chromates, dichromate, and arsenates have been found effective as inhibitors of metal corrosion, but a major disadvantage is their toxicity, and as such, their use has become intolerant for its adverse effect to our societal health in the long run [12].
Corrosion inhibition occurs via adsorption of their molecules on the corroding metal surface, and the efficiency of inhibition depends on the mechanical, structural, and chemical characteristics of the adsorption layers formed under particular condition [13]. Research activities in recent times are geared towards finding alternative corrosion inhibitors to replace the inorganic and organic compounds. Naturally occurring substances have been found to readily satisfy this need. Apart from being readily available, cheap, and a renewable source of materials, naturally occurring substances are eco-friendly and ecologically acceptable. The successful use of naturally occurring substances to inhibit the corrosion of metals in acidic and alkaline environment has been reported by some authors: Ebenso et al. [14], Eddy and Ebenso [15], etc.
Polymers as corrosion inhibitors
In recent years, many branches of national economy for the struggle with corrosion are finding broad application for nonmetallic and chemically stable materials, obtained on the basis of polymers, in form of individual structural materials or linings, protective coatings, coatings, etc. These materials are used for the purpose of economizing on nonferrous metals and highly alloyed steels used by various industries in the role of anticorrosion materials [16]. Several factors such as cost and amount needed, safety to environment and its species, and most importantly, easy availability are needed to be considered when choosing an inhibitor.
An anticorrosion coating for the corrosion protection of metals, liquid Nairit (chloroprene rubber; Yerevan, Armenia), allows us to use these materials widely for the replacement of deficit metals in various branches of national economy. This coating was tested, especially, in ship construction and for the protection of screws (propellers), subjected not only to corrosion but to erosion and cavitations in thermal power equipment and others [16].
They are all, obtainable mainly on the basis of organic products, suitable not only for the preparation of independent structures but also for the lining of devices and equipment, and in the role of compositions and protective coatings. Polymeric materials possessing a practically unlimited chemical stability exceeding even platinum appear to be plastics obtained on the basis of fluorocarbonic compounds, which can be used as independent and lining materials, and in the role of coatings.
The polytetrafluoroethylene or fluoroplast-4 plastics manufactured in the USSR suffer no destruction under the effect of almost all known aggressive media, including such strong oxidizers as fuming nitric acid, aqua regia, at temperatures of up to 2,500°C. Fluoroplast-4 is not void of very essential specific deficiencies: its adhesion to metals and other materials is inconsiderable, which limits its utilization for the lining of devices. Its use for coating is difficult in connection with the insolubility of polytetrafluoroethylene neither in any one of the known organic solvents.
A number of polymers possessing high heat resistance for preservation of their anticorrosion qualities and mechanical strength belong so-called polyorganosiloxanes. Polyorganosiloxane coatings are stable also to the effect of oxygen, ozone, humid atmosphere, ultraviolet rays, etc. and in combination with various fillers (powder aluminum, titanium, boron, etc.) up to 500°C to 550°C and briefly to 700°C to 800°C. Polyorganosiloxane coatings are suitable for the protection against corrosion of smoke pipes, pumps for pumping hot liquids, cracking installations, and other equipment under conditions of high temperatures and under the action of aggressive media [16]. This reputation as well as the presence of heteroatoms, for example O, N, and S, has been found to increase basicity and electron density in polymers and plant extracts, thus intensifying their corrosion potential. O, N, and S are the active centers for the process of adsorption on the metal surface. The inhibition efficiency should follow the sequence O < N < S < P.
Mechanism of action of polymers in a corrosion inhibitor
Many theories to substantiate the mode of action of these green inhibitors have been put forth by several workers. It was suggested that organic substances, which form onium ions in acidic solutions, are adsorbed on the cathodic sites of the metal surface and interfere with the cathodic reaction.
Various mechanisms of action have been postulated for the corrosion-inhibition property of the natural products. The use of organic compounds containing oxygen, sulfur, and especially nitrogen to reduce corrosion attack on steel has been studied in some detail. The existing data show that most organic inhibitors adsorbed on the metal surface by displacing water molecules on the surface and forming a compact barrier. Availability of non-bonded (lone pair) and p electrons in inhibitor molecules facilitates electron transfer from the inhibitor to the metal. A coordinate covalent bond involving transfer of electrons from inhibitor to the metal surface may be formed. The strength of the chemisorption bond depends upon the electron density on the donor atom of the functional group and the polarizability of the group. When an H atom attached to the C in the ring is replaced by a substituent group (−NH2, –NO2, –CHO, or –COOH), the electron density in the metal at the point of attachment changes, resulting in the retardation of the cathodic or anodic reactions. Electrons are consumed at the cathode and are furnished at the anode. Thus, corrosion is retarded. Straight chain amines containing between three and 14 carbons have been examined. Inhibition increases with carbon number in the chain to about 10 carbons, but with higher members, little increase or decrease in the ability to inhibit corrosion occurs. This is attributed to the decreasing solubility in aqueous solution with increasing length of the hydrocarbon chain. However, the presence of a hydrophilic functional group in the molecule would increase the solubility of the inhibitors. The performance of an organic inhibitor is related to the chemical structure and physicochemical properties of the compound-like functional groups, electron density at the donor atom, p orbital character, and the electronic structure of the molecule. The inhibition could be due to the following: (1) adsorption of the molecules or its ions on anodic and/or cathodic sites, (2) increase in cathodic and/or anodic over voltage, and (3) the formation of a protective barrier film.
Several researches have indicated that some polymers can be used as corrosion inhibitors because, through their functional groups, they form complexes with metal ions and on the metal surfaces. These complexes occupy a large surface area, thereby blanketing the surface and protecting the metals from corrosive agents present in the solution [17]. The inhibitive power of these polymers is related structurally to the cyclic rings, heteroatom (oxygen and nitrogen), that are the major active centers of adsorption.
Natural polymers, for example gum arabic, were reported by Umoren et al. [8] as potential corrosion inhibitors for aluminum in alkaline medium. The inhibition of aluminum corrosion by gum Arabic was attributed to the presence of arabinogalactan, oligosaccharides, polysaccharides, and glucoproteins since these compounds contain oxygen and nitrogen atoms which are the centers of adsorption.
The inhibitive effect of exudate gum from D. edulis in the corrosion of aluminum in HCl solutions was studied using weight loss and thermometric methods at 30°C to 60°C by Umoren et al. [10]. The results revealed that the exudate gum acts as an inhibitor for corrosion of aluminum in HCl solution. The inhibition efficiency increases with an increase in the concentration of the exudate gum but decreases with increase in temperature. The Temkin adsorption isotherm was tested for its fit to the experimental data. The result confirms that the corrosion inhibition of the exudate gum from D. edulis was attributed to the adsorption of molecules of phytochemicals present in the exudate gum on the surface of the metal. The free energies and equilibrium constant for the adsorption process were determined. A mechanism of physical adsorption was proposed.
Some natural polymers have also been found to be good corrosion inhibitors. According to Finkenstadt et al. [18], agricultural polymers composed of extra-cellular polysaccharides secreted by Leuconostoc mesenteroides have been shown to inhibit corrosion on corrosion-sensitive metals. The substantially pure exopolysaccharide has a general structure consisting of alpha(1–6)-linked d-glucose backbone and approximately 3% to 4% branching of alpha(1–3) linkages. Corrosion behavior was determined to be strain-dependent. Pore resistance and corrosion rate were calculated from electrochemical impedance spectroscopy (EIS) and polarization studies. The corrosion rate was at least 50% lower, and the pore resistance was twice as high as the control samples, indicating good inhibitory processes. Nanoscale aqueous coatings were examined using AFM and QCM and showed self-repair properties. These materials may be useful in anti-corrosive coating applications.
Natural-occurring polymers
Green corrosion inhibitors are biodegradable and do not contain heavy metals or other toxic compounds. Some research groups have reported the successful use of naturally occurring substances to inhibit the corrosion of metals in acidic and alkaline environments. The effect of addition of halides (KCl, KBr, and KI) was also studied, and the results obtained indicated that the increase in efficiency was due to synergism [10]. Buchweishaija and Mhinzi [19] studied the inhibition efficiency of gum exudates from Acacia seyal var. seyal using potentiodynamic polarization and EIS technique at a 30°C, and it was found to be a good anodic inhibitor for drinking water with increasing temperature. Umoren et al. [10] also investigated the corrosion properties of R. hookeri exudates gum-halide mixtures for aluminum corrosion in acidic medium. R. hookeri exudate gum obeys Freundlich, Langmuir, and Temkin adsorption isotherms. Phenomenon of physical adsorption is proposed.
Abdallah [11] also tested the effect of guar gum on carbon steel. It is proposed that it acts as a mixed type inhibitor. The mechanism of action of C steel by guar gum is due to the adsorption at the electrode/solution interface. Guar gum is a polysaccharide compound containing repeated heterocyclic pyrane moiety. The presence of hetero-oxygen atom in the structure makes its adsorption possible by coordinate type linkage through the transfer of lone pairs of electron of oxygen atoms to the steel surface, giving a stable chelate five-membered ring with ferrous ions.
The behavior of corrosion inhibition by various cationic polymers such as polyethyleneimine derivative, polyacrylamide derivative, and polydicyanodiamide derivative, and anionic polymers such as polymaleic-acid derivative, polyacrylic-acid derivative, and polyacrylic acid, was investigated by Sekine et al. [1]. Polymers having the COOH group are one of the effective polymer-based systems. There are several carboxylic acids of low molecular weight, which are used as corrosion inhibitors to many inhibitor formulations. Muller et al. [2] has studied the corrosion inhibition of polymethacrylic acid and styrene maleic acid co-polymer on zinc pigments in aqueous alkaline medium. Abdel Rehim et al. [3] have discussed that the amino polycarboxylic acids, such as diethylenetriaminepentaacetic acid, polyacrylic acid, and polymethacrylic acid, have been used as inhibitors for metal corrosion. Sedahmed et al. [20] have studied the use of the formulation containing polyethylene oxide, polyacrylamide, and CMC as corrosion inhibitors for iron in acidic and neutral media using electrochemical methods. Khairou and Sayed [5] have evaluated the inhibiting action of polyacrylamide, PVA, sodium polyacrylate, poly(ethylene glycol), pectin, and carboxymethyl cellulose (CMC) on the corrosion of Cd in 0.5 M HCl solution. Meena et al. [6] have examined the synergistic effect between CMC and Zn2+ on the corrosion inhibition of carbon steel in NaCl solutions.
Synthetic polymers
Synthetic polymers are polymers synthesized in the laboratory to serve as a proficient substitute for the natural ones; they are made mostly to check the demerits and enhance the qualities of natural polymers depending on their use. Plastic materials which are suitable for use in combating corrosion and several which have suitable strength characteristics as to serve in place of scarce expensive metals and alloys include vinyl for piping, sheet graphite for parts of heat exchangers, and liquid Nairit (chloroprene rubber) for anticorrosion protection of ship propellers.
Plastics have also been used successfully to fabricate parts of armatures. Plastics of fluorocarbonic compounds have been found especially chemically stable, resisting ever fuming nitric acid and aqua regia. Antegmit, an anticorrosion and antifriction heat conductive material made from phenolformaldehyde resin and graphite, differs from saturated graphites by impermeability and much lower heat conductivity, but its strength is approximately twice higher than that of saturated graphite.
High chemical stability is possessed by chlorosulfonated polyethylenes, hypalones, used for linings of chemical devices as well as coatings, applied by a brush, by immersion, and dusting. Hypalon-20 at room temperature is affected only by fuming nitric acid, carbon tetrachloride, gasoline, and nitrobenzene. Thermo and fire resistance of Hypalon (DuPont, DE, USA) allow the same role of coating conveyer belts for the transportation of hot materials.
Polypropylene is a highly perspective, polymeric anticorrosion material which is derived from petroleum gasses. Its melting point is 170°C, it is stable against organic solvent as well as in 80% sulfuric acid and caustic sodium, and is frost-resistant. Films from polypropylene possess lower gas permeability than polyethylene films.
Polyorganosiloxanes have proven their value in preventing corrosion. Fiber glass has also found many uses in industrial plant installations. Epoxy resins, epoxy lacquers, and nylon have been found to be of remarkable importance in countering metal corrosion. Umoren et al. [21] studied the corrosion inhibition of mild steel in H2SO4 in the presence of gum arabic (naturally occurring polymer) and polyethylene glycol (PEG) (synthetic polymer). It was found that PEG was more effective than gum arabic and acetylthiourea chitosan polymer (ATUCS).Corrosion inhibition study on mild steel using potentiodynamic polarization, EIS measurements, and SEM technique, and ATUCS has shown very good IE in 0.5 M sulfuric acid solution which reaches to 94.5% for 0.76 M concentration [22].
Poly(styrenesulfonic acid)-doped polyaniline has been synthesized, and the influence of this polymeric compound on the inhibition of corrosion of mild steel in 1 M HCI has been investigated using weight loss measurements, galvanostatic polarization studies, electropermeation studies and AC impedance measurements by Manickavasagam et al. [23]. The polymer acts predominantly as an anodic inhibitor.
The inhibitive performance of novel synthesized water soluble triblock copolymers2-(diethylamino)ethyl methacrylate-block-2-(dimethylamino)ethyl methacrylate-block-2-(N-morpholino)ethyl methacrylate (PDEA-PDMA-PMEMA) and 2-(diisopropylamino)ethyl methacrylate-block-2-(dimethylamino)ethyl methacrylate-block-2-(N-morpholino)ethyl methacrylate (PDPA-PDMA-PMEMA) of two different molecular weights on the corrosion behavior of mild steel in 0.5 M HCl has been reported by Yurt et al. [24].
The inhibitive action of orthomethoxy-substituted polyaniline (poly(o-methoxyaniline), a new class of conducting polymer on the corrosion of iron in acidic chloride solution, has been evaluated by EIS, linear polarization resistance, weight loss, and by logarithmic polarization technique. Inhibition efficiencies of nearly 80% to 88% have been observed even at 25-ppm concentration. Double-layer capacitance studies indicate a strong adsorption of the polymer following Temkin adsorption isotherm is largely responsible for its inhibitive action [25]. Volatile corrosion inhibitors (VCIs) are unique. They are organic compounds that protect metal surfaces by emitting a vapor such as an amine-based compound. The nitrogen on the amine has two electrons that are attracted to the polar metal surface.
Once it is attracted to the metal, the rest of the molecule is very hydrophobic and repels water to significantly retard corrosion. Miksic et al. [26], Kuznetsov [27], and Andreev and colleagues [28] studied several amines, their derivatives, and imines used as VCIs. VCIs migrate from the coated area to the unprotected metal area. Metals coated with these VCI paints corrode very little in scribed areas since the films are self healing. The VCI evaporates and redeposits itself where the coating has been scratched. Many VCI coatings also contain other corrosion inhibitors to enforce the corrosion-resistance process [29].
Replacement of nonferrous metals in the manufacture of synthetic fibers with paint and varnish coatings for ferrous metals gave a greater economical effect. Investigations of various coatings obtained on the basis of high molecular epoxy resins with the use of organic amines and mineral acids in the role of hardener have shown that epoxy coatings possess excellent physicomechanical properties and greater acid resistance.
Paint and varnish materials prepared on the basis of epoxy resin differ also in alkaline resistance. Alkaline resistance is characteristic also for perchlorovinyl enamel, which represents a suspension of pigments and plasticizer in a solution of dry perchlorovinyl resin in organic solvents. Enamel obtained from phenolformaldehyde resin is used as benzostable material for the protection of metal surfaces, subjected to corrosion effect of hot lubricating materials. Tests of coatings, prepared on the basis of chlorovinyl copolymers with vinyliden chloride, anticorrosion enamel and non-overgrowing paint, obtained on the basis of a chlorovinyl copolymer with vinylacetate, are suitable for the protection of metal against corrosion and against the effects of sea water.
Advantages and limitations
Polymeric materials are environmentally friendly, non toxic, and relatively less expensive. Most polymers are not easily biodegradable which, in merits, allow for their long time storage and usage on corrosion protection of metals and alloys. To protect against corrosion on most metals, it is necessary to apply a coating with a thickness of 250 to 400 μm. As is known, majority of polymeric materials are suitable to a temperature of not more than 150°C, and many of them up to 40°C to 50°C (vinylplast, polyisobutylene, etc.).
Majority of polymeric materials have low mechanical strength; that is why their use is limited. Although sheet vinyl has high mechanical strength and can withstand a temperature of about 100°C for a while, a clear reason why its need is high in recent times is that it can be used for making faolite columns and pumps as well as lining metal pipes. Warning from plant scientists: If polymeric materials, some of which are directly gotten from a plant source such as plant gums, are excessively used as corrosion inhibitors to prevent the corrosion of metals, the plant kingdom will slowly diminish; metals will be protected at the cost of destruction of plant kingdom.
Coatings applied directly to metals normally use conventional corrosion inhibitor pigments such as zinc, aluminum, zinc oxide (ZnO), modified ZnO, and calcium ion-exchanged amorphous silica gel. Using corrosion inhibitor pigments has several disadvantages. Some pigments contain metals that are toxic. Several, including metallic zinc, have high densities and settle. A number of pigments react with the resins in the coating. Additional pigmentation also requires added wetting agents that may affect corrosion resistance. A list of various polymeric materials that have been used as corrosion inhibitors is given in Table 1.
Conclusions
It has been shown that polymers especially the water soluble ones are efficient corrosion inhibitors in different aqueous media. Mechanism of inhibition are mainly attributed to adsorption and depends on the metal, physicochemical properties of the molecule such as functional groups, steric factors, aromaticity at the donor atom and p orbital character of donating electrons, as well as the electronic structure of the molecules. In other words, the efficiency of polymers as corrosion inhibitor depends not only on the characteristics of the environment in which it acts, the nature of the metal surface, and electrochemical potential at the interface, but also on the structure of the inhibitor itself, which includes the number of adsorption active centers in the molecule, their charge density, the molecular size, the mode of adsorption, the formation of metallic complexes, and the projected area of the inhibitor on the metallic surface. The results of the series of investigations have revealed that the processes involved in corrosion inhibition are not uniform with respect to all classes of compounds so far investigated and are not even constant or consistent with one inhibitor in a given system. Indeed, the overall process is a function of the metal, corrodent, inhibitor structure, and concentration, as well as temperature.
Authors’ information
DEA was born in Minna in 1986 and was raised in a Christian home. He is a Physical Chemist and a graduate of Ahmadu Bello University, Zaria, where he obtained his bachelors and masters degrees in chemistry. As a scientist, his desire is to further his carrier by accepting challenging responsibilities in a dynamic progressive course that will create and maintain a friendly working environment and offer opportunity for growth.
References
Sekine I, Sanbongi M, Hagiuda H, Oshibe T, Yuasa M, Imahama V, Shibata Y, Wake T: J ElectrochemSoc. 1992,139(11):3167. 10.1149/1.2069050
Muller B, Forster I, Klager W: Corrosion inhibition of zinc pigments in alkaline media by polymers. Progr Org Coating 1997,31(3):229–233. 10.1016/S0300-9440(97)00042-8
Abdel Rehim SS, Tohommy FM, Seleet MM: Effect of some polyaminopolycarboxylic acids on the corrosion of steel in sulphate solutions. Surf Tech 1984,21(2):169–177. 10.1016/0376-4583(84)90160-2
Sedahmed GH, Nagy Soliman M, El-Kholy NS: J Appl Electrochem. 1982,12(4):479. 10.1007/BF00610490
Khairou KS, El Sayed A: Inhibition effect of some polymers on the corrosion of Cd in a hydrochloric acid solutions. J Appl Polymer Sci 2003,88(4):866–871. 10.1002/app.11663
Meena BV, Anthony N, Mangayarkarasi K, Jayaram P, Rajendran S: Proc. of Tenth National Congress on Corrosion Control. NCC and CECRI, Karaikudi; 2000:241–247.
Umoren SA, Ogbobe O, Ebenso EE, Ekpe UJ: Effect of halides on the corrosion inhibition of mild steel in acidic medium using polyvinyl alcohol. Pigm Resin Technol 2006,35(5):284–292. 10.1108/03699420610692896
Umoren SA, Ebenso EE, Okafor PC, Ekpe UJ, Ogbobe O: Effect of halides on the corrosion inhibition of mild steel in alkaline medium using polyvinyl alcohol. J Appl Polymer Sci 2006, 103: 2810–2816.
Umoren SA, Obot IB, Ebenso EE: Corrosion inhibition of aluminium using exudate gum from Pacchylobus edulis in the presence of halide ions in HCl. Electron J Chem 2008,5(2):355–364.
Umoren SA, Ogbobe O, Igwe IE, Ebenso EE: Inhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halide additives. Corrosion Sci 2008. 10.1016/j.corsci.2008.04.015
Abdallah M: Guar gum as corrosion inhibitor for carbon steel in sulphuric acid solutions. Portugaliae Electrochimica Acta 2004, 22: 161–175. 10.4152/pea.200402161
Obot IB, Obi-Egbedi NO, Umoren SA, Ebenso EE: Synergistic and antagonistic effects of anions and Ipomoea invulcrata as green corrosion inhibitor for aluminium dissolution in acidic medium. Int J Electrochem Sci 2010,5(7):994–1007.
Eddy NO, Ibok UJ, Ebenso EE: Adsorption, synergistic inhibitive effect and quantum chemical studies on ampicillin and halides for the corrosion of mild Steel. J Appl Electrochem 2009. 10.1007/s10800-009-0015-z
Ebenso EE, Ekpe UJ, Ibok UJ: Studies on the inhibition of mild steel corrosion by some plant extracts in acidic medium. Discov Innovat 1998, 10: 52–59.
Eddy NO, Ebenso EE: Corrosion inhibition and adsorption properties of ethanol extract of Gongronema latifolium on mild steel in H2SO4. Pigm Resin Technol 2010,39(2):77–83. 10.1108/03699421011028653
Klinov IY: New polymeric materials in anticorrosion technology. Trudy. Vsesoyuznoy Mezhvuzovskoy Nauchnoy Konferentsii po Voprosam Bor’by s Korroziyey (Translations of the Inter-Higher Educational Institute Scientific Conference on Questions of Fighting Corrosion). Gostoptekhizdat, Moskva; 1962:296–303.
Rajendran S, Sridevi SP, Anthony N, John Amalraj A, Sundearavadivelu M: Corrosion behaviour of carbon steel in polyvinyl alcohol. Anti-Corros Method M 2005,52(2):102–107. 10.1108/00035590510584816
Finkenstadt VL, Bucur CB, Cote GL, Evans KO: Agricultural polymers as corrosion inhibitors [abstract]. International Chemical Congress of Pacific Basin Societies 2011, 8: 1.
Buchweishaija I, Mhinzi GS: Natural products as a source of environmentally friendly corrosion inhibitors: the case of gum exudates from Acacia seyal var. seyal. Portugaliae. Electrochim Acta 2008,26(3):257–265.
Sedahmed GH, Soliman M, el Kholy NS N: Electropolishing of vertical copper cylinders in phosphoric acid under natural convention conditions. Surf Technol 1980, 11: 67–71. 10.1016/0376-4583(80)90020-5
Umoren SA, Obot IB, Ebenso EE, Okafor PC, Ogbobe O, Oguzie EE: Gum arabic as a potential corrosion inhibitor for aluminium in alkaline medium and its adsorption characteristics. Anti-Corros Method M 2006,53(5):277–282. 10.1108/00035590610692554
Fekry AM, Riham MR: Acetyl thiourea chitosan as an eco-friendly inhibitor for mild steel in sulphuric acid medium. Electrochim Acta 2009,55(6):1933–1939.
Manickavasagam R, Jeya K, Paramasivam M, Venkatakrishnalyer S: Poly(styrene sulphonic acid) doped polyaniline as an inhibitor for the corrosion of mild steel in hydrochloric acid. Anti-Corros Method M 2002,49(1):19–26. 10.1108/00035590210413566
Yurt A, Butun V, Duran B: Effect of the molecular weight and structure of some novel water soluble triblock copolymers on the electrochemical behaviour of mild steel. Mater Chem Phys 2007, 105: 114–121. 10.1016/j.matchemphys.2007.04.009
Sathiyanarayanan S, Balkrishnan K: Prevention of corrosion of iron in acidic media using poly(o-methoxy aniline). Electrochem Acta 1994,39(6):831–836. 10.1016/0013-4686(94)80032-4
Miksic BA, Tarvin M, Sparrow GR: Surface analytical techniques in evaluation of the effects of VCI organic corrosion inhibitors on the surface chemistry of metals. Corrosion 1989, 607: 1–10.
Kuznetsov YI: Inhibiting action and absorption of beta-aminoketones on metals. Zasshchita Metallov 1996,32(5):528–533.
Andreev NN: To simplified methods of estimation of saturated vapor pressure of volatile corrosion inhibitors. Eurocorr 97 conference, Trodheim, Norway; 1997:22–25. September 1997
Prenosil M: Volatile corrosion inhibitor coatings. Cortec Corporation Technical Publications, New Orleans; 1998.
Amin MA, Abd El-Rehim SS, El-Sherbini EEF, Hazzazi OA, Abbas MN: Polyacrylic acid as a corrosion inhibitor for aluminium in weakly alkaline solutions. J Corrosion Sci 2008, 51: 658–667.
Nnabuk Okon E, Paul A, Gimba CE, Ebenso EE: GCMS studies On Anogessus leocarpus (Al) gum and their corrosion inhibition potential for mild steel in 0.1 M HCl. Int J Electrochem Sci 2011, 6: 5815–5829.
Obot IB, Obi-Egbedi NO: Ginseng root: CA new efficient and effective eco-friendly corrosion inhibitor for aluminium alloy of type AA 1060 in hydrochloric acid solution. Int J Electrochem Sci 2009,4(9):1277–1288.
Kamal Y, Wan Aizan WAR Thesis. In Effectiveness of acrylic acid based terpolymer as corrosion inhibitor in water treatment. Department of Chemical Engineering, University College of Engineering and Technology, Malaysia; 2003.
Berkovic K, Kovac S, Vorkapic-Furac J: Natural compounds as environmentally friendly corrosion inhibitors of aluminium. Acta Alimentaria 2004,33(3):237–247. 10.1556/AAlim.33.2004.3.4
Chetouani A, Medjahed K, Benabadji KE, Hammouti B, Kertit S, Mansri A: Poly (4-vinylpyridine isopentyl bromide) as inhibitor for corrosion of pure iron in molar sulphuric acid. Prog Org Coating 2003,46(4):312–316. 10.1016/S0300-9440(03)00019-5
El-Dahan HA: Natural products as corrosion inhibitors for copper in saline water in presence of sulphide. Egypt J Chem 2006,49(5):589–600.
Soror TK: New naturally occurring product extract as corrosion inhibitor for 316 stainless steel in 5% HCl. J Mater Sci Tech 2004,20(4):463–466.
Abdallah A, Khaled M, El Ali B, Emad M: Corrosion inhibition of steel in cooling water system by 2-phosphonobutane-1,2,4-tricaboxylic acid and polyvinyl pyrrolidone (PVP). Arab J Sci Eng 2007, 33: 1A.
Arukalam OI, Obidiegwu MU: The inhibition of aluminum corrosion in hydroxyl ethyl cellulose. Acad Res Int 2011,1(3):2223–9553.
Arukalam OI: The inhibitive effect of hydroxyl cellulose on mild steel corrosion in hydrochloric acid solution. Acad Res Int 2012,2(1):SS35-SS42.
Mazen MK: The effect of molecular weight on corrosion protection properties of polyvinyl pyrrolidone polymers on stainless steel. Arab J Sci Eng 2007,35(1A):29–39.
Kinleni KJ, Hayes S, Doering F, Welker S, Koustis G, Hannah B Tri-service corrosion conference. In Electroactive polymers and smart coatings for corrosion inhibition of aluminum alloys. Orlando; 2005. 14–18November 2005 14–18November 2005
Bressy-Brondino C, Boutevin B, Hervaud Y, Gaboyard M: Adhesive and anticorrosive properties of poly (vinylidene fluoride) powders blended with phosphonated copolymers on galvanized steel plates. J Appl Polym Sci 2002,83(11):2277–2287. 10.1002/app.2320
Attar MM, Scantlebury JD: Polyaniline as a possible inhibitor for the corrosion of mild steel. J Corrosion Eng 2005,8(1):97–99.
Bereket GA, Yurt A, Turk H: Inhibition of corrosion of low carbon steel in acidic solution by selected polyelectrolytes and polymers. Anti-Corros Method M 2003,50(6):422–535. 10.1108/00035590310501585
Morooka M, Sekine I, Tanaki T, Hirosett N, Yuasa M: Effects of polymer-polymer complexes on the corrosion of mild steel in cooling water system (part 2): corrosion investigation in polymethacrylic acid/polyacrylamide system. Corrossion Eng 2001,50(3):106–114.
Jianguo J, Lin W, Otieno-Alego V, Schweinsberg DP: Polyvinylpyrrolidone and polyethylenimine as corrosion inhibitors for the corrosion of a low carbon steel in phosphoric acid. Corrosion Sci 1995,37(6):975–985. 10.1016/0010-938X(95)00008-8
Dubey AK, Singh G: Corrosion inhibition of mild steel in sulphuric acid solution by using polyethylene glycol methyl ether (PEGME). Port Electrochim Acta 2007, 25: 221–235. 10.4152/pea.200702221
Hirai T, Yameki J, Okada T, Yamaji A: Inhibitive effects of Al corrosion by polymer ammonium chloride in alkaline electrolyte. Electrochim Acta 1985,30(1):61–67. 10.1016/0013-4686(85)80059-1
El-khair A, Mostata B: Inhibition of iron corrosion by soluble monomeric and polymeric vinyl pyrrolidone. Corrosion Prev Control 1983,30(6):14–16.
Manivel P, Venkatachari G: Inhibitive effect of p-aminobenzoic acid and its polymer on corrosion of iron in 1 mol/L HCl solution. J Mater Sci Technol 2006,22(3):301–305. 10.1179/174328406X86155
Jeyaprabha C, Sathiyanarayanan S, Phani KLN, Venkatachari G: Investigation of the inhibitive effect of poly (diphenylamine) on corrosion of iron in 0.5 M H2SO4 solutions. J Electroanal Chem 2005,585(2):250–255. 10.1016/j.jelechem.2005.08.017
Chetouani A, Medjahed K, Sid-Lakhdar KE, Hammouti B, Benkaddour M, Mansri A: Poly(4-vinylpyridine-poly(3-oxideethylene) tosyle) as an inhibitor for iron in sulphuric acid at 80°C. Corrosion Sci 2004,6(10):2421–2430.
Sathiyanarayanan SK, Balakrishnan K, Dhawan SK, Trivedi DC: Influence of poly(aminoquinone) on corrosion inhibition of iron in acid media. Applied Surf Sci 2005, 252: 966–975. 10.1016/j.apsusc.2005.01.098
Pawar P, Gaikwad AB, Patil PP: Corrosion protection aspects of electrochemically synthesized poly (o-anisidine-co-o-toluidine) coatings on copper. Electrochim Acta 2007, 52: 5958–5967. 10.1016/j.electacta.2007.03.043
Xue G, Lu Y, Shi G: SERS studies of synergistic effect of corrosion inhibition of Cu by two component inhibitor systems of polyvinyl imidazole and benzimdazole. Appl Surf Sci 1994,74(1):37–41. 10.1016/0169-4332(94)90097-3
Tuken T, Tansug G, Yazici B, Erbil M: Poly(N-methyl pyrrole) and its copolymer with pyrrole for mils steel protection. Surf Coating Technol 2007, 2002: 146–154.
Tuken T, Yazici B, Erbil M: Polypyrrole/polythiophene coating for copper protection. Prog Org Coating 2005, 53: 38–45. 10.1016/j.porgcoat.2004.11.008
Schweinsberg DP, Hope GA, Trueman A, Otieno-Alego V: An electrochemical and SERS study of the action of polyvinylpyrrolidone and polyethylenimine as inhibitor for copper in aerated H2SO4. Corrosion Sci 1996, 38: 587–599. 10.1016/0010-938X(95)00148-D
Acknowledgments
We would like to acknowledge the editor Joseph Gerard Bautista of the Journal Editorial Office, Springer Open for his help and gift as a brilliant editor as well as his contribution to the final form and beauty of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
DA, AJ, PA, and CA participated in the design of the study. DA conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Arthur, D.E., Jonathan, A., Ameh, P.O. et al. A review on the assessment of polymeric materials used as corrosion inhibitor of metals and alloys. Int J Ind Chem 4, 2 (2013). https://doi.org/10.1186/2228-5547-4-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5547-4-2