Abstract
In the present work, the decolourization of azo dye Acid Yellow 99 (AY99) in an aqueous solution by Fenton oxidation process was investigated. The results indicated that the initial pH, the nature of the acid to adjust pH, the temperature and the initial concentrations of the Fenton’s reagent FeSO4 of H2O2 and of the AY99 played an important role on the dye decolourization. It was found that under optimum conditions, which have been determined to be pH = 3, [H2O2]0 = 1.8 mmol/L, [Fe2+]0 = 0.09 mmol/L, [AY]0 = 0.06 mmol/L and T = 30°C, efficiency of degradation obtained after 40 min of reaction was about 95%. The decolourization of AY99 by the Fenton process followed the second-order reaction kinetics. The empirical kinetic equation for AY99 decolourization under the conditions of 0.03 to 0.15 mmol/L of Fe2+, 0.9 to 1.8 mmol/L of H2O2, 0.03 to 0.15 mmol/L of Acid Yellow and pH = 3 was found to follow the relation:
K = 6.39 × 107 [AY] −2.8[H2O2]2.29[FeSO4]0.837 exp (−7021.3/T).
The decolourization efficiency Ef (in percent) was 88.36%, 87.93%, 85.98% and 39.61% in the presence of H2SO4, HNO3, HCl and H3PO4 acids, respectively. We can deduce that H2SO4, HNO3 and HCl acids lead almost to the same efficiency (Ef), but in the presence of H3PO4, it is too low:
Ef (H2SO4) ~ Ef (HNO3) > Ef (HCl) > > Ef (H3PO4).
Effects of additives such as inorganic salts (sodium chloride, calcium chloride, sodium sulphate and calcium sulphate) and cations (sodium sulphate, calcium sulphate, copper sulphate, manganese sulphate and magnesium sulphate) on the efficiency (Ef) and on the rate constant (k2) of AY99 degradation were also studied under optimum conditions.
Ef (Na2SO4) > Ef (CaSO4) > Ef (NaCl) > Ef (CaCl2)
k2 (CaCl2) < k2 (NaCl) < k2 (CaSO4) < k2 (Na2SO4)
Ef (ZnSO4) > Ef (MgSO4) > Ef (Na2SO4) > Ef (CaSO4) > Ef (CuSO4) > Ef (MnSO4)
k2 (Na2SO4) < k2 (MnSO4) < k2 (CuSO4) < k2 (CaSO4) < k2 (ZnSO4) < k2 (MgSO4).
The presence of anions and cations in the aqueous solution decreases the decolourization efficiency and the rate constant of the degradation of AY99. This inhibition may be due to a complexation and a radical scavenging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Azo dyes are synthetic organic compounds widely used in textile dyeing, paper printing and other industrial processes such as the manufacture of pharmaceutical drugs, toys and foods.
Yearly, 800,000 tonne of dyes are produced in the world, and about 50% of them are azo dyes[1]. Coloured wastewaters from azo dye production processes and utilisation industries pose a major threat to the surrounding ecosystems due to the documented health hazards caused by toxicity and a potentially carcinogenic nature of such organic pollutants[2].
The traditional treatment techniques applied in textile wastewaters, such as coagulation/flocculation, membrane separation (ultra-filtration and reverse osmosis) or elimination by activated carbon adsorption[3–8], only do a phase transfer of the pollutant, and biological treatment is not a complete solution to the problem due to biological resistance[9, 10]. Recent progress in the removal of dyes has led to the development of advanced oxidation processes (AOPs). These processes have attracted wide interests in wastewater treatment since 1990. In principle, AOPs such as photocatalytic degradation[11–15], ozonation[16, 17], H2O2 photolysis (UV/H2O2)[17–21], Fenton’s reaction (H2O2/Fe2+)[22, 23] and photo-Fenton (H2O2/Fe2+/UV)[24–26] are based on the generation of the hydroxyl radical ·OH in water. ·OH is a highly reactive and non-selective oxidant which can initiate oxidative degradation reactions of refractory synthetic and natural organic compounds and is capable of mineralizing them ultimately to CO2 and H2O owing to their high oxidation potential (+2.80 eV versus NHE) in an aqueous solution[27, 28]. In recent years, different combinations of these methods were used to obtain the complete mineralization of pollutants[29–33].
The Fenton process is a homogeneous catalytic AOP using a mixture of hydrogen peroxide and ferrous ions[34]. Compared with other AOPs, Fenton’s reagent is relatively inexpensive, and the process is easy to operate. The mechanism that describes a Fenton’s process mainly includes the following reactions[35]:
This paper reports the findings of the decolourization of Acid Yellow 99 using Fenton’s reagent. Effects of the pH, the nature of the acid to adjust pH and the temperature were examined. The influence of the concentration of H2O2, Fe2+ and the dye were also explored. The relation between the reaction rate coefficient and the initial concentration of Acid Yellow 99, ferrous sulphate concentrations, hydrogen peroxide concentrations and operating temperature will subsequently be elucidated based on the obtained experimental data.
The influences of salts (such as sodium chloride, calcium chloride, sodium sulphate and calcium sulphate) on the degradation of Acid Yellow 99 (AY99) were examined. The effects of cations from salts (sodium sulphate, calcium sulphate, copper sulphate, manganese sulphate, zinc sulphate and magnesium sulphate) were also studied.
Methods
Materials
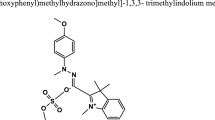
Acid Yellow 99 was purchased from Aldrich (Sigma-Aldrich Corporation, St. Louis, MO, USA) and was used as received. The molecular structure of Acid Yellow (C.I. 62055) is shown in Figure1. Hydrogen peroxide (30% w/w) was obtained from Merck (Merck & Co., Inc., White house Station, NJ, USA). Ferrous sulphate heptahydrate (≥99%) was supplied by Sigma-Aldrich. H2SO4, HCl, H3PO4 and HNO3 were all obtained from Shanghai Chemical Reagents Co. (Shanghai, China). Sodium chloride, calcium chloride, sodium sulphate and calcium sulphate were supplied by Acros Organics (Geel, Belgium). Copper sulphate, manganese sulphate and magnesium sulphate were procured from Sigma-Aldrich.
Procedure
The aqueous solutions of AY99 were prepared by dissolving the required amount in distilled water. The pH of the solution was adjusted by adding sulphuric acid (H2SO4) and measured by a pH meter (Eutech Instruments Pte. Ltd., Rajah Crescent, Singapore).
The experimental device consisted of a perfectly agitated reactor (reactor batch with a capacity of 1,000 mL) in which a volume (500 mL) of solution was studied. The temperature was controlled by a thermostatic bath (TECTRON BIO, SELECTA, Barcelona, Spain), and the agitation was carried out using a mechanical stirrer (Janke & Kunkel RW 20, Janke & Kunkel GmbH & Co. KG IKA-Labortechnik, Staufen, Germany). The dye decolourization was made by a Fenton’s reagent, which was composed of a mixture of FeSO4 and H2O2. The necessary quantities of Fe2+ and H2O2 were added in the dye solution simultaneously. The concentration of the dye at different reaction times was determined by measuring the absorption intensity at using a UV-visible spectrophotometer (BOECOS24).
The efficiency of AY99 decolourization was calculated by using Equation 4:
where C0 is the initial concentration of AY99 and C t is the concentration of AY99 at time t.
Results and discussion
Effect of operational parameters
Effect of initial pH
The pH of the wastewater controls the production rate of hydroxyl radical and the nature of iron species in the solution. Hence, pH is an important parameter for Fenton processes. In order to find the optimal pH at which the reaction mixture for the decolourization of AY99 can give the best efficiency, a series of experiments at different pH were conducted. The pH values used were 2, 2.5, 3, 3.5 and 4, the initial concentration of the dye was 0.06 mmol/L, the temperature was maintained at 30°C and the pH of the solutions was adjusted by concentrated (98%) sulphuric acid.
The effect of the initial pH on the treatment of AY99 is shown in Figure2. It was observed that the decolourization of AY99 was significantly influenced by the solution’s pH value, and the highest decolourization efficiency (88.36%) was achieved at pH = 3.0. These observations are in good agreement with published results[36–38].
When pH is below 3, HO· could be consumed by the scavenging effects of H+, which will limit the decolourization rate (Equation 5)[34]. According to Equations 1 and 2, the reaction could be slowed down because hydrogen peroxide can probably be stable by acquiring a proton to form an oxonium ion as H3O2+ in Equation 6.
The oxonium ion makes hydrogen peroxide electrophilic to enhance its stability and presumably to reduce substantially the reactivity with ferrous ion[34]. For pH above 3, the generation of HO· decreases due to the formation of iron ions, which tend to precipitate in the form Fe(OH)3, as shown by reaction 7, and that is favoured at higher pH[35].
Following this precipitation, regeneration of Fe2+ from Fe3+ stops; then, the production of HO· radicals decreases. However, the initial pH value should be between 2.5 and 3 to generate the maximum of radicals to oxidise organic matter.
Effect of temperature
The effect of temperature on the decolourization of AY99 was studied at 30°C, 40°C, 50°C, 60°C and 70°C with other test conditions at [AY]0 = 0.06 mmol/L, [H2O20 = 0.9 mmol/L, [Fe2+0 = 0.09 mmol/L and pH = 3.0. The results are shown in Figure3. It can be seen that the temperature exerts a strong effect on the decolourization efficiency of AY99, and the decolourization was accelerated by a rise in temperature. The decolourization efficiency increases from 45.00% to 89.88% as a consequence of increasing the temperature from 30°C to 70°C within 6 min. This is because higher temperature increased the reaction rate between hydrogen peroxide and any form of ferrous/ferric ion, thus increasing the rate of generation of oxidising species such as HO· radical or high-valence iron species[36].
Effect of FeSO 4
To elucidate the role of Fe2+ ion on the degradation of AY99, a series of experiments was performed by varying the concentration of Fe2+ ions from 0.03 to 0.15 mmol/L for an adjusted H2O2 concentration of 0.9 mmol/L at pH = 3. The effect of Fe2+ on the decolourization of AY99 by Fenton is show in Figure4.
It was observed that the decolourization efficiency increases from 25.74% to 69.51% as a consequence of increasing Fe2+ dosage from 0.03 to 0.15 mmol/L within 10 min. This is due to Fe2+ playing the role of a catalyst and initiating the decomposition of H2O2 to generate a very reactive HO· in the process. Therefore, additional HO· radicals were produced along with the increase of Fe2+ concentration. The maximum degradation efficiency (about 88.36%) was achieved at 0.09 mmol/L of Fe2+ within 70 min. The influence of higher Fe2+ concentration upon the degradation behaviour of AY99 may be explained by the redox reactions since HO· radicals may be scavenged by the reaction with hydrogen peroxide or with another Fe2+ molecule, as shown below in Equation 8[35].
Effect of H 2 O 2 concentration
Hydrogen peroxide plays the role of an oxidising agent in the Fenton process. The effect of H2O2 dosage on the degradation of AY99 by Fenton process was investigated by varying initial concentration of H2O2 from 0.9 to 2.1 mmol/L, the results are shown in Figure5. It was observed that the decolourization efficiency increases from 85% to 95% as a consequence of increasing H2O2 dosage from 0.9 to 1.8 mmol/L within 40 min. This is due to the oxidation power of the Fenton process, which was improved by increasing the amount of HO· radicals in the solution obtained from the decomposition of increasing hydrogen peroxide. The maximum decolourization efficiency of about 96% was achieved with 1.5 mol/L of H2O2 within 70 min.
Beyond critical concentrations, the degradation rate of AY99 decreased with the increasing H2O2 concentration due to the hydroxyl radical scavenging effect of H2O2[37] and the recombination of hydroxyl radicals according to Equations 9 and 10.
And the incremental generation of HOO· also consumed HO·.
Effect of the Acid Yellow 99 concentration
The pollutant concentration is one of the important parameters in Fenton processes. Therefore, the effect of dye concentration on the degradation efficiency was investigated at different concentrations of AY99 and presented in Figure6. It was observed that the increase of dye concentration from 0.03 to 0.15 mmol/L decreases the decolourization from 97.5% to 67.26% within 25 min. This phenomenon can be explained by the fact that an increase in the initial concentration leads to an increase of the number of dye molecules and not the concentration of HO· radicals, which is why the decolourization efficiency decreases. These results are in agreement with those found in the literature[36, 38].
Effect of the iron ion type
The nature of the catalyst ensures the smooth running of catalytic cycle. It is responsible for the production of HO· radicals whose metal ions are the main actors. The AY99 decolourization has been studied in the presence of three different catalysts: FeSO4, FeCl3 and Fe(NO3)3. These experiments were carried out to compare the performance of Fe2+/H2O2 and Fe3+/H2O2 catalytic systems. The results presented in Figure7 show that the decolourization efficiency of Acid Yellow 99 at 30°C reached 98.5% within 120 min when the Fe3+ system was used and 95% within 40 min for the Fe2+ system. It was noted that these two systems were effective, but their kinetic speeds were different. The decolourization of Acid Yellow 99 by the system Fe2+/H2O2 was faster than that obtained by the system Fe3+/H2O2.
The degradation of the dye was caused by hydroxyl radicals in the case of Fe2+/H2O2 (Fenton) and hydroperoxyl radicals in the case of the system Fe3+/H2O2. It is known that the radicals have a hydroperoxyl oxidising lower than that of hydroxyl radicals.
Several studies have shown that the oxidation of organic compounds is faster with the system Fe2+/H2O2 (Fenton) than with the system Fe3+/H2O2 (Fenton-like), and this concords with our results. This is due to the immediate formation of hydroxyl radicals in the case of Fe2+/H2O2 system. The mechanism of the oxidation of organic compounds[39] is shown in Figure8.
Effect of anions
The inorganic ions inhibit Fenton and photo-Fenton oxidations of organic compounds; their reaction on iron makes complexes of ferrous and ferric ions less reactive for the Fenton reaction, and they can decrease the overall efficiency. In the presence of anions such as SO42−, NO3−, Cl− and PO43−, the rate of the reaction of H2O2 with ferrous ion is different. To evaluate the influence of these ions, the pH of an AY99 aqueous solution by different acids (H2SO4, HNO3, HCl and H3PO4) was adjusted in similar operating conditions to those established previously. After 50 min of contact time, the decolourization efficiency was 88.36%, 87.93%, 85.98% and 39.61% in the presence of H2SO4, HNO3, HCl and H3PO4 acids, respectively. We can deduce that H2SO4, HNO3 and HCl acids lead almost to the same efficiency, but in the presence of H3PO4, it is too low. The inhibitory effect of phosphate ions may be due to a complexation and a radical scavenging as shown in Equations 14 and 15[39–41]. Phosphate ions create competition between hydroxyl radicals and organics, leading to the inhibition of oxidation.
According to complexation, effect of phosphate ions may undergo a complex reaction with ferrous and ferric ions, which impedes the reaction, causing hydroxyl radicals to be produced. The complexation reactions are shown by Equations 16 and 17.
Kinetics study
Kinetics order
The degradation of dye by Fenton’s process was a complicated process due to the involvement of many side reactions and intermediates. The process could not be easily depicted by simple reaction kinetics. Several investigators have found that the Fenton reaction for dye degradation follows a pseudo-first-order kinetics[10]. Malik and Saha[36] investigated the oxidation of direct dyes via the Fenton reaction and found that the entire degradation reaction could be divided into a two-stage reaction. In the first stage, referred to as Fe2+/H2O2 stage, it was considered that the dyes decomposed quickly. The second stage of reaction was referred to as the Fe3+/H2O2 stage. The two stages have a pseudo-first-order kinetics.
Chan and Chu[42] have studied the degradation of atrazine by Fenton reagent. They showed that the reaction of degradation occurs in two stages and that these steps have a pseudo-second-order kinetics. Sheng-Pen Sun et al.[43] found that the degradation of the azo dye (orange G) by Fenton oxidation process in aqueous solutions occurs in one step and that the reaction kinetics follows a pseudo-second order.
Subsequently, the reaction order for the degradation of AY99 by Fenton reagent will be determined, and kinetics model will consider that it involves a single step. The reaction kinetics of a Fenton reaction on the degradation of dye can be described as:
where, C is AY99 concentration, m is the order of the reaction, t is the time and k is the reaction rate constant.
For a first-order reaction, the above equation after integration becomes:
In which, C0 is the initial AY99 concentration and C t is the concentration at reaction time t. For a second-order reaction, the integrated equation becomes:
The first- and second-order kinetics rate constants for the degradation of AY99 at different reaction conditions were obtained, and the results were shown in Table1. It was observed that the correlation coefficients obtained for the two models are different. The second-order kinetics shows higher correlation (R2 > 0.85) than the first-order kinetics (R2 > 0.7). It can be concluded that the degradation of AY99 by Fenton oxidation fits the second-order reaction kinetic of the type:
To verify the feasibility of the proposed model, results derived from the model and those from raw data were compared and confirmed.
Rate equation for the degradation of AY99 using the homogeneous Fenton’s reagent
According to Equation 18, when plotting the function 1/C versus t, it is possible to determine the rate constant (k) (second order). Since Fenton’s reaction remains dependent on the initial concentrations of H2O2, Fe2+ and C0, then these dependencies can be included in the expression for the rate constant (k2) according to Equation 22:
where A is the frequency factor, Ea is the apparent activation energy, T is the absolute temperature (in kelvin) and the constant R is the universal constant of ideal gazes (8.314 J/mol/K). [AY]0, [H2O2]0 and [FeSO4]0 are the three initial concentrations in millimole per liter and n, m and p are the orders of concentration dependence. These results show that the reaction rate is more dependent on the concentration of hydrogen peroxide than on the concentration of Fe2+. It is interesting to note that the order of concentration dependence on [H2O2]0 is significantly larger than that on [FeSO4]0 for AY99 and that the frequency factor A depends on the pH of the aqueous medium in the Fenton process.
The Arrhenius expression showing the relationship between the reaction temperature and the specific reaction rate constant k is expressed as the following:
where A is the pre-exponential (or frequency) factor, E a is the activation energy (in Joule per mole), R is the ideal gas constant (8.314 J/mol/K) and T is the absolute temperature (in kelvin). Experimental data are plotted according to ln(k2) versus 1/T. Good linear relationships exist between the plots of ln(k2) and 1/T because the regression coefficient was higher than 0.99, as shown in Equation 24.
Based on the slope (−Ea/R) and intercepts (ln A), Ea and A in Arrhenius form (Equation 23) were determined, i.e.,Ea = 58.375 kJ/mol and A = 1.937 × 1,010/min. Finally, the expression for the calculation of the constant of speed (k2) for this process is:
This equation is valid for the concentration range from 0.03 to 0.15 mmol/L for Fe2+, from 0.9 to 1.8 mmol/L for H2O2 and from 0.03 to 0.15 mmol/L for AY99. The resulting parity plot is shown in Figure9, giving a good agreement between the data computed from both equations.
Effects of additives
Effects of inorganic anions
In the present study and under the optimum conditions (initial pH = 3, [H2O2]0 = 1.8 mmol/L, [Fe2+]0 = 0.09 mmol/L, concentration of dye [AY]0 = 0.06 mmol/L and temperature = 303 K), the degradation of AY99 was investigated in the presence of certain inorganic anions over the range 0 to 5 mmol/L, which were added to the dye solution. Among different salts, sodium chloride, calcium chloride, sodium sulphate and calcium sulphate were chosen for this purpose. It was observed that the increase in sodium chloride and calcium chloride concentrations from 0 to 5 mmol/L decreases the decolourization efficiency from 88.36% to 52.18% and 40.91% within 20 min, respectively. At 40 min, the decolourization efficiency decreases about 33% for sodium chloride and about 45% for calcium chloride. This inhibition may be due to complexation and radical scavenging. As shown in Figure10, chloride ions create competition between hydroxyl radicals and AY99 dye, leading to the inhibition of degradation. Table2 shows the degradation rate constant of AY99 in the absence and in the presence of sodium chloride and calcium chloride. It can be seen that the rate constants decrease about 80% for increasing sodium chloride dosage from 0 to 5 mmol/L and about 90% for calcium chloride. This phenomenon can be explained by:
-
a)
The ferric reacts with chlorine radical Cl·, which reacts rapidly with Fe(II) K = 5.9 × 109/mol/s) to produce Cl− and ferrous ions (Equation 26) [44–46].
(26)
The reaction rate constant (in per mole per second) of ferrous ions reacting with hydrogen peroxide to produce hydroxyl radicals is 53/mol/s, and the reaction rate constant of ferric ions reacting with hydrogen peroxide to produce ions is 0.02/mol/s.
-
b)
A decrease in the rate of generation of hydroxyl radicals because of the formation of chloro-Fe(III) complexes decreases the formation of perxo complexes [Fe(III)H2O2]2+ and consequently decreases the generation rates of Fe(II) (Figure 11).
It should be noted that the formation of the dichloride anion radicals (Cl2·−) will not affect the oxidation rate of Fe(II) because this radical is a strong oxidant (E0 = 2.09 V), which reacts rapidly with Fe(II) species, and the formation of HO· by the reaction of H2O2 with Fe(II) is the limiting step in the overall reaction rate of Fe(II) oxidation[44–46]. If we assume that FeCl+ can also be oxidised by H2O2 (Figure10), the rate constants for the reaction of H2O2 with FeCl+ and Fe2+ will be identical (55/mol/s) because the addition of chloride (and consequently the complexation of Fe(II) by Cl−) has no effect on the overall oxidation rate of Fe(II). The overall rate constant equations for AY99 degradation by Fenton process in the presence of sodium chloride and calcium chloride are:
The effect of different sodium sulphate and calcium sulphate concentrations ranging from 0 to 5 mmol/L on the decolourization of 0.06 mmol/L AY99 solution is shown in Table2. Increasing of sodium sulphate and calcium sulphate concentrations will result in the decrease of the constant rate. This enhancement can be explained by the formation of SO4−Fe(III) complex (Equations 29 and 30).
Sulphate ions can also complex ferrous and ferric ions, which are responsible for the production of HO· radicals and the regeneration of Fe2+ ions. As compared to the sulphate ion, the inhibiting effect of the chloride ion for the formation of the peroxocomplexes is less important (Figure11). The increase in sodium sulphate and calcium sulphate concentrations from 0 to 5 mmol/L decreases the decolourization efficiency from 88.36% to 81.86% and 79.48% within 20 min, respectively. The effect of various inorganic anions (concentration, 5 mmol/L) on the degradation of AY99 is in the following decreasing order:
the efficiency of degradation: Ef (Na2SO4) > Ef (CaSO4) > Ef (NaCl) > Ef (CaCl2)
the rate constant of degradation: k2 (CaCl2) < k2 (NaCl) < k2 (CaSO4) < k2 (Na2SO4)
Effect of cations
In order to compare the performance of different cations, additional experiments were performed with different sulphates. The cations of concern were Na+, Ca2+, Cu2+, Mn2+, Zn2+ and Mg2+ while maintaining the other operational conditions constant (hydrogen peroxide and AY99 concentrations were kept constant at 1.8 mmol/L and 0.06 mmol/L, respectively, initial pH = 3, temperature = 303 K and concentration of Fe2+ = 0.09 mmol/L).
Effect of copper sulphate
The influence of copper sulphate on the degradation of AY99 was studied for concentration in the range 0 to 8 mmol/L. The results are shown in Table2. It can be seen that the copper sulphate exerts a strong effect on the rate constant degradation of AY99.
The decolourization efficiency decreases from 88.36% to 60.63% as a consequence of increasing the copper sulphate concentration from 0 to 5 mmol/L within 20 min and from 95.08% to 80.34% within 40 min. The results showed that the difference in overall efficiency within 300 min for the different concentrations were negligible (<5%).
The rate constant in the absence of copper sulphate is three times larger than in the presence of 5 mmol/L copper sulphate and four times larger than in the presence of 8 mmol/L copper sulphate. The reaction mechanism and the catalytic cycle become complicated in presence of copper sulphate. This could be explained as Cu2+ ions reacting with H2O2 to produce less oxidative radicals such as H2O· in reactions 31 and 32[47, 48]:
The overall rate constant equation for AY99 degradation by Fenton process in the presence of copper sulphate is:
Effect of manganese sulphate
The degradation of AY99 by Fenton process was investigated in the presence of different concentrations of manganese sulphate ranging from 0 to 8 mmol/L. The obtained results are presented in Table2. It was observed that the degradation efficiency and rate constant decreased with the increase in manganese sulphate concentration. The decolourization efficiency decreases from 88.36% to 49.36% as a consequence of increasing the manganese sulphate from 0 to 5 mmol/L within 20 min and from 95.08% to 83.59% within 140 min. It might to be that Mn2+ either reacts with H2O2 to decrease the amount of H2O2 available for HO· radicals production or that it quenches the HO· radicals directly. The overall rate constant equation for AY99 degradation by Fenton process in the presence of manganese sulphate is:
Effect of zinc sulphate and magnesium sulphate
In order to investigate the effect induced from the addition of zinc sulphate and magnesium sulphate, the degradation of AY99 in the presence of 0 to 8 mmol/L zinc sulphate and 0 to 20 mmol/L magnesium sulphate was performed. It was observed that the increase of zinc sulphate and magnesium sulphate concentrations decreases the decolourization efficiency from 88.36% to 80.74% and 78.61%, respectively, within 20 min. After 80 min, for the different concentrations, the difference in overall efficiency was negligible.
Effect of sodium sulphate and calcium sulphate
As mentioned above, the increase in sodium sulphate and calcium sulphate concentrations from 0 to 5 mmol/L decreases the decolourization efficiency from 88.36% to 81.86% and 79.48% within 20 min, respectively. The effect of all these cations (concentration, 5 mmol/L) on the efficiency of the degradation of AY99 is listed in the following decreasing order:
Ef (ZnSO4) > Ef (MgSO4) > Ef (Na2SO4) > Ef (CaSO4) > Ef (CuSO4) > Ef (MnSO4).
k2 (Na2SO4) < k2 (MnSO4) < k2 (CuSO4) < k2 (CaSO4) < k2 (ZnSO4) < k2 (MgSO4).
Conclusions
The present study has investigated the degradation of Acid Yellow 99 by Fenton oxidation process. The test results showed that the solution’s pH value, the acid’s nature to adjust the pH, the initial H2O2 concentration, the initial Fe2+ concentration, the initial AY99 concentration and the temperature were the main factors that have strong influence on the degradation efficiency of Acid Yellow 99. The optimal values of the operating parameters during the oxidation of the Acid Yellow dye by Fenton process were found to be pH = 3, [H2O2]0 = 1.8 mmol/L, [Fe2+]0 = 0.09 mmol/L, [AY]0 = 0.06 mmol/L and T = 30°C. The Fenton process for AY99 was found to follow pseudo-second-order kinetics, and the reaction rate constant was dominated by initial [H2O2]0, [Fe2+]0 and [AY]0 concentrations. A kinetic model was proposed in the range of the operating conditions studied. The presence of cations and some inorganic anions in an aqueous solution decreases the decolourization efficiency and the rate constant of the degradation of AY99. This inhibition may be due to a complexation and a radical scavenging. In real wastewater, the phenomena will be more complicated; many anions and cations may be present simultaneously, and different interactions may occur.
Competing’ interest
The authors declare that they have no competing interests.
Authors’ information
Prof. Fadhel Ismail, Department of Process Engineering, University of Annaba, Annaba, Algeria Email: ismail.fadhel@univ-annaba.org.
References
Chung KT, Stevens SE: Degradation of azo dyes by environmental microorganisms and helminths. Environ Toxicol Chem 1993,12(11):2121–2132.
Nilsson R, Nordlinder R, Wass U, Meding B, Belin L: Asthma, rhinitis, and dermatitis in workers exposed to reactive dyes. Br J Ind Med 1993, 50: 65–70.
Correia VM, Stephenson T, Judd SJ: Characterization of textile wastewaters. Environ Technol 1994, 15: 917–929. 10.1080/09593339409385500
Porter JJ: Treatment of textile waste with activated carbon. Am Dyestuff Rep 1972, 61: 8–12.
Mckay G: Colour removal by adsorption. Am Dyestuff Rep 1980, 69: 38–66.
Yeh RYL, Liu R, Chiu HM, Hung YT: Comparative study of adsorption capacity of various adsorbents for treating dye wastewaters. Int J Environ Stud Environ Sci Technol 1993, 44: 259–284. 10.1080/00207239308710867
Yeh RYL, Thomas A: Colour removal for dye wastewaters by adsorption using powdered activated carbon: mass transfer studies. J Chem Technol Biotechnol 1995, 63: 48–54. 10.1002/jctb.280630107
Yeh RYL, Thomas A: Colour difference measurement and colour removal from dye wastewaters using different adsorbents. J Chem Technol Biotechnol 1995, 63: 55–59. 10.1002/jctb.280630108
Uygur A, Kök E: Decolourisation treatments of azo dyes waste waters including dichlorotriazinyl reactive groups by using advanced oxidation method. J Soc Dyers Colour 1999, 115: 350–354.
Chen Y, Chen L: Study on the relationship between structure of synthetic organic chemicals and their biodegradability. Environ Chem 1995, 14: 354–367.
Liu Y, Chen X, Li J, Burda C: Photocatalytic degradation of azo dyes by nitrogen-doped TiO2 nanocatalysts. Chemosphere 2005, 61: 11–18. 10.1016/j.chemosphere.2005.03.069
Baran W, Makowski A, Wardas W: The separation of catalyst after photocatalytic reactions conducted in the presence of TiO2/FeCl3/UV. Chemosphere 2005, 59: 853–859. 10.1016/j.chemosphere.2004.10.059
Stylidi M, Kondarides DI, Verykios XE: Visible light-induced photocatalytic degradation of Acid Orange 7 in aqueous suspensions. Appl Catal B: Environ 2004, 47: 189–201. 10.1016/j.apcatb.2003.09.014
Konstantinou IK, Albanis TA: TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Appl Catal B: Environ 2004, 49: 1–14. 10.1016/j.apcatb.2003.11.010
Xie YB, Li XZ: Interactive oxidation of photoelectrocatalysis and electro-Fenton for azo dye degradation using TiO2–Ti mesh and reticulated vitreous carbon electrodes. Mater Chem Phys 2006, 95: 39–50. 10.1016/j.matchemphys.2005.05.048
Neamtu M, Yediler A, Siminiceanu I, Macoveanu M, Kettrup A: Decolorization of disperse red 354 azo dye in water by several oxidation processes - a comparative study. Dyes and Pigments 2004, 60: 61–68. 10.1016/S0143-7208(03)00129-3
Shu HY, Chang MC: Decolorization effects of six azo dyes by O3, UV/O3 and UV/H2O2 processes. Dyes and Pigments 2005, 65: 25–31. 10.1016/j.dyepig.2004.06.014
Lipczynska-Kochany E: Hydrogen peroxide mediated photodegradation of phenol as studied by a flash photolysis/HPLC technique. Environ Pollution 1993, 80: 147–152. 10.1016/0269-7491(93)90140-J
Aleboyeh A, Moussa Y, Aleboyeh H: The effect of operational parameters on UV/H2O2 decolourisation of Acid Blue 74. Dyes and Pigments 2005, 66: 129–134. 10.1016/j.dyepig.2004.09.008
Aleboyeh A, Aleboyeh H, Moussa Y: Critical effect of hydrogen peroxide in photochemical oxidative decolorization of dyes: Acid Orange 8, Acid Blue 74 and Methyl Orange. Dyes and Pigments 2003, 57: 67–75. 10.1016/S0143-7208(03)00010-X
Daneshvar N, Rabbani M, Modirshahla N, Behnajady MA: Photooxidative degradation of Acid Red 27 in a tubular continuous-flow photoreactor: influence of operational parameters and mineralization products. J Hazard Mater 2005, B118: 155–160.
Xu XR, Li HB, Wang WH, Gu JD: Degradation of dyes in aqueous solutions by the Fenton process. Chemosphere 2004, 57: 595–600. 10.1016/j.chemosphere.2004.07.030
Kusic H, Koprivanac N, Srsan L: Azo dye degradation using Fenton type processes assisted by UV irradiation: a kinetic study. J Photochem Photobiol A: Chem 2006, 181: 195–202. 10.1016/j.jphotochem.2005.11.024
Lipczynska-Kochany E: Novel method for a photocatalytic degradation of 4-nitrophenol on homogeneous aqueous solution. Environ Technol 1991, 12: 87–92. 10.1080/09593339109384985
Rodriguez M, Sarria V, Esplugas S, Pulgarin C: Photo-Fenton treatment of a biorecalcitrant wastewater generated in textile activities: biodegradability of the photo-treated solution. J Photochem Photobiol A: Chem 2002, 151: 129–135. 10.1016/S1010-6030(02)00148-X
Hsueh CL, Huang YH, Wang CC, Chen CY: Photoassisted Fenton degradation of nonbiodegradable azo-dye (Reactive Black 5) over a novel supported iron oxide catalyst at neutral pH. J Mol Catal A: Chem 2006, 245: 78–86. 10.1016/j.molcata.2005.09.044
Buxton GV, Greenstock CL, Helman WP, Ross AB: Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/.O−) in aqueous solution. J Phys Chem 1988, 17: 513–886.
Kim SM, Geissen SU, Vogelpohl A: Landfill leachate treatment by a photoassisted fenton reaction. Water Sci Technol 1997, 35: 239–248.
Balcioghu IU, Arslan I: Partial oxidation of reacture dyestuffs and synthetic textile duebeth by the O3 and O3/H2O2 processes. Water Sci Technol 2004, 43: 221–228.
Shu HY, Huang CR: Degradation of commercial azo dyes in water using azonation and UV enhanced ozonation process. Chemosphere 1995, 31: 3813–3825. 10.1016/0045-6535(95)00255-7
Marechal AM, Slokar YM, Tanfer T: Decoloration of chlorotriazine reactive azo dyes with H2O2/UV. Dyes and Pigments 1997, 33: 281–298. 10.1016/S0143-7208(96)00057-5
Daneshvar N, Salari D, Khataee AR: Photocatalytic degradation of azo dye Acid Red 14 in water; investigation of the effect of operational parameters. J Photochem Photobiol A Chem 2003,157(1):111–116. 10.1016/S1010-6030(03)00015-7
Neamtu M, Yediler A, Siminiceanu I, Kettrup A: Oxidation of commercial reactive azo dye aqueous solution by the photo-Fenton and Fenton-like processes. J Photochem Photobiol A Chem 2003, 161: 87–93. 10.1016/S1010-6030(03)00270-3
Walling C: Fenton’s reagent revisited. Acc Chem Res 1975, 8: 125–131. 10.1021/ar50088a003
Pignatello JJ, Oliveros E, Mackay A: Advanced oxidation processes for organic contaminants destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 2006, 66: 1–84.
Malik PK, Sk S: Oxidation of direct dyes with hydrogen peroxide using ferrous ion as catalyst. Sep Purif Technol 2003, 31: 241–250. 10.1016/S1383-5866(02)00200-9
Modirshahla N, Behnajady MA, Ghanbary F: Decolorization and mineralization of C.I. Acid Yellow 23 by Fenton and photo-Fenton processes. Dye and Pigments 2007, 73: 305–310. 10.1016/j.dyepig.2006.01.002
Sun JH, Sun SP, Wang GL, Qiao LP: Degradation of azo dye Amido black 10B in aqueous solution by Fenton oxidation process. Dyes and Pigments 2007, 74: 647–652. 10.1016/j.dyepig.2006.04.006
Bouasla C, Samar MEH, Ismail F: Degradation of methyl violet 6B dye by the Fenton process. Desalination 2010,254(1–3):35–41.
Maruthamuthu P, Neta P: Phosphate radicals. Spectra, acid-base equilibria, and reactions with inorganic compounds. J Phys Chem 1978, 82: 710–713. 10.1021/j100495a019
Lipczynska-Kochany E, Sprah G, Harms S: Influence of some groundwater and surface waters constituents on the degradation of 4-chlorophenol. Chemosphere 1995,30(1):9–20. 10.1016/0045-6535(94)00371-Z
Chan KH, Chu W: The system design of atrazine oxidation by catalytic oxidation process through a kinetic approach. Water Research 2003, 37: 3997–4003. 10.1016/S0043-1354(03)00316-6
Suna SP, Li CJ, Sunb JH, Shib SH, Fand MH, Zhoua Q: Decolorization of an azo dye Orange G in aqueous solution by Fenton oxidation process: effect of system parameters and kinetic study. J Hazard Mater 2009, 161: 1052–1057. 10.1016/j.jhazmat.2008.04.080
Lu MC, Chang YF, Chen IM, Huang YY: Effect of chloride ions on the oxidation of aniline by Fenton’s reagent. J Environmental Management 2005, 75: 177–182. 10.1016/j.jenvman.2004.12.003
Le TG, Laat JD: Effects of chloride ions on the iron (III)- catalyzed decomposition of hydrogen peroxide and on the efficiency of the Fenton-like oxidation process. Appl Catal B: Environ 2006, 66: 137–146. 10.1016/j.apcatb.2006.03.008
Truong GL, Laat DJ, Legube B: Effects of chloride and sulphate on the rate of oxidation of ferrous ion by H2O2. Water Research 2004, 38: 2384–2394. 10.1016/j.watres.2004.01.033
Sun JH, Sun P, Sun JY, Sun RX, Qiao LP, Guo HQ, Fan MH: Degradation of azo dye Acid black 1 using low concentration iron of Fenton process facilitated by ultrasonic irradiation. Utrasonics Sonochemistry 2007,14(6):761–766. 10.1016/j.ultsonch.2006.12.010
Burlando B, Viarengo A: Ca2+ is mobilized by hydroxyl radical but not by superoxide in RTH-149 cells: the oxidative switching-on of Ca2+ singnaling. Cell Calcium 2005, 38: 507–513. 10.1016/j.ceca.2005.07.004
Acknowledgements
We acknowledge the editor for his investigation to make significant revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors’ contributions
All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bouasla, C., Ismail, F. & Samar, M.EH. Effects of operator parameters, anions and cations on the degradation of AY99 in an aqueous solution using Fenton’s reagent. Optimization and kinetics study. Int J Ind Chem 3, 15 (2012). https://doi.org/10.1186/2228-5547-3-15
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5547-3-15