Abstract
Background
In un-resuscitated rodent models of septic shock, the peroxisome proliferator-activated receptor-β/δ (PPAR-β/δ) agonist GW0742 improved visceral organ function. Therefore, we tested the hypothesis whether GW0742 would attenuate kidney injury during long-term, resuscitated, porcine polymicrobial septic shock.
Methods
Six, 12, and 18 h after the induction of fecal peritonitis by inoculation of autologous feces, anesthetized, mechanically ventilated, and instrumented male pigs with pre-existing atherosclerosis resulting from familial hypercholesteremia and atherogenic diet randomly received either vehicle (dimethyl sulfoxide, n = 12) or GW0742 (n = 10). Resuscitation comprised hydroxyethyl starch and norepinephrine infusion titrated to maintain mean arterial pressure at baseline values.
Results
Despite aggressive fluid resuscitation, fecal peritonitis was associated with arterial hypotension requiring norepinephrine infusion, ultimately resulting in progressive lactic acidosis and acute kidney injury. GW0742 did not beneficially affect any parameter of systemic and regional hemodynamics, gas exchange, metabolism, or organ function. The parameters of inflammation, oxidative and nitrosative stress, and organ injury (post-mortem analysis for histomorphology and markers of apoptosis) were not influenced either. Immunohistochemistry of pre-shock kidney biopsies from a previous study in this swine strain showed markedly lower PPAR-β/δ receptor expression than in healthy animals.
Conclusions
In swine with pre-existing atherosclerosis, the PPAR-β/δ agonist GW0742 failed to attenuate septic shock-induced circulatory failure and kidney dysfunction, most likely due to reduced receptor expression coinciding with cardiovascular and metabolic co-morbidity.
Similar content being viewed by others
Background
Ample evidence is available that the activation of the peroxisome proliferator-activated receptors (PPAR), ligand-activated transcription factors of the nuclear hormone receptor family, presenting as PPAR-α, PPAR-γ, and PPAR-β/δ, has beneficial effects in various shock models. The highly selective synthetic PPAR-β/δ agonist GW0742 [1] blunted shock-induced organ injury as a result of attenuated inflammation and oxidative and nitrosative stress and decreased activation of the nuclear transcription factor κB (NF-κB) [2–8]. These organ-protective properties were also present in animals with obesity [9] and diabetes [10], most likely as a result of enhanced insulin sensitivity and, consequently, improved glucose utilization [11], as well as attenuated endothelial dysfunction [12]. However, all these data originate from short-term, un-resuscitated rodent models characterized by hypotension and low cardiac output. Therefore, we tested the hypothesis whether GW0742 may attenuate kidney dysfunction during long-term, resuscitated, porcine fecal peritonitis-induced septic shock [13, 14]. Given the beneficial effects of GW0742 on glucose homoeostasis [11] and vascular function [12], we investigated swine with hyperlipidemia and ubiquitous atherosclerosis [15].
Methods
The University of Ulm Animal Care Committee and the Federal authorities for animal research had approved the experiments, which were performed in adherence to National Institutes of Health Guidelines on the Use of Laboratory Animals. Twenty-two adult, castrated, male pigs (age 15 to 30 months, median (interquartile range) body weight of 72 (65 to 81) kg) were used. The pig strain is a cross-breed of Rapacz farm pigs homozygous for the R84C low-density lipoprotein (LDL) receptor mutation with smaller strains ('FBM’), with hypercholesteremia due to an atherogenic diet [15].
Animal preparation
Anesthesia and surgical instrumentation have been described in detail previously [13–15]. Briefly, anesthesia was induced with atropine, propofol, and ketamine to allow endotracheal intubation and was maintained thereafter with pentobarbitone, buprenorphine, and pancuronium. Ventilator settings were fraction of inspired O2 (FiO2) 0.35, positive end-expiratory pressure (PEEP) 10 cmH2O, tidal volume 8 mL·kg-1, respiratory rate 10 to 12 breaths·min-1 adjusted to maintain arterial PCO2 = 35 to 40 mmHg, inspiratory (I)/expiratory (E) ratio 1:1.5, peak airway pressure <40 cmH2O, and modified to I/E ratio 1:1 and PEEP 12 or 15 cmH2O, respectively, if the ratio of arterial O2 partial pressure (PaO2)/FiO2 is <300 or <200 mmHg [13–15]. The right jugular vein and carotid artery were exposed for the insertion of a central venous catheter sheath and the placement of a balloon-tipped pulmonary artery catheter to measure central venous (CVP), pulmonary arterial (MPAP), and pulmonary artery occlusion pressures (PAOP), and a thermistor-tipped arterial catheter for blood pressure (MAP) recording and transpulmonary single indicator thermodilution-cardiac output (CO) measurement. The right kidney and a femoral vein were surgically exposed, and a catheter was advanced into the inferior vena cava and manually guided into a right renal vein under visual control [14, 15]. A catheter in the urinary bladder allowed urine collection. Two tubes were placed through the abdominal wall for peritonitis induction. Ringer's solution was continuously infused as maintenance fluid (10 mL·kg-1·h-1). As needed, animals received hydroxyethyl starch to maintain cardiac filling pressures during surgery.
Experimental protocol
After instrumentation and an 8-hour recovery, baseline data were collected. Thereafter, the supernatant (3 mL·kg-1) of 1.0 g·kg-1 autologous feces incubated in 500 mL 0.9% saline for 12 h at 38°C was injected into the abdominal cavity via the abdominal drainage tubes. Hydroxyethyl starch (10 mL∙kg-1∙h-1, 5 mL∙kg-1∙h-1 if CVP or PAOP is >18 mmHg) allowed maintaining hyperdynamic hemodynamics. If necessary, norepinephrine was infused and titrated to maintain MAP at baseline values (no further increase if the heart rate is ≥160 min-1 to avoid tachycardia-induced myocardial ischemia) [13–16]. Animals randomly received i.v. GW0742 (0.03 mg∙kg-1, n = 10, body weight 71 (66 to 80) kg) or vehicle (DMSO; n = 12, body weight 75 (66 to 81) kg) at 6, 12, and 18 h after the induction of peritonitis. The same GW0742 dose attenuated renal dysfunction in murine endotoxic shock [2] and organ injury after kidney ischemia/reperfusion injury in diabetic rats [10]. The timing of the GW0742 administration was chosen, because10-day survival was doubled in mice injected with this dose at 6.5 and 12.5 h after cecal ligation and puncture-induced sepsis [2]. After additional data collection at 12 and 24 h after the induction of peritonitis, animals were sacrificed under deep anesthesia.
Measurements and calculations
Hemodynamics, gas exchange (calorimetric O2 uptake and CO2 production, arterial and mixed venous blood gases), glucose, lactate, creatinine, renal venous nitrite + nitrate (NO2 - + NO3 -), tumor necrosis factor-α (TNFα), and interleukin-6 (IL-6) concentrations were determined as described previously [13–16]. Endogenous glucose production and glucose oxidation were derived from plasma 1,2,3,4,5,6-13C6-glucose and the mixed expiratory 13CO2 isotope enrichment, respectively, during continuous glucose isotope infusion [13, 14]. Urinary and blood creatinine and Na+ levels were analyzed to calculate creatinine clearance and fractional Na+-excretion [9] together with blood neutrophil gelatinase-associated lipocalin (NGAL) [15]. At the end of the experiment, immediate post-mortem kidney tissue samples were analyzed for the expression of the inducible nitric oxide synthase (iNOS), heme oxygenase-1 (HO-1), and cleaved caspase-3 as well as for the activation of the nuclear transcription factor κB (NF-κB) as described in detail previously [15, 16]. Pyramid-shaped kidney specimens showing kidney cortex, medulla, renal papilla, and the corresponding renal calyx were dissected for histopathological examination, performed by an experienced pathologist (A.S.) blinded for the sample grouping [15]. Histopathological alterations were analyzed for the degree of 'glomerular tubularization’, dilatation of Bowman's space, and swelling of Bowman's capsule, cellular edema of the proximal tubule, distal tubular dilatation and elongation, tubular protein cylinders, and tubular necrosis as described in detail previously [15].
Immunohistochemistry allowed quantifying the formation of nitrotyrosine (rabbit anti-Nitrotyrosine, Millipore, Schwalbach, Germany) [16] and the expression of the PPAR-β/δ (rabbit anti-PPAR delta antibody #ab23673, Abcam plc, Cambridge, UK), the method being described in detail in the supplement. The latter was determined on formalin-fixed, paraffin-embedded kidney biopsies, which had been taken during surgical instrumentation both in FBM and young and healthy German Landswine in a previous study [15], as well as age-matched FBM pigs that had not been fed with the atherogenic diet (n = 5 in each group). Results are presented as mean densitometric sum red.
Statistical analysis
Data are presented as median (quartiles). After the exclusion of normal distribution using the Kolmogorov-Smirnoff test, differences within groups were analyzed by a Friedmann analysis of variance on ranks and a subsequent Dunn's test with Bonferroni correction. Inter-group differences were tested using a Mann–Whitney rank sum test.
Results
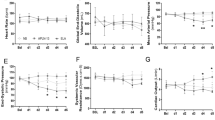
One animal in the control group died 15 h after the induction of peritonitis; therefore, data at the end of the experiment originate from 11 vehicle-treated animals only. Colloid and norepinephrine requirements were comparable in the two groups (Additional file 1: Table A). MAP progressively decreased and CO increased despite aggressive circulatory support, ultimately resulting in impaired pulmonary gas exchange and lactic acidosis, however, without inter-group difference (Table 1). Sepsis caused a progressive deterioration of renal function, coinciding with increased renal venous concentrations of pro-inflammatory cytokines, NO metabolites, and isoprostanes, again without any inter-group difference (Table 2). Western blotting confirmed the findings on blood biomarkers, while tissue expression of HO-1, iNOS, and activated caspase 3 were comparable to those from animals that had undergone surgical instrumentation only, sepsis nearly tripled NF-κB activation (Additional file 1: Table B). There was, however, no treatment effect.
Kidney histopathology showed only mild to moderate glomerular and tubular damage without inter-group difference (Additional file 1: Table C). Immunohistochemistry showed marked nitrotyrosine formation, again without inter-group difference (Additional file 1: Table B). Immunohistochemistry of the renal PPAR-β/δ showed that expression was nearly ten times lower than both in young and healthy German Landswine and age-matched FBM pigs that had not been fed with the atherogenic diet (Figure 1).
Examples and results of the quantitative image analysis of the PPAR-β/δ immunohistochemistry in biopsies. Examples (A) (magnification ×20; left panel: German Landswine, right panel: FBM swine) and results of the quantitative image analysis (B) of the PPAR-β/δ immunohistochemistry in biopsies taken during surgical instrumentation in comparison to biopsies taken in otherwise young and healthy German Landswine undergoing a similar surgical instrumentation. German Landswine (open box-and-whisker plots), FBM swine with (dark gray box-and-whisker plots), and without hyperchloesteremic diet (light gray box-and-whisker plots). All data are median (quartiles, range; n = 5 in each group).
Discussion
This study was to test the hypothesis whether the PPAR-β/δ agonist GW0742 would attenuate kidney injury during long-term, resuscitated, polymicrobial porcine septic shock. Since GW0742 had been effective in animals with obesity and diabetes, we studied swine with hyperlipidemia and ubiquitous atherosclerosis [15]. The major findings were that (1) GW0742 failed to attenuate sepsis-induced organ dysfunction and histological damage and (2) did not affect the parameters of inflammation and oxidative and nitrosative stress.
The lacking efficacy of GW0742 is in contrast to previous studies in polymicrobial sepsis [2, 7], which, however, report data from young and otherwise healthy rodents. We studied septic shock in FBM swine, a strain presenting with ubiquitous hypercholesteremia-induced atherosclerosis [15]. In addition, these animals showed a several-fold reduction of the kidney PPAR-β/δ expression as compared to young and healthy German Landswine. In obese, insulin-resistant mice, weight loss increased PPAR-γ expression [17], and both starvation and endurance training activated PPAR-δ in healthy animals [18, 19]. PPAR-δ knockout mice are glucose-intolerant [11], and PPAR-β/δ activation normalized the diabetes-related endothelial dysfunction [12]. Our FBM swine presented with both hypercholesteremia as well as impaired glucose homoeostasis; at baseline, direct, aerobic glucose oxidation was significantly lower (<30% vs. 60% to 75% of glucose production) when compared to young (age 3 to 4 months) and healthy German Landswine undergoing the same protocol of resuscitated fecal peritonitis [13, 14]. Hence, the pre-existing atherosclerosis together with the 'metabolic syndrome’ may have caused GW0742 inefficacy due to PPAR-β/δ down-regulation. It could be argued in this context that GW0742 did attenuate ischemia/reperfusion injury in obese and diabetic rats [9, 10]. These data, however, originate from un-resuscitated, short-term models in rats, whereas we studied long-term, fully resuscitated porcine septic shock.
Our findings of reduced PPAR-β/δ expression were obtained from biopsies taken during surgical instrumentation, i.e., prior to any inflammatory challenge [15]. Since the abdominal cavity had been closed again after the surgical instrumentation, we could not obtain kidney biopsies after induction of peritonitis. Therefore, we cannot exclude that sepsis further aggravated PPAR-β/δ down-regulation and thus contributed to the lacking efficacy of GW0742. In rodents, any shock-related effect on PPAR expression seems to be organ-specific; hemorrhage, injection of endotoxin, or cecal ligation and puncture significantly reduced pulmonary [20], myocardial [21], and hepatic [22] PPAR-α, -γ, or -δ protein expression and/or mRNA. However, endotoxemia only decreased renal PPAR-α mRNA, whereas PPAR-γ and PPAR-δ mRNA remained unchanged [23].
Limitations of the study
Since we did not study the effect of GW0742 in young and healthy animals, we do not have direct evidence that the PPAR-β/δ down-regulation associated with the underlying hypercholesteremia and atherosclerosis rather than other potentially confounding factors contributed to our findings, e.g., the use of adult swine per se, the integration of standard intensive care procedures into the experimental design, the timing and dosing of the treatment, and/or the duration of the study. To our knowledge, GW0742 has only been studied in rodents. Nevertheless, GW501516, another highly specific PPAR-β/δ agonist with a chemical structure very close to that of GW0742 [1], attenuated endotoxin-induced NF-κB activation, and cytokine release not only in rodent [24–26] but also in human tissues [26, 27]. So far, a modulation of the PPAR-β/δ expression related to nutritional and/or metabolic interventions has only been studied in rodents as well [18, 19]. However, in swine, a similar diet-induced hypercholesteremia as in our experiments [28] was associated with a markedly reduced PPAR-α and PPAR-γ expression [29], coinciding with impaired glucose tolerance [28, 29], arterial hypertension [28], increased transaminase activities [29], and aggravated oxidative stress [28]. Treatment with resveratrol restored PPAR-α and PPAR-γ expression [29], going along with improved left heart perfusion and function during chronic myocardial ischemia [30, 31]. It could be argued that all rodent data on GW0742 were obtained in young animals, while we studied adult swine. In fact, ischemia/reperfusion injury and hemorrhagic shock induced PPAR-γ expression only in young but not in adult or old rodents [32, 33], which in turn impaired adaptive autophagy [33]. There are no data available on PPAR-β/δ expression under these conditions, but the above-mentioned studies on hypercholesteremia-induced PPAR-α and PPAR-γ down-regulation also originate from adult swine. Moreover, tissue PPAR-β/δ expression in kidneys from adult FBM swine that had not been fed with cholesterol-enriched diet was similar to that in young and healthy German Landswine (Figure 1B). We only studied the effect of 0.03 mg∙kg-1 of GW0742. This dose was chosen because it attenuated kidney dysfunction in murine endotoxin- and cecal ligation and puncture-induced septic shock at plasma concentrations selectively activating the PPAR-β/δ without any cross-reactivity on the other PPAR isoforms [2]. Moreover, in rats, this dose reduced organ injury after kidney ischemia/reperfusion injury [10], and a ten times higher dose did not further influence myocardial ischemia/reperfusion injury [3]. It should be noted in this context that equipotent drug doses are usually much lower on a per kilogram basis in large species (e.g., swine, dogs) than in rodents [34]. FBM swine present with reduced creatinine clearance and moderate histological damage of the kidney already under baseline conditions [15]. Nevertheless, any pre-existing organ dysfunction most likely did not influence our results; due to fluid resuscitation and catecholamine infusion, creatinine clearance was comparable to that in young and healthy German Landswine undergoing the same protocol of resuscitated fecal peritonitis [13, 14].
Conclusions
In swine with pre-existing atherosclerosis, the PPAR-β/δ agonist GW0742 failed to attenuate septic shock-induced kidney dysfunction and organ damage, most likely due to reduced receptor expression associated with cardiovascular and metabolic co-morbidity.
References
Sznaidman ML, Haffner CD, Maloney PR, Fivush A, Chao E, Goreham D, Sierra ML, LeGrumelec C, Xu HE, Montana VG, Lambert MH, Willson TM, Oliver WR Jr, Sternbach DD: Novel selective small molecule agonists for peroxisome proliferator-activated receptor δ delta (PPARδ) - synthesis and biological activity. Bioorg Med Chem Lett 2003, 13: 1517–1521. 10.1016/S0960-894X(03)00207-5
Kapoor A, Shintani Y, Collino M, Osuchowski MF, Busch D, Patel NS, Sepodes B, Castiglia S, Fantozzi R, Bishop-Bailey D, Mota-Filipe H, Yaqoob MM, Suzuki K, Bahrami S, Desvergne B, Mitchell JA, Thiemermann C: Protective role of peroxisome proliferator-activated receptor-β/δ in septic shock. Am J Respir Crit Care Med 2010, 182: 1506–1513. 10.1164/rccm.201002-0240OC
Kapoor A, Collino M, Castiglia S, Fantozzi R, Thiemermann C: Activation of peroxisome proliferator-activated receptor-β/δ attenuates myocardial ischemia/reperfusion injury in the rat. Shock 2010, 34: 117–124. 10.1097/SHK.0b013e3181cd86d6
Galuppo M, Di Paola R, Mazzon E, Genovese T, Crisafulli C, Paterniti I, Cuzzocrea E, Bramanti P, Kapoor A, Thiemermann C, Cuzzocrea S: Role of PPAR-delta in the development of zymosan-induced multiple organ failure: an experimental mice study. J Inflamm (Lond) 2010, 7: 12. 10.1186/1476-9255-7-12
Di Paola R, Crisafulli C, Mazzon E, Esposito E, Paterniti I, Galuppo M, Genovese T, Thiemermann C, Cuzzocrea S: GW0742, a high affinity PPAR-β/δ agonist, inhibits acute lung injury in mice. Shock 2010, 33: 426–435. 10.1097/SHK.0b013e3181b8f2fb
Di Paola R, Esposito E, Mazzon E, Paterniti I, Galuppo M, Cuzzocrea S: GW0742, a selective PPAR-β/δ agonist, contributes to the resolution of inflammation after gut ischemia/reperfusion injury. J Leukoc Biol 2010, 88: 291–301. 10.1189/jlb.0110053
Zingarelli B, Piraino G, Hake PW, O’Connor M, Denenberg A, Fan H, Cook JA: Peroxisome proliferator-activated receptor δ regulates inflammation via NF-κB signaling in polymicrobial sepsis. Am J Pathol 2010, 177: 1834–1847. 10.2353/ajpath.2010.091010
Paterniti I, Mazzon E, Riccardi L, Galuppo M, Impellizzeri D, Esposito E, Bramanti P, Cappellani A, Cuzzocrea S: Peroxisome proliferator-activated receptor β/δ agonist GW0742 ameliorates cerulein- and taurocholate-induced acute pancreatitis in mice. Surgery 2012, 152: 90–106. 10.1016/j.surg.2012.02.004
Yue TL, Nerurkar SS, Bao W, Jucker BM, Sarov-Blat L, Steplewski K, Ohlstein EH, Willette RN: In vivo activation of peroxisome proliferator-activated receptor-δ protects the heart from ischemia/reperfusion-injury in Zucker fatty rats. J Pharmacol Exp Ther 2008, 325: 466–474. 10.1124/jpet.107.135327
Collino M, Benetti E, Miglio G, Castiglia S, Rosa AC, Aragno M, Thiemermann C, Fantozzi R: Peroxisome proliferator-activated receptor β/δ agonism protects the kidney against ischemia/reperfusion injury in diabetic rats. Free Radical Biol Med 2011, 50: 345–353. 10.1016/j.freeradbiomed.2010.10.710
Lee CH, Olson P, Hevener A, Mehl I, Chong LW, Olefsky JM, Gonzalez FJ, Ham J, Kang H, Peters JM, Evans RM: PPARδ regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci U S A 2006, 103: 3444–3449. 10.1073/pnas.0511253103
Quintela AM, Jiménez R, Gómez-Guzmán M, Zarzuelo MJ, Galindo P, Sánchez M, Vargas F, Cogolludo A, Tamargo J, Pérez-Vizcaíno F, Duarte J: Activation of peroxisome proliferator-activated receptor-β-δ (PPARβ/δ) prevents endothelial dysfunction in type 1 diabetic rats. Free Radical Biol Med 2012, 53: 730–741. 10.1016/j.freeradbiomed.2012.05.045
Barth E, Bassi G, Maybauer DM, Simon F, Gröger M, Öter S, Speit G, Nguyen CD, Hasel C, Möller P, Wachter U, Vogt JA, Matejovic M, Radermacher P, Calzia E: Effects of ventilation with 100% oxygen during early hyperdynamic porcine fecal peritonitis. Crit Care Med 2008, 36: 495–503. 10.1097/01.CCM.0B013E318161FC45
Simon F, Giudici R, Scheuerle A, Gröger M, Asfar P, Vogt JA, Wachter U, Ploner F, Georgieff M, Möller P, Laporte R, Radermacher P, Calzia E, Hauser B: Comparison of cardiac, hepatic and renal effects of arginine vasopressin and noradrenaline during porcine fecal peritonitis: a randomized, controlled trial. Crit Care 2009, 13: R113. 10.1186/cc7959
Matějková S, Scheuerle A, Wagner F, McCook O, Matallo J, Gröger M, Seifritz A, Stahl B, Vcelar B, Calzia E, Georgieff M, Möller P, Schelzig H, Radermacher P, Simon F: Carbamylated erythropoietin-FC fusion protein and recombinant human erythropoietin during porcine kidney ischemia/reperfusion injury. Intensive Care Med 2013, 39: 497–510. 10.1007/s00134-012-2766-y
Gröger M, Scheuerle A, Wagner F, Simon F, Matallo J, McCook O, Seifritz A, Stahl B, Wachter U, Vogt JA, Asfar P, Matejovic M, Möller P, Lampl L, Bracht H, Calzia E, Georgieff M, Radermacher P, Stahl W: Effects of pre-treatment hypothermia during resuscitated porcine hemorrhagic shock. Crit Care Med 2013, 41: e105-e117. 10.1097/CCM.0b013e31827c0b1f
Verreth W, De Keyzer D, Pelat M: Weight loss-associated induction of peroxisome proliferator-activated receptor-α and peroxisome proliferator-activated receptor-γ correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation 2004, 110: 3259–3269. 10.1161/01.CIR.0000147614.85888.7A
Holst D, Luquet S, Nogueira V, Kristiansen K, Leverve X, Grimaldi PA: Nutritional regulation and role of proliferator-activated receptor δ in fatty acid catabolism in skeletal muscle. Biochim Biophys Acta 2003, 1633: 43–50. 10.1016/S1388-1981(03)00071-4
Luquet S, Lopez-Soriano J, Holst D, Fredenrich A, Melki J, Rassoulzadegan M, Grimaldi PA: Proliferator-activated receptor δ controls muscle development and oxidative capacity. FASEB J 2003, 17: 2299–2301.
Chima RS, LaMontagne T, Piraino G, Hake PW, Denenberg A, Zingarelli B: C-peptide, a novel inhibitor of lung inflammation following hemorrhagic shock. Am J Physiol Lung Cell Mol Physiol 2011, 300: L730-L739. 10.1152/ajplung.00308.2010
Feingold K, Kim MS, Shigenaga J, Moser A, Grunfeld C: Altered expression of nuclear hormones and coactivators in mouse heart during the acute-phase response. Am J Physiol Endocrinol Metab 2004, 286: E201-E207.
Zhou M, Wu R, Dong W, Jacob A, Wang P: Endotoxin downregulates peroxisome proliferator-activated receptor-γ via the increase in TNF-α release. Am J Physiol Regul Integr Comp Physiol 2004, 94: R84-R92.
Feingold KR, Wang Y, Moser A, Shigenaga J, Grunfeld C: LPS decreases fatty oxidation and nuclear hormone receptors in the kidney. J Lipid Res 2008, 49: 2179–2187. 10.1194/jlr.M800233-JLR200
Rodríguez-Calvo R, Serrano L, Coll T, Moullan N, Sánchez RM, Merlos M, Palomer X, Laguna JC, Michalik L, Wahli W, Vázquez-Carrera M: Activation of peroxisome proliferator-activated receptor β/δ inhibits lipolysaccharide-induced cytokine production in adipocytes by lowering nuclear factor-κB activity via extracellular signal-related kinase 1/2. Diabetes 2008, 57: 2149–2157. 10.2337/db08-0176
Defaux A, Zurich MG, Braissant O, Honegger P, Monnet-Tschudi F: Effects of the PPAR-β agonist GW501516 in an in vitro model of brain inflammation and antibody-induced demyelination. J Neuroinflamm 2009, 6: 15. 10.1186/1742-2094-6-15
Álvarez-Guardia D, Palomer X, Coll T, Serrano L, Rodríguez-Calvo R, Davidson MM, Merlos M, El Kochairi I, Michalik L, Wahli W, Vázquez-Carrera M: PPAR β/δ activation blocks lipid-induced inflammatory pathways in mouse heart and human cardiac cells. Biochim Biophys Acta 2011, 1811: 59–67. 10.1016/j.bbalip.2010.11.002
Thulin P, Wei T, Werngren O, Cheung L, Fisher RM, Grandér D, Corcoran M, Ehrenborg E: MicroRNA-9 regulates the expression of peroxisome proliferator-activated receptor δ in human monocytes during the inflammatory response. Int J Mol Med 2013, 31: 1003–1010.
Robich MP, Osipov RM, Chu LM, Han Y, Feng J, Nezafat R, Clements RT, Manning WJ, Sellke FW: Resveratrol modifies risk factors for coronary artery disease in swine with metabolic syndrome and myocardial ischemia. Eur J Pharmacol 2011, 664: 45–53. 10.1016/j.ejphar.2011.04.059
Burgess TA, Robich MP, Chu LM, Bianchi C, Sellke FW: Improving glucose metabolism with resveratrol in a swine model of metabolic syndrome through alteration of signaling pathways in the liver and skeletal muscle. Arch Surg 2011, 146: 556–564. 10.1001/archsurg.2011.100
Robich MP, Osipov RM, Nezafat R, Feng J, Clements RT, Bianchi C, Boodhwani M, Coady MA, Laham RJ, Sellke FW: Resveratrol improves myocardial perfusion in a swine model of hypercholesteremia and chronic myocardial ischemia. Circulation 2010,122(11 suppl):S142-S149.
Robich MP, Chu LM, Burgess TA, Feng J, Han Y, Nezafat R, Leber MP, Laham RJ, Manning WJ, Sellke FW: Resveratrol preserves myocardial function and perfusion in remote nonischemic myocardium in a swine model of metabolic syndrome. J Am Coll Surg 2012, 215: 681–689. 10.1016/j.jamcollsurg.2012.06.417
Zingarelli B, Hake PW, O’Connor M, Burroughs TJ, Wong HR, Solomkin JS, Lentsch AB: Lung injury after hemorrhage is age dependent: role of peroxisome proliferator-activated receptor γ. Crit Care Med 2009, 37: 1978–1987. 10.1097/CCM.0b013e31819feb4d
Shin T, Kuboki S, Huber N, Eismann T, Galloway E, Schuster R, Blanchard J, Pritts TA, Lentsch AB: Activation of peroxisome proliferator-activated receptor-γ during hepatic ischemia is age-dependent. J Surg Res 2008, 147: 200–205. 10.1016/j.jss.2008.02.004
Reagan-Shaw S, Nihal M, Ahmad N: Dose translation from animal to human studies revisited. FASEB J 2007, 22: 659–661. 10.1096/fj.07-9574LSF
Acknowledgements
This study was supported by the Else-Kröner-Fresenius-Stiftung (AZ 2011_A18). Dr. M. Matejovic was supported by the Charles University Research Fund (project number P36). Very special thanks are dedicated to Rosemarie Mayer, Rosa Maria Engelhardt, Tanja Schulz, and Ingrid Eble for their skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PR, EC, CT, AK, and MM conceived the study and designed the experiment. MW, SH, FS, and JM were responsible for anesthesia and surgery and, together with MR, MGr, FG, and AS, for data collection and statistical analysis. FW and BS carried out the immune biology measurements. OM, ASch, and PM were responsible for histology and immunohistochemistry. UW and JV performed the isotope measurements. PR, MGe, CT, and PM drafted the manuscript. All authors read and approved the final manuscript.
Martin Wepler, Sebastian Hafner contributed equally to this work.
Electronic supplementary material
40635_2013_28_MOESM1_ESM.docx
Additional file 1: Supplementary information. Table A: Norepinephrine infusion rate, hydroxyethyl starch infusion rate and urine output. Table B: Activation of NF-κB, protein expression of the iNOS, HO-1, cleaved caspase-3, and nitrotyrosine in post mortem kidney specimen. Table C: Kidney histopathology score. Figure A: Representative examples of the histopathological items analyzed. (DOCX 1 MB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wepler, M., Hafner, S., Scheuerle, A. et al. Effects of the PPAR-β/δ agonist GW0742 during resuscitated porcine septic shock. ICMx 1, 9 (2013). https://doi.org/10.1186/2197-425X-1-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2197-425X-1-9