Abstract
Myconanotechnology is a study of nanoparticle synthesis using fungi and their application mainly in medicine. Myconanotechnology has great advantage due to wide range and diversity of the fungi. Recently, fungal-mediated synthesis of nanoparticles is a reliable and ecofriendly method. The present work investigates the synthesis of silver nanoparticles using Trichoderma viride which is a non-pathogenic fungus. The cell filtrate of T. viride was used for the reduction of silver nitrate to silver nanoparticles. The pH of the cell filtrate was changed, and the effect of the pH was monitored on the synthesis of the silver nanoparticles. While changing the pH of the medium, the shape and size of the nanoparticles were controlled. The silver nanobowls were synthesized at first time using T. viride. The synthesized silver nanoparticles were characterized using UV spectrophotometer, X-ray diffractometer, Fourier transform infrared spectrophotometer, scanning Electron microscope (SEM), and transmission electron microscope (TEM). The SEM and TEM images showed the shape and size of the nanoparticles, and the synthesized nanoparticles were nanobowl in shape and polydispersed in size. The antibacterial activity of silver nanoparticles was carried out against gram-positive and gram-negative organisms. The maximum zone of inhibition occurred at 50 μl, while the concentration of the nanoparticles increased the inhibition activity also increased.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Myconanotechnology is the connecting term of mycology and nanotechnology, and it has a significant potential in the production of nanoparticles. The synthesis of nanoparticles using fungi has gained much importance because they are easy to culture in bulk, and the extracellular secretion of enzymes has an added advantage in the downstream processing. The fungi produce higher amount of protein compared to bacteria, thus resulting in the higher production of nanoparticles[1–3]. Because of their properties, fungi might be extensively used for the rapid and eco-friendly biosynthesis of metal nanoparticles. It has been monitored that the fungal-mediated synthesized nanoparticles have good monodispersity and good dimensions[2, 4]. The intracellular and extracellular methods are being used for the synthesis of nanoparticles via a biological route. The extracellular method is more advantageous than intracellular method because the intracellular method needs an additional step to obtain the purified nanoparticles[5, 6].
Synthesis of noble metals has become mandatory issue in the present decade due to the abnormal increase of their market values[7]. Metals such as gold, silver, and copper have been widely used for the synthesis of stable dispersion of nanoparticles, which are being useful in the area of photography, biological labeling, photonics, optoelectronics, and surface-enhanced Raman scattering detection[8–10]. Various physical and chemical methods are used for the synthesis of nanoparticles; these methods are intended at controlling the physical properties of particles. Still, most of these methods are in the development stage, and problems are often solved through the stability of the nanoparticle preparation, control of the crystal growth, and aggregation of the particles[11]. As a result of some drawbacks in physical and chemical methods, biological synthesis method has been developed to obtain biocompatible, inexpensive, eco-friendly, and size-controlled nanoparticles[12]. Nowadays, noble metal nanoparticles play a vital role in the fields of medicine, biology, physics, chemistry, and materials science. Among noble metal nanoparticles, silver is of particular interest because of its distinctive properties, such as good electrical conductivity, chemical stability, and catalytic and antibacterial activities[6, 13, 14].
Trichoderma viride is a rhizosphere competent strain found in all type of soils; it is a non-pathogenic fast-growing organism which is used as a biocontrol agent[15, 16]. The present work demonstrated the synthesis of silver nanoparticles using T. viride. The synthesized silver nanoparticles were characterized using UV spectrophotometer analysis, X-ray diffractometer to analyze the nature of the nanoparticles, and scanning electron microscope to analyze the surface morphology of the nanoparticles, transmission electron microscope to analyze the size of the nanoparticles, and selected area electron diffraction (SAED) pattern to confirm the crystalline nature of the nanoparticles. Fourier transform infrared spectroscopy (FTIR) is used to identify the functional groups of the silver nanoparticles. The bactericidal activity of silver nanoparticles was carried out using disc diffusion method.
Results and discussion
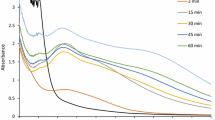
The UV absorption spectral studies were carried out to confirm the formation of silver nanoparticles using T. viride (Figure 1). The absorption peak in UV spectrum equivalent to the surface plasmon resonance, the peak found at 400 nm, and the maximum synthesis of silver nanoparticles occurred at 96 h of incubation. The UV spectral absorption reading confirmed that the well-dispersed, but not aggregated silver nanoparticles, were formed using T. viride cell filtrate[5]. The silver nanoparticles were formed very rapidly within 30 min. Fayaz et al.[16] have reported the extracellular synthesis of silver nanoparticles using T. viride after 4 h of incubation. The inset in Figure 1 shows the conical flasks containing T. viride filtrate (a) before and (b) after reaction with silver ions. The aqueous solution of the biomass is green in color before the reaction with silver ions, which turned to brown after the reaction with silver ions. The change of color indicates the formation of silver nanoparticles using T. viride. The color change occurs due to the excitation of surface plasmon resonance vibration of silver; hence, it confirmed the reduction of silver ions extracellulary[17, 18].
Figure 2 shows the UV spectrum of pH effect on the synthesis of silver nanoparticles using T. viride. The sharp peak was observed at 400 nm, and the maximum production of silver nanoparticles occurred at pH 9. The inset picture (Figure 2) showed that the color intensity of the aqueous medium increased, while the pH of the fungal cell filtrate increased. The color of the solution varied from yellow to dark brown. The pH of the medium has a great effect on the control of the shape and size of the nanoparticles. The color variation from yellow to dark brown of the solution occurred because of the increase amount of nanoparticle production in the fungal cell filtrate medium[17, 19]. Veerasamy et al.[20] explained that the particles are larger at lower pH, and a large amount of smaller diameter particles were formed at higher pH due to the large number of functional groups obtainable for nanoparticle binding. The nanoparticles which are synthesized using pH 9 were used for further characterization studies.
The X-ray diffraction studies were carried out to confirm the crystalline nature of the nanoparticles, and the XRD pattern (Figure 3) of the synthesized silver nanoparticles shows the crystalline nature of silver nanoparticles synthesized using T. viride. The XRD pattern shows strong peaks in the entire spectrum of 2θ values ranging from 20 to 80. The silver nanoparticles synthesized in this experiment were in nanocrystal form as evidenced by the peaks at 2θ values of 38.24°, 44.11°, 64.64°, and 77.47° analogous to (111), (200), (220), and (311) planes for silver. The unassigned peaks present in spectrum indicate the presence of organic matters which are present in the fungal cell filtrate.
FTIR spectrum is used to identify the possible interactions among silver salts and proteins (present in the fungal cell filtrate) which are responsible for the reduction of silver ions and stabilization of nanoparticles. The FTIR spectrum of the biosynthesized silver nanoparticles using T. viride (Figure 4) shows the absorption peaks at 3,396, 2,925, 2,855, 1,740, 1,651, 1,548, and 1,380 cm−1. The peak at 3,396 and 2,925 cm−1 reveals the presence of N-H bend, indicating the primary and secondary amine groups of protein. Likewise, the bands at 1,651 and 1,548 cm−1 correspond to the primary and secondary amine groups of N-H bending and carbonyl stretching vibrations of protein, respectively[21]. Therefore, the FTIR study has shown that the carbonyl group of amino acid residues and peptides of proteins has a stronger metal-binding capability. Most possibly, the proteins could form a coat to cover the nanoparticles and act as capping agents for silver nanoparticle formation to avert the agglomeration of the nanoparticles; therefore, the particles are stabilized[22]. The two bands at 2,855 and 1,380 cm−1 represent the C-H of symmetric and asymmetric stretching vibrations of aromatic and aliphatic modes, and the C-H stretching vibrations of aromatic and aliphatic amines, respectively. The band at 1,740 cm−1 corresponds to C=O stretching vibrations of aldehyde group. Hence, the observations indicate that the bioreductions of silver nitrate are tied to the oxidation of hydroxyl groups in fungal cell filtrate[23]. The whole observation suggests that the biological molecules might possibly be the reason for the formation and stabilization of metal nanoparticles in the fungal cell filtrate.
After 120 h of incubation, the biosynthesized silver nanoparticles were air dried and examined under scanning electron and transmission electron microscopes. The SEM image (Figure 5a,b) of the silver nanoparticles shows the well-dispersed nanoparticles, and the biosynthesized silver nanoparticles have bowl-like shapes. The TEM image (Figure 6a,b) revealed the size of the synthesized silver nanoparticles. These nanoparticles were polydispersed, and their sizes were in the range from 28 to 59.17 nm. The selected area of electron diffraction pattern (inset in Figure 6) of the silver nanoparticles shows their face centered cubic structure, and the synthesized silver nanoparticles are in crystalline nature. This is the first study to report on silver nanoparticle synthesis using T. viride resulting in nanobowl-shaped nanoparticles. The definite reaction mechanism for the synthesis of silver nanoparticles using fungal cell filtrate is not yet to be elucidated. The fungal biomass secretes proteinic compounds in liquid medium which may be accountable for the synthesis of nanoparticles, and consequently, the proteins attach and improve the stability of the nanoparticles[5]. Two protein bands were identified with molecular weights of 45 and 39 KDa, respectively, in silver nanoparticle sample, which may be responsible for the synthesis of silver nanoparticles (Figure 7). The extracellular proteins have great advantage in the synthesis of nanoparticles because they provide large amount of nanoparticles in pure form, free from other cell components[3].
Well diffusion method was used to provide the evidence for the antibacterial activity of biosynthesized silver nanoparticles against Bacillus subtilis and Klebsiella planticola. Different concentrations (10 to 50 μl) of silver nanoparticles were used to confirm the antibacterial efficiency of the silver nanoparticles. The maximum zone of inhibition was observed in K. planticola followed by B. subtilis (Figure 8). The antibacterial activity of silver nanoparticles was indicated by the formation of the zone. The diameter of the inhibition zone was measured in millimeters. The maximum zone of inhibition occurred at 50 μl concentration of silver nanoparticles. The possible mechanism for the bactericidal activity of silver nanoparticles may be the penetration of the nanoparticles into the cell wall and entry inside the cells, compressing the DNA which simultaneously inactivates the cellular proteins[24, 25].
Conclusion
An ecological, cost-effective method was employed for the extracellular synthesis of silver nanoparticles using T. viride. X-ray diffractometry revealed the crystalline nature of the nanoparticles. Scanning electron microscopy revealed the bowl-shaped nanoparticles. Transmission electron microscope shows the average size of the nanoparticles to be around 28 nm, and the SAED confirms the crystalline nature of the nanoparticles. The antibacterial activity occurred due to the penetration of the silver nanoparticles into the cell walls, reducing the function of the organisms.
Methods
Preparation of silver nanoparticles
T. viride was obtained from MTCC-800 Mumbai, India. The culture was sub-cultured in SDA media. Then, the T. viride was inoculated in a liquid SDA media and grown aerobically at 35°C and agitated at 150 rpm on orbital shaker. The biomass was harvested after 72 h of growth by filtration using Whatman No. 1 filter paper followed by extensive washing with distilled water to remove any medium component from the biomass. The 10 g of biomass was brought in contact with 100 ml of double distilled water for 48 h at 35°C and agitated as described earlier. After 48 h of incubation, the cell filtrate was obtained by passing it through Whatman filter paper No. 1. The silver nitrate (1 mM) was added in 100 ml of cell filtrate, and the reaction was carried out at room temperature. The UV spectroscopic absorbance was taken at various time intervals.
Effect of pH on the synthesis of nanoparticles
The cell filtrate was obtained using the above-stated process, and then the pH (5, 7, and 9) of the cell filtrate was adjusted using 0.1 N of NaOH and O.1 N of HCl followed by the addition of 1 mM of silver nitrate to the cell filtrate. After 24 h of reaction, the UV spectrophotometric reading was taken at different wavelengths.
Characterization of the synthesized silver nanoparticles
The synthesized silver nanoparticles were characterized using UV (Perkin Elmer Inc., MA, USA) visible absorbance spectra, absorbance taken at various time intervals at different wavelength, powder X-ray diffractometer (Bruker D8 Advance using CuKα radiation, at 40 keV in the range of 10 to 80, Bruker Corporation, Ettlingen, Germany), scanning electron microscope (S-3400N, Hitachi, Tokyo, Japan), Fourier transform infrared spectrometer (6700, Thermo Nicolet, MA, USA), transmission electron microscope (CM200, Koninklijke Philips Electronics N.V., Amsterdam, Netherlands), and selected area electron diffractometer to characterize the nature, shape, functional groups, and size of the synthesized silver nanoparticles.
SDS-PAGE gel electrophoresis
The proteins which might be the reason for the synthesis of nanoparticles were determined using SDS-PAGE gel electrophoresis. Silver nitrate (1 mM) was added to the T. viride cell filtrate. After 24 h of incubation, it was precipitated with ammonium sulfate (80% saturation). Then, the pellet was dialyzed against phosphate buffer (0.05 M) using dialysis bag. The sample was analyzed on SDS-Page, and the molecular weight of the protein was determined using marker protein.
Antibacterial activity of silver nanoparticles
The bactericidal activity of the synthesized silver nanoparticles was observed against B. subtilis (3053) and K. planticola (2277). The bactericidal activity was carried out using well diffusion method. Mueller-Hinton agar medium (MHA) was prepared and poured into plates, and wells were made in the agar medium. Then, 24-h-old test bacterial cultures were swabbed in MHA medium to form a confluent lawn of bacterial cultures. Various concentrations (10 to 50 μl) of silver nanoparticles were loaded into the wells. Then, the plates were incubated at 35°C for 24 h. After 24 h of incubation, the plates were observed for zone of inhibition.
References
Rai M, Yadav A, Bridge P, Gade A, Rai MK, Bridge PD: Myconanotechnology: a new and emerging science. In Applied Mycology. Edited by: Rai M, Bridge PD. New York: CAB International; 2009:258–267.
Rai M, Yadav A, Gade A: Mycofabrication, mechanistic aspect and multifunctionality of metal nanoparticls – where are we? And where should we go. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Edited by: Mendez-Vilas A. Badajoz, Spain: Formatex Research Center; 2010:1343–1354.
Sastry M, Ahmad A, Khan MI, Kumar R: Biosynthesis of metal nanoparticles using fungi and actinomycetes. Current Sci 2003, 85: 162–170.
Mandal D, Bolander ME, Mukhopadhyay D, Sarkar G, Mukherjee P: The use of microorganisms for the formation of metal nanoparticles and their application. Appl. Microbiol. Biotechnol. 2008, 69: 485–492. 10.1007/s00253-005-0179-3
Kuber C, Bhainsa SF, Souza D: Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus . Colloids Surf B: Biointerfaces 2006, 47: 160–164. 10.1016/j.colsurfb.2005.11.026
Prathna TC, Chandrasekaran N, Raichur AM, Mukherjee A: Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle size. Colloids Surf B: Biointerfaces 2011, 82: 152–159. 10.1016/j.colsurfb.2010.08.036
Binupriya AR, Sathiskumar M, Yun Sl: Biocrystallization of silver and gold ions by inactive cell filtrate of Rhizopus stolonifer . Colloids Surf B: Biointerfaces 2010, 79: 531–534. 10.1016/j.colsurfb. 2010.05.021
Sharma VK, Yingard RA, Lin Y: Silver nanoparticles: green synthesis and their antimicrobial activities. Adv. Colloid. Interface. Sci. 2009, 145: 83–96. 10.1016/j.cis.2008.09.002
Simth AM, Duan H, Rhyner MN, Ruan G, Nie SA: A systematic examination of surface coatings on the optical and chemical properties of semiconductor quantum dots. Phys. Chem. Chem. Phys. 2006, 8: 3895–3903. 10.1039/b606572b
Kearns GJ, Foster EW, Hutchison JE: Substrates for direct imaging of chemically functionalized SiO 2 surfaces by transmission electron microscopy. Anal. Che. 2006, 78: 298–303. 10.1021/ac051459k
Gericke M, Pinches A: Biological synthesis of metal nanoparticles. Hydrometallurgy 2006, 83: 132–140. 10.1016/j.hydromet.2006.03.019
Sadhasivum S, Shanmugam P, Yun K: Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids and Surface B: Biointerfaces 2010, 81: 358–362. 10.1016/j.colsurfb.2010.07.036
Wang J, Wang Z: Rapid synthesis of hexagon-shaped gold nanoplates by microwave assistant method. Mater. Lett. 2007, 61: 4149–4151. 10.1016/j.matlet.2007.01.043
Yokoyama K, Welchons K: The conjugation of amyloid beta protein on the gold colloidal nanoparticles surfaces. Nanotechnology 2007, 18: 105101. 10.1088/0957-4484/18/10/105101
Sathiyaseelan K, Sivasakthivelan P, Lenin G: Evaluation of antagonistic activity and shelf life study of Trichoderma viride . Bontany. Res. Int. 2009, 2: 195–197.
Fayaz M, Tiwary CS, Kalaichelvan PT, Venkatesan R: Blue orange light emission from biogenic synthesized silver nanoparticles using Trichoderma viride . Colloids Surf B: Biointerfaces 2010, 75: 175–178. 10.1016/j.colsurfb.2009.08.028
Ahmad A, Mukherjee P, Senapathi S, Mandal D, Khan MI, Kumar R, Sastry M: Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum . Colloids Surf B: Biointerfaces 2003, 28: 313–318. 10.1016/SO927-7765 (02)001741
Balaji DS, Basavaraja S, Deshpande R, Mahesh DB: Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf B: Biointerfaces 2009, 68: 88–92. 10.1016/j.colsurfb.2008.09.022
Kathiresan K, Manivannan S, Nabeel MA, Dhivya B: Studies on silver nanoparticles synthesized by a marine fungus Penicillium fellutanum isolated from coastal mangrove sediment. Colloids and Surface B: Biointerf 2009, 71: 133–137. 10.1016/j.colsurfb.2009.01.016
Veerasamy R, Xin TZ, Gunasagaran S, Xiang TFW, Yang EFC, Jeyakumar N, Dhanaraj SA: Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antibacterial activities. J. Saudi. Chem. Soc. 2010, 15: 113–120. 10.1016/j.jscs.2010.06.004
Sawle BD, Salimath B, Deshpande R, Bedre MD, Prabhakar BK, Venkataraman A: Biosynthesis and stabilization of Au and Au-Ag alloy nanoparticles by fungus Fusarium semitectum . Sci. Technol. Adv. Mater. 2008, 9: 035012. 10.1088/1468-6996/9/3/035012
Vigneshwaran N, Ashtapure M, Varadarajan PV, Nachane RP, Paralikar KM, Balasubramanya RH: Biological synthesis of silver nanoparticles using the fungus Aspergillus flaves . Mater. Lett. 2007, 61: 1413–1418. 10.1016/j.matlet.2006.07.042
Sangi R, Verma P: Biomimetic synthesis and characterization of protein capped silver nanoparticles. Biores. Technol. 2009, 100: 501–504. 10.1016/j.biortech.2008.05.048
Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO: A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus . J. Biomed. Mater. Res. 2000, 52: 662–668. 10.1002/1097-4636(20001215)
Sondi I, Sondi BS: Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid. Interface. Sci 2004, 275: 177–182. 10.1016/j.jcis.2004.02.012
Acknowledgments
Authors gratefully acknowledge the DST-FIS-sponsored program from the Department of Science Technology, New Delhi, India for funding this research (ref. no. S/FST/ESI-101/2010). Authors convey their thanks to the Central Instrumentation Facility Pondicherry for providing the FTIR and SEM facilities, the Sophisticated Analytical Instrumentation Facility IIT Bombay for providing TEM, and the School of Advanced Sciences (Characterization Facility Wing) VIT University Vellore for providing the XRD facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KC and GGA provided the same contributions in this article. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Chitra, K., Annadurai, G. Bioengineered silver nanobowls using Trichoderma viride and its antibacterial activity against gram-positive and gram-negative bacteria. J Nanostruct Chem 3, 9 (2013). https://doi.org/10.1186/2193-8865-3-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-9