Abstract
Visible-light-activated Cr2O3/SnO2 nanocomposite photocatalyst was prepared by coprecipitation method and characterized by X-ray diffraction, transmission electron microscopy, X-ray photoelectron spectroscopy, N2 adsorption-desorption measurement, and UV–vis diffuse reflectance spectroscopy. The results show that phase composition, crystallite size, Brunauer-Emmett-Teller surface area, and optical absorption of samples varied significantly with the heat treatment temperatures. The Cr2O3/SnO2 photocatalyst (the molar ratio Cr to Sn is 1:2) calcined at 400°C for 2 h exhibited maximum photocatalytic activity because it has a smaller particle size of 10.05 nm and a higher surface area of 38.75 m2/g. Under visible-light (λ > 400 nm) irradiation, the degradation rate of Rhodamine B reached 98.0% in 60 min, which is about 3.5 times higher than that of the standard P25 photocatalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
In recent years, photocatalytic degradation of various kinds of organic–inorganic pollutants using semiconductor powder as a photocatalyst has been extensively studied[1, 2]. Among various oxide semiconductor photocatalysts, TiO2 was intensively investigated because of its biological and chemical inertness, strong oxidizing power, nontoxicity, and long-term stability[3–5]. However, the photocatalytic activity of TiO2 (the bandgap is 3.2 eV, and it can be excited by photons with wavelengths below 387 nm) is limited to irradiation wavelengths in the UV region so that the effective utilization of solar energy is limited to about 3% to 5% of the total solar spectrum. Furthermore, the fast recombination of photo-generated electron–hole pairs hinders the commercialization of this technology[6]. The decomposition of adsorbed organic compound is closely correlated with the density of space charge-separated electron–hole pair on TiO2. Therefore, it is of great interest to separate the electron–hole pairs effectively to increase the photon efficiencies and develop new visible-light photocatalyst to extend the absorption wavelength range into the visible-light region. In this sense, an interesting approach to deal with the issue is carried out by coupled semiconductor technique. Recently, there are a number of studies related to the photocatalytic activity of coupled semiconductor photocatalyst such as ZnO/SnO2[6], SnO2-TiO2[7], Au/TiO2-CeO2[8], and TiO2-CdS[9]. The results show that nearly all the composite semiconductors have presented higher photocatalytic activity than single ones. Indeed, this effect could be due to the synergistic effect of the coupled semiconductor photocatalyst. It was also reported that the chromium-doped TiO2 has been found to exhibit superior photocatalytic activity under visible-light irradiation because chromium atom can effectively narrow the energy bandgap of TiO2[10, 11]. Chromium-based catalysts have been widely examined for polymerization, partial oxidation, and aromatization reaction because of the peculiar characteristics of Cr oxide species on the surface of the support, including oxidation state and coordination environment[12]. However, there have been few studies clearly elucidating the roles of the surface chromate species and supporting the oxidation reaction, particularly the decomposition of environmental pollutants.
In this study, a series of Cr2O3/SnO2 photocatalysts with different calcination temperatures were prepared by coprecipitation method, and their phase compositions, crystalline structures, and particle sizes were studied. We also explored their photocatalytic degradation performance in treating Rhodamine B solution under visible-light (λ > 400 nm) irradiation.
Results and discussion
XRD analysis
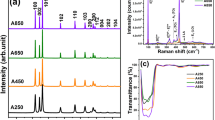
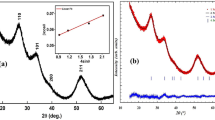
Figure 1 shows X-ray diffraction (XRD) patterns of the Cr2O3/SnO2 photocatalysts calcined at different temperatures for 2 h. It can be seen that the diffraction pattern of the sample prepared at 300°C is indistinct because of the amorphous configuration. While the sample calcined at 400°C, the diffraction peaks clearly show that the Cr2O3 and SnO2 nanocrystals coexisted in the samples. With the increase of calcination temperature (at 500°C), the phase composition is still the mixture of Cr2O3 and SnO2 phase, but the peak intensity of the sample obviously increases due to the growth of crystallites and enhancement of crystallization. Table 1 summarizes the Brunauer-Emmett-Teller (BET) surface area and grain size of the samples. The average grain sizes estimated from the highest intensity diffraction peaks of SnO2 (2θ = 33.37) using Scherrer’s equation were 6.80, 10.05, and 23.15 nm for samples a, b, and c, respectively. It is obvious that the grain sizes of the samples improved with the increase of heat treatment temperature, while the BET surface areas decreased.
TEM analysis
The grain sizes of the Cr2O3/SnO2 nanocomposite photocatalysts varied with calcination temperature, which has been confirmed in the transmission electron microscopy (TEM) images (Figure 2a,b,c,d). It can be seen that the sample calcined at 300°C appeared amorphous because of its weak crystallization, and the grain edge of the sample was a little dim. The TEM images also indicated that the grain size was homogenous and fairly small (about 10 nm) when the sample was calcined at 400°C. In contrast, the sample calcined at 500°C displayed greater particle size due to the enhancement of crystallization or sintering between smaller particles, which is estimated to be about 25 nm. The increase of heat treatment temperature caused the catalyst grains to become larger. The result was in agreement with that of XRD. Figure 2d shows a high-resolution (HR) TEM image of Cr2O3/SnO2 powder calcined at 400°C and its corresponding fast Fourier transform (FFT) pattern. The space between adjacent lattice planes is 0.32 nm, corresponding to 110 planes of rutile SnO2. Combined with the results of FFT analysis, the preferential growth direction is (112).
UV–vis DR spectral analysis
Figure 3 gives the UV–vis diffuse reflectance spectra (DRS) of the Cr2O3/SnO2 photocatalysts calcined at 300°C, 400°C, and 500°C. A progressive red shift in the bandgap absorption is noticed with the increase of calcination temperature. This implies that the absorption edges of the samples shift to the longer wavelength range. When calcined at 300°C, the sample has a little absorption in the range of visible light (λ > 400 nm) because the catalyst is mainly composed of amorphous SnO2 powder (shown in XRD patterns), which is only effective under ultraviolet irradiation. With the increase of calcination temperature (reaching 400°C), the absorption edge of the sample has some red shift. The red shift is presumably ascribed to the formation of the homogeneous Cr2O3 nanocrystals. The absorption onsets were determined by linear extrapolation from the infection point of the curve to the baseline. The edges of the absorption of the Cr2O3 incorporated SnO2 samples were shifted to approximately 500 nm, corresponding to a bandgap energy of 2.25 eV.
The bandgap energy of Cr2O3 is 2.5 eV and can be activated by the light below 560 nm[13], when it couples with SnO2 semiconductor, the conduction band of SnO2 acts as a sink for photo-generated electrons. The photo-generated holes move in the opposite direction and accumulate in the valence band of the Cr2O3 particle, which leads to increasing the charge separation efficiency and extending the photo-responding range to visible light. When the heat treatment temperature reaches 500°C, the crystallite sizes become larger, and the absorption edge shows more red shift.
XPS studies
Figure 4a,b,c shows the high-resolution X-ray photoelectron spectroscopy (XPS) spectra of the Cr2p3/2, O 1s, and Sn 3d regions, taken on the surface of Cr2O3/SnO2 photocatalyst calcined at 400°C. The Cr2p3/2 level (Figure 4a) is broad and showed asymmetry towards higher binding-energy side of the main peak. The deconvolution of the Cr2p3/2 signals based upon the previous studies[14] and the comparison to the reference samples including CrO3 and Cr2O3 indicate that spectra for the present catalytic systems contain two major peaks at 579.1 eV for Cr(VI) and 576.1 eV for Cr(III). The change in oxidation state of chromium from sixth to third might be due to oxide/oxide support interaction. From high-resolution XPS spectra of O 1s region (Figure 4b), it can be seen that oxygen on the sample surface exists at least in three forms: at the binding energy of 530.4 eV which corresponds to oxygen in SnO2 lattice; 531.7 eV which corresponds to oxygen in SnO2 surface adsorption of (−OH); and 533.5 eV which corresponds to oxygen in SnO2 surface adsorption of H2O. It is just because so much adsorption oxygen exists on the SnO2 surface that they become captives of photo-generated electron–hole pairs directly or indirectly. So, the recombination of photo-generated electron–hole pairs is suppressed, and therefore, the quantum efficiency of photocatalytic reaction is improved. This may be the possible reason for the enhancement of photocatalytic activity. The binding energy of Sn3d5/2 (Figure 4c) was 486.36 eV, slightly lower than that of SnO2 (>486.4 eV), might be due to oxide/oxide support interaction.
Photocatalytic activity studies
The characteristic patterns of XRD, TEM, and UV–vis DRS displayed that the Cr2O3/SnO2 photocatalyst calcined at 400°C for 2 h (the molar ratio of Cr to Sn is 1:2) has better crystallization, smaller crystal size, and stronger response to visible light. In order to explore the photocatalytic activity of the catalyst in the degradation of Rhodamine B solution (0.5 g/L) under visible light (λ > 400 nm), the photocatalytic behavior of the standard photocatalyst Degussa P25 was also measured as a reference. Results indicate that the Cr2O3/SnO2 photocatalyst displayed significantly high degradation ability for Rhodamine B, the degradation rate reached 98.0% in 60 min, which is obviously about 3.5 times higher than the value of 27.5% over Degussa P25 (Figure 5). The enhancement may be explained in terms of the synergetic effect on the specific adsorption property and efficient electron–hole separation at the Cr2O3/SnO2 nanocomposite photocatalyst interfaces and surfaces. Cr2O3-doped SnO2 photocatalyst have stronger reduction/oxidation capabilities, probably due to the high polymerized chromium oxide species on the catalyst surface[15]. As shown in the XPS results, formed polychromate species can substitute Ti to form a stable solid that creates a charge imbalance and must be satiated therefore; more hydroxide ions would be adsorbed on the surface to balance the charge. These hydroxide ions on the surface can accept holes generated by visible-light illumination to form hydroxyl radicals which oxidize adsorbed organic molecule.
Factors influencing the photocatalytic activity
Effect of catalyst loading
The effect of catalyst loading on the photocatalytic degradation of Rhodamine B was studied by varying the amount of Cr2O3/SnO2 nanocomposite calcined at 400°C. Figure 6 showed the degradation profile of Rhodamine B with an initial concentration of 0.5 mg/L under various catalyst loadings from 0.2 to 4 g/L. It could be seen that photocatalytic degradation efficiency has increased up to 0.5 g/L and then declined with increasing catalyst loading. This could be attributed to the shadowing effect, wherein high turbidity due to high Cr2O3/SnO2 nanocomposite concentration decreased the penetration depth of solar radiation. Hence, the optimal catalyst loading of 0.5 g/L was employed throughout the present study.
Effect of pH
Figure 7 presented the effect of pH value in the suspension on photocatalytic activity. The variation of pH value showed its strong influence on the Rhodamine B photodegradation. The photodegradation efficiency as a function of pH value decreased in the order of 10 > 7 > 3. It was generally accepted that the pH-dependent photodecomposition was mainly ascribed to the variation of surface charge properties of a photocatalyst. Consequently, this changed the adsorption behavior of a dye on the catalyst surface. Since Rhodamine B had a cationic configuration, its adsorption was favored in alkaline solution as demonstrated in Figure 8. The increase of pH value resulted in a higher adsorption amount of Rhodamine B on the Cr2O3/SnO2 nanoparticle surface.
Conclusions
The novel visible-light-activated Cr2O3/SnO2 nanocomposite photocatalyst was prepared by coprecipitation method. The characteristic patterns of XRD, BET, TEM, and UV–vis DRS displayed that the sample calcined at 400°C for 2 h (the molar ratio of Cr to Sn is 1:2) has better crystallization, smaller crystal size, and stronger response to visible light. The Cr2O3/SnO2 photocatalyst showed remarkable photocatalytic activity compared with the standard P25 photocatalyst. Rhodamine B (98.0%) can be degraded in 60 min under illumination of the visible light (λ > 400 nm). At high catalyst loadings, penetration of the light inside the reaction medium was reduced because of the light scattering and shielding effect by catalyst particles. In addition, basic pH level of suspension was found to be beneficial for photocatalytic degradation.
Methods
Preparation of photocatalysts
The reagent grade chemicals used in preparing the samples, CrCl3·6H2O and SnCl4·5H2O were used as the starting materials. CrCl3·6H2O and SnCl4·5H2O were mixed (the molar ratio of Cr to Sn is 1:2) and dissolved in minimum amount of ethanol. The cationic surfactant cetyltrimethylammonium bromide (5%; 20 mL) in ethanol was dropped into the solution. The system was kept under constant stirring and sustaining the pH of 7 by simultaneous addition of ammonium hydroxide to form the precipitate. The precipitate was filtered and washed with deionized water until no Cl¯ was found in the filtrates. Then, the wet powder was dried at about 100°C in air to form the precursor of the Cr2O3/SnO2 photocatalyst. Finally, the precursors were calcined for 2 h at different temperatures in air to prepare the photocatalyst powders.
Characterization of photocatalysts
To determine the crystallite sizes and identities of the Cr2O3/SnO2 nanocomposite photocatalysts, XRD analysis was carried out at room temperature using a model D8 Bruker AXS (Madison, WI, USA)with monochromatic Cu radiation (40 kV and 30 mA), over the 2θ collection range of 20° to 80°. The shapes of the samples were tested using transmission electron microscopy; FEI, Tecnai F30 HRTEM, FEG (FEI, Hillsboro, OR, USA) operated at 300 kV. The BET surface areas were determined using a Micromeritics ASAP 2010 N2 adsorption apparatus (Norcross, GA, USA). XPS was used to study the chemical composition of the sample. The monochromatic X-ray beams of Al Kα (hν = 1486.6 eV) and Mg Kα (hν = 1253.6 eV) radiations were used as the excitation source. A hemispherical sector analyzer and multi channel detectors were used to detect the ejected photoelectrons as a function of their kinetic energies. XPS spectra were recorded at pass energy of 50 eV, 5-mm slit width, and a take-off angle of 55°. The spectrometer was calibrated by determining the binding energy values of Au 4f7/4 (84.0 eV), Ag 3d5/2 (368.4 eV), and Cu 2P3/2 (932.6 eV) levels using spectrograde materials. The instrumental resolution under these conditions was 1.6 eV full-width at half-maximum for Au 4f7/4 level. The Cls (285 eV) and Au 4f7/4 (84.0 eV) were used as internal standards whenever needed.
UV–vis DRS were recorded in air at room temperature in the wavelength range of 200 to 800 nm using a PE LAMBDA35 spectrophotometer (PerkinElmer, Waltham, MA, USA).
Rhodamine B adsorption experiment
All batch equilibrium experiments were conducted in the dark. The study of Rhodamine B adsorption has been performed at room temperature. In each test, 1.5 g of Cr2O3/SnO2 sample calcined at 400°C were added to 50 mL of 50 mg/L Rhodamine B solution. The equilibrium concentration was determined using centrifugation and filtration, through a Millipore filter of the suspension. The amounts of Rhodamine B adsorbed were calculated as follows:
where n(ads) was the number of moles adsorbed; ΔC was the difference between the initial concentration, C0 and equilibrium concentration, Cc; and V was the volume (50 mL).
Photocatalytic activity measurements
Photocatalytic degradation of Rhodamine B in aqueous solution (0.5 g/L) was carried out using a Quartz reactor under visible-light irradiation (tungsten lamp, 500 W). Air was bubbled into the solution throughout the entire experiment. A cutoff filter was placed outside the Quartz jacket to completely remove all wavelengths less than 400 nm to ensure irradiation with visible light (λ > 400 nm). About 0.5 g of photocatalyst was immersed into a 50-mL aqueous Rhodamine B. Prior to irradiation, the suspensions were magnetically stirred in the dark for 30 min to ensure establishment of an adsorption-desorption equilibrium among the photocatalyst, Rhodamine B, and atmospheric oxygen. At given irradiation time intervals, 10 mL of the suspensions are collected, and then filtered through a Millipore filter to separate the photocatalyst particles. The changes in Rhodamine B concentration were analyzed by UV-visible spectroscopy.
References
Hoffmann MR, Martin ST, Choi W, Bahnemann DW: Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95: 69–96. 10.1021/cr00033a004
Khan SU, Al-shahry M, Lngler WB Jr: Efficient photochemical water splitting by a chemically modified n-TiO 2 . Science 2002, 297: 2243–2245. 10.1126/science.1075035
Zhang T, Oyama T, Horikoshi S, Zhao J, Serpone N, Hidaka H: Photocatalytic decomposition of the sodium dedecylbenzene sulfonate surfactant in aqueous titania suspensions exposed to highly concentrated solar radiation and effects of additives. Appl. Catal. B: Environ. 2003, 42: 13–24. 10.1016/S0926-3373(02)00189-3
San N, Hatipoglu A, Kocturk G, Cinar Z: Photocatalytic degradation of 4-nitrophenol in aqueous TiO 2 suspensions, theoretical prediction of the intermediates. J. Photochem. Photobiol. A: Chem. 2002, 146: 189–197. 10.1016/S1010-6030(01)00620-7
Chen S, Cao G: Study on the photocatalytic reduction of dichromate and photocatalytic oxidation of dichlorvos. Chemosphere 2005, 60: 1308–1315. 10.1016/j.chemosphere.2005.01.056
Cun W, Jincai Z, Xinming W, Bixian M, Guoying S, Ping’an P, Jiamo F: Preparation, characterization and photocatalytic activity of nano-sized ZnO/SnO 2 coupled photocatalysts. Appl. Catal. B: Environ. 2002, 39: 269–279. 10.1016/S0926-3373(02)00115-7
Khan R, Kim TJ: Preparation and application of visible-light-responsive Ni-doped and SnO 2 -coupled TiO 2 nanocomposite photocatalysts. J. Hazardous Mater. 2009, 163: 1179–1184. 10.1016/j.jhazmat.2008.07.078
Guzman C, del Angel G, Gomez F, Galindo-Hernandez F, Angeles-Chavez C: Degradation of the herbicide 2,4-dichlorophenoxyacetic acid over Au/TiO 2 -CeO 2 photocatalysts: effect of the CeO 2 content on the photoactivity. Catal. Today 2011, 166: 146–151. 10.1016/j.cattod.2010.09.009
Kanai N, Nuida T, Ueta K, Hashimoto K, Watanabe T, Ohsaki H: Photocatalytic efficiency of TiO 2 /SnO 2 thin film stacks prepared by DC magnetron sputtering. Vaccum 2004, 74: 723–727. 10.1016/j.vacuum.2004.01.056
Wang C, Shi H, Li Y: Synthesis and characterization of natural zeolite supported Cr-doped TiO 2 photocatalysts. Appl. Surf. Sci. 2012, 258: 4328–4333. 10.1016/j.apsusc.2011.12.108
Hamadanian M, Jabbari V, Gravand A: Dependence of energy conversion efficiency of dye-sensitized solar cells on the annealing temperature of TiO 2 nanoparticles. Mater. Sci. Semicond. Process. 2012, 15: 371–379. 10.1016/j.mssp.2011.12.004
Weckhuysen B, Wachs I, Schoonheydt R: Surface chemistry and spectroscopy of chromium in inorganic oxides. Chem. Rev. 1996, 96: 3327–3350. 10.1021/cr940044o
Radecka M, Zakrzewska K, Wierzbicka M, Gorzkowska A, Komornicki S: Study of the TiO 2 -Cr 2 O 3 system for photoelectrolytic decomposition of water. Solid State Ion. 2003, 157: 379–386. 10.1016/S0167-2738(02)00236-9
Yim SD: Nam, I–S: Characteristics of chromium oxides supported on TiO 2 and Al 2 O 3 for the decomposition of perchloroethylene. J. Catal. 2004, 221: 601–611. 10.1016/j.jcat.2003.09.026
Hakuli A, Harlin ME, Backman LB, Krause AOI: Dehydrogenation of i -butane on Cr 2 O 3 /SiO 2 catalysts. J. Catal. 1999, 184: 349–356. 10.1006/jcat.1999.2468
Acknowledgements
This work is financed by the University Grants Commission, New Delhi, India (Grant no. 47–2028/11).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RB carried out the photocatalytic degradation study, SP carried out XRD characterization of samples, BP synthesized and calcined the samples, GM carried out XPS analysis, and AG drafted the manuscript and rechecked the whole manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bhosale, R., Pujari, S., Muley, G. et al. Visible-light-activated nanocomposite photocatalyst of Cr2O3/SnO2. J Nanostruct Chem 3, 46 (2013). https://doi.org/10.1186/2193-8865-3-46
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-46