Abstract
In the present study, an attempt has been made to impart antimicrobial finishing to the cellulose fabric using nano silver solution at various concentrations (5, 10, 15, 20 and 25 ml) and an eco-friendly crosslinking agent by the exhaustion technique. Curing conditions were varied, keeping curing temperatures at 110°C and 100°C and curing times to 1 and 2 min. To assess the quality of the finished fabric, various properties such as washing fastness and zone of inhibition were studied. The zones of inhibition have been studied using Escherichia coli bacteria to determine antimicrobial activity of the fabric; the surface characteristics of these fabrics have been studied by scanning electron microscopy (SEM).
In the case of the treated commercial cotton fabric product, the zones of inhibition are at a minimum of 14 mm and a maximum of 18 mm for Gram-negative bacteria. SEM study and antibacterial tests of the silver-finished fabrics indicated that, generally, silver nanoparticles were well dispersed on the fabric surface. Antibacterial test was used to estimate the biological activity of the treated fabrics, and Gram-negative bacteria (Escherichia coli) were used for this purpose. The washing fastness of finished textiles was investigated in terms of ISO 105 CO3-1982 standard. The results obtained proved the good and long-lasting bacteriostatic efficacy of silver nanoparticles applied during the finishing of cotton and viscose.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Background
In the last few years, the market for antimicrobial textiles has shown double-digit growth. This growth has been fuelled by the increased need of consumers for fresh, clean and hygienic clothing. Extensive research is taking place to develop new antimicrobial finishes. This paper reports, in detail, the mechanism of antimicrobial activity and the principles of antimicrobial finishing of textiles. Antimicrobial finishes add value to textiles and garments by providing protection in different ways.
There are different types of fungicides or bactericides such as metal salt, organic metallics, quaternary ammonium salts, formaldehyde, amines, urea, phenols and antibiotics, which have the ability to interrupt the usual metabolism of microorganism and inhibit their growth thereby imparting antibacterial and antifungal activity to cellulosic fibers. Since antibacterial method to textiles is the chemical method, antibacterial finishing via nanotechnology is used instead[1].
Nanoscale particles provide a narrow size distribution, which is required to obtain a uniform material response. Materials such as paints, pigments, electronic inks and ferrofluids, as well as advanced functional and structural ceramics, require that the particles be uniform in size and stable against agglomeration. Fine particles, particularly nanoscale particles with significant surface areas, often agglomerate to minimize the total surface or interfacial energy of the system. Although the process of using solution chemistry can be a practical route for the synthesis of both sub-micrometer and nanoscale particles of many materials, issues such as the control of size, distribution of particles, morphology, crystallinity, particle agglomeration during and after synthesis and separation of these particles from the reactant need further investigation.
Druids used silver to preserve food. American settlers put silver dollars in milk to stop spoilage. Silver leaf was used during World War I to combat infection in wounds. Human skin has many surface bacteria present at any time; that is not a bad thing[1, 2].
Antimicrobial activity of silver
The antimicrobial activity of silver has been recognised by clinicians for over 100 years. However, it is only in the last few decades that the mode of action of silver as an antimicrobial agent has been studied with any rigour.
Metallic silver is relatively unreactive; however, when exposed to aqueous environments, some ionic silver (Ag+) is released. Certain salts (e.g. silver nitrate) are readily soluble in water and have been exploited as antiseptic agents for many decades. The generation of silver ions can also be achieved through ion exchange using complexes of silver with other inorganic materials (e.g. silver-zeolite complexes; silver nanoparticles have also been demonstrated to exhibit antimicrobial properties against both bacteria and viruses with close attachment to the microbial cell/virus particles being demonstrated with activity being size dependent. Despite this, the principle activity of silver is as a result of the production of silver ions within an aqueous matrix. This therefore implies that for silver to have an antimicrobial effect, free water must be present. Silver ions interact with a number of components of both bacterial, protozoal and fungal cells. Toxicity to microbial cells is exhibited at very low concentrations with masses within the range of a few fg cell-1s being associated with bactericidal activity. The kinetics of kill vary depending on the source of silver ions with silver derived from ion-exchange processes demonstrating delayed activity compared with that derived from soluble salts. Activity appears to increase with temperature and pH. Studies have demonstrated that silver ions interact with sulfydryl (−SH) groups of proteins as well as the bases of DNA leading either to the inhibition of respiratory processes or DNA unwinding. Inhibition of cell division and damage to bacterial cell envelopes are also recorded, and interaction with hydrogen-bonding processes has been demonstrated to occur. Interruption of cell wall synthesis resulting in the loss of essential nutrients has been shown to occur in yeasts and may well occur in other fungi.
Antiviral activity of silver ions has been recorded, and interaction with -SH groups has been implicated in the mode of action. The association of silver nanoparticles with the envelope of certain viruses has been suggested to prevent them from being infective. Much of the research into the mechanism of action of silver ions has been associated with its use as a therapeutic agent especially as a topical dressing on burns. The concentration employed in and released from treated articles is significantly lower than in these applications. Under such conditions, it has been suggested that in many cases, the concentration of silver ions available following hydration of the surface of a treated article is too low to produce antimicrobial activity associated with many of the mechanisms described above. However, silver ions have been demonstrated to interact with the proteins and possibly phospholipids associated with the proton pump of bacterial membranes. This results in a collapse of the membrane proton gradient causing a disruption of many of the mechanisms of cellular metabolism and hence cell death. Silver ions clearly do not possess a single mode of action. They interact with a wide range of molecular processes within microorganisms resulting in a range of effects from the inhibition of growth, loss of infectivity to cell death. The mechanism depends on both the concentration of silver ions present and the sensitivity of the microbial species to silver. Contact time, temperature, pH and the presence of free water all impact on both the rate and extent of antimicrobial activity[3–16].
Bound type antimicrobial agents
Another set of antimicrobials with a completely different mode of action is one that bonds molecularly to the textile. This product makes the substrate surface antimicrobially active and works by rupturing the cell membrane of the microorganism when it comes into direct contact. These give durable antimicrobial properties to textiles.
Antimicrobial agent based on silver
Silver kills bacteria by strangling them in a warm and moist environment. Highly bioactive silver ions bind with proteins inside and outside bacterial cell membranes thus inhibiting cell respiration and reproduction. Silver is three to four times more active at pH 8 than at pH 6. Silver products are effective against bacteria but not as effective against other organisms like fungi, mould and mildew; they can be used with polyester where many other products cannot. Alginate and chitosan have also been used to make novel antimicrobial materials in combination with silver. Various techniques have been explored to attach silver to textile materials. To prepare antimicrobial fabrics suitable for sterilisation of air, cellulose was grafted with acrylic acid and treated with silver nitrate to bind the silver ions to the COOH group of graft copolymer. To develop a durable finish on wool, it was treated with a complexing agent such as tannic acid or ethylene diaminetetraaceticdianhydride. Thus, treated wool can react easily with copper and silver and inhibit the propagation of S. aureus and intestinal bacteria effectively. Deposition or interstitial precipitation of tetrasilver tetroxide crystals within the interstices of fibres, yarns and/or fabrics has also been reported in a US patent[17–21].
Results and discussion
Antimicrobial efficiency
From Table 1 and Figure 1, it is clear that the higher the concentration of silver and the higher the curing temperature and time, the better the zone of inhibition. Here, for Gram-negative bacteria (E. coli), the minimum zone of inhibition is 14 mm and the maximum is 18.6 mm.
Scanning electron microscopy (SEM)
The antimicrobial finished samples were observed visually, and the topography or morphology of the fabric samples was analysed by high-resolution SEM. The photographs are shown in Figure 2. From this figure, it is thoroughly possible to recognize the silver particles on cotton.
Washing fastness
Textiles are subjected to frequent washing during their use, and the requirement of durability is a very important parameter. According to Table 2, after washing, bacteria reduction of fabrics treated with nano silver gives good results.
Conclusion
The results of both fabrics applied in this work to determine the silver content in fabrics for microbiological estimation of bactericidal efficacy show that silver nanoparticles are well coupled with the fabric, indicating the long-lasting durability of such a finish against washing. The results obtained with the SEM image of cotton fabrics, and the results derived before and after finishing demonstrate that both fabrics are protected from Escherichia coli. The silver nanoparticles used in this work were well dispersed in the finishing bath nanoparticles, even in very small amounts, can provide the final product with bacteriostatic properties due to the fact that nanoscaled materials have a high surface area-to-volume ratio.
Methods
Materials
Preparation of fabric
Viscose and woven cotton fabrics of plain weave with a surface mass of 100 g/m2 underwent
-
a)
Desizing. The grey fabric is desized with 5 gpl enzyme and 10 gpl common salt at 60°C for 2 h.
-
b)
Scouring. The desized fabric is treated with 2.5% sodium hydroxide, 1.5% sodium carbonate and 0.5% NP - at 100°C boiling point for 6 h.
-
c)
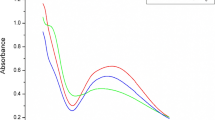
Bleaching. The scoured fabric is bleached with 1% H2O2 (50%) at 80°C for 1 h. Silver nanoparticles, produced by Sigma-Aldrich (St. Louis, MO, USA), for crosslink nano silver with viscose and cotton 20.5 cm3 were added to the finishing bath according to the fabric treatment graph in Figure 3.
Antibacterial finishing of textiles
Escherichia coli (Gram-negative bacterium internal name: LOCK 0836 1998) was used for the evaluation of antimicrobial properties. The agar diffusion test is a method of testing the effectiveness of estimated antimicrobial activity of AATCC6538 (Figure 1). Colonies of bacteria recovered on the agar plate were counted, and the reduction percent of bacteria (R) was calculated using the following equation:
where A is the number of bacteria colonies from the treated specimen after inoculation over a 24-h contact period and B is the number of bacteria colonies from untreated control specimen after inoculation at 0 contact time.
Scanning electron microscopy (SEM)
The antimicrobial finished samples were observed visually, and the topography or morphology of the fabric samples was analysed by high-resolution SEM.
Washing fastness test
To evaluate the durability of the antibacterial effect after washing, the treated fabrics were cut into the size of 7.5 × 13.5 cm and washed according to the standard ISO 105 CO3-1982 test method.
References
Burnision N, Bygott C, Stratton J: Nano Technology Meets TiO 2 . Surface Coating International Part A 2004, 179–814.
Fei B, Deng Z, Xin JH, Xhang Y, Pang G: Room temperature synthesis of rutile nanorods and their applications on cloth. Nanotechnol 2006, 17: 1927–1932. 10.1088/0957-4484/17/8/021
Brown MRW, Anderson RA: The bactericidal effect of silver ions on Pseudmonas aeruginosa . J. Pharm. Pharacol. 1968,20(Supplement):1–3.
Im K, Takasaki Y, Endo A, Kuriyama M: Antibacterial activity of A-type zeolite supporting silver ions in distilled water. J. Antibacterial. Antifungal. Agents 1996, 24: 269–274.
Sondi I, Salopek-Sondi B: Silver nanoparticles as antimicrobial agent: a case study on E coli as a model for gram-negative bacteria. J. Colloid Interface Sci. 2004, 275: 177–182. 10.1016/j.jcis.2004.02.012
Elchiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH, Yacaman MJ: Interaction of silver nanoparticles with HIV-I. J. Nanobiotechnology 2005, 3: 6. 10.1186/1477-3155-3-6
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ: The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16: 2346–2353. 10.1088/0957-4484/16/10/059
McDonnell G, Denver R: Antiseptics and disinfectants: activity, action and resistance. Clinical. Microbiol. Reviews. 1999,12(1):147–179.
Matsumura Y, Yoshikata K, Kunisaki S-I, Tsuschido T: Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. App. Env. Micro. 2003,69(7):4278–4281. 10.1128/AEM.69.7.4278-4281.2003
Bragg PD, Rannie DJ: The effect of silver ions on the respiratory chain of E coli . Can J Microbiol 1974, 20: 883–889. 10.1139/m74-135
Batarseh KI: Anomaly and correlation of killing in the therapeutic properties of silver (I) chelation with glutamic and tartaric acids. J. Antimicrobial Chemotherapy 2004, 54: 546–548. 10.1093/jac/dkh349
Richards RME, Taylor RB, Xing DKL: Effect of silver on whole cells and speroplasts of a silver resistant Pseudomonas aeruginosa . Microbios 1984, 39: 151–158.
Russell AD, Hugo WB: Antimicrobial activity and action of silver. Prog. Med. Chem. 1994, 31: 351–371.
Wells TN, Scully P, Paravicini G, Proudfoot AE, Payton MA: Mechanism of irreversible inactivation of phosphomannose isomerases by silver ions and flamazine. Biochemistry 1995,34(24):896–903.
Thurmann RB, Gerba CP: The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. Crit. Rev. Environ. Control. 1989, 18: 295–315. 10.1080/10643388909388351
Dibrov P, Dzioba J, Gosink KK, Hase CC: Chemiosmotic mechanism of antimicrobial activity of Ag+ in Vibrio cholerae . Antimicrob. Agents Chemother. 2002,46(8):2668–2670. 10.1128/AAC.46.8.2668-2670.2002
Achwal WB: Antimicrobial finishes and their modifications. Colourage. 2003,50(1):58–59.
Gulrajani ML: Cold plasma an environment friendly textile processing medium. Asian Dyer 2004, 39–41.
Qin Y: Novel antimicrobial fibres. Textile Magazine. 2004,31(2):14–17.
Freddi G, Arai T, Colonna GM, Boschi A, Tsukada M: Binding of metal cations to chemically modified wool and antimicrobial properties of the wool–metal complexes. J. Appl. Polym. Sci. 2001, 82: 3513–3519. 10.1002/app.2213
Antelman MS: High performance silver (I,III) oxide antimicrobial textile articles. US Patent 6,436,420, 20 August 2002 US Patent 6,436,420, 20 August 2002
Acknowledgements
The authors want to thank Prof. Omid Moradi and the editor Sherlyn C. Machica for correcting the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MM (Mohammad) designed the experiments and revised the manuscript. NY studied the literature and helped MM (Marjan) to fabricate the specimens and perform the tests. Both of them drafted the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Mirjalili, M., Yaghmaei, N. & Mirjalili, M. Antibacterial properties of nano silver finish cellulose fabric. J Nanostruct Chem 3, 43 (2013). https://doi.org/10.1186/2193-8865-3-43
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-43